Published online Jun 25, 2025. doi: 10.5527/wjn.v14.i2.104760
Revised: February 26, 2025
Accepted: March 10, 2025
Published online: June 25, 2025
Processing time: 96 Days and 1.9 Hours
Renal tubular acidosis (RTA) refers to a group of kidney disorders characterized by defective acid excretion or bicarbonate reabsorption, leading to metabolic acidosis. This case series presents three cases of RTA with distinct etiologies and clinical manifestations. These cases emphasize the necessity of a comprehensive evaluation of RTA, considering both renal and systemic origins.
The first case describes a female patient with osteopetrosis-related RTA, diag
These cases underscore the interdisciplinary approach essential in RTA management. Understanding the diverse pathophysiology of RTA aids in tailored therapeutic strategies and improved patient outcomes.
Core Tip: Renal tubular acidosis (RTA) is characterized by systemic acidosis due to impaired ability of kidneys to excrete acid or absorb bicarbonate. The etiology is varied with both renal and extrarenal causes. Here we present three unique cases of RTA due to rare etiologies. Associated features may provide a clue to diagnosis in these cases such as osteopetrosis, thyrotoxicosis and renal failure. Whole exome sequencing may help. These cases emphasize the importance of multi-disciplinary approach to such cases for evaluation and management.
- Citation: Bhandarkar A, Varmudy A, Boro H, Bhat S. Renal tubular acidosis: Varied aetiologies and clinical presentations: Three case reports. World J Nephrol 2025; 14(2): 104760
- URL: https://www.wjgnet.com/2220-6124/full/v14/i2/104760.htm
- DOI: https://dx.doi.org/10.5527/wjn.v14.i2.104760
Renal tubular acidosis (RTA) is a heterogeneous group of disorders characterized by the impaired ability of the kidneys to excrete acid in the urine or absorb filtered bicarbonate (HCO3−)[1]. RTA is of four types: Type 1 (distal), type 2 (proximal), type 3 (mixed), and type 4 (hyporeninemic hypoaldosteronism)[2]. RTA may result from varying etiologies, including genetic factors, autoimmune diseases, nephrotoxins, and miscellaneous causes such as amyloidosis, sarcoidosis, obstructive uropathy, interstitial nephritis, and pyelonephritis[2]. RTA exhibits a varied spectrum of clinical manifestations, including impaired growth, failure to thrive, polyuria, polydipsia, preference for savory foods, refractory rickets, renal calculi, unexplained hypertension, and recurrent episodes of hypokalemic periodic paresis[3].
In this case series, we present three distinct cases of RTA with diverse underlying etiologies and clinical presentations: Guibaud-Vainsel syndrome with osteopetrosis, focal segmental glomerulosclerosis (FSGS), and Graves’ disease. Through these cases, we aim to emphasize the need for a comprehensive evaluation in patients presenting with RTA and the importance of considering both renal and extra-renal causes. Furthermore, this case series highlights the pertinence of a multidisciplinary team in assessing and managing RTA.
Case 1: 30-year-old woman presented with pain and redness around her right eye, which persisted for 3 months despite treatment for conjunctivitis.
Case 2: 56-year-old man presented with progressive weakness in his upper and lower limbs over a period of 2 years. He had a right femoral neck fracture and multiple wedge compression fractures in the thoracolumbar spine. He was admitted to the orthopedics department because of these complaints.
Case 3: 54-year-old man presented with recurrent episodes of hypokalemic periodic paralysis over a period of 10 months, which was initially triggered by physical exertion.
Case 1: The symptoms escalated to swelling on her right cheek and mucopurulent discharge from the right side of her nose. These were accompanied by dental caries. A contrast-enhanced computed tomography (CT) scan indicated an enhancing soft tissue lesion in her right maxilla that eroded into adjacent structures. Biopsy implied osteomyelitis, and extremely hardened bones were observed intraoperatively, which was consistent with osteopetrosis.
Case 2: The patient’s medical history included recurring episodes of weakness, which were treated symptomatically elsewhere. There was no history of exposure to nephrotoxic medication or any gastrointestinal disturbance. An autoimmune history was absent.
Case 3: Despite potassium correction, the patient’s symptoms worsened. He experienced anxiety, weight loss, palpitations, tremulousness of hands, heat intolerance, and easy fatiguability, which preceded the paralysis attacks by 6 months.
Case 1: The patient had experienced global developmental delay, recurrent fractures, and hypokalemic periodic paresis since childhood. However, there was no history of hearing loss. She exhibited severe intellectual impairment and required assistance for routine daily activities.
Case 1: Family history revealed second-degree consanguineous parentage. Nonetheless, similar complaints were not evident in other family members. The patient had fully developed secondary sexual characteristics, and her menstrual cycles were regular.
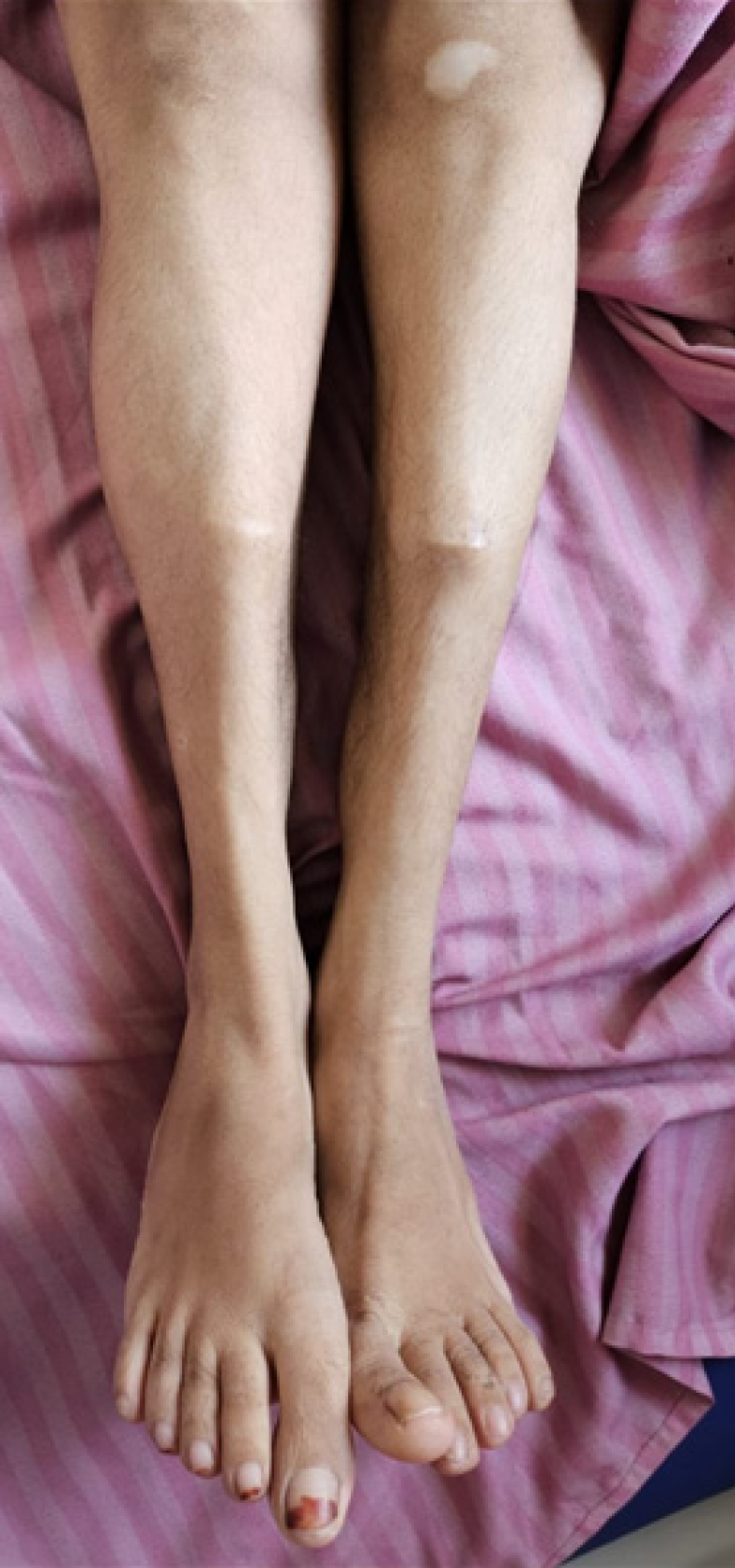
Case 1: Clinical examination showed a short stature (146 cm, below the 3rd centile), high arched palate, dental crowding, low-set ears, and malunited fractures of the bilateral shin (Figure 1).
Case 2: Physical examination revealed a fracture in the right femoral neck and multiple wedge compression fractures in the thoracolumbar spine. Despite normal vital signs and mental functions, motor weakness was noted in proximal upper and lower limbs (power 3/5), with reduced deep tendon reflexes.
Case 3: Upon admission to our clinic, physical examination suggested quadriparesis (power 2/5 in both upper and lower limbs) and acute urinary retention. However, higher mental functions, cranial nerves, and sensory examination were normal.
