INTRODUCTION
Cirrhosis is a complex disease characterized by necroinflammation and fibrogenesis of the liver caused by different mechanisms, such as drug-induced hepatotoxicity, hepatitis B or C infections, excessive alcohol consumption, primary metabolic diseases, obesity, nonalcoholic fatty liver disease (NAFLD), autoimmune diseases, cholestatic diseases, and iron or copper overload[1-3].
In the course of the disease, hepatocytes and collagen deposition are continuously destroyed, replacing healthy liver parenchyma with fibrotic tissue and regenerative nodules. These events cause a pronounced distortion of the hepatic vascular architecture, which results in increased resistance to portal blood flow and, therefore, portal hypertension (PH), in addition to loss of liver function[3,4].
One of the main consequences in the advanced stage of cirrhosis is the development of hepatorenal syndrome (HRS), one of the most common types of kidney damage that affects patients with severe and decompensated liver disease and is characterized by decreased renal perfusion and vasoconstriction[5-7].
Few studies have analyzed the effect of treatment with antioxidant substances on the kidneys of cirrhotic animals since there is a consensus that renal dysfunction in cirrhosis results from hemodynamic changes and the activation of compensatory hormonal mechanisms. Previous studies have already demonstrated that, although there are no detectable structural changes in the kidney tissue of cirrhotic animals that underwent biliary duct ligation (BDL), there is a loss of integrity and cellular function in the kidney, with apoptosis, activation of caspase-3, oxidative damage to cell membranes and DNA damage[8].
BDL is a well-established model for cirrhosis development in 4 wk after surgery. The cirrhosis observed in animals shows damage in the liver parenchyma, the presence of fibrosis, PH, and ascites[9,10]. These conditions, mainly the presence of PH, are what lead to renal changes, with systemic hypotension, reduced glomerular filtration, and activation of the renin–angiotensin–aldosterone system. These data are widely recorded in current scientific literature, allowing the BDL model to study the HRS commonly seen in cirrhotic patients[11-15].
Omega-3 polyunsaturated fatty acids modulate the immune response by altering membrane phospholipids and preventing a proinflammatory state[16]. The benefit of omega-3 supplementation is well established in cardiovascular risk protection, mainly due to the reduction in triglycerides and low-density lipoproteins associated with omega-3 intake[17,18]. Other benefits regarding regular omega-3 intake are also reported, including a reduction in reactive oxygen species (ROS) and C-reactive protein, as well as the decreased release of cytokines and other inflammatory mediators[16,19]. Another factor directly related to HRS is PH, characterized by an excessive increase in portal venous pressure and the development of portosystemic collaterals, diverting portal blood flow to the systemic circulation. Several factors can trigger PH; however, liver cirrhosis is the leading cause, comprising > 90% of cases of PH. Among the main complications resulting from PH is upper gastrointestinal bleeding due to the rupture of gastroesophageal varices. Another less critical complication is the presence of ascites[20,21].
In liver diseases, some studies have reported a beneficial action of omega-3 polyunsaturated fatty acids, reducing sepsis and inflammatory markers in patients with severe acute hepatitis[22], reducing inflammation and fibrosis in patients with biliary atresia[23], and decreasing the percentage of fat liver in patients with nonalcoholic steatohepatitis treated with purified docosahexaenoic acid (DHA)[24]. A significant reduction in the risk of liver disease, particularly NAFLD, has been demonstrated with regular omega-3 supplementation[19].
In this sense, regular use of omega-3 is closely related to preventing and reducing the severity of several diseases. Omega-3 fatty acids are found in the largest amounts in walnuts, flaxseed, chia, sardines, salmon, and tuna[25]. Omega-3 supplementation may represent an additional therapy for individuals affected by HRS since the effectiveness of vasoconstrictor and albumin treatment is limited to less than half of patients with HRS-acute kidney injury (AKI)[7], and liver transplantation may not be a reality for all patients. However, little is known about the role of these fatty acids in patients with HRS, including the effects on the concentrations of inflammatory cytokines and the improvement in the disease’s course.
This study aimed to investigate the effects of supplementation with omega-3 fatty acids on oxidative stress and apoptosis in the kidney tissue of cirrhotic animals.
MATERIALS AND METHODS
Animals
All experimental procedures carried out on animals are by the recommendations of the Arouca Law (Law n° 11794, of 10/08/2008) and were approved by the Federal University of Health Sciences of Porto Alegre (UFCSPA) Ethics Committee with number 146/12. The animals were acclimatized to laboratory conditions (22 ± 2 °C, 12 h/12 h light/dark, 50% humidity, ad libitum access to food and water) for 2 wk before experimentation. BDL was performed under anesthesia, and the animals received analgesia for 48 h after surgery. Intragastric gavage administration was carried out with conscious animals, using straight gavage needles appropriate for the animal size. All animals were killed by an overdose of anesthetics.
