Published online Nov 6, 2013. doi: 10.5527/wjn.v2.i4.103
Revised: October 10, 2013
Accepted: October 19, 2013
Published online: November 6, 2013
Processing time: 57 Days and 11.2 Hours
Primary focal and segmental glomerulosclerosis (FSGS) may be due to genetic or acquired etiologies and is a common cause of nephrotic syndrome with high morbidity that often leads to end-stage renal failure. The different available therapeutic approaches are unsuccessful, in part due to partially deciphered heterogeneous and complex pathophysiological mechanisms. Moreover, the term FSGS, even in its primary form, comprises a histological description shared by a number of different causes with completely different molecular pathways of disease. This review focuses on the latest developments regarding the pathophysiology of primary acquired FSGS caused by soluble factor urokinase type plasminogen activator receptor, a circulating permeability factor involved in proteinuria and edema formation, and describes recent advances with potential success in therapy.
Core tip: Primary acquired focal and segmental glomerulosclerosis is a frequent cause of nephrotic syndrome with no specific treatment. New discoveries in its pathophysiolohy have revealed that a podocyte permeability factor named soluble urokinase plasminogen activator receptor (suPAR) may be involved in the development of proteinuria and edema formation. This effect is supposed to be achieved by its interaction with podocyte integrins and subsequent cell contraction. Moreover, suPAR also activates water and sodium retention in this disease. Interestingly, plasmin mediates both effects. Amiloride is postulated to interfere with suPAR proteinuric actions.
- Citation: Trimarchi H. Primary focal and segmental glomerulosclerosis and soluble factor urokinase-type plasminogen activator receptor. World J Nephrol 2013; 2(4): 103-110
- URL: https://www.wjgnet.com/2220-6124/full/v2/i4/103.htm
- DOI: https://dx.doi.org/10.5527/wjn.v2.i4.103
Focal and segmental glomerulosclerosis (FSGS) is a major cause of chronic kidney disease in children and adults[1-3]. It can occur as a primary disorder (called primary acquired FSGS), as a consequence of genetic mutations in podocyte-specific proteins (also called primary genetic FSGS) or as a secondary disorder[4,5]. In recent years, much of the progress obtained in unraveling the pathophysiological events in FSGS has been focused primarily on the identification of genetic mutations of membrane and podocyte slit diaphragm proteins and on immune factors, but the real identity of the primary acquired variant apparently caused by circulating permeability factors remains elusive. In this regard, the role of these permeability factors in the pathogenesis of proteinuria has also shown progress in recent years. The soluble factor urokinase type plasminogen activator receptor (suPAR) has become one of the most studied permeability factors with potential involvements in FSGS. It is supposed to be responsible for the contraction of podocytes and its eventual detachment from the glomerular basement membrane, denuding it and causing proteinuria in the majority of primary acquired cases of FSGS[6]. However, this phenomenon is not shared by others, who question whether elevated levels of suPAR are indeed pathogenic, or just a mere marker of a split urokinase-type plasminogen activator (uPAR) (CD87) molecule. Moreover, in other clinical situations in which suPAR is elevated, proteinuria does not occur[7-9]. It is not a specific marker of FSGS, as in other glomerulopathies suPAR levels are also high; in addition, after FSGS post-transplant recurrence elevated suPAR levels are not always encountered[7-9]. Finally some authors state that is not the plasmatic but the urinary presence of suPAR the real culprit of primary acquired FSGS[9].
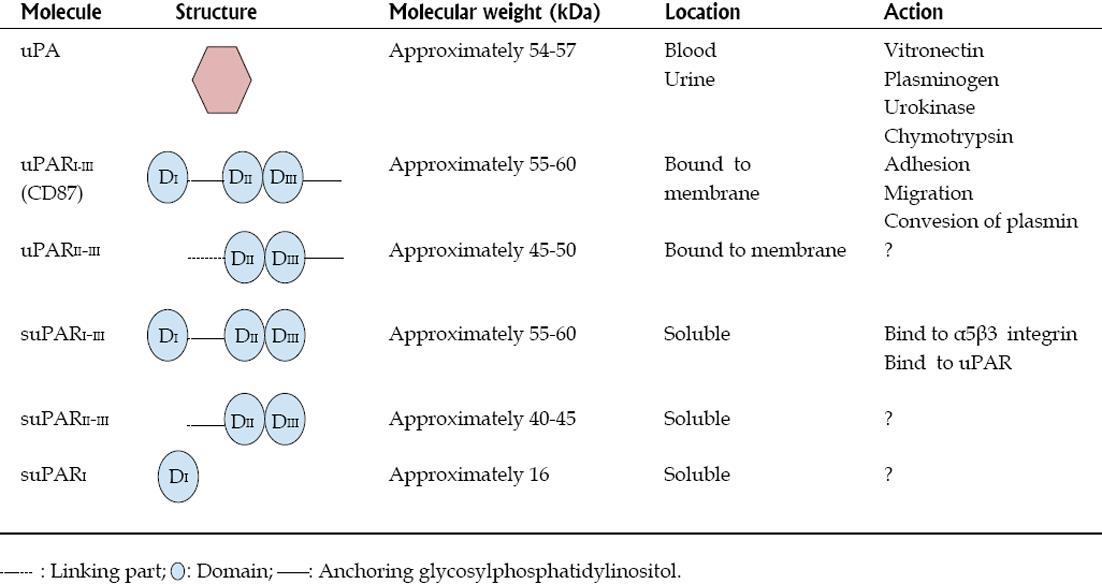
Urokinase receptors, expressed on the cell surface of various cells, are committed to the pericellular proteolysis of plasminogen, are essential for the remodeling of the extracellular matrix, and are involved in vasculogenesis and cell migration processes[10]. The urokinase receptor, also known as uPAR (urokinase-type plasminogen activator) is a membrane bound protein linked to glycosylphosphatidylinositol (GPI) of about 45-55 kDa (Figure 1)[10,11]. UPAR consists of three domains (DI, DII and DIII) and is present in various immunologically active cells, including monocytes, macrophages and activated T cells, and also in endothelial cells, keratinocytes, fibroblasts, smooth muscle cells, megakaryocytes, certain cells tumor, podocytes and renal tubular cells[12-18]. It therefore follows that suPAR is not a specific marker, although in the context of high circulating levels in a nephrotic patient with FSGS, it suggests a leading role as a permeability factor[8]. UPAR can be cleaved not only at the portion of the GPI-anchored protein to the cell membrane, but also in the inner part of the receptor itself (for example, in the connection region between DI and DII-III), giving rise to various soluble forms of suPAR with different molecular weights (Figure 1). The most common form of soluble suPAR originates from the cleavage and release of membrane-bound uPAR, detaching the membrane anchoring compound GPI, and is present in plasma, urine and cerebrospinal fluid in different concentrations depending on the level of activation of the immune system[19-22] (Figure 1). It has also been documented the existence of the whole molecule of suPAR in serum from healthy individuals and of two truncated soluble forms of the entire molecule (suPARI and suPARII-III) in the urine[23] (Figure 1).
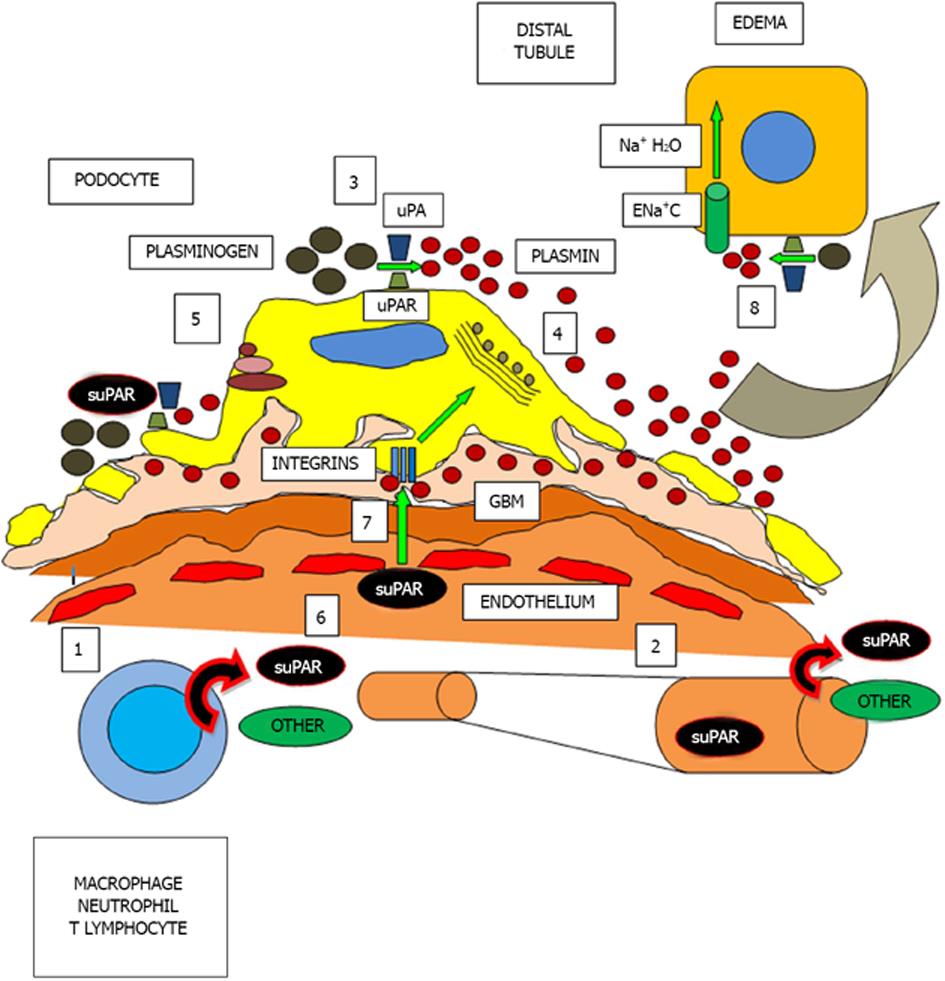
UPAR can be activated by various molecules, such as uPA (urokinase-type plasminogen activator, or simply urokinase), plasminogen, chymotrypsin, various metalloproteinases and some elastases[24-27]. Studies are generally based on the action of these molecules on the uPAR, but as SuPAR barely shares the same structure as uPAR, these proteases are also likely to cleave suPAR fragments. Furthermore, once activated, suPAR or uPAR are capable of catalyzing the conversion of plasminogen to plasmin, an important molecule in fibrinolytic processes and in the activation of several matrix metalloproteinases, in the recycling and degradation of the extracellular matrix, in cell activation, migration, contraction, vasculogenesis and in vitronectin degradation[10,28-32]. This phenomenon may occur in plasma, on the podocyte surface or in renal distal tubular cells[16,17] (Figure 2).
SuPAR whole molecule ( suPARI-III) consists of three domains (DI, DII and DIII) of uPAR , as mentioned previously but lacks the GPI anchor protein; however, the I-III portion of suPAR can compete with uPARI-III for Upa[33] (Figure 1). Another agonist of UPAR is vitronectin, the main antagonist of plasminogen activator inhibitor type-1 (PAI-1), the most important physiological inhibitor of tissue plasminogen activator and urokinase (uPA)[10]. Thus, vitronectin can accomplish its adherent and fibrinolytic actions increasing plasminogen and plasmin levels by two independent pathways: blocking PAI-1 and activating uPAR. Furthermore, vitronectin achieves its adherent action to the cell matrix through integrins, particularly those that possess the α5 domain[10]. While this point will be addressed below, it is worth to mention that patients with nephrotic syndrome present elevated serum levels of plasminogen and plasmin[34]. In turn, after being filtered, urinary plasminogen is converted to plasmin by podocyte or distal renal tubular epithelial uPA/uPAR; at this distal location, plasmin has been reported to function as a regulator of water and sodium absorption, a key event in the pathogenesis of edema in nephrotic syndrome, and also as a mediator in calcium tubular transport[17,35,36].
Cell migration across the endothelium and into tissues is a critical component in inflammation, in immune responses against infections, and in tissue repair and remodeling after injury. The UPA/uPAR system is directly involved in these mechanisms of adhesion, migration and chemotaxis[18,31]. For example, the adhesion and migration of monocytes involves a functional interaction between cellular uPAR and matrix integrins[37] and in uPAR-dependent changes in integrin-mediated adhesion to fibrinogen, collagen and vitronectin[10,38,39]. It is known that uPAR is needed to activate the integrin α5β3 in podocytes, which promotes cell motility and activation of small GTPases that control cell division, as Cdc4240. If α5β3 integrin is activated, the podocyte contracts and proteinuria ensues. However, it is believed that suPAR has inhibitory properties on adhesion uPAR dependent migration but not on cell contraction. Thus, it would be able to interact with α5β3 integrin, vitronectin or plasmin[18,40]. Finally, it has been shown that suPARII-III is a chemotactic agent[41,42], and its circulating levels reflect the activation status of the immune system[18].
Abnormally high circulating levels of suPAR have been associated with the pathogenesis of acquired primary FSGS, since approximately two thirds of patients with acquired FSGS have increased circulating levels of suPAR[6]; suPAR would then bind to and activate α5β3 integrin in podocytes by a lipid-dependent mechanism[16], leading to alterations in the morphology and dynamics of the metabolism of podocytes and foot process effacement, detachment and podocyturia, finally resulting in proteinuria and the beginning of glomerulosclerosis, nephrotic syndrome and renal insufficiency[16,43].
What is the cellular origin of this increased membrane uPAR and circulating suPAR in FSGS? Wei et al[16] suggest that neutrophils and monocytes may be culprits, but another possibility lies in circulating lymphocyte T cells, since there is an association between T-cell activation and systemic proteinuria. In turn, as mentioned previously, not in all cases of idiopathic acquired FSGS circulating levels of suPAR have been increased. This is another confirmation that the mere histologic FSGS description is not a disease but a form of kidney damage characterized by common histopathological features but with completely different pathophysiological pathways. Even within the primary FSGS scenario, and even more, within the primary FSGS circulating factors, more than one peptide may cause damage to the glomerular basement membrane. In this regard, other described permeability factors are angiopoetin-4 and vascular endothelial growth factor (VEGF), both secreted by the podocyte, operating in autocrine or paracrine fashions[43-45]. In addition, plasma and urinary levels of CD80 from T cells due to a lymphocyte-podocyte interaction, are elevated in primary acquired FSGS; CD80 could potentially contribute to the diagnosis and serve as a potential marker of damage in FSGS, being another potential tool to help distinguish clinically primary FSGS from minimal change nephropathy at the initial steps of the disease. In minimal change nephropathy, hemopexin may be the main permeability factor[46,47]. Another molecule that has been identified in primary acquired recurrent FSGS is CLC-1 (cardiotrophin type-1 cytokine), a member of the family of interleukin (IL)-6, and which is present in the plasma of patients with active disease. CLC-1 decreases the expression of nephrin in glomeruli and cultured podocytes, and CLC-1 concentration in the circulation of patients with recurrent FSGS can be up to 100 times higher than in normal subjects[48]. To make matters more difficult to understand in primary acquired FSGS, suPAR activity has been identified in recurrence after kidney transplantation in some patients with concomitant genetic mutations in podocyte proteins[49,50]. One can only speculate on the relationship between mutations and the coexistence of podocyte permeability circulating factors in this setting. It may be that the occurrence of both phenomena is attributable just to mere coincidence, or that genetic abnormalities in podocytes may cause subsequent structural local damage and inflammation inducing leukocyte stimulation via the uPAR, ending with the secretion of molecules with permeability actions, giving rise to severe kidney recurrent disease[48].
Is there any relationship between the etiology of minimal change nephropathy and that of primary focal segmental sclerosis? If FSGS is of genetic origin, the link would be none. If chronic minimal change nephropathy leads to an inflammatory state that induces focal sclerosis histological changes, this morphology would be of secondary origin and have no connotation with primary acquired FSGS. If a causal factor it is to be established as a primary permeability factor in minimal change nephropathy, hemopexin would be the first candidate. Hemopexin is a protease which activates protein kinase B and the small GTPase RhoA (ras homolog gene family, member A) and induces a nephrin-dependent reorganization of the actin cytoskeleton in cultured podocytes[51]; reduces endothelial glycocalyx and increases the albumin diffusion through glomerular endothelial cell monolayers[51]. Hemopexin injection in rats causes proteinuria and glomerular changes characteristic of minimal change nephropathy[48,52]. Another candidate is vascular permeability factor (VPF). VPF is a lymphokine that is produced by T lymphocytes stimulated by concanavalin A of patients with idiopathic nephrotic syndrome. VPF acts on systemic capillary glomerular basement membrane[53]. Its secretion is enhanced by IL-2, IL-15, IL-12, and IL-18 is inhibited by transforming growth factor-β1[54] and causes a histological damage identical with minimal change nephropathy[48]. Whether two or more factors such as the suPAR permeability can coexist in these situations has not been reported. Finally, in the early course of idiopathic nephrotic syndrome, histological changes may not be present even at the ultrastructural level, in turn making more problematic and difficult the distinction between minimal change nephropathy and primary FSGS. The histology of primary FSGS caused by a permeability factor compared to that caused by a mutation (podocytopathy) is indistinguishable at early stages, although in the latter focal ultrastructural microscopic damage may be seen first. Moreover, none of the permeability factors mentioned in the case of minimal change nephropathy or FSGS are currently measured in clinical grounds. In the future, samples of blood or urine may be part of a diagnostic panel.
As to treatment, to date no randomized controlled trials of sufficient numbers of patients are available to provide robust information so as to guide us in the treatment of primary FSGS in native kidneys or in renal allografts. Current treatment results in complete or partial remissions in approximately 50% of cases. Treatment approaches that have been used to date include corticosteroids with or without cyclophosphamide[55,56], cyclosporine[57], mycophenolate[58], rituximab[59,60] and plasmaphresis[61,62]. When proteinuria is reduced by these agents or by non-specific drugs as angiotensin converting enzyme inhibitors, angiotensin II receptor blockers, statins, antiaggregants and/or reduction of salt intake, the progression of renal dysfunction is slowed[63,64]. Regardless of the debates that arise about the true etiology of nephrotic syndrome in primary FSGS, current and proposed therapies include strategies such as the identification and reversal of the primary cause of renal injury (usually not possible), the decrease in proteinuria by interventions related to hemodynamic factors, and retarding renal fibrosis by the action of nonspecific agents (Figure 2).
In a study by Wei et al[6] in which blood samples of 164 pediatric and adult patients with primary steroid-resistant FSGS were analyzed and suPAR concentrations were measured, the main conclusions arrived by the authors were that circulating suPAR levels were significantly elevated in most patients with primary FSGS in both groups; 84.3% of patients in the American cohort (CT) and 55.3% of those belonging to the European group (PodoNet) had elevated suPAR levels; high suPAR levels were not associated with systemic inflammatory phenomena according to C-Reactive Protein titers as did not differ from controls, treatment with mycophenolate/dexamethasone was associated with lower circulating suPAR levels in comparison to those treated with cyclosporine A; a sustained decrease in suPAR levels over the course of 26 wk of treatment was associated with a reduction in proteinuria and more likely to accomplish complete remission; suPAR serum levels were higher in the familial cases, including those with a genetic disorder, as in the diagnosis of a podocin mutation (podocytopathy in coexistence with elevated suPAR levels)[6]. The fact that in 15%-45% of patients in both groups had normal levels of suPAR shows that primary FSGS is a heterogeneous disorder which additional factors contributing to the renal damage and to proteinuria. It is possible that patients with primary FSGS express higher levels of suPAR in response to a certain pathological stimulus with independent features or related to a primary inflammatory instigator[6].
An agreed cut-off level of suPAR is another controversial issue. Gao et al[65] proposed 3000 pg/mL as the cut-off level for the population with primary FSGS, since in a previous study of a normal population the cut-offs level was set at 2710 pg/mL. As therapies, chronic plasmapheresis and plasma adsorption of suPAR are supporting treatments that can help maintain normal suPAR blood levels, which would lead to lower podocyte damage and partial resolution of nephrotic syndrome with a possible slowing of progression to renal failure[61,62]. Cyclosporine may be useful to stabilize the podocyte by inhibiting synaptopodin dephosphorylation; therefore, synaptopodin interaction with actin would be blocked, and the podocyte contraction abrogated[66]. Salomon et al found cyclosporine trough levels between 250 and 300 ng/mL suffice to obtain a rapid remission in proteinuria (average intravenous dose 3 mg/kg per day)[67], although the treatment is generally cyclosporine-dependent and may lead to chronic renal damage[68-70]. Rituximab may be another option in refractory cases, not only due to its action by decreasing the population of CD20 lymphocytes, but also because it would bind other podocyte molecules as protein SMPDL-3b (sphingomyelin phosphodiesterase acid-like 3b). In primary FSGS, this molecule (acting on the remodeling of podocyte actin) is decreased. Rituximab levels would increase SMPDL-3b concentrations, stabilizing the podocyte[71].