Case 1: The results of laboratory examinations signified that the patient had hypokalemia with a potassium level of 2.8 mmol/L [normal range (N): 3.5-5.5 mmol/L], hyperchloremia with a serum chloride level of 115 mmol/L (N: 96-106 mmol/L), a normal anion gap of 11 mmol/L (N: 4-12 mmol/L), metabolic acidosis [pH of 7.24 (N: 7.38-7.42), serum
Case 2: During admission for femoral fracture fixation, hypokalemia (potassium level of 2.6 mmol/L, N: 3.5-5.5 mmol/L), elevated serum creatinine (203.3 μmol/L, N: 52.2-91.9 μmol/L), and blood urea (6.6 mmol/L, N: 1.8-7.1 mmol/L) were observed. Blood gas analysis signified a normal anion gap [9 mmol/L (N: 4-12 mmol/L)], hyperchloremia [117 mmol/L (N: 96-106 mmol/L)], metabolic acidosis (pH: 7.27, HCO3−: 14 mmol/L), and an alkaline urine pH of 7.0, consistent with distal RTA. Proteinuria or glucosuria was not noted. Calcium, magnesium, and phosphorus levels were normal, and cortisol and thyroid tests were also normal. Antinuclear antibody (ANA) and anti-SSA and anti-SSB antibodies for Sjogren’s syndrome were negative.
Case 3: The patient exhibited hypokalemia with a potassium level of 2.4 mmol/L (N: 3.5-5.5 mmol/L), hyperchloremia with a chloride level of 109 mmol/L (N: 96-106 mmol/L), and a normal serum sodium level of 137 mmol/L (N: 135-145 mmol/L). Arterial blood gas analysis indicated metabolic acidosis with a pH of 7.29 (N: 7.38-7.42), a serum HCO3− level of 17.9 (N: 22-29 mmol/L)], and a normal anion gap of 10.1 mmol/L (N: 4-12 mmol/L). The urine pH was 7.2. Proteinuria or glucosuria was not observed. Thyroid function tests showed an elevated free tri-iodothyronine level of 10.34 pmol/L (N: 4-8.3 pmol/L), an increased free thyroxine level of 50.22 pmol/L (0.25-5.0 pmol/L), and a suppressed thyroid stimulating hormone (TSH) level of < 0.05 mIU/mL (N: 0.25-5.0 mIU/mL). The TSH receptor antibody level was determined to confirm the etiology of thyrotoxicosis, which was elevated at 19.3 U/L (< 1 U/L: Negative), verifying the diagnosis of Graves’ disease. Furthermore, the patient was diagnosed with diabetes and had a fasting blood glucose level of 8.5 mmol/L (N: 3.9-5.6 mmol/L), a postprandial blood glucose level of 12.9 mmol/L (n < 7.8 mmol/L), and a glycated hemoglobin level of 6.9% (N: < 6.5%). Arterial blood gas analysis indicated systemic metabolic acidosis with a pH of 7.29 (N: 7.38-7.42), serum HCO3− level of 17.9 (N: 22-29 mmol/L)], a normal anion gap of 10.1 mmol/L (N: 4-12 mmol/L), and an alkaline urine pH of 7.2. Proteinuria or glucosuria was not observed. Complete hemogram; liver and kidney function tests; calcium, magnesium, creatinine kinase, and total prostate-specific antigen levels; and urine analysis were normal. ANA testing using immunofluorescence was negative. The ophthalmic evaluation was unremarkable.
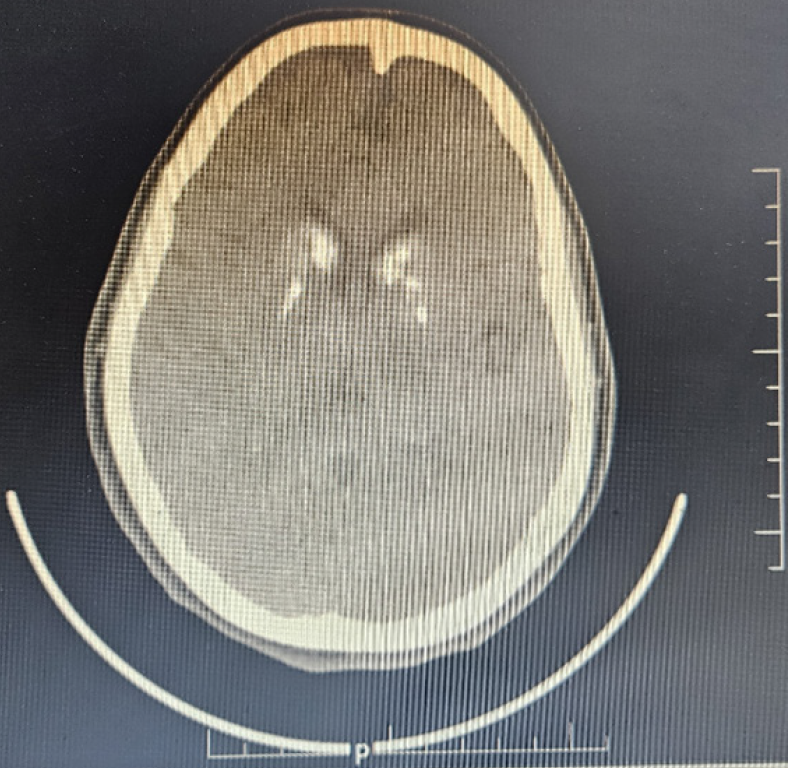
Case 1: Skull and limb radiographs showed features suggestive of osteopetrosis, including substantial sclerosis involving the skull and facial bones. A CT scan of the brain revealed basal ganglia calcification (Figure 2), and ultrasonography of the kidneys ruled out nephrocalcinosis.
Case 2: An ultrasound examination of the kidneys revealed bilateral medullary nephrocalcinosis.
Case 3: Thyroid ultrasound indicated that both lobes were enlarged but had normal vascularity. Technetium 99 (99mTc) thyroid scintigraphy showed uniformly increased uptake in both lobes, with uptake being 16.2% (normal 0.3%-3%), which agreed with Graves’ disease.
Guibaud-Vainsel syndrome was suspected because of the presence of the above features of RTA, severe intellectual impairment, and osteopetrosis. Homozygous deletion variant in intron 3 of the anhydrase II (CA 2) gene on chromosome 8 of autosomal recessive (AR) inheritance was observed on whole-exome sequencing, confirming the diagnosis of marble brain disease. A final diagnosis of Guibaud-Vainsel syndrome/Marble brain disease due to AR mutation of CA 2 gene.
Whole-exome sequencing identified an autosomal dominant, heterozygous missense mutation in exon 2 of the transient receptor potential channel 6 (TRP6) gene (chr11: g.101504735C>A; depth: 92x, typical of FSGS 2). Hence, the patient was finally diagnosed with FSGS due to TRP6 mutation and secondary RTA.
The final diagnosis was periodic paresis caused by hypokalemia and normal anion gap metabolic acidosis due to distal RTA secondary to Graves’ disease.
The treatment regimen involved a tablet of sodium bicarbonate 300 mg twice daily and a tablet of potassium citrate 1080 mg (equivalent to 10 mmol) twice daily.
The patient was started on oral potassium citrate as per recommendations. Over the next 4 days, the HCO3− levels increased and the serum potassium levels normalized. Upper limb strength improved significantly in a week. Subsequently, fixation of the right femoral fracture was performed. A denosumab injection was administered for osteoporosis, which was evident on bone densitometry. As denosumab injection is a safe option when creatinine levels are elevated, it was considered for treating osteoporosis. Renal biopsy was not performed.
The patient received intravenous potassium correction, resulting in the rapid resolution of quadriparesis. He was started on oral sodium bicarbonate at 3 g per day, oral potassium citrate to supplement 60 mmol/day of potassium, carbimazole 30 mg in divided doses, and propranolol 80 mg in divided doses. A venous blood gas bicarbonate level of 22 mmol/L and a serum potassium level of > 3.5 mmol/L were targeted.
After a month of follow-up, metabolic acidosis and serum potassium levels improved (arterial blood pH: 7.33, serum HCO3− level: 20 mmol/L, and serum potassium level: 3.5 mmol/L).
Muscular weakness improved considerably after 3 months of oral potassium citrate treatment, and the patient could walk with limited support. However, owing to financial constraints, the family could not afford Sanger sequencing.
For Graves’ disease, the patient was subjected to radioiodine ablation after 6 months of carbimazole therapy, following which he developed hypothyroidism and was switched to levothyroxine. His oral sodium bicarbonate and potassium citrate were tapered after 6 months once the thyrotoxicosis was reversed, with monthly testing of serum potassium, pH and bicarbonate levels in venous blood gas. There were no further episodes of quadriparesis during the 10 months of follow-up since diagnosis.
In this case series, we have described three distinct cases of RTA involving diverse etiologies and clinical presentations (Table 1).
| Case | Age (years)/sex | Presenting complaint | Significant medical history | Lab findings | Imaging findings | Genetic testing | Diagnosis | Therapy |
| 1 | 30/F | Pain, redness around right eye, swelling on cheek, mucopurulent discharge from nose, dental caries | Consanguineous parentage, developmental delay, severe intellectual impairment, recurrent fractures, hypokalemic periodic paresis since childhood | Hypokalemia, hyperchloremic metabolic acidosis, normal anion gap, alkaline urine pH | Osteopetrosis in radiographs of the skull and limbs, basal ganglia calcification in CT scan | Homozygous deletion variant in intron 3 of CA 2 gene | Proximal RTA (type 2) | Oral sodium bicarbonate and potassium citrate |
| 2 | 56/M | Progressive weakness in limbs, femur neck fracture, thoracolumbar spine compression fractures | Proximal muscle weakness, inability to walk without support | Hypokalemia, elevated creatinine and urea, hyperchloremic metabolic acidosis, normal anion gap | Bilateral medullary nephrocalcinosis on kidney ultrasound | Heterozygous missense mutation in TRP6 gene suggestive of FSGS 2 | Distal RTA (type 1) | Oral potassium citrate, right femur fracture fixation, denosumab |
| 3 | 54/M | Quadriparesis, acute urinary retention | Recurrent episodes of hypokalemic quadriparesis | Hypokalemia, hyperchloremic metabolic acidosis, alkaline urine pH, Normal anion gap, Thyrotoxicosis, Elevated TSH receptor antibody, normal ANA, Elevated FBS and HbA1C | On ultrasound diffuse enlargement of thyroid with normal vascularity. Technetium 99 (99mTc) thyroid scintigraphy showed uniformly increased uptake in both lobes, 16.2% (normal 0.3%-3%), consistent with Graves’ disease | Nil | Distal RTA secondary to Graves’ disease | Oral sodium bicarbonate and potassium citrate for distal RTA. For Graves’ disease carbimazole, propranolol followed by radio-iodine ablation |
Carbonic anhydrase II (CA2) deficiency is a rare disease classically characterized by osteopetrosis, RTA, and cerebral calcification. The first case involves a woman with a history of short stature, developmental delay, and intellectual impairment presenting with maxillofacial swelling, which on surgery, revealed features of osteopetrosis consistent with hardened bones. Osteopetrosis, or marble bone disease, is a group of rare, heritable diseases of the bone marked by an elevated bone density[4]. When combined with RTA, osteopetrosis is known as Guibaud-Vainsel syndrome or marble brain disease[5]. It follows an AR pattern of inheritance, and the clinical manifestations comprise sclerotic bones, growth failure, mental retardation, facial dysmorphism, intracerebral calcification, and conductive hearing loss. The most common cause is a CA2 gene mutation, as observed in our patient. RTA is usually of the mixed type (type 3) in such patients[5]. Other features that can be associated with this syndrome such as optic nerve and retinal atrophy and hematological features were absent in the index case.
CA II is a zinc metalloproteinase enzyme found in various cell types, such as osteoclasts and proximal and distal tubular cells. In the proximal renal tubules, its primary role is to convert carbon dioxide (CO2) into protons (H+) and bicarbonate ions (HCO3−) to enable the absorption of HCO3− into the basal membrane of proximal tubular cells and subsequently into the systemic circulation[6]. The reabsorption of HCO3− in the proximal renal tubule requires the activity of membranous carbonic anhydrase type 4 (CA IV) and intracellular CA II[7]. When filtered HCO3− from the glomerular filtrate is reabsorbed into CO2 by membranous CA IV, the cellular enzyme CA II converts CO2 back into HCO3− and H+, thereby completing the process of HCO3− absorption. A deficiency in CA II may impede HCO3− reabsorption, potentially contributing to acidosis.
In addition, CA II plays an indirect role in the distal acidification of urine. This enzyme is present in the intercalated cells of the distal tubule. When H+ is secreted into the lumen by the H+-ATPase, CA II efficiently removes the hydroxide (OH−) produced. An intracellular acidic pH and a suitable gradient for acid secretion are thus maintained. CA II deficiency may disrupt acid secretion, resulting in systemic acidosis[8]. Hence, CA II deficiency can be linked to both proximal and distal RTA, with proximal RTA being milder than the more prominent distal RTA. In the index case, either might have been predominant which would probably become evident on follow up.
Osteoclasts in the bone play a vital role in bone resorption and remodeling to maintain bone mineral homeostasis. This activity necessitates an acidic pH generated by H+ resulting from the conversion of CO2 by CA II. The deficiency of this enzyme can hamper bone resorption by osteoclasts, potentially leading to osteopetrosis[9].
FSGS is a collection of podocytopathies characterized by scarring in parts of some glomeruli, with various causes that manifest as nephrotic or sub-nephrotic proteinuria. There are recognised primary, hereditary, and secondary/maladaptive types. Primary, secondary, genetic and undetermined are the categories into which a recently proposed clinicopathological categorisation separated FSGS according to aetiology[10].
Nephrotic syndrome is a common presentation of primary FSGS. The glomerular filtration barrier is disrupted in genetic forms when genes producing proteins with structural and signalling functions in the podocyte or glomerular basement membrane are mutated. The TRP6 mutation (a mutation in the TRPC6 gene, which encodes for a calcium channel) can lead to podocyte dysfunction, contributing to proteinuria and progressive kidney damage and is one of the genetic causes of FSGS[11]. Nonetheless, a few cases might present tubular dysfunction prior to the development of glomerular proteinuria. In this case, the tubular acidosis is secondary to the podocyte dysfunction and the glomerular damage seen in FSGS. The type of RTA seen in FSGS due to TRP6 mutations is often type 2 (proximal). Such an association of RTA with FSGS has been reported in Dent disease, where low molecular (tubular) proteinuria rather than albuminuria was identified on urine protein electrophoresis[12].
The co-occurrence of distal RTA and FSGS has been found in Alagille and Sjogren syndrome cases[13,14]. In the index case, a variant of the TRPC6 mutation was associated with distal RTA, which has not been reported previously. The exact mechanism of RTA in these cases is yet to be elucidated. In salt-losing tubulopathies such as Bartter syndrome, chronic stimulation of the renin–angiotensin–aldosterone axis has been hypothesized to cause secondary FSGS. In contrast, distal RTA may cause secondary FSGS and glomerular proteinuria. Distal RTA is linked to medullary nephrocalcinosis either as a cause or effect[15].
RTA secondary to FSGS associated with a TRP6 mutation presents a complex diagnostic challenge, as it involves both glomerular and tubular dysfunction requiring a combination of clinical, histopathological and genetic testing. Presence of normal anion gap metabolic acidosis, urine pH greater than 5.5, hypokalemia, kidney stones and bone disease would suggest a diagnosis of distal RTA. If a kidney biopsy is performed, it would confirm FSGS by showing segmental sclerosis and hyalinosis in the glomeruli. There may be interstitial fibrosis and tubular atrophy, which are indicative of tubular dysfunction and chronic renal disease. Although it is not required, genetic testing for mutations might identify the underlying genetic mutation of FSGS and provide further information for prognostication.
Patients with TRP6 mutations often have a more aggressive disease course, potentially progressing to end-stage renal disease if not managed appropriately. The combination of FSGS due to TRP6 mutation and secondary RTA leads to a challenging clinical scenario, with a high risk of kidney decline, complications from metabolic acidosis, and a potentially poor long-term prognosis. As kidney function declines, high blood pressure may develop, further contributing to kidney damage. Chronic acidosis can cause osteomalacia, leading to bone pain and fractures. Early diagnosis and treatment are crucial for slowing the progression of the disease.
Supplementing with potassium and bicarbonate is a crucial treatment approach for distal RTA. Metabolic alkalosis may result from excessive and protracted bicarbonate administration. Prolonged alkalosis can lead to abnormalities in the calcium and potassium balance, which can impact muscle and bone health. It can also impair renal function and affect the body's potassium, sodium, and chloride levels. Arrhythmias may result from elevated potassium levels. Prolonged use of oral potassium and bicarbonate can affect nutrient absorption and digestion in general, and excessive use can cause gastrointestinal problems like bloating and nausea.
At times, distal RTA occurs secondary to autoimmune disorders such as Graves’ disease, Hashimoto’s thyroiditis, Sjogren’s syndrome, rheumatoid arthritis, systemic lupus erythematosus, and primary biliary cirrhosis[2]. In the first reported association between Graves’ disease and RTA in 1959, hypercalciuria resulting from hyperthyroidism causing tubular damage was proposed to be the cause of the RTA[16]. Zisman et al[17] subsequently reported a patient with such a co-existence and examined acid excretion in another five patients of hyperthyroidism and no known renal disease. Not finding any abnormal acid excretion, they concluded that the apparent association of hyperthyroidism and RTA was merely coincidental. Jaeger et al[18] summarized the association of thyroid disease including hypothyroidism and hyperthyroidism with RTA in about ten cases. He observed that the RTA was not always associated with nephrocalcinosis and in some cases persisted despite resolution of the altered thyroid function, suggesting a possible immunological mechanism rather than a metabolic one. The exact mechanism of damage to distal and collecting tubules in Graves’ disease is not established as no antibodies against renal tubules have been detected to date. However, TSH receptor antibodies have been suggested to cross-react with CA II, epithelial sodium channels, intercalated cells, or acid-base transporters[19]. Sparse literature has shown good long-term outcomes as reversal to euthyroid state causes improvement of hypercalciuria, thus correcting the RTA.
This case series highlights the varied etiologies and clinical presentations of RTA. This report underscores the importance of considering systemic diseases while evaluating and managing RTA. Comprehensive evaluation, including detailed medical history and laboratory investigations such as genetic studies and imaging studies, is crucial for identifying the underlying cause and providing personalized care to patients (Table 2). A collaborative multidisciplinary approach involving nephrologists, endocrinologists, geneticists, and other specialists is essential for treating patients with RTA and the associated comorbidities. Further research is needed to decipher the underlying mechanisms linking RTA to various systemic conditions and to identify optimal therapeutic strategies for improving patient outcomes.
| Step | Description |
| Step 1: Clinical suspicion | Evaluate for symptoms: Growth retardation, non-healing rickets/osteomalacia, bone deformities, polyuria, nocturia, salt craving, muscle weakness |
| Step 2: Laboratory evaluation | Determination of arterial pH and anion gap. Check for non-anion gap metabolic acidosis |
| Measure serum electrolytes (hypokalemia or hyperkalemia) | |
| Assess urine pH (< 5.5 or > 5.5) | |
| Calculate urine anion gap (Na + K) –Cl | |
| Measure serum bicarbonate levels | |
| Step 3: Classification of RTA | Type 1 (distal) RTA |
| Non-anion gap metabolic acidosis with urine pH > 5.5 | |
| Hypokalemia, hypercalciuria, nephrocalcinosis, nephrolithiasis common | |
| Causes of distal RTA | |
| (1) Autoimmune causes (Sjogren’s, SLE, Graves’ disease, Primary biliary cholangitis, autoimmune hepatitis) | |
| (2) Genetic [sporadic gene mutations (SLC4A4, ATP6B1), Wilson’s disease, hereditary fructose intolerance, primary hyperoxaluria] | |
| (3) Drugs (amphotericin B, trimethoprim, analgesic abuse, toluene, amiloride, pentamidine) | |
| (4) Miscellaneous (sarcoidosis, amyloidosis, obstructive uropathy, interstitial nephritis, pyelonephritis, primary hyperparathyroidism, intravascular volume depletion of any cause, CKD of any cause, focal segmental glomerulosclerosis) | |
| Treatment: Alkali therapy [Bicarbonate supplement (2-3 mEq/kg/day)/Potassium Citrate] | |
| Thiazide diuretics (if hypercalciuria) | |
| Type 2 (Proximal RTA) | |
| Non-anion gap metabolic acidosis with urine pH < 5.5 | |
| Associated with Fanconi’s syndrome | |
| Low molecular weight proteinuria | |
| Low serum phosphate levels | |
| Generalized aminoaciduria | |
| Glucosuria | |
| Causes of proximal RTA | |
| (1) Autoimmune (Sjogren’s, SLE) | |
| (2) Genetic [Sporadic gene mutations (SLC4A4, ATP6B1, ATP6NA1B), Wilson’s disease, Cystinosis, Lowe’s syndrome, Galactosemia] | |
| (3) Drugs (Amphotericin B, Trimethoprim, Analgesic abuse, toluene, amiloride, pentamidine, vanadium) | |
| (4) Miscellaneous causes (Amyloidosis, multiple myeloma, monoclonal gammopathy, light chain deposition disease, obstructive uropathy, nephrotic syndrome, medullary cystic kidney disease) | |
| Treatment | |
| High-dose alkali therapy (bicarbonate supplementation 5-20 mEq/kg/day) | |
| Phosphate supplementation | |
| Type 4 RTA (Hyporeninemic hypoaldosteronism) | |
| Non-anion gap metabolic acidosis | |
| Urine pH < 5.5 | |
| Hyperkalemia | |
| Low serum aldosterone | |
| Low direct renin concentration | |
| Causes of type 4 RTA: | |
| Diabetic kidney disease | |
| CKD of any cause | |
| Drugs (NSAIDs, ACE inhibitors, ARBs, Heparin) | |
| Treatment | |
| Treat the underlying cause | |
| Dietary potassium restriction | |
| Fludrocortisone, if aldosterone deficiency | |
| Bicarbonate supplementation, if acidotic | |
| Type 3 RTA (Mixed RTA) | |
| Features of both distal and proximal RTA | |
| Causes (Rare, autosomal recessive osteopetrosis, carbonic anhydrase deficiency) | |
| Treatment: Similar to that of distal and proximal RTA with bicarbonate supplementation and electrolyte management | |
| Sometimes, features of both proximal and distal RTA may be present initially as a transient phenomenon, and on follow-up after treatment, one form may become predominant. This transient mixed presentation can occur in severe early cases of distal RTA, immature renal tubules in infants, or acquired conditions with widespread tubulopathy (autoimmune or toxic insults) | |
| Step 4: Monitoring and follow-up | Regular monitoring of serum bicarbonate and potassium levels. Follow-up of nephrocalcinosis/nephrolithiasis, hypercalciuria, and renal functions. To adjust treatment doses based on clinical and biochemical parameters |
We acknowledge the contributions of Dr. Subodha H R towards the surgical management of the first patient.