In vivo studies
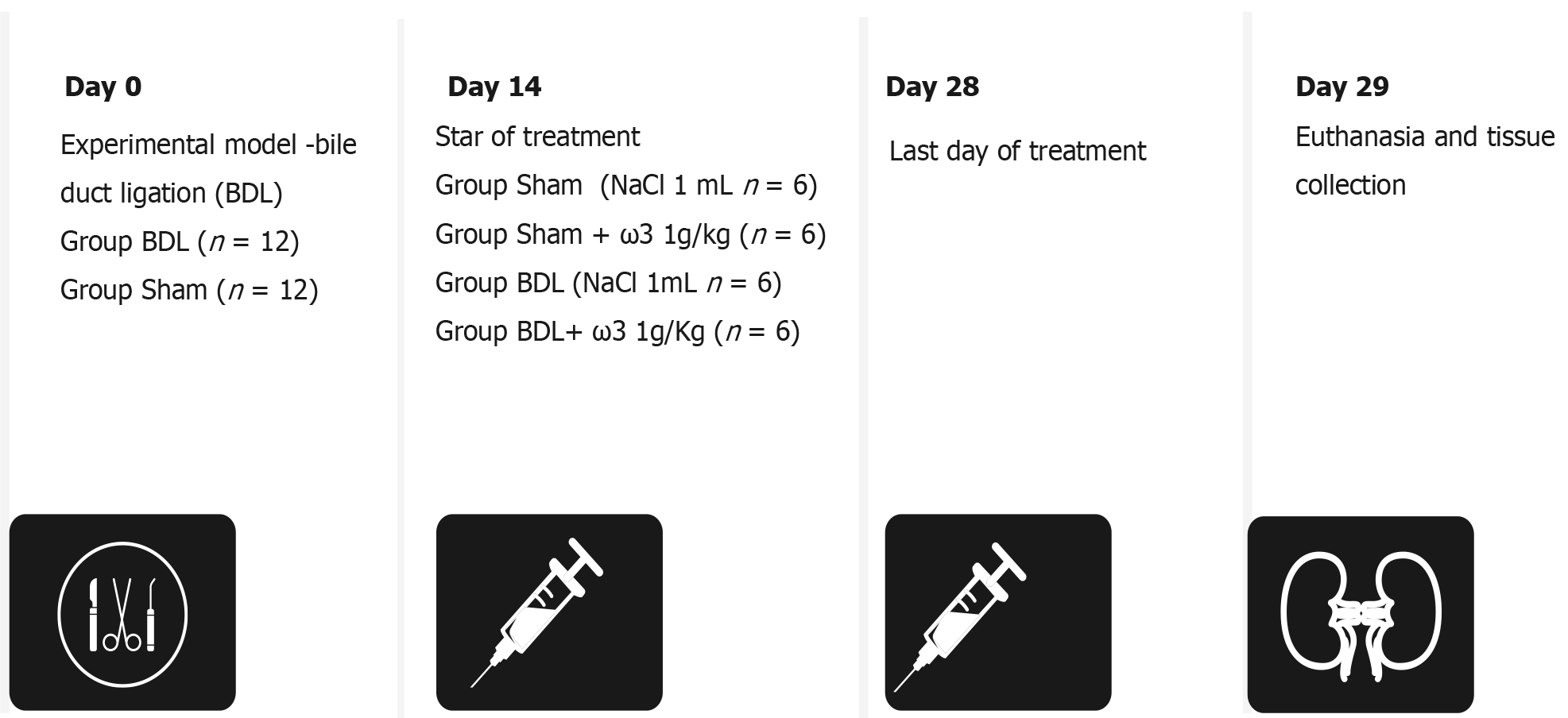
Twenty-four adults male Wistar rats with an average weight of 300 g were used. Liver cirrhosis was induced by the BDL model. Rats were anesthetized with 2% xylazine hydrochloride (50 mg/kg) and ketamine hydrochloride (100 mg/kg) (i.p.). Secondary biliary cirrhosis was induced in animals by double ligation and a total section of the common bile duct. The animals were killed 28 d after obstruction; a time in which there is complete development of both cholestasis and fibrosis and clear establishment of the biochemical changes characteristic of liver cirrhosis[26]. Animals were divided into four groups (n = 6): I (control treated with saline); II (treated with omega-3, 1 g/kg/d); III (BDL treated with omega-3, 1 g/kg/d); and IV (BDL treated with saline) (Figure 1). Daily omega-3 supplementation began on day 14 after BDL and continued for 2 wk until the death of the animals on day 28 after BDL.
Figure 1 Experimental protocol of omega-3 administration.
Histological analysis
Five-micrometer sections of formalin-fixed and paraffin-embedded kidney slices were routinely processed with hematoxylin–eosin (performed by the Laboratory of Pathology at UFCSPA). A single pathologist, blinded to the experimental protocol, analyzed all kidney fragments using light microscopy.
Tissue and homogenate preparation
Animals were killed by an overdose of anesthetic. The frozen kidneys from each rat were homogenized in an ice-cold phosphate buffer and diluted in 10 volumes (1:10 w/v) of 20 mmol/L sodium phosphate buffer, pH 7.4, containing 140 mmol/L KCl. Homogenates were centrifuged at 750 × g for 10 min at 4 °C, the pellet was discarded, and the supernatant was immediately separated and used for the measurements.
Free radical levels
Homogenates were overlayed with 100 μL 25 μM dichlorofluorescein diacetate (DCFDA) and placed back in the incubator for 30 min at 37 °C. At the end of the incubation period, plates were removed, and the fluorescence of the homogenates was measured on a SpectraMax M2e Microplate Reader (Molecular Devices, MDS Analytical Technologies, Sunnyvale, CA, USA). The excitation/emission wavelengths for DCFDA were 480/520 nm. Relative fluorescence (RFU) values were expressed as RFU/mg protein[27].
Thiobarbituric acid reactive substances
Thiobarbituric acid reactive substances (TBARS), a measure of lipid peroxidation, were determined according to Esterbauer and Cheeseman[28]. Homogenates were mixed with 10% trichloroacetic acid and 0.67% TBARS and heated in a water bath for 25 min. The absorbance determined TBARS at 535 nm. Results were reported as nmol TBARS/mg protein.
Superoxide dismutase assay
Superoxide dismutase (SOD) activity was evaluated by quantifying the inhibition of superoxide-dependent autoxidation of epinephrine and verifying the absorbance of the samples at 480 nm[29]. Briefly, 20 µL homogenate was added to 170 µL of a mixture containing 50 mmol/L glycine buffer pH 10.2 and 10 mmol/L catalase (CAT). After that, 10 µL epinephrine was added, and the absorbance was immediately recorded every 30 s for 12 min at 480 nm in a SpectraMax M2e Microplate Reader (Molecular Devices). The inhibition of autoxidation of epinephrine occurs in the presence of SOD, whose activity can then be indirectly assayed spectrophotometrically. One SOD unit was defined as the amount of SOD necessary to inhibit 50% of epinephrine autoxidation, and the specific activity is reported as SOD units/mg protein.
CAT
CAT activity was assayed according to the method described by Chance and Machley[30], based on the disappearance of at 240 nm. Briefly, 10 µL homogenate was added to 180 µL 20 mmol/L potassium phosphate buffer, pH 7.2. Subsequently, 10 µL 5 mmol/L H2O2 was added, and the absorbance was immediately recorded every 30 s for 10 min using a SpectraMax M2e Microplate Reader (Molecular Devices. One CAT unit was defined as 1 µmol H2O2 consumed per minute, and the specific activity is calculated as CAT units/mg protein.
