Revised: April 15, 2013
Accepted: May 1, 2013
Published online: May 6, 2013
AIM: To determine survival parameters as well as characteristics of patients with this syndrome.
METHODS: The investigation was conducted over a period of eight years, as a prospective, non-randomized, clinical study which included 204 patients, treated by chronic hemodialysis. Most patients received hemodialysis 12 h per week. As vascular access for hemodialysis all subjects had an arteriovenous fistulae. Based on surveys the respondents were divided into groups of patients with and without digital hypoperfusion ischemic syndrome. Gender, demographic and anthropometric characteristics, together with comorbidity and certain habits, were recorded. During this period 34.8% patients died.
RESULTS: Patients with digital hypoperfusion ischemic syndrome were older than those without ischemia (P = 0.01). Hemodialysis treatment lasted significantly longer in the patients with digital hypoperfusion ischemic syndrome (P = 0.02). The incidence of cardiovascular disease (P < 0.001) and diabetes mellitus (P = 0.01), as well as blood flow through the arteriovenous fistula (P = 0.036), were higher in patients with digital hypoperfusion ischemic syndrome. Statistically significant differences also existed in relation to oxygen saturation (P = 0.04). Predictive parameters of survival for patients with digital hypoperfusion ischemic syndrome were: adequacy of hemodialysis (B = -3.604, P < 0.001), hypertension (B = -0.920, P = 0.018), smoking (B = -0.901, P = 0.049), diabetes mellitus (B = 1.227, P = 0.005), erythropoietin therapy (B = 1.274, P = 0.002) and hemodiafiltration (B = -1.242, P = 0.033). Kaplan-Meier survival analysis indicated that subjects with and without digital hypoperfusion ischemic syndrome differed regarding the length of survival (P < 0.001), i.e., patients with confirmed digital hypoperfusion ischemic syndrome died earlier.
CONCLUSION: Survival was significantly longer in the patients without digital hypoperfusion ischemic syndrome.
Core tip: In order to prevent the occurrence of distal hypoperfusion syndrome, is important identification of risk factors. Problem is the absence of objective indicators of distal ischemia, which is main reason why large proportion our patients have of this symptoms. Our patients with these symptoms were significantly older, which confirms older age as a factor that characterizes patients with distal hypoperfusion syndrome. We have confirmed greater incidence of diabetes mellitus and smokers among the patients with distal hypoperfusion syndrome. Quality of dialysis, diabetes mellitus, erythropoietin therapy, smokers and hemodiafiltration has predictive value for survival of patients with distal ischemia.
- Citation: Stolic RV, Trajkovic GZ, Miric DJ, Kisic B, Djordjevic Z, Azanjac GL, Stanojevic MS, Stolic DZ. Arteriovenous fistulas and digital hypoperfusion ischemic syndrome in patients on hemodialysis. World J Nephrol 2013; 2(2): 26-30
- URL: https://www.wjgnet.com/2220-6124/full/v2/i2/26.htm
- DOI: https://dx.doi.org/10.5527/wjn.v2.i2.26
Digital hypoperfusion ischemic syndrome (DHIS) refers to hand ischemia due to peripheral hypoperfusion, induced by an arteriovenous fistula (AVF) for hemodialysis (HD). Its older name, arterial steal syndrome, is less accurate, because most patients with vascular access, have clinically silent arterial steal due to retrograde flow. The etiology of DHIS is a combination of true steal from the distal arteries, arterial stenosis of the arm and distal arteriopathy. Patients commonly present with a cold hand, numbness and hand pain on and/or off dialysis. In advanced cases, there may be ischemic ulcers and dry gangrene[1-3].
Clinically significant distal extremity ischemia occurs in 1.6%-8% of all individuals with a functioning access[4,5]. Due to the increasing age of patients on HD, as well as the rising number of comorbidities, the incidence of hand ischemia is becoming more common. A prerequisite for the occurrence of hand ischemia is reduced blood flow through the arterial system, excessive blood flow through distended blood vessels, inadequate vascular adaptation and reduced collateral perfusion. It is assumed that the incidence of ischemia could rise in the future due to popularization of vascular access in the proximal region of the elbow and the growing number of elderly people on dialysis[6,7].
The goal of this study was to determine clinical characteristics and survival parameters in patients with DHIS.