A recent study has shown that podocyte uPAR expression can be reduced using amiloride. Amiloride plays a significant role in reducing podocyte cell motility in vitro and proteinuria in mice[72]. Amiloride inhibits the synthesis of uPAR and uPAR mRNA and consequently the α5β3 integrin activation mediated by uPAR. The reduced uPAR pool would translate in a lower suPAR concentration. Amiloride capacity to inhibit uPAR synthesis and suPAR secretion by T lymphocytes should be of particular interest in FSGS, because blocking their activation would inhibit α5β3 integrin activation and the development of proteinuria with final renal dysfunction[16,73]. Furthermore, amiloride may further decrease proteinuria by acting on the distal nephron in ENaC channels, as nephrotic range proteinuria stimulates the activity of these channels by promoting the reabsorption of sodium and water[17]. Tubular plasmin, already high in patients with nephrotic syndrome, would act as the mediator in sodium and water reabsorption and amiloride may inhibit its action by blocking uPAR[17,34,72,74] (Figure 2). This would be another additional and relevant non-immunosuppressive strategy contributing to the fall in proteinuria, if tolerated hemodynamically and no hyperkalemia ensues.
In summary, these observations aim to explain the possibility that circulating suPAR is the most prominent factor in the pathophysiology of primary acquired FSGS due to the encountered high levels in blood and urine, activating α5β3 integrin, contracting the podocyte and causing the proteinuria, and acting on the water and sodium reabsorption at the distal tubular. Moreover, it explains the importance of urokinase and its receptor uPAR play in cell adhesion and migration, being plasmin the final effector. Whether suPAR causes a rise in plasminogen and plasmin levels, and the consequent final action on the podocyte integrins and renal distal tubular cell, in primary acquired FSGS both proteinuria and edema would have suPAR as the trigger for plasmin activation, a final effector, and amiloride as a potential novel adjunct antiproteinuric agent in this complex nephropathy.
P- Reviewers: Tanaka H, Watanabe T S- Editor: Cui XM L- Editor: A E- Editor: Yan JL
| 1. | Benchimol C. Focal segmental glomerulosclerosis: pathogenesis and treatment. Curr Opin Pediatr. 2003;15:171-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Korbet SM. Treatment of primary focal segmental glomerulosclerosis. Kidney Int. 2002;62:2301-2310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 55] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Boyer O, Moulder JK, Somers MJ. Focal and segmental glomerulosclerosis in children: a longitudinal assessment. Pediatr Nephrol. 2007;22:1159-1166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Barisoni L, Schnaper HW, Kopp JB. Advances in the biology and genetics of the podocytopathies: implications for diagnosis and therapy. Arch Pathol Lab Med. 2009;133:201-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 5. | Santín S, Bullich G, Tazón-Vega B, García-Maset R, Giménez I, Silva I, Ruíz P, Ballarín J, Torra R, Ars E. Clinical utility of genetic testing in children and adults with steroid-resistant nephrotic syndrome. Clin J Am Soc Nephrol. 2011;6:1139-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 158] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 6. | Wei C, Trachtman H, Li J, Dong C, Friedman AL, Gassman JJ, McMahan JL, Radeva M, Heil KM, Trautmann A. Circulating suPAR in two cohorts of primary FSGS. J Am Soc Nephrol. 2012;23:2051-2059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 167] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 7. | Maas RJ, Deegens JK, Wetzels JF. Serum suPAR in patients with FSGS: trash or treasure? Pediatr Nephrol. 2013;28:1041-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 8. | Naesens M, Meijers B, Sprangers B. suPAR and FSGS: the gap between bench and bedside. Transplantation. 2013;96:368-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Franco Palacios CR, Lieske JC, Wadei HM, Rule AD, Fervenza FC, Voskoboev N, Garovic VD, Zand L, Stegall MD, Cosio FG. Urine but not serum soluble urokinase receptor (suPAR) may identify cases of recurrent FSGS in kidney transplant candidates. Transplantation. 2013;96:394-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 10. | Wei Y, Waltz DA, Rao N, Drummond RJ, Rosenberg S, Chapman HA. Identification of the urokinase receptor as an adhesion receptor for vitronectin. J Biol Chem. 1994;269:32380-32388. [PubMed] |
| 11. | Ploug M, Rønne E, Behrendt N, Jensen AL, Blasi F, Danø K. Cellular receptor for urokinase plasminogen activator. Carboxyl-terminal processing and membrane anchoring by glycosyl-phosphatidylinositol. J Biol Chem. 1991;266:1926-1933. [PubMed] |
| 12. | de Bock CE, Wang Y. Clinical significance of urokinase-type plasminogen activator receptor (uPAR) expression in cancer. Med Res Rev. 2004;24:13-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 124] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 13. | Estreicher A, Mühlhauser J, Carpentier JL, Orci L, Vassalli JD. The receptor for urokinase type plasminogen activator polarizes expression of the protease to the leading edge of migrating monocytes and promotes degradation of enzyme inhibitor complexes. J Cell Biol. 1990;111:783-792. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 339] [Cited by in RCA: 360] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 14. | Florquin S, van den Berg JG, Olszyna DP, Claessen N, Opal SM, Weening JJ, van der Poll T. Release of urokinase plasminogen activator receptor during urosepsis and endotoxemia. Kidney Int. 2001;59:2054-2061. [PubMed] |
| 15. | Grøndahl-Hansen J, Lund LR, Ralfkiaer E, Ottevanger V, Danø K. Urokinase- and tissue-type plasminogen activators in keratinocytes during wound reepithelialization in vivo. J Invest Dermatol. 1988;90:790-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 167] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 16. | Wei C, Möller CC, Altintas MM, Li J, Schwarz K, Zacchigna S, Xie L, Henger A, Schmid H, Rastaldi MP. Modification of kidney barrier function by the urokinase receptor. Nat Med. 2008;14:55-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 423] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 17. | Svenningsen P, Bistrup C, Friis UG, Bertog M, Haerteis S, Krueger B, Stubbe J, Jensen ON, Thiesson HC, Uhrenholt TR, Jespersen B, Jensen BL, Korbmacher C, Skøtt O. Plasmin in nephrotic urine activates the epithelial sodium channel. J Am Soc Nephrol. 2009;20:299-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 203] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 18. | Thunø M, Macho B, Eugen-Olsen J. suPAR: the molecular crystal ball. Dis Markers. 2009;27:157-172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 213] [Reference Citation Analysis (0)] |
| 19. | Huai Q, Mazar AP, Kuo A, Parry GC, Shaw DE, Callahan J, Li Y, Yuan C, Bian C, Chen L. Structure of human urokinase plasminogen activator in complex with its receptor. Science. 2006;311:656-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 227] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 20. | Sier CF, Sidenius N, Mariani A, Aletti G, Agape V, Ferrari A, Casetta G, Stephens RW, Brünner N, Blasi F. Presence of urokinase-type plasminogen activator receptor in urine of cancer patients and its possible clinical relevance. Lab Invest. 1999;79:717-722. [PubMed] |
| 21. | Stephens RW, Pedersen AN, Nielsen HJ, Hamers MJ, Høyer-Hansen G, Rønne E, Dybkjaer E, Danø K, Brünner N. ELISA determination of soluble urokinase receptor in blood from healthy donors and cancer patients. Clin Chem. 1997;43:1868-1876. [PubMed] |
| 22. | Ostergaard C, Benfield T, Lundgren JD, Eugen-Olsen J. Soluble urokinase receptor is elevated in cerebrospinal fluid from patients with purulent meningitis and is associated with fatal outcome. Scand J Infect Dis. 2004;36:14-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 60] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Sidenius N, Sier CF, Blasi F. Shedding and cleavage of the urokinase receptor (uPAR): identification and characterisation of uPAR fragments in vitro and in vivo. FEBS Lett. 2000;475:52-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 87] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 24. | Andersen O, Eugen-Olsen J, Kofoed K, Iversen J, Haugaard SB. Soluble urokinase plasminogen activator receptor is a marker of dysmetabolism in HIV-infected patients receiving highly active antiretroviral therapy. J Med Virol. 2008;80:209-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 110] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 25. | Cunningham O, Andolfo A, Santovito ML, Iuzzolino L, Blasi F, Sidenius N. Dimerization controls the lipid raft partitioning of uPAR/CD87 and regulates its biological functions. EMBO J. 2003;22:5994-6003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 103] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 26. | Fazioli F, Resnati M, Sidenius N, Higashimoto Y, Appella E, Blasi F. A urokinase-sensitive region of the human urokinase receptor is responsible for its chemotactic activity. EMBO J. 1997;16:7279-7286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 176] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 27. | Høyer-Hansen G, Ploug M, Behrendt N, Rønne E, Danø K. Cell-surface acceleration of urokinase-catalyzed receptor cleavage. Eur J Biochem. 1997;243:21-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 84] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Beaufort N, Leduc D, Rousselle JC, Magdolen V, Luther T, Namane A, Chignard M, Pidard D. Proteolytic regulation of the urokinase receptor/CD87 on monocytic cells by neutrophil elastase and cathepsin G. J Immunol. 2004;172:540-549. [PubMed] |
| 29. | Ossowski L, Aguirre-Ghiso JA. Urokinase receptor and integrin partnership: coordination of signaling for cell adhesion, migration and growth. Curr Opin Cell Biol. 2000;12:613-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 283] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 30. | Chapman HA. Plasminogen activators, integrins, and the coordinated regulation of cell adhesion and migration. Curr Opin Cell Biol. 1997;9:714-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 353] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 31. | Blasi F. uPA, uPAR, PAI-1: key intersection of proteolytic, adhesive and chemotactic highways? Immunol Today. 1997;18:415-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 179] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 32. | Waltz DA, Natkin LR, Fujita RM, Wei Y, Chapman HA. Plasmin and plasminogen activator inhibitor type 1 promote cellular motility by regulating the interaction between the urokinase receptor and vitronectin. J Clin Invest. 1997;100:58-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 207] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 33. | Behrendt N, Ploug M, Patthy L, Houen G, Blasi F, Danø K. The ligand-binding domain of the cell surface receptor for urokinase-type plasminogen activator. J Biol Chem. 1991;266:7842-7847. [PubMed] |
| 34. | Vaziri ND, Gonzales EC, Shayestehfar B, Barton CH. Plasma levels and urinary excretion of fibrinolytic and protease inhibitory proteins in nephrotic syndrome. J Lab Clin Med. 1994;124:118-124. [PubMed] |
| 35. | Tudpor K, Laínez S, Kwakernaak AJ, Kovalevskaya NV, Verkaart S, van Genesen S, van der Kemp A, Navis G, Bindels RJ, Hoenderop JG. Urinary plasmin inhibits TRPV5 in nephrotic-range proteinuria. J Am Soc Nephrol. 2012;23:1824-1834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 36. | Andersen RF, Buhl KB, Jensen BL, Svenningsen P, Friis UG, Jespersen B, Rittig S. Remission of nephrotic syndrome diminishes urinary plasmin content and abolishes activation of ENaC. Pediatr Nephrol. 2013;28:1227-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 37. | May AE, Kanse SM, Lund LR, Gisler RH, Imhof BA, Preissner KT. Urokinase receptor (CD87) regulates leukocyte recruitment via beta 2 integrins in vivo. J Exp Med. 1998;188:1029-1037. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 200] [Cited by in RCA: 203] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 38. | Wei Y, Yang X, Liu Q, Wilkins JA, Chapman HA. A role for caveolin and the urokinase receptor in integrin-mediated adhesion and signaling. J Cell Biol. 1999;144:1285-1294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 307] [Cited by in RCA: 313] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 39. | Wei Y, Eble JA, Wang Z, Kreidberg JA, Chapman HA. Urokinase receptors promote beta1 integrin function through interactions with integrin alpha3beta1. Mol Biol Cell. 2001;12:2975-2986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 187] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 40. | Welsh GI, Saleem MA. The podocyte cytoskeleton--key to a functioning glomerulus in health and disease. Nat Rev Nephrol. 2012;8:14-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 196] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 41. | Resnati M, Guttinger M, Valcamonica S, Sidenius N, Blasi F, Fazioli F. Proteolytic cleavage of the urokinase receptor substitutes for the agonist-induced chemotactic effect. EMBO J. 1996;15:1572-1582. [PubMed] |
| 42. | Resnati M, Pallavicini I, Wang JM, Oppenheim J, Serhan CN, Romano M, Blasi F. The fibrinolytic receptor for urokinase activates the G protein-coupled chemotactic receptor FPRL1/LXA4R. Proc Natl Acad Sci U S A. 2002;99:1359-1364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 276] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 43. | Shankland SJ, Pollak MR. A suPAR circulating factor causes kidney disease. Nat Med. 2011;17:926-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 44. | Sison K, Eremina V, Baelde H, Min W, Hirashima M, Fantus IG, Quaggin SE. Glomerular structure and function require paracrine, not autocrine, VEGF-VEGFR-2 signaling. J Am Soc Nephrol. 2010;21:1691-1701. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 231] [Cited by in RCA: 220] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 45. | Clement LC, Avila-Casado C, Macé C, Soria E, Bakker WW, Kersten S, Chugh SS. Podocyte-secreted angiopoietin-like-4 mediates proteinuria in glucocorticoid-sensitive nephrotic syndrome. Nat Med. 2011;17:117-122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 268] [Cited by in RCA: 235] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 46. | Garin EH, Diaz LN, Mu W, Wasserfall C, Araya C, Segal M, Johnson RJ. Urinary CD80 excretion increases in idiopathic minimal-change disease. J Am Soc Nephrol. 2009;20:260-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 123] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 47. | Garin EH, Mu W, Arthur JM, Rivard CJ, Araya CE, Shimada M, Johnson RJ. Urinary CD80 is elevated in minimal change disease but not in focal segmental glomerulosclerosis. Kidney Int. 2010;78:296-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 129] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 48. | McCarthy ET, Sharma M, Savin VJ. Circulating permeability factors in idiopathic nephrotic syndrome and focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 2010;5:2115-2121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 231] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 49. | Ghiggeri GM, Aucella F, Caridi G, Bisceglia L, Ghio L, Gigante M, Perfumo F, Carraro M, Gesualdo L. Posttransplant recurrence of proteinuria in a case of focal segmental glomerulosclerosis associated with WT1 mutation. Am J Transplant. 2006;6:2208-2211. [PubMed] |
| 50. | Srivastava T, Garola RE, Kestila M, Tryggvason K, Ruotsalainen V, Sharma M, Savin VJ, Jalanko H, Warady BA. Recurrence of proteinuria following renal transplantation in congenital nephrotic syndrome of the Finnish type. Pediatr Nephrol. 2006;21:711-718. [PubMed] |
| 51. | Lennon R, Singh A, Welsh GI, Coward RJ, Satchell S, Ni L, Mathieson PW, Bakker WW, Saleem MA. Hemopexin induces nephrin-dependent reorganization of the actin cytoskeleton in podocytes. J Am Soc Nephrol. 2008;19:2140-2149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 96] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 52. | Bakker WW, Borghuis T, Harmsen MC, van den Berg A, Kema IP, Niezen KE, Kapojos JJ. Protease activity of plasma hemopexin. Kidney Int. 2005;68:603-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 53. | Lagrue G, Xheneumont S, Branellec A, Hirbec G, Weil B. A vascular permeability factor elaborated from lymphocytes. I. Demonstration in patients with nephrotic syndrome. Biomedicine. 1975;23:37-40. [PubMed] |
| 54. | Matsumoto K, Kanmatsuse K. Transforming growth factor-beta1 inhibits vascular permeability factor release by T cells in normal subjects and in patients with minimal-change nephrotic syndrome. Nephron. 2001;87:111-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 55. | Tune BM, Mendoza SA. Treatment of the idiopathic nephrotic syndrome: regimens and outcomes in children and adults. J Am Soc Nephrol. 1997;8:824-832. [PubMed] |
| 56. | Fine RN. Recurrence of nephrotic syndrome/focal segmental glomerulosclerosis following renal transplantation in children. Pediatr Nephrol. 2007;22:496-502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 69] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 57. | Cattran DC, Alexopoulos E, Heering P, Hoyer PF, Johnston A, Meyrier A, Ponticelli C, Saito T, Choukroun G, Nachman P. Cyclosporin in idiopathic glomerular disease associated with the nephrotic syndrome : workshop recommendations. Kidney Int. 2007;72:1429-1447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 132] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 58. | Moudgil A, Bagga A, Jordan SC. Mycophenolate mofetil therapy in frequently relapsing steroid-dependent and steroid-resistant nephrotic syndrome of childhood: current status and future directions. Pediatr Nephrol. 2005;20:1376-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 59. | Nozu K, Iijima K, Fujisawa M, Nakagawa A, Yoshikawa N, Matsuo M. Rituximab treatment for posttransplant lymphoproliferative disorder (PTLD) induces complete remission of recurrent nephrotic syndrome. Pediatr Nephrol. 2005;20:1660-1663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 99] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 60. | Guigonis V, Dallocchio A, Baudouin V, Dehennault M, Hachon-Le Camus C, Afanetti M, Groothoff J, Llanas B, Niaudet P, Nivet H. Rituximab treatment for severe steroid- or cyclosporine-dependent nephrotic syndrome: a multicentric series of 22 cases. Pediatr Nephrol. 2008;23:1269-1279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 182] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 61. | Keith DS. Therapeutic apheresis rescue mission: recurrent focal segmental glomerulosclerosis in renal allografts. Semin Dial. 2012;25:190-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 62. | Ponticelli C. Recurrence of focal segmental glomerular sclerosis (FSGS) after renal transplantation. Nephrol Dial Transplant. 2010;25:25-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 119] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 63. | Gipson DS, Chin H, Presler TP, Jennette C, Ferris ME, Massengill S, Gibson K, Thomas DB. Differential risk of remission and ESRD in childhood FSGS. Pediatr Nephrol. 2006;21:344-349. [PubMed] |
| 64. | Troyanov S, Wall CA, Miller JA, Scholey JW, Cattran DC. Focal and segmental glomerulosclerosis: definition and relevance of a partial remission. J Am Soc Nephrol. 2005;16:1061-1068. [PubMed] |
| 65. | Gao W, Wang Z, Bai X, Xi X, Ruan C. Detection of soluble urokinase receptor by immunoradiometric assay and its application in tumor patients. Thromb Res. 2001;102:25-31. [PubMed] |
| 66. | Faul C, Donnelly M, Merscher-Gomez S, Chang YH, Franz S, Delfgaauw J, Chang JM, Choi HY, Campbell KN, Kim K. The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat Med. 2008;14:931-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 704] [Cited by in RCA: 763] [Article Influence: 44.9] [Reference Citation Analysis (0)] |
| 67. | Salomon R, Gagnadoux MF, Niaudet P. Intravenous cyclosporine therapy in recurrent nephrotic syndrome after renal transplantation in children. Transplantation. 2003;75:810-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 60] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 68. | Raafat RH, Kalia A, Travis LB, Diven SC. High-dose oral cyclosporin therapy for recurrent focal segmental glomerulosclerosis in children. Am J Kidney Dis. 2004;44:50-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 57] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 69. | Ingulli E, Tejani A, Butt KM, Rajpoot D, Gonzalez R, Pomrantz A, Ettenger R. High-dose cyclosporine therapy in recurrent nephrotic syndrome following renal transplantation. Transplantation. 1990;49:219-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 70. | Schwarz A, Krause PH, Offermann G, Keller F. Recurrent and de novo renal disease after kidney transplantation with or without cyclosporine A. Am J Kidney Dis. 1991;17:524-531. [PubMed] |
| 71. | Fornoni A, Sageshima J, Wei C, Merscher-Gomez S, Aguillon-Prada R, Jauregui AN, Li J, Mattiazzi A, Ciancio G, Chen L. Rituximab targets podocytes in recurrent focal segmental glomerulosclerosis. Sci Transl Med. 2011;3:85ra46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 400] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 72. | Zhang B, Xie S, Shi W, Yang Y. Amiloride off-target effect inhibits podocyte urokinase receptor expression and reduces proteinuria. Nephrol Dial Transplant. 2012;27:1746-1755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 73. | Wei C, El Hindi S, Li J, Fornoni A, Goes N, Sageshima J, Maiguel D, Karumanchi SA, Yap HK, Saleem M. Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nat Med. 2011;17:952-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 623] [Cited by in RCA: 647] [Article Influence: 46.2] [Reference Citation Analysis (0)] |
| 74. | Passero CJ, Mueller GM, Rondon-Berrios H, Tofovic SP, Hughey RP, Kleyman TR. Plasmin activates epithelial Na+ channels by cleaving the gamma subunit. J Biol Chem. 2008;283:36586-36591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 149] [Article Influence: 8.8] [Reference Citation Analysis (0)] |