Measurement of nitric oxide production
The production of nitric oxide (NO) was estimated by measuring the amount of nitrite, a stable metabolite of NO[31]. Briefly, 100 µL homogenate was mixed with 100 µL Griess reagent (1% sulfanilamide; 0.1% naphthyl ethylenediamine; 2.5% H3PO4) at ambient temperature. After 20 min, absorbance was measured at 540 nm using a SpectraMax M2e Microplate Reader (Molecular Devices). A nitrite calibration curve was used to convert absorbance to µM nitrite.
Alkaline comet assay
The alkaline comet assay was performed as previously described by Singh et al[32]. Briefly, 10 µL cells were mixed with 90 µL LMP agarose, spread on a standard agarose precoated microscope slide, and placed at 4 °C for 5 min to allow for solidification. Cells were lysed in high salt and detergent concentrations (2.5 M NaCl, 100 mmol/L Na2EDTA, 10 mmol/L Tris with 1% Triton X-100 and 10% DMSO freshly added) for 2 h. Slides were removed from the lysing solution and washed three times with PBS. Subsequently, cells were exposed to alkali conditions (300 mmol/L NaOH/1 mmol/L Na2EDTA, pH > 13, 30 min, 4 °C) to allow DNA unwinding and expression of alkali-labile sites. Electrophoresis was conducted for 25 min at 25 V and 300 mA (94 V/cm). After electrophoresis, the slides were neutralized and silver-stained[33]. One hundred cells were scored visually according to the tail length and the amount of DNA in the tail. Each comet was given an arbitrary value of 0–4 (0, undamaged; 4, maximally damaged), as described by Collins et al[34]. Damage score was thus assigned to each sample and ranged from 0 (completely undamaged: 100 cells 0) to 400 (with maximum damage: 100 cells 4). International guidelines and recommendations for the comet assay consider that visual scoring of comets is a well-validated evaluation method, as it highly correlates with computer-based image analysis[33,34].
Cell viability assay
Cell viability was determined by a trypan blue dye-exclusion assay (TBDE) used as a cytotoxic measurement[8]. Trypan blue staining is a long-standing and widely used method to identify dead cells. Only cells with intact membranes can effectively exclude the dye, so dead cells with compromised membranes become stained. Homogenates were mixed with 0.4% trypan blue solution for each group, which was then added. Cytotoxicity (cellular growth inhibitory rate) was determined from the number of viable cells (no color) in treated samples as a percentage of the PBS control. We used the Countess® Automated Cell Counter (Invitrogen, Carlsbad, CA, USA).
Assessment of apoptosis by flow cytometric analysis
Annexin V-PE was used with a vital dye, 7-AAD, to distinguish apoptotic (Annexin V-PE positive, 7-AAD negative) from necrotic (Annexin V-PE positive, 7-AAD positive) cells. After treatment, cells were collected and resuspended in 40 µL binding buffer with 2 µL Annexin V-PE. Cells were incubated for 15 min in the dark at room temperature. After incubation, 160 µL binding buffer and 2 µL 7-AAD were added. Cells were incubated for 5 min, and an additional 200 µL binding buffer was added. Before analysis, cells were filtered through a cell strainer cap fitted to a polystyrene round bottom flow cytometric tube. Data were collected and analyzed by a FACS Calibur flow cytometer with CellQuest software in a total of 10 000 events per sample; fluorescence was measured, and the percentage of viable, early apoptotic, late apoptotic, and necrotic cells was determined[35].
Quantification of cleaved caspase 9 by flow cytometric analysis
After treatment, cells were harvested and resuspended in 25 μL PBS and fixed with 4% formaldehyde. After permeabilization and blocking (0.2% Triton X-100 in PBS and 1% BSA), cells were incubated with the primary antibodies diluted 1:1000 for 1 h at room temperature, followed by incubation with anti-rabbit FITC secondary antibody at 1:1000 for 1 h at room temperature in the dark. Fluorescence intensity in arbitrary units was plotted in histograms, and the mean fluorescence intensity was calculated using CellQuest software.
Assay of electrochemical potential
After treatment, cells were incubated with 2 mmol/L Rh123 (rhodamine 123) for 30 min at 37 °C, washed, and resuspended in 100 μL PBS. The mitochondrial electrochemical potential was correlated to the fluorescence intensity of Rho123 (with decreased fluorescence signifying loss of the mitochondrial electrochemical potential)[36]. Flow cytometry was performed using a FACS Calibur (Becton Dickinson, San Jose, CA, USA) with excitation at 488 nm and emission read using a 525–550 nm filter (FL1).
Protein determination
Protein was measured using the method of Lowry et al[37], using bovine serum albumin as a standard.
Statistical analysis
Data were analyzed by one-way analysis of variance (ANOVA), and means were compared using the Tukey test, with P ≤ 0.05 considered statistically significant, using GraphPad Prism version 5.0 Program (Intuitive Software for Science, San Diego, CA, United States). All data were expressed as mean ± SEM. All analyses were performed in duplicate. The statistical methods of this study were reviewed by the statistical office of the Federal University of Health Sciences of Porto Alegre-NUPESQ-UFCSPA (https://ufcspa.edu.br/pesquisa-e-inovacao/apoio-a-pesquisa/assessoria-estatistica).
RESULTS
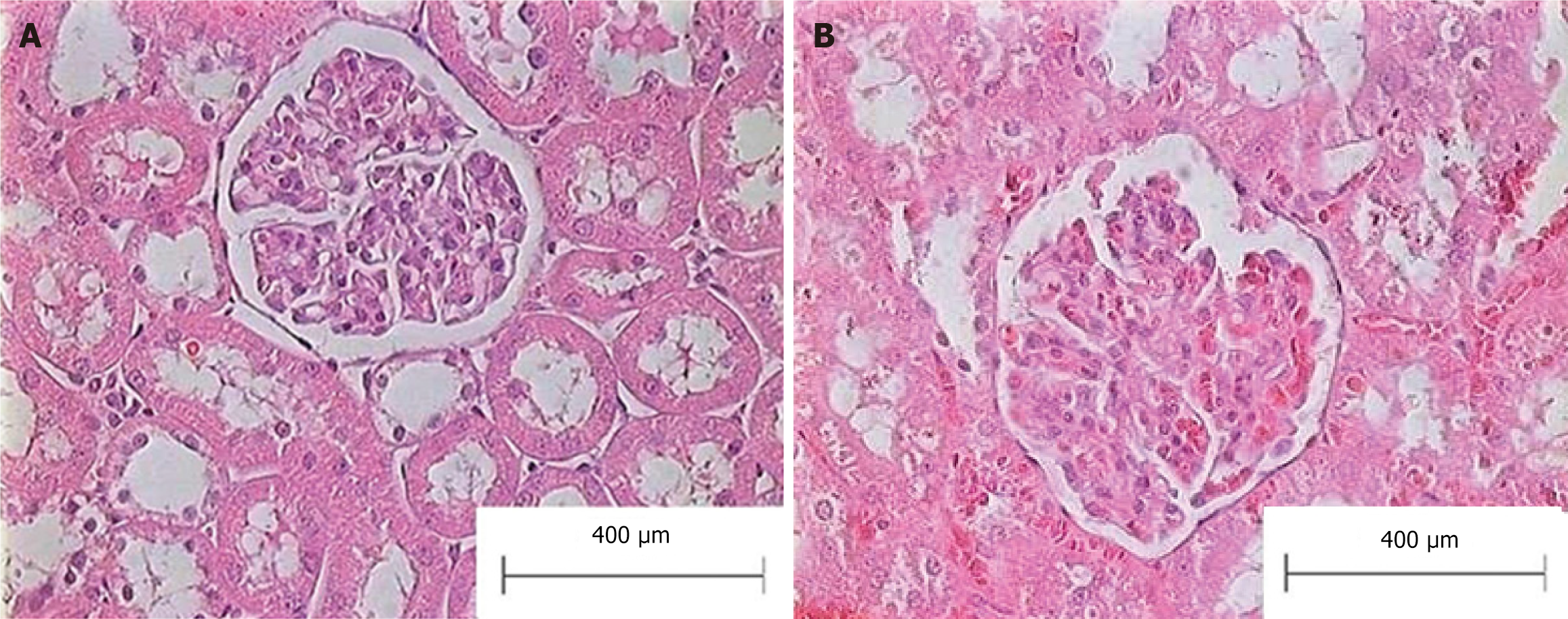
Histological evaluation of the liver of animals with BDL showed a loss of typical architecture and the presence of regenerative nodules, cellular necrosis, and fibrosis (data not shown). No alterations in renal histology were observed in bile duct ligated rats compared to sham-operated animals, as shown in Figure 2. The exclusion of structural kidney damage is a crucial component in HRS diagnosis. We did not perform other markers of renal function assessment, as the BDL model is widely accepted and validated for the experimental study of HRS.
Figure 2 Representative micrographs of the renal slices (hematoxylin and eosin) from bile duct ligated and sham-operated (control) rats.
A: Control group; B: Bile-duct-ligated group.
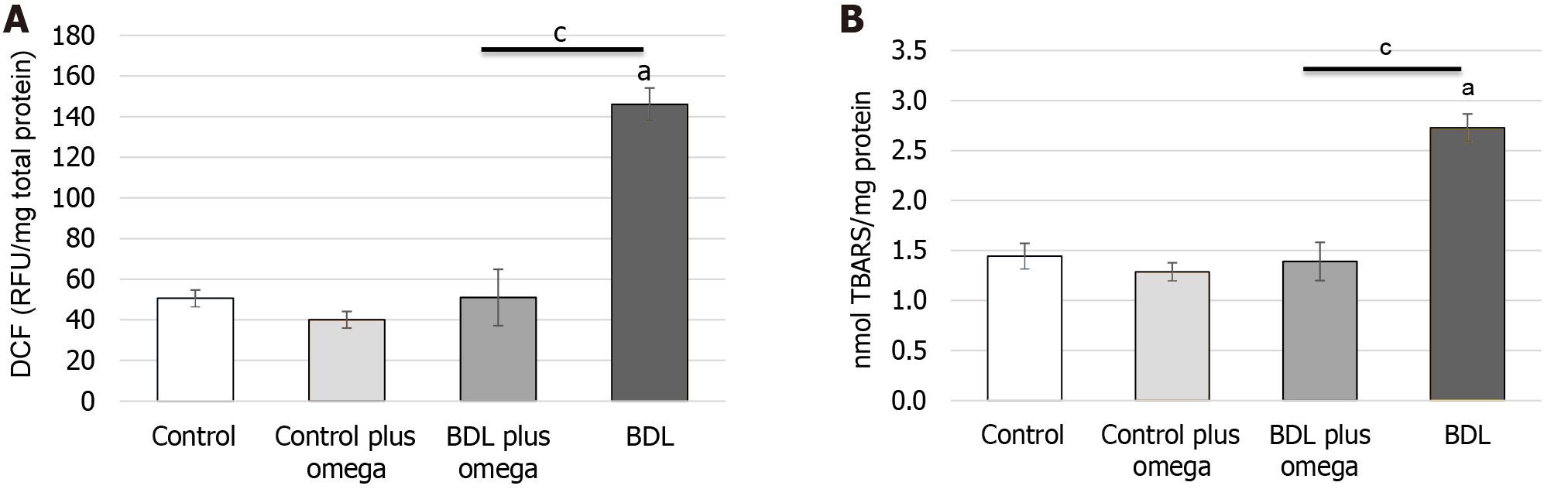
Initially, we investigated the effect of exposure to omega-3 on some parameters of oxidative stress in rat kidneys. As shown in Figure 3, omega-3 decreased ROS production significantly (P < 0.001), and TBARS level was reduced by omega-3 (P < 0.001) in HRS.
Figure 3 Effect of omega-3 on the generation of free radicals and lipoperoxidation in the kidneys of cirrhotic rats.
A: In vivo effect of omega-3 on flow cytometry detection of reactive oxygen species in renal homogenates. Mean data for dichlorofluorescein fluorescence; B: Effect of omega-3 on lipoperoxidation measured by thiobarbituric acid reactive substances in the kidney of rats. Values represent the mean ± SD of n = 6 animals per group. aP ≤ 0.001 vs control group; cP ≤ 0.001 vs BDL with BDL plus omega group. DCF: Dichlorofluorescein; TBARS: Thiobarbituric acid reactive substances; BDL: Bile duct ligation.
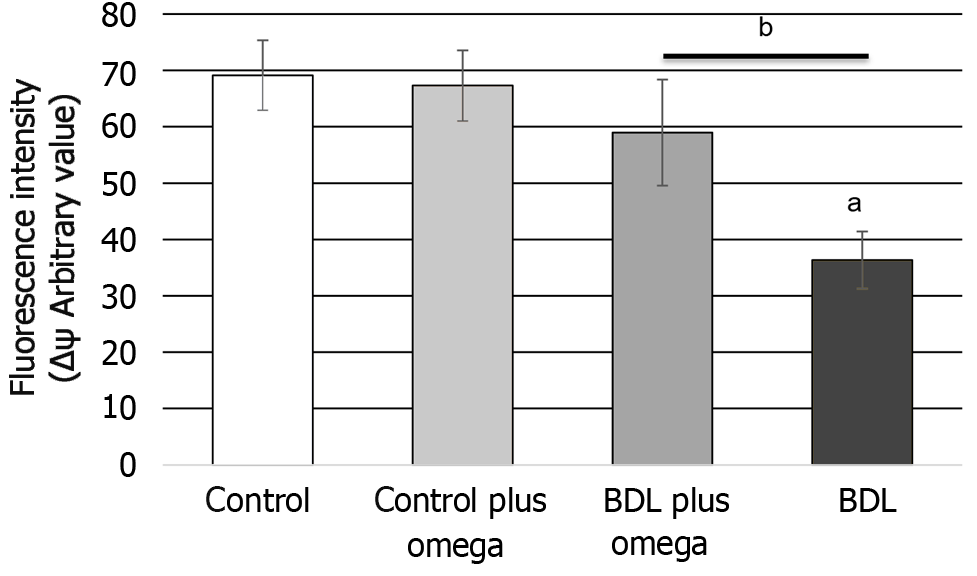
We also observed an increase in mitochondrial electrochemical potential by omega-3 (P < 0.01) in HRS (Figure 4).
Figure 4 In vivo effect of omega-3 on mitochondrial membrane potential in the kidney of rats.
Values represent the mean ± SD of n = 6 animals per group. aP ≤ 0.001 versus control group, bP ≤ 0.01 versus BDL with BDL plus omega group. BDL: Bile duct ligation.
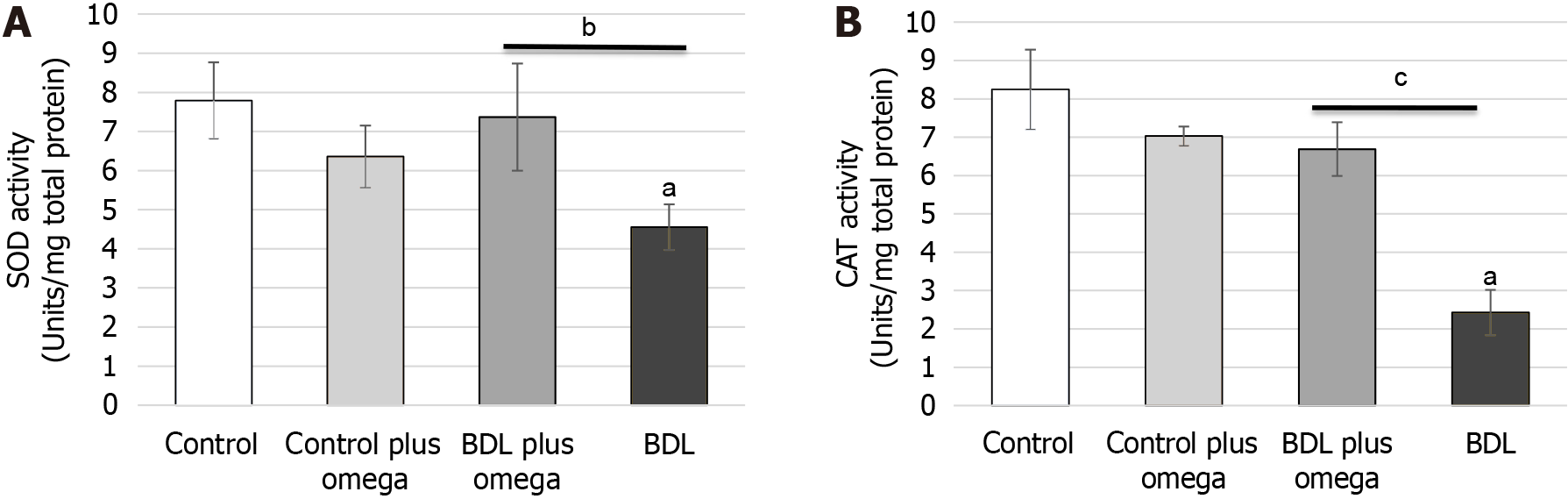
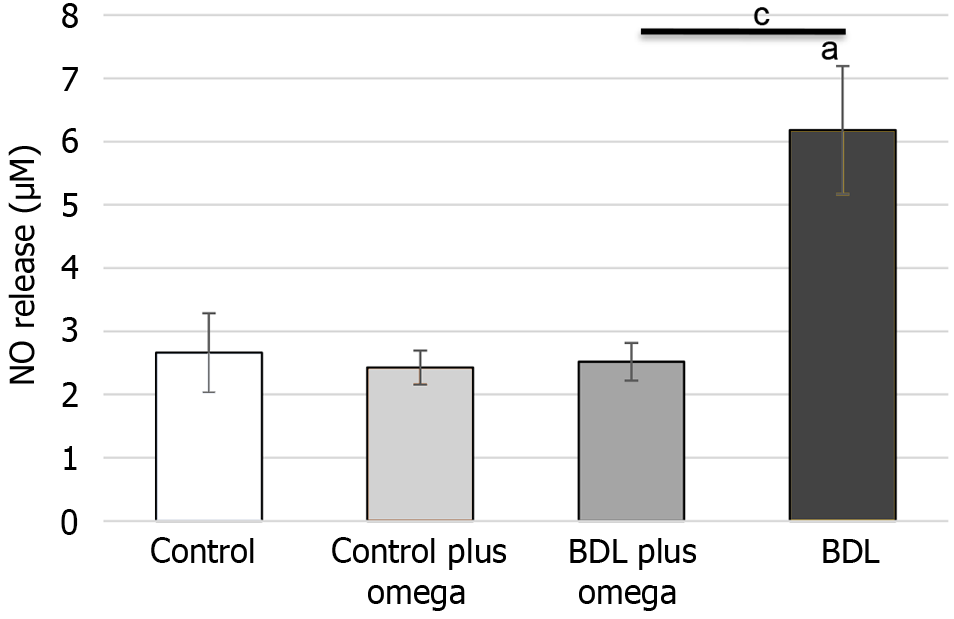
Regarding antioxidant enzymes (Figure 5), omega-3 increased SOD activity (P < 0.01). Control plus omega-3 did not alter activity of this enzyme (P > 0.05). CAT activity was significantly increased by omega-3 (P < 0.001). Figure 6 shows omega-3 inhibited NO production (P < 0.05).
Figure 5 Effect of omega-3 on the activity of antioxidant enzymes measured in the kidneys of cirrhotic rats.
A: In vivo effect of omega-3 on superoxide dismutase activities in the kidneys of rats. B: In vivo effect of omega-3 on catalase activities in the kidneys of rats. Values represent the mean ± SD of n = 6 animals per group. aP ≤ 0.001 versus control group, bP ≤ 0.01 and cP ≤ 0.001 versus BDL with BDL plus omega group. BDL: Bile duct ligation; SOD: Superoxide dismutase; CAT: Catalase.
Figure 6 In vivo effects of omega-3 on nitric oxide production.
Values represent the mean ± SD of n = 6 animals per group. aP ≤ 0.001 versus control group, cP ≤ 0.001 versus BDL with BDL plus omega group. BDL: Bile duct ligation; NO: Nitric oxide.
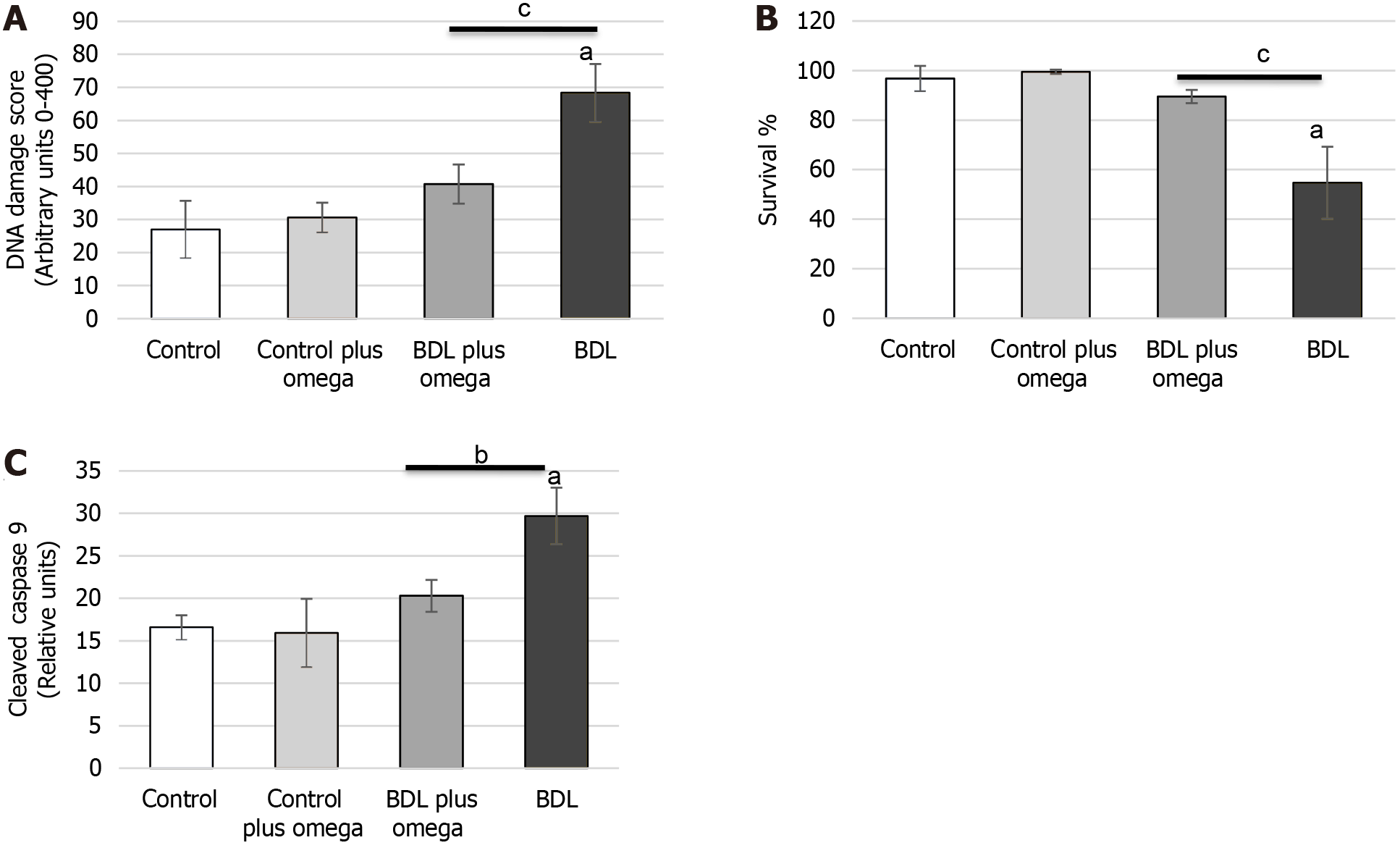
To assess the DNA damage, we performed an alkaline comet assay. Figure 7A shows that omega-3 significantly decreased DNA damage (P < 0.001). No changes in HRS omega-3 were observed compared to the control group (P > 0.05).
Figure 7 Effect of omega-3 on DNA damage and cell survival in the kidney of cirrhotic rats.
A: In vivo effect of omega-3 on DNA damage in the kidneys of rats; B: Effect of omega-3 on cell viability determined by trypan blue dye-exclusion assay; C: Effect of omega-3 on caspase 9 cleaved. Values represent the mean ± SD of n = 6 animals per group. aP ≤ 0.001 versus control group, bP ≤ 0.01 and cP ≤ 0.001 versus BDL with BDL plus omega group. BDL: Bile duct ligation.
Omega-3 treatment preserved cellular integrity in the kidneys of cirrhotic animals, as observed by cell viability determined by a TBDE (Figure 7B).
To delineate the mechanism of omega-3 after treatment, we monitored the caspase-9 activity (Figure 7C). Caspase-3 did not change at 1 h (P > 0.05). Caspase-9 was decreased by omega-3 in HRS (P < 0.001).
DISCUSSION
Decompensated liver cirrhosis in an advanced stage is difficult to treat, as it affects several systems and organs, causing numerous complications such as PH, ascites, hepatic encephalopathy, spontaneous bacterial peritonitis, and HRS[4]. In HRS, deterioration of renal function is mainly caused by systemic circulatory dysfunction[3]. There is a significant constriction of the blood vessels that irrigate the kidneys due to splanchnic vasodilation, which increases central blood volume and interferes with renal reflex mechanisms in response to PH[7]. HRS is understood as a poor prognosis for liver disease patients, as it presents high mortality rates, especially in decompensated patients and noncandidates for liver transplantation[6,38].
The functional nature of HRS means that improvement in renal function is expected with liver transplantation, which remains the optimal treatment of AKI-HRS whenever feasible. However, kidney recovery is not universal and depends on multiple factors, particularly the duration of kidney injury. In such cases, simultaneous liver–kidney transplantation is recommended rather than liver transplantation alone. Despite best efforts, almost 10% of patients with either acute kidney disease or chronic kidney disease who receive a liver alone may have persistent or progressive renal failure after transplantation[7]. In this context, searching for complementary treatments that can protect the kidney from the damage caused by liver cirrhosis is essential to guarantee the quality of life of the post-transplant patient.
The use of omega-3 polyunsaturated fatty acids has aroused researchers' interest in recent decades due to their various roles in treating and reducing the risk of diseases. The main types of omega-3 are eicosapentaenoic acid (EPA) and DHA, found in cold-water fish. Omega-3 can also be found in seeds and oilseeds, such as walnuts, chestnuts, flaxseeds, and sunflower seeds, in the form of linolenic acid, a precursor to the endogenous synthesis of EPA and DHA. However, this bioconversion is limited[39]. Numerous studies have highlighted the benefits of using omega-3 in cardiovascular diseases[40], diabetes[41], immune system and inflammatory modulation[42], and neurodegenerative diseases[43].
In the present study, we used animal model rats subjected to bile duct ligation for 28 d, according to the protocol by Kountouras et al[26], which allows the development of the clinical picture of secondary biliary cirrhosis triggered by the accumulation of bile in the liver parenchyma. In this model, from 4 wk of BDL, it is possible to identify HRS characteristics with ascites, fluid imbalance, sodium retention, and increased creatinine[10]. Renal failure observed in the chronic BDL model shares pathophysiological similarities with HRS[44].
Animal models of human diseases are important tools for studying the cellular and molecular mechanisms involved and allow the possibility of using invasive methods and testing new therapies that would be unfeasible in human patients, whether for technical or ethical reasons. From this perspective, we inquire about the benefits of omega-3 supplementation in light of the complex and difficult-to-manage scenario that HRS presents to patients affected by the disease.
In this model, we evaluated the effect of supplementation with 1 g/kg/d of omega-3 for 2 wk from the day 14 of BDL on the kidney tissue of cirrhotic rats. We observed a significant reduction in markers of oxidative stress with a decrease in the production of ROS, lipoperoxidation measured by TBARS, improvement in mitochondrial dysfunction assessed by mitochondrial electrochemical potential, and an increase in the activity of SOD and CAT.
Both EPA and DHA have a range of antioxidant and anti-inflammatory effects. Inflammation and oxidative stress are interrelated, and oxidative stress can activate inflammatory signaling pathways, while inflammation induces oxidative stress. Therefore, agents that act to reduce oxidative stress can also have anti-inflammatory action and vice versa[45].
In subjects with cirrhosis and PH, oxidative stress plays an essential role in the pathogenesis of several complications. These complications include hyperdynamic circulation, cirrhotic cardiomyopathy, and AKI/HRS. Antioxidants can reverse or mitigate these processes and thus may have potential therapeutic effects on cardiovascular and renal abnormalities in cirrhosis[46].
Omega-3 polyunsaturated fatty acids can act as antioxidants and modulate inflammatory processes[47]. Intervention studies have demonstrated that increased intake of EPA + DHA results in increased concentrations of these fatty acids in cell membranes, which may be partly responsible for reducing membrane lipoperoxidation. Furthermore, they decrease the production of eicosanoids derived from arachidonic acid (AA)[25,48,49]. Increased omega-3 content is associated with reduced levels of other inflammatory markers, including several cytokines and chemokines, acute phase proteins, and adhesion molecules[50,51].
The use of omega-3 in an animal model of hepatitis was also able to reduce the production of ROS[52]. In contrast, in patients with advanced NAFLD, the effects of omega-3 supplementation are not evident. However, in the early stages of liver disease, omega-3 supplementation may be effective in counteracting oxidative stress and steatosis[53,54] since there is a correlation between NAFLD and depletion of polyunsaturated fatty acids[55]. In an animal model of alcoholic liver disease, omega-3 supplementation decreased steatosis and alcohol-induced liver injury through multiple mechanisms, including decreased de novo lipogenesis and lipid mobilization from adipose tissue, increased beta-oxidation of mitochondrial fatty acids, reduction of liver inflammation and oxidative stress[56]. The reduction in inflammation results from the well-described effects of EPA and mainly DHA in competing with AA for the delta-6-desaturase enzyme. This event forms metabolites with anti-inflammatory action, such as resolvins, maresins, and protectins. Decreased lipid accumulation in the liver is mediated through modulation of the activity of nuclear transcription factors such as peroxisome proliferator-activated receptors and sterol regulatory element-binding protein 1c. Responsive carbohydrate binding involves inflammatory pathways and liver lipid metabolism[24,25]. The use of omega-3 in liver disease improves the condition of the liver and thus reduces damage to kidney tissue since the kidney changes observed in HRS are due to complications of cirrhosis. Previous studies have already demonstrated that the use of antioxidants improves and/or protects the liver from the development of cirrhosis and reduces disease complications[57-59]. Cirrhotic animals treated with melatonin showed a reduction in hepatic transaminases in the blood, a decrease in lipoperoxidation, and an increase in SOD activity in the liver, which led to reduced liver damage[58]. Also, in the BDL cirrhosis model, melatonin treatment improved biochemical and histological parameters and liver inflammatory markers. A significant reduction in lipoperoxidation, SOD activity, and NO levels was observed[59]. In a model of severe acute hepatitis, the combination of resveratrol + ε-viniferin had a hepatoprotective effect, reducing DNA damage, exhibiting a protective role in the antioxidant pathway by altering the activities of SOD, CAT, and glutathione s-transferase, negatively regulating tumor necrosis factor (TNF)-α, cyclo-oxygenase (COX)-2, and inducible NO synthase (iNOS) and increasing interleukin (IL)-10[57].
In a chronic kidney injury model with C57BL/6 mice treated with omega-3 for 7 d, lower oxidative stress, inflammation, and fibrosis were demonstrated compared to untreated mice[60]. It has already been shown that omega-3 derivatives, maresins, and protectins reduce inflammation and protect the kidneys in AKI[61]. In a model of AKI associated with sepsis, pretreatment with maresin-1, a lipid mediator derived from DHA, reduced the production of malondialdehyde and improved the activity of SOD in renal tissues while inhibiting the production of ROS and protected mitochondria[62]. In a clinical trial with hemodialysis patients who received 3 g of omega-3 for 2 mo, an increase in the antioxidant enzymes SOD and glutathione peroxidase and a reduction in lipoperoxidation were observed, indicating an improvement in the antioxidant status of hemodialysis patients[63]. When added to cell membranes, polyunsaturated fatty acids act as antioxidants and regulate antioxidant signaling pathways. Mitochondrial membranes of eukaryotic cells have a high content of DHA, indicating that DHA is a fundamental phospholipid for synthesizing ATP by oxidative phosphorylation. In mitochondria, DHA acts by reducing oxidative stress and the activity of cytochrome-c oxidase (complex IV), as well as increasing the activity of manganese-dependent SOD[47,64]. The protective effect of omega-3 on cardiovascular disease is well known, and dietary supplementation has been shown to reduce mitochondrial dysfunction related to oxidative stress and endothelial cell apoptosis, an effect that occurs through an increase in the activity of endogenous antioxidant enzymes[47].
Systemic inflammation plays an important role in the pathophysiology of HRS but the exact mechanisms by which systemic inflammation leads to HRS remain to be elucidated[65].
Another important finding in our study was the reduction in NO metabolites in the kidney tissue of animals treated with omega-3. NO is considered an ambiguous molecule that can act beneficially, such as in providing vasodilation or harmfully, in situations of oxidative stress and deficiency in the antioxidant system[66]. Our research showed a significant increase in NO metabolites in the kidney tissue of cirrhotic animals, and omega-3 attenuated NO production in treated animals, highlighting an antioxidant effect of omega-3 supplementation. This effect was also observed by Bosco et al[67] in the lung tissue of cirrhotic animals in the hepatopulmonary syndrome model when treated with melatonin. It is known that an increase in oxidative stress induces endothelial dysfunction since ROS reduces the bioavailability of NO and increases the synthesis of more toxic oxidative species, such as peroxynitrite[68]. In cirrhosis, the presence of PH increases the release of NO through increased expression and activity of iNOS, and renal vasoconstriction, typical of HRS, also increases the NO content resulting from the activation of iNOS. In this condition, the increase in NO levels is directly related to the oxidative damage to tissues resulting from peroxynitrite formation[69]. Omega-3 supplementation reduces NO levels in kidney tissue and thus reduces the damage caused by nitrosative stress. Omega-3 stimulates the expression and activity of endothelial NO synthase (eNOS), which is one of the important effects of the cardiovascular protective action exerted by omega-3. EPA determines greater NO synthesis by increasing the activation of eNOS induced by AMP-activated protein kinase and the dissociation of eNOS from the inhibitory structure protein caveolin. eNOS activity is also stimulated by DHA, which favors the connection between eNOS and heat shock protein 90, with activation of the PKB/Akt pathway[70,71]. Treatment with omega-3 was also able to reduce the rate of DNA damage assessed by the comet assay in kidney tissue, inhibit the expression of caspase-9, and increase cell viability in the kidneys of treated cirrhotic animals. DHA pretreatment in lipopolysaccharide-stimulated bone-marrow-derived macrophages blocked caspase-1 activation and IL-1 secretion[72]. EPA treatment also reduced oxidative damage and expression of caspase-9 and caspase-3 in a mouse model of diabetic kidney injury[73]. In a vancomycin renal injury model, DHA inhibited oxidative stress and inactivated the MAPK signaling pathway, which was associated with upregulation of Bcl-2 and downregulation of caspase-9, caspase-3, cytochrome-c, p38 and C-Jun N-terminal kinase (JNK)[74]. In a model of liver injury caused by valproate, treatment with DHA prevented hepatocellular apoptosis by reducing the expression of cleaved caspase-9 and the number of positive hepatocytes by the TUNEL technique[75]. Caspase-9 acts as an apoptotic trigger stimulated by factors such as NADPH oxidase (NOX)/ROS, extracellular signal-regulated kinase (ERK)1/2, or COX-2, which leads to oxidative and inflammatory damage in liver tissue. Omega-3, in addition to its important antioxidant effect, is capable of increasing anti-inflammatory prostaglandin E3 and inhibiting IL-1 and TNF-α, in addition to negatively regulating MAPK and ERK and thereby reducing inflammation and apoptosis[75]. Using bioactive metabolites derived from EPA and DHA, such as maresins, resolvins, and protectins, has demonstrated promising results in improving many inflammatory diseases[76]. In a model of AKI associated with sepsis, pretreatment with maresin-1, a lipid mediator derived from DHA, inhibited the expression of Bax and cleaved caspase-3 while increasing the expression of Bcl-2 in renal tissues. This effect appears to occur through inhibiting the NOX4/ROS/NF-κB p65 signaling pathway[64]. Maresin-1 also improved inflammation, reduced necrotic areas, and increased cell proliferation, as assessed by the mitotic activity index and Ki-67 expression in the livers of NAFLD animals[77]. Pretreatment with RvD1, another lipid mediator derived from DHA, attenuated apoptosis induced by endoplasmic reticulum stress and also decreased caspase 3 activity in HepG2 cells, an effect mediated through the JNK pathway[78].
In summary, the use of omega-3 treatment for 2 wk in cirrhotic animals from day 14 of BDL showed protective effects on renal tissue, as we demonstrated an antioxidant effect through the reduction in the formation of ROS, decreased lipoperoxidation measured by TBARS, decreased in NO metabolites. Furthermore, there was improvement in mitochondrial electrochemical potential and an increase in the activity of the antioxidant enzymes SOD and CAT in the kidneys of treated cirrhotic animals. We also demonstrated the protection of kidney tissue by reducing DNA damage, apoptosis, and cell death in animals with BDL treated with omega-3.