The investigation was conducted at the Clinic of Urology and Nephrology, Clinical Center, Kragujevac, Serbia, over a period of eight years, as a prospective, non-randomized, clinical study. It included 204 patients, 71 (34.8%) women and 133 (65.2%) men, who had been treated by chronic HD for about 4.5 years. Most patients received HD for 12 h per week, using commercially available dialysers (Fresenius Medical Care, Bad Homburg, Germany, and Gambro AB, Lund, Sweden) providing bicarbonate HD and hemodiafiltration (HDF). The range of the blood pump during dialysis was 250-320 mL/min giving a dialysis fluid flow of 500 mL/min. As vascular access for HD all subjects had an AVF. During this period, 71 (34.8%) patients died.
All patients responded to the questionnaire, which revealed clinical symptoms for DHIS, namely, a positive response to the questions: Do you feel cold or numbness of the hand with the AVF and do you feel pain during or after dialysis treatment? This was defined as the existence of DHIS[7,8].
Age, sex, length of time spent on HD (mo), erythropoietin therapy, oxygen saturation determined for the arm with the AVF and the contralateral side, and the presence of various forms of cardiovascular disease and/or diabetes mellitus were recorded for all participants. Dialysis procedure is defined as bicarbonate and hemodiafiltration. Adequacy of HD was calculated by the urea kinetic model (Kt/V), according to the formula of Daugirdas[9]. Body mass index was calculated using the formula: the ratio between body weight in kg and the square of body height in square meters. Flow through the AVF (mL/L) was measured by Doppler ultrasound examination on a LOGIQ P5 apparatus with a camera (GE Healthcare, 9900 Innovation Drive, Wauwatosa, WI, United States 53226). We also examined the use of alcohol and smoking habits (former and current smokers).
The protocol was approved by the Ethics Committee of the Clinical Center, Kragujevac, Serbia and informed consent was obtained from all the patients, according to the Helsinki declaration.
Descriptive statistics, Student’s t-test, Mann-Whitney test, χ2 test for homogeneity, and Fisher’s exact test were used when indicated. Survival was estimated using the Kaplan-Meier product-limit method. The criterion for statistical significance was P < 0.05.
Digital hypoperfusion ischemic syndrome was confirmed in the most part of our patients. Patients with symptoms of distal ischemia were significantly older, the average length of dialysis treatment is longer, have higher rates of cardiovascular disease and diabetic nephropathy, and blood flow through the arteriovenous fistula is higher compared to those without the syndrome. It was also found that there is a statistical difference in relation to oxygen saturation (Table 1).
| Digital hypoperfusion ischemic syndrome | P | ||
| Yes | No | ||
| (n = 120) | (n = 84) | ||
| Age (yr), mean ± SD | 61 ± 11.0 | 58.0 ± 11 | 0.011 |
| Gender, | 0.76 | ||
| Male | 77 (64.2) | 56 (66.7) | |
| Female | 43 (35.8) | 28 (33.3) | |
| Length of dialysis treatment (mo), median (range) | 60 (3–312) | 43 (3–263) | 0.021 |
| BMI, mean ± SD | 23.1 ± 5.4 | 23.5 ± 4.7 | 0.62 |
| Smoking | 59 (49.2) | 43 (51.2) | 0.88 |
| Alcohol consumption | 27 (25) | 21 (27) | 0.78 |
| Cardiovascular diseases | 77 (64.2) | 33 (39.3) | < 0.0011 |
| Diabetes mellitus | 30 (25) | 8 (9.5) | < 0.0011 |
| AVF blood flow (mL/min), median (range) | 760 | 630 | 0.031 |
| (100–2000) | (100–1500) | ||
| Oxygen saturation, mean ± SD (%) | 97 ± 20 | 97 ± 3.2 | 0.041 |
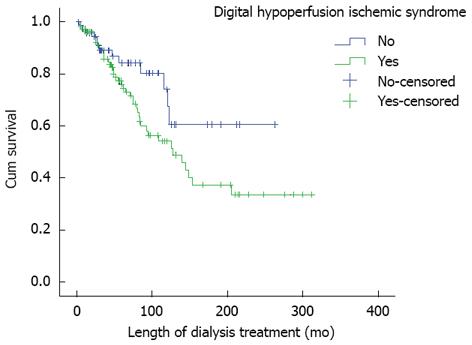
The average survival of the patients with DHIS was 160 mo, they died earlier, in the correlation of the patients without this syndrome (Figure 1).
Predictive parameters for the survival of patients with DHIS are: Kt/V index, smokers, diabetes mellitus, erythropoietin therapy and HDF, as well as mode of dialysis (Table 2).