| 1. | Yaxley J, Pirrone C. Review of the Diagnostic Evaluation of Renal Tubular Acidosis. Ochsner J. 2016;16:525-530. [PubMed] |
| 2. | Boro H, Khatiwada S, Alam S, Kubihal S, Dogra V, Mannar V, Khadgawat R. Renal Tubular Acidosis Manifesting as Severe Metabolic Bone Disease. touchREV Endocrinol. 2021;17:59-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 3. | Bagga A, Bajpai A, Menon S. Approach to renal tubular disorders. Indian J Pediatr. 2005;72:771-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Stark Z, Savarirayan R. Osteopetrosis. Orphanet J Rare Dis. 2009;4:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 277] [Cited by in RCA: 308] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 5. | Whyte MP. Carbonic anhydrase II deficiency. Bone. 2023;169:116684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 6. | Alayed Y, Alghamdi W, Alyousef R. Carbonic Anhydrase II Deficiency: Unusual Presentation of the Arabic Mutation. A Case Report. Glob Pediatr Health. 2024;11:2333794X241230873. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Ring T, Frische S, Nielsen S. Clinical review: Renal tubular acidosis--a physicochemical approach. Crit Care. 2005;9:573-580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Batlle D, Haque SK. Genetic causes and mechanisms of distal renal tubular acidosis. Nephrol Dial Transplant. 2012;27:3691-3704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 124] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 9. | Sly WS, Sato S, Zhu XL. Evaluation of carbonic anhydrase isozymes in disorders involving osteopetrosis and/or renal tubular acidosis. Clin Biochem. 1991;24:311-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Bonilla M, Efe O, Selvaskandan H, Lerma EV, Wiegley N. A Review of Focal Segmental Glomerulosclerosis Classification With a Focus on Genetic Associations. Kidney Med. 2024;6:100826. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 11. | Bezdíčka M, Langer J, Háček J, Zieg J. Dent Disease Type 2 as a Cause of Focal Segmental Glomerulosclerosis in a 6-Year-Old Boy: A Case Report. Front Pediatr. 2020;8:583230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Yadla M, Reddy S, Sriramnaveen P, Krishnakishore C, Sainaresh VV, Sivakumar V. An unusual association of primary focal and segmental glomerulosclerosis, distal renal tubular acidosis and secondary erythrocytosis. Saudi J Kidney Dis Transpl. 2011;22:1028-1029. [PubMed] |
| 13. | Boro H, Goyal A, Naik SS, Tandon N. Primary Sjögren's syndrome manifesting as sclerotic metabolic bone disease. BMJ Case Rep. 2021;14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Balogun RA, Adams ND, Palmisano J, Yamase H, Chughtai I, Kaplan AA. Focal segmental glomerulosclerosis, proteinuria and nephrocalcinosis associated with renal tubular acidosis. Nephrol Dial Transplant. 2002;17:308-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Fogo AB. Causes and pathogenesis of focal segmental glomerulosclerosis. Nat Rev Nephrol. 2015;11:76-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 248] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 16. | HUTH EJ, MAYOCK RL, KERR RM. Hyperthyroidism associated with renal tubular acidosis; discussion of possible relationship. Am J Med. 1959;26:818-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 33] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Zisman E, Buccino RA, Gorden P, Bartter F. Hyperthyroidism and renal tubular acidosis. Arch Intern Med. 1968;121:118-122. [PubMed] |
| 18. | Jaeger P, Portmann L, Wauters JP, Hürlimann J, Bill G, Scazziga B, Burckhardt P. Distal renal tubular acidosis and lymphocytic thyroiditis with spontaneously resolving hyperthyroidism. Report of 1 case without nephrocalcinosis. Am J Nephrol. 1985;5:116-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 19. | Both T, Zietse R, Hoorn EJ, van Hagen PM, Dalm VA, van Laar JA, van Daele PL. Everything you need to know about distal renal tubular acidosis in autoimmune disease. Rheumatol Int. 2014;34:1037-1045. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |