Published online Jun 25, 2024. doi: 10.5527/wjn.v13.i2.93976
Revised: May 4, 2024
Accepted: May 21, 2024
Published online: June 25, 2024
Processing time: 108 Days and 10.7 Hours
Acute kidney injury (AKI) due to interstitial nephritis is a known condition primarily attributed to various medications. While medication-induced interstitial nephritis is common, occurrences due to non-pharmacological factors are rare. This report presents a case of severe AKI triggered by intratubular oxalate crystal deposition, leading to interstitial nephritis. The aim is to outline the case and its management, emphasizing the significance of recognizing uncommon causes of interstitial nephritis.
A 71-year-old female presented with stroke-like symptoms, including weakness, speech difficulties, and cognitive impairment. Chronic hypertension had been managed with hydrochlorothiazide (HCTZ) for over two decades. Upon admis
This case underscores the importance of a thorough diagnostic approach in identifying and addressing uncommon causes of interstitial nephritis. The occurrence of interstitial nephritis due to oxalate crystal deposition, especially without typical risk factors, emphasizes the need for vigilance in clinical practice.
Core Tip: We have submitted a case report detailing a rare instance of acute kidney injury presenting as interstitial nephritis due to oxalate crystal deposition. While cases of thiazide-induced interstitial nephritis are documented, occurrences after 20 years of treatment are uncommon. This underscores the necessity of considering oxalate crystal deposition when evaluating patients on long-term thiazide diuretics without other risk factors for interstitial nephritis, emphasizing the importance of a comprehensive diagnostic approach.
- Citation: Lathiya MK, Errabelli P, Roy S, Mareedu N. Severe acute kidney injury due to oxalate crystal induced severe interstitial nephritis: A case report. World J Nephrol 2024; 13(2): 93976
- URL: https://www.wjgnet.com/2220-6124/full/v13/i2/93976.htm
- DOI: https://dx.doi.org/10.5527/wjn.v13.i2.93976
Acute kidney injury (AKI) due to interstitial nephritis is a well-known entity. Interstitial nephritis can be acute or chronic, leading to AKI or chronic kidney disease depending on the duration of exposure to the offending agent and severity of insult[1,2]. Interstitial nephritis usually occurs due to exposure to various drugs. The list of drugs available for interstitial nephritis is quite large. However, interstitial nephritis due to causes other than medications is uncommon[2]. Here, we describe a case of severe AKI due to interstitial nephritis triggered by intratubular oxalate crystal deposition and its management.
Progressive weakness in lower extremities, intermittent slurring of speech, dementia, decreased appetite, severe fatigue.
A 71-year-old female presented with stroke-like symptoms including weakness, speech difficulties, and cognitive impairment. She reported a recent episode of diarrhea but denied urinary symptoms including dysuria, urinary frequ
History of obstructive sleep apnea, peripheral vascular disease, common iliac artery stenting, Gillian Barre syndrome, Long-standing hypertension managed with HCTZ and metoprolol succinate, and hyperlipidemia. No history of renal calculi.
No relevant family history.
Afebrile, heart rate approximately 70 beats/min, respiratory rate 16 breaths per minute, blood pressure 153/128 mmHg, and weight 76 kg.
She had normal kidney function at baseline as per the patient’s previous baseline range, with a serum creatinine level of approximately 0.7 mg/dL and an estimated glomerular filtration rate (eGFR) greater than 60 mL/minute. The basic metabolic panel (BMP) on admission showed a creatinine concentration of 10 mg/dL and an eGFR concentration less than 15 mL/minute. She had moderate acidosis with an anion gap of 17, a serum bicarbonate concentration of 24 millimoles/L, a sodium concentration of 136 millimoles/L, a chloride concentration of 95 millimoles/L and a blood urea nitrogen concentration of 69 mg/dL (Table 1).