In order to prevent the occurrence of DHIS, identification of risk factors is very important. However, one problem is the absence of a simple and practical classification of this syndrome and objective indicators of distal ischemia, which is the main reason why such a large proportion of our patients showed clinical symptoms of this syndrome[8]. It is practically impossible to predict the occurrence of distal hypoperfusion in the preoperative period, but the likelihood of it developing has been related to a number of risk factors, which primarily include old age, female sex and conditions that lead to arterial occlusion[10]. Women, in the our research, have no higher risk for developing distal hypoperfusion, probably because they accounted for only one-third of our respondents. On the other hand, patients who had symptoms of distal ischemia were significantly older than those without this syndrome, which confirms old age as a factor that characterizes patients with DHIS[6,11]. Half of our respondents were smokers and this habit has been established as a significant feature in patients with pronounced symptoms of distal ischemia. We confirmed this trend for greater incidence of diabetes mellitus and more smokers among the patients with DHIS[2].
We have found that the patients differed significantly in relation to oxygen saturation on the hand with the AVF and the contralateral hand. On the other hand, atherosclerotic disease, caused by insufficient collateral perfusion, reduce the flow through the AVF in our patients with DHIS. Because, in the literature is known, that the low vascular resistance in fistulated veins, and arterial stenosis, in the proximal parts, encourages arm ischemia, and reduces blood flow in distal parts of the vascular bed[12-14]. An AVF can cause significant local and general changes in the bloodstream. In fact, retrograde flow in the radial artery occurs in more than 70% of patients with AVF, which, however, does not cause ischemia in subjects with normal blood circulation[15]. Ischemic syndrome in physiological conditions results from a fall in blood pressure in the periphery and failure of compensatory vasodilatation[16].
Heart failure in HD patients has a multifactorial character[14,17-19]. We found an important trend concerning the incidence of cardiovascular disease in the patients with DHIS, most likely due to expressed atherogenesis in the HD patients. Longer survival of the patients treated by HDF was probably due to increased clearance of small and medium-sized molecules, which improved hemodynamic stability[20-23]. Any improvement of the biocompatibility of dialysis systems will reduce their inflammatory character and eventually be reflected by a lower atherogenic profile in patients on HD.
Predictive factors for survival patients with distal ischemia.
Adequacy of the HD, diabetes mellitus, erythropoietin therapy, smokers and HDF had predictive value for survival of patients with distal ischemia. In fact, survival patients with DHIS was associated with the erythropoietin therapy, better quality HD, those who do not smoke and HDF, as well as the absence of diabetes mellitus.
In addition to arterial insufficiency, arm pain may be due to carpal tunnel syndrome and various forms of tendopathy and arthropathy, which can occur in patients on chronic HD. Moreover, pain in the hand after creation of vascular access may be described as so-called. Reflex sympathetic dystrophic syndrome, characterized by pain and swelling of the extremities. Patients with diabetes mellitus often feel neurogenic pain, which is symmetric and includes both extremities. This situation should be distinguished from so-called ischemic neuropathy as a complication of vascular access in diabetic patients, especially those who already have pronounced neuropathic changes characterized by acute pain, muscle weakness and paralysis of arms at rest, as a consequence of somatic-sensory disorders[2,24-27]. Such a differential-diagnostic dilemma for the subjective assessment of distal ischemia is a limiting factor in our study, making it difficult to place our respondents in research sub-categories. We were also unable to establish a time sequence between the outcome of the examined patients and the time of appearance of symptoms of distal ischemia.
In the our respondents with DHIS we have found significant number of the atherogenic factors risk. Consequently, in the every patient with DHIS needs a thorough evaluation of extremity anatomy and access hemodynamics, because one of the causes of DHIS is pathology within the arterial inflow to the arteriovenous fistula[1]. Due to a lack of adequate compensatory flow to the extremity to accommodate both the low-resistance outflow of the access and the high-resistance vascular bed of the distal party can predispose patients to DHIS. Access flow is determined by the interaction of the resistances associated with the access outflow, distal vascular bed, and collaterals[28,29]. Also, diabetic neuropathy may play a role in a predilection for significant ischemic symptoms, particularly with pain and motor that dysfunction that are often seen in patients with significant symptoms of the ischemia[1].
In conclusion, survival was significantly longer in the patients without DHIS. The incidence of cardiovascular disease and diabetes mellitus increased the mortality rate in the patients with DHIS. Digital hypoperfusion ischemic syndrome was significantly more serious in the elderly. Absence of diabetes mellitus and cardiovascular disease, adequacy of the HD, nonsmokers, erythropoietin therapy and HDF are predictive parameters for survival of patients with DHIS. Finally, from crucial is to pay attention to the risky population for DHIS, especially in the elderly patients on dialysis who were smokers, with established cardiovascular disease or diabetes mellitus, with HD poor quality, which are not in the erythropoietin therapy.
Digital hypoperfusion ischemic syndrome is ischemia due to peripheral hypoperfusion, induced by an arteriovenous fistula for hemodialysis. Manifested by a cold hand, numbness and hand pain on and/or off dialysis, and can contribute to the development dry gangrene of the hand.
In the research areas of this topic is very important serious complications, in the patients with distal ischemia, that may arise if not timely disclose.
To pay attention to the risky patients on hemodialysis for development distal ischemia, caused by vascular access for hemodialysis, especially in the elderly patients on dialysis, smokers, with cardiovascular disease or diabetes mellitus, and patients with poor hemodialysis, and in patients who not have erythropoietin therapy.
The application of these findings will contribute to improving the quality of life in hemodialysis patients.
The absence of a simple and practical classification of this syndrome is the main reason for dilemma relating to the the clinical picture distal ischemia.
This is study in which analyzed complications as is distal ischemia, as one important cause, rare but very serious complication in the hemodialysis population. Progression of these symptoms can lead to gangrene. It is assumed that, in the future, the incidence of ischemia could rise due to popularization of vascular access in the proximal region of the elbow, and the growing number of elderly people on dialysis.
P- Reviewers Eirini G, Friedman E S- Editor Gou SX L- Editor A E- Editor Lu YJ
| 1. | Berman SS, Gentile AT, Glickman MH, Mills JL, Hurwitz RL, Westerband A, Marek JM, Hunter GC, McEnroe CS, Fogle MA. Distal revascularization-interval ligation for limb salvage and maintenance of dialysis access in ischemic steal syndrome. J Vasc Surg. 1997;26:393-402; discussion 402-404. [PubMed] [Cited in This Article: ] |
| 2. | Leon C, Asif A. Arteriovenous access and hand pain: the distal hypoperfusion ischemic syndrome. Clin J Am Soc Nephrol. 2007;2:175-183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 3. | Bachleda P, Utikal P, Kojecky Z, Drac P, Köcher M, Cerna M, Zadrazil J. Autogenous arteriovenous elbow fistula for haemodialysis and upper extremity ischemia. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2007;151:129-132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Wixon CL, Mills JL. Hemodynamic basis for the diagnosis and treatment of angioaccess-induced steal syndrome. Advances in vascular surgery. St. Louis: Mosby 2000; 147–159. [Cited in This Article: ] |
| 5. | Polimanti AC, Galego SJ, de Carvalho Fürst RV, da Silveira Moraes G, da Silva Barbosa RC, Silveira SR, Kawhage MCSN, Correa JA. Treatment of hemodialysis access steal syndrome by distal revascularization arterial ligature: report of three cases. J Vasc Bras. 2012;11:158-161. [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 6. | Scheltinga MR, van Hoek F, Bruijninckx CM. Time of onset in haemodialysis access-induced distal ischaemia (HAIDI) is related to the access type. Nephrol Dial Transplant. 2009;24:3198-3204. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Mickley V. Steal syndrome--strategies to preserve vascular access and extremity. Nephrol Dial Transplant. 2008;23:19-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 8. | Kirksey L. Ischemic monomelic neuropathy: an underappreciated cause of pain and disability following vascular access surgery. J Vasc Access. 2010;11:165-168. [PubMed] [Cited in This Article: ] |
| 9. | Daugirdas JT. Chronic haemodialysis prescription: A urea kinetics approach. Handbook of Dialysis. Boston: Little Brown 1994; 92-120. [Cited in This Article: ] |
| 10. | Stern AB, Klemmer PJ. High-output heart failure secondary to arteriovenous fistula. Hemodial Int. 2011;Jan 12; [Epub ahead of print]. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Davidson D, Louridas G, Guzman R, Tanner J, Weighell W, Spelay J, Chateau D. Steal syndrome complicating upper extremity hemoaccess procedures: incidence and risk factors. Can J Surg. 2003;46:408-412. [PubMed] [Cited in This Article: ] |
| 12. | Regalado S, Navuluri R, Vikingstad E. Distal revascularization and interval ligation: a primer for the vascular and interventional radiologist. Semin Intervent Radiol. 2009;26:125-129. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Schanzer A, Nguyen LL, Owens CD, Schanzer H. Use of digital pressure measurements for the diagnosis of AV access-induced hand ischemia. Vasc Med. 2006;11:227-231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Tordoir JH, Dammers R, van der Sande FM. Upper extremity ischemia and hemodialysis vascular access. Eur J Vasc Endovasc Surg. 2004;27:1-5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 199] [Cited by in F6Publishing: 176] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 15. | Zamani P, Kaufman J, Kinlay S. Ischemic steal syndrome following arm arteriovenous fistula for hemodialysis. Vasc Med. 2009;14:371-376. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 16. | Stolic R. Most important chronic complications of arteriovenous fistulas for hemodialysis. Med Princ Pract. 2013;22:220-228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 88] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 17. | Pedrini LA, De Cristofaro V, Pagliari B, Samà F. Mixed predilution and postdilution online hemodiafiltration compared with the traditional infusion modes. Kidney Int. 2000;58:2155-2165. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 51] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Cheung AK, Sarnak MJ, Yan G, Dwyer JT, Heyka RJ, Rocco MV, Teehan BP, Levey AS. Atherosclerotic cardiovascular disease risks in chronic hemodialysis patients. Kidney Int. 2000;58:353-362. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 512] [Cited by in F6Publishing: 526] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 19. | Himmelfarb J, Stenvinkel P, Ikizler TA, Hakim RM. The elephant in uremia: oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int. 2002;62:1524-1538. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 825] [Cited by in F6Publishing: 847] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 20. | Locatelli F, Marcelli D, Conte F, Limido A, Malberti F, Spotti D. Comparison of mortality in ESRD patients on convective and diffusive extracorporeal treatments. The Registro Lombardo Dialisi E Trapianto. Kidney Int. 1999;55:286-293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 164] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 21. | van der Weerd NC, Penne EL, van den Dorpel MA, Grooteman MP, Nube MJ, Bots ML, ter Wee PM, Blankestijn PJ. Haemodiafiltration: promise for the future? Nephrol Dial Transplant. 2008;23:438-443. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Vilar E, Fry AC, Wellsted D, Tattersall JE, Greenwood RN, Farrington K. Long-term outcomes in online hemodiafiltration and high-flux hemodialysis: a comparative analysis. Clin J Am Soc Nephrol. 2009;4:1944-1953. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 23. | Penne EL, Blankestijn PJ, Bots ML, van den Dorpel MA, Grooteman MP, Nubé MJ, van der Tweel I, Ter Wee PM. the CONTRAST study group: Effect of increased convective clearance by on-line hemodiafiltration on all cause and cardiovascular mortality in chronic hemodialysis patients – the Dutch CONvective TRAnsport STudy (CONTRAST): rationale and design of a randomised controlled trial [ISRCTN38365125]. Curr Control Trials Cardiovasc Med. 2005;6:8. [Cited in This Article: ] |
| 24. | Sidawy AN, Gray R, Besarab A, Henry M, Ascher E, Silva M, Miller A, Scher L, Trerotola S, Gregory RT. Recommended standards for reports dealing with arteriovenous hemodialysis accesses. J Vasc Surg. 2002;35:603-610. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 676] [Cited by in F6Publishing: 665] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 25. | Papasavas PK, Reifsnyder T, Birdas TJ, Caushaj PF, Leers S. Prediction of arteriovenous access steal syndrome utilizing digital pressure measurements. Vasc Endovascular Surg. 2003;37:179-184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 65] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 26. | Reifsnyder T, Arnaoutakis GJ. Arterial pressure gradient of upper extremity arteriovenous access steal syndrome: treatment implications. Vasc Endovascular Surg. 2010;44:650-653. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Awais M, Nicholas J, Al-Saleh A, Dyer J. Ischaemic monomelic neuropathy (IMN) following vascular access surgery for haemodialysis: an under-recognized complication in non-diabetics. Clin Kidney J. 2012;5:140–142. [DOI] [Cited in This Article: ] |
| 28. | Wixon CL, Hughes JD, Mills JL. Understanding strategies for the treatment of ischemic steal syndrome after hemodialysis access. J Am Coll Surg. 2000;191:301-310. [PubMed] [Cited in This Article: ] |
| 29. | Knox RC, Berman SS, Hughes JD, Gentile AT, Mills JL. Distal revascularization-interval ligation: a durable and effective treatment for ischemic steal syndrome after hemodialysis access. J Vasc Surg. 2002;36:250-255; discussion 256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 155] [Article Influence: 7.0] [Reference Citation Analysis (0)] |