| Diagnostic test | Normal value | At presentation | Hospital day 2 | Hospital day 6 | On discharge | Follow-up |
| Hemoglobin, g/dL | 11.6-15.0 | 9.0 | 7.6 | 9.1 | 7.3 | |
| White blood cell count, × 109 /L | 3.4-9.6 | 6.0 | 4.3 | 16.8 | 9.2 | |
| Platelet count, × 109/L | 157-371 | 243 | 207 | 168 | 124 | |
| Serum urea nitrogen, mg/dL | 6-21 | 69 | 62 | 55 | 34 | 23 |
| Serum creatinine, mg/dL | 0.59-1.04 | 10.15 | 9.10 | 8.03 | 5.03 | 1.6 |
| Serum sodium | 135-145 mmol/L | 136 | 143 | 143 | 144 | 137 |
| Serum potassium | 3.6-5.2 mmol/L | 2.7 | 3.6 | 3.9 | 3.4 | 3.9 |
| Serum chloride | 98-107 mmol/L | 95 | 106 | 108 | 109 | 98 |
| Serum calcium | 8.8-10.2 mg/dL | 9.4 | 8.8 | 8.6 | 8.4 | 9.7 |
| Serum phosphorus | 2.5-4.5 mg/dL | 5.5 | 5.5 | 5.2 | 3.8 | |
| Serum magnesium | 1.7-2.3 mg/dL | 2.3 | 2.0 | 1.6 | ||
| Serum bicarbonate | 22-29 mmol/L | 24 | 21 | 21 | 22 | 26 |
| eGFR | ≥ 60 mL/min/BSA | < 15 | < 15 | < 15 | < 15 | 34 |
| Anion gap | 7-15 | 17 | 16 | 14 | 13 | 13 |
| Serum albumin | 3.5-5.0 g/dL | 3.7 |
Urinalysis on admission revealed large blood, 30 mg/dL protein, 4-10 white blood cell (WBC) counts per high-power field, no red blood cell (RBC) count, and no granular casts. The creatinine kinase level was 862 U/L. Her complete blood count was 9.8 g/dL, her platelet count was 243000/mL, and her leukocyte count was 6000/mL. She had severe hypokalemia; her potassium concentration was 2.7 millimoles/L at admission, and she received IV and oral potassium chloride (KCl) supplements. The magnesium concentration was 2.3 mg/dL.
She underwent extensive serology investigations for cryoglobulins, antineutrophil cytoplasmic antibody vasculitis, anti-GBM antibody disease, antinuclear antibody screening, monoclonal protein studies to detect paraproteinemia, serum free light chains, viral hepatitis panels, and complement agents, which were all negative (Table 2).
| Diagnostic test | Normal value | Result |
| HCV Ab screen, S | Negative | Negative |
| HBs antibody, S | Negative | Negative |
| HBs antigen, S | Non-reactive | Non-reactive |
| HBc total ab w/reflex, S | Negative | Negative |
| Complement, C3 | 75-175 mg/dL | 143 mg/dL |
| Complement, C4 | 14-40 mg/dL | 27 mg/dL |
| Cryoglobulins | Negative | Negative |
| GBM, IgG Ab | < 0.1 (Negative) U | < 0.2 U |
| MPO, Ab | < 0.4 (Negative) U | < 0.2 U |
| PR 3, Ab | < 0.4 (Negative) U | < 0.2 U |
| SPEP | No monoclonal protein | - |
| Haptoglobin, S | 30-200 mg/dL | 297 |
| Kappa free light Chain, S | 0.3300-1.94 mg/dL | 13.7 |
| Lambda free light chain, S | 0.5700-2.63 mg/dL | 8.08 |
| Kappa/Lambda FLC ratio | 0.2600-1.65 | 1.70 |
| DNA double stranded Ab, IgG, S | ≤ 4 (Negative) IU/mL | 2 |
| Sm. Ab, IgG, S | < 1.0 (Negative) U | < 0.2 |
| CRP | ≤ 8.0 mg/L | < 3.0 mg/L |
| Bilirubin, total | ≤ 1.2 mg/L | 0.8 |
| ALT, S | 7-45 U/L | 11 |
| AST, S | 8-43 U/L | 17 |
| Alkaline phosphatase | 35-104 U/L | 60 |
| Protein, total | 6.3-7.9 g/dL | 6.9 g/dL |
| Albumin | 3.5-5.0 g/dL | 3.7 g/dL |
Repeating BMP after one month showed that her serum creatinine concentration improved to 1.5 mg/dL, and her eGFR was close to 35 mL/minute. Her prednisolone dose slowly tapered over 6 wk (approximately 1 and a half months). Her potassium concentration stabilized, and she was given potassium supplements at the follow-up visit. Repeat urinalysis did not reveal any WBCs, RBCs, or proteins. Blood pressure had normalized and was hovering at approximately 120 to 130/70 to 80 mmHg during the clinic visits.
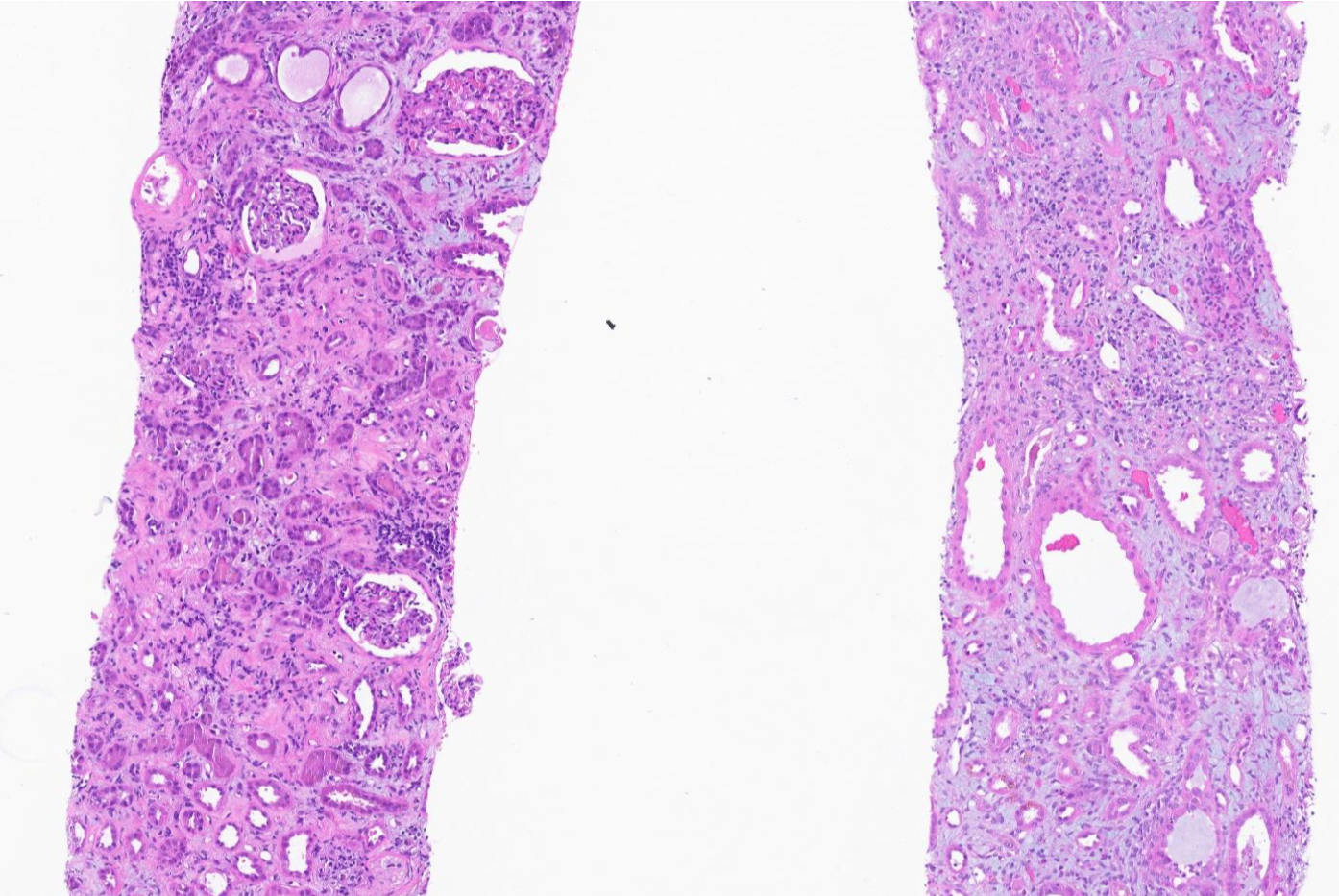
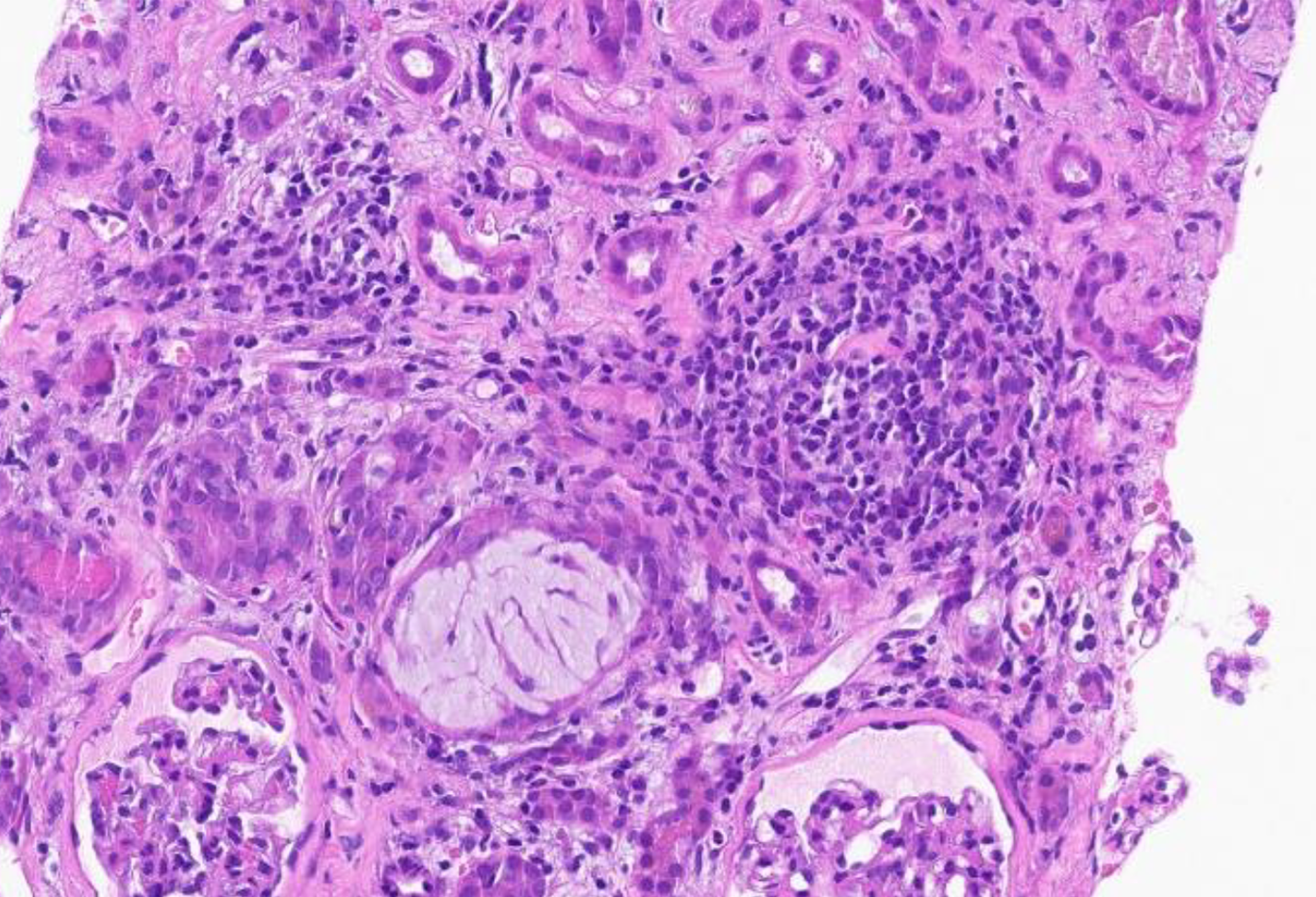
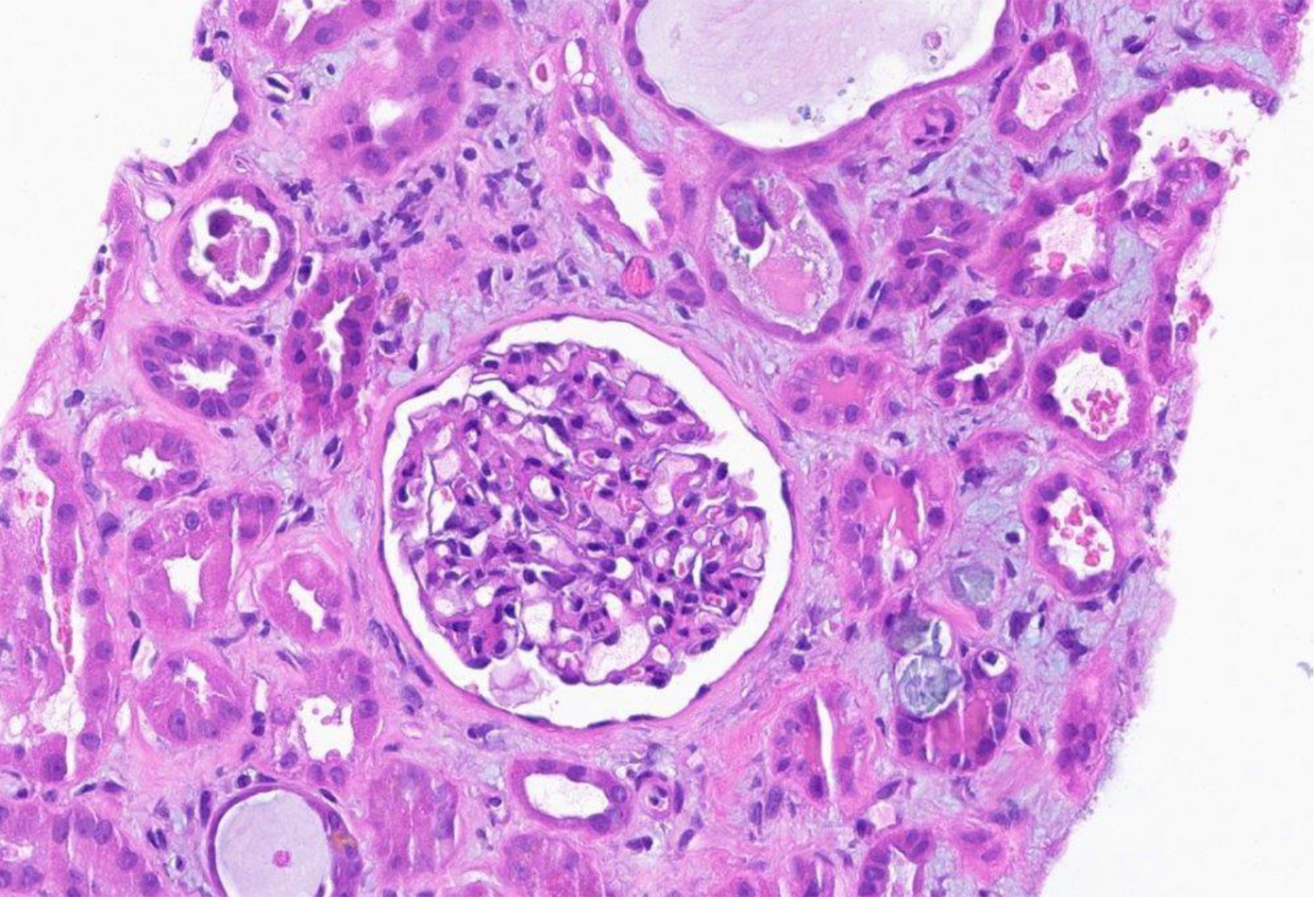
Kidney biopsy: (1) Final diagnosis: Acute onset of severe interstitial nephritis; (2) Light microscopy: The glomeruli were normal in size and had a normal mesangial matrix. There was no mesangial or endocapillary hypercellularity. Special stains do not demonstrate spikes, craters, or basement membrane remodeling; (3) Tubules and interstitium (Figures 1 and 2): Severe diffuse interstitial edema involving the cortex and medulla was observed. Severe tubular epithelial cell injury occurs with luminal ectasia, fraying of the brush border, and simplification of the lining epithelium. Tubular lumina contain necrotic debris, and some lumina contain hypereosinophilic ropy casts. The interstitium contains dense infiltrates of lymphocytes. Some areas contained aggregates of eosinophils. Mild tubulitis was observed. There are intratubular oxalate crystals (Figure 3); (4) Vessels: The visualized arteries show severe intimal fibrosis. There was no vasculitis, thrombi, or atheroembolic lesions; (5) Electron microscopy: Normal cellularity and mildly expanded mesangial regions were confirmed. No immune complex or paraprotein-related deposits were observed, and the glomerular basement membranes showed wrinkling of several of the segments; however, other regions showed no ultrastructural abnormalities. There was mild foot process effacement present. An examination of the tubulointerstitial compartment revealed interstitial edema, severe interstitial inflammation, and multifocal tubulitis. No tubular basement membrane deposits were observed; (6) Impression: Kidney, needle biopsy: Acute interstitial nephritis; and (7) Immunofluorescence: There was no significant glomerular staining for albumin, IgA, IgG, IgM, C1q, C3, fibrinogen, kappa, or lambda light chains. The cast was observed to be stained equally with IgA, kappa, and lambda light chains.
Computed tomography of the abdomen pelvis did not reveal any hydronephrosis but showed a 3 mm (approximately 0.12 in) stone in the distal left ureter. A renal artery Doppler study revealed normal-sized kidneys bilaterally with normal velocities in both renal arteries and no occlusion in the renal arteries.
Severe acute interstitial nephritis with oxalate crystal deposits leading to AKI.
She received normal saline and potassium chloride supplementation and had a normal urine output. However, her renal function did not significantly improve. HCTZ was discontinued due to hypokalemia and AKI. She was started on Nifedipine and continued to use metoprolol. After 48 h (approximately 2 d) of continuous IV fluid infusion, her creatinine level remained at approximately 9 mg/dL, and her eGFR level remained below 15 mL/minute.
Serum creatinine and eGFR improved with prednisolone treatment. Potassium stabilized, blood pressure normalized, and urinalysis normalized. Prednisolone tapered over 6 wk. Follow-up showed continued improvement in renal function and resolution of symptoms. Biopsy confirmed oxalate crystal deposits as the etiology of acute interstitial nephritis.
Interstitial nephritis is a renal disease characterized by inflammation and scarring of the kidney's tubular and interstitial components. It manifests in three primary types: immune-mediated, infection-mediated, and idiopathic. Immune-mediated interstitial nephritis can be caused by drug reactions or due to immunological diseases. Many drugs, including antibiotics, antacids, analgesics, immunotherapies, diuretics (including thiazide diuretics), antivirals, anticonvulsants, lithium, allopurinol, etc., have been linked to interstitial nephritis[2]. The mechanism through which drugs induce interstitial nephritis varies[3]. As mentioned, drug-induced interstitial nephritis is a well-documented entity associated with thiazide diuretics, and our case report adds a complex layer to this well-known entity[1,2,4].
In our patient, the complexity was enhanced when multiple oxalate crystals were identified via kidney biopsy along with interstitial nephritis. Oxalate nephropathy is a rare pathology that can be difficult to diagnose clinically and requires a biopsy. This presentation aligns with crystalline nephropathy, a condition marked by crystal precipitation in kidney tubules[2,3]. Crystalline nephropathy poses risks of both acute and chronic kidney injuries. Various factors contribute to the risk of crystal deposition, encompassing intravascular volume depletion, underlying kidney disease, and metabolic imbalances that alter urinary pH[2,3]. The intricate interplay of supersaturation, urine pH, and crystallization inhibitors influences intratubular crystal deposition. Drug-induced crystal precipitation, often associated with supersaturation in low urine volume or drug insolubility in acidic or alkaline urine pH, can exacerbate renal complications. Metabolic disturbances, including systemic acidosis or alkalosis and renal tubular acidosis, play a role in worsening intrarenal crystal deposition. Patient characteristics linked to medication intake predispose individuals to intratubular crystal deposition and subsequent tubular obstruction[2,3].
Severe volume depletion, prevalent in conditions such as chronic diarrhea, anorexia, excessive diuresis, febrile illnesses, adrenal insufficiency, and renal salt wasting, is a crucial factor in the development of AKI. Conditions leading to effective intravascular volume depletion, such as pancreatitis, ascites, heart failure, pleural effusions, and nephrotic syndrome, contribute to renal hypoperfusion, heightening the risk of tubular crystal deposition[2,3]. Urine pH further modulates crystallization, with certain drugs exhibiting varying solubilities in acidic or alkaline urine. Crystal precipitation within the kidneys obstructs tubular lumens in the distal nephron, involving crystals mixed with cellular debris and proteinaceous material[4,5]. Gastrointestinal disorders involving small bowel dysfunction or defective fat and bile acid absorption contribute to enteric hyperoxaluria, which is characterized by increased gastrointestinal oxalate absorp
Numerous case reports have described drug-induced crystallopathy[6-10]. In our case, the patient had been on a thiazide diuretic for more than two decades without any previous kidney injury or associated complications. The continued exposure to a thiazide diuretic throughout the period during which she had diarrhea raises the possibility of severe volume depletion and enteric hyperoxaluria as contributing factors to her crystal formation. Prolonged exposure to thiazide diuretics without any adverse events ruled out the possibility that interstitial nephritis was solely due to the thiazide diuretic. However, it is quite possible that thiazide diuretic use in the setting of diarrhea might aggravate volume depletion, leading to oxalate crystal deposition, which triggers the onset of interstitial nephritis.
Recommendations to reduce the risk of developing crystal nephropathy emphasize maintaining adequate volume status and avoiding concurrent use of diuretics, angiotensin-converting-enzyme inhibitors, angiotensin receptor blockers, and NSAIDs[2,3]. In cases where crystal nephropathy occurs, sustaining a high urine flow rate is crucial for minimizing further crystal precipitation and dislodging obstructing crystals. Early detection and withdrawal of the causative agent can be achieved through timely examination of urine sediment in individuals experiencing AKI, which can sometimes demonstrate crystals. Monitoring facilitates the implementation of supportive measures, including volume repletion, urine alkalinization and, rarely, hemodialysis, in severe cases of AKI[2,3]. Case reports have highlighted various treatments for oxalate crystallopathy, often tailored to underlying causes. For food-induced crystallopathy, a low-oxalate diet, high fluid intake, and calcium acetate have shown efficacy[11]. Treatment for oxalate disorders depends on whether they're primary or secondary. Enteric hyperoxaluria patients benefit from a low-fat, low-oxalate diet. Those with fat malabsorption may need calcium supplements or pancreatic enzyme supplementation (in case of pancreatic insufficiency). Lanthanum carbonate shows promise for secondary hyperoxaluria. Primary hyperoxaluria (PH) requires strategies to reduce endogenous oxalate production, with PH1 patients possibly benefitting from pyridoxine supplementation. Dialysis is essential if renal function declines, with transplantation preferred for severe systemic oxalosis in PH patients. In severe cases requiring dialysis, high flux or continuous hemodialysis is crucial for removing excess oxalate[12].
In this case, we treated the patient with a prolonged prednisolone tapering agent via a strategy targeting the inflammatory response associated with interstitial nephritis. There was a significant improvement in renal function, which provides insight into the role of corticosteroids in the treatment of oxalate crystallography-induced interstitial nephritis.
In addition, oxalate crystal deposition is associated with kidney epithelial cell damage, suggesting that crystals induce epithelial injury and progressive inflammation, leading to interstitial nephritis[13]. Our case study revealed that prolonged exposure to diuretics can lead to volume depletion, triggering oxalate crystal deposition, which can induce severe interstitial nephritis.
It is important to fully assess the impact of oxalate crystal deposition on renal function to provide comprehensive patient care. The frequency of interstitial nephritis due to oxalate crystal deposition, especially in the context of long-term thiazide diuretic use in the absence of other risk factors for interstitial nephritis, highlights the importance of a comprehensive diagnostic approach. This case contributes to the expanding body of knowledge on renal pathology, highlighting the clinical importance of identifying and addressing uncommon causes of interstitial nephritis. Further research and case studies will play an important role in improving our understanding of such unusual presentations and optimizing treatment strategies.
| 1. | Magil AB, Ballon HS, Cameron EC, Rae A. Acute interstitial nephritis associated with thiazide diuretics. Clinical and pathologic observations in three cases. Am J Med. 1980;69:939-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 39] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | Perazella MA, Rosner MH. Drug-Induced Acute Kidney Injury. Clin J Am Soc Nephrol. 2022;17:1220-1233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 154] [Article Influence: 51.3] [Reference Citation Analysis (0)] |
| 3. | Yarlagadda SG, Perazella MA. Drug-induced crystal nephropathy: an update. Expert Opin Drug Saf. 2008;7:147-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 94] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 4. | Karimzadeh I, Barreto EF, Kellum JA, Awdishu L, Murray PT, Ostermann M, Bihorac A, Mehta RL, Goldstein SL, Kashani KB, Kane-Gill SL. Moving toward a contemporary classification of drug-induced kidney disease. Crit Care. 2023;27:435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Perazella MA, Herlitz LC. The Crystalline Nephropathies. Kidney Int Rep. 2021;6:2942-2957. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 6. | Ansari FA, Manuel S, Dwivedi R, Boraiah SK, Raju SB, Uppin M, Sharma A. A Rare Case of Acute Kidney Injury Due to Levofloxacin-induced Crystal Nephropathy. Indian J Nephrol. 2019;29:424-426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Garneau AP, Riopel J, Isenring P. Acute Methotrexate-Induced Crystal Nephropathy. N Engl J Med. 2015;373:2691-2693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | Goli R, Mukku KK, Raju SB, Uppin MS. Acute Ciprofloxacin-Induced Crystal Nephropathy with Granulomatous Interstitial Nephritis. Indian J Nephrol. 2017;27:231-233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Farge D, Turner MW, Roy DR, Jothy S. Dyazide-induced reversible acute renal failure associated with intracellular crystal deposition. Am J Kidney Dis. 1986;8:445-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Nasr SH, Milliner DS, Wooldridge TD, Sethi S. Triamterene crystalline nephropathy. Am J Kidney Dis. 2014;63:148-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Clark B, Baqdunes MW, Kunkel GM. Diet-induced oxalate nephropathy. BMJ Case Rep. 2019;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Lorenz EC, Michet CJ, Milliner DS, Lieske JC. Update on oxalate crystal disease. Curr Rheumatol Rep. 2013;15:340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 13. | Geraghty R, Wood K, Sayer JA. Calcium oxalate crystal deposition in the kidney: identification, causes and consequences. Urolithiasis. 2020;48:377-384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |