Published online Jan 25, 2023. doi: 10.5527/wjn.v12.i1.10
Peer-review started: August 12, 2022
First decision: October 12, 2022
Revised: October 14, 2022
Accepted: December 31, 2022
Article in press: December 31, 2022
Published online: January 25, 2023
Processing time: 150 Days and 14.4 Hours
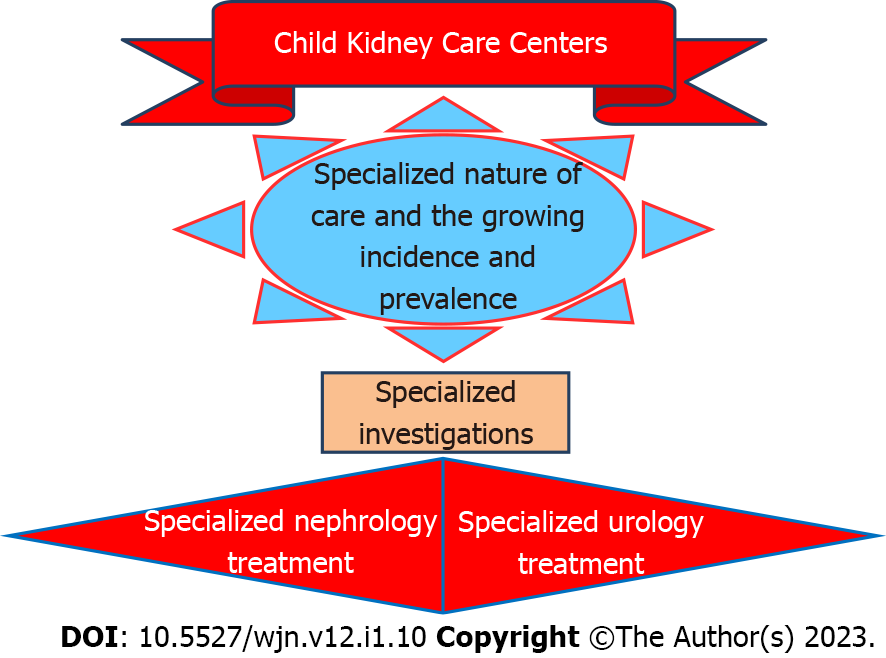
Specialized centers are needed for nephrology and urology care of children. The justifications are the specialized nature of care needed and the growing incidence and prevalence. Children with chronic kidney disease (CKD) are at risk of morbidity, mortality, and decreased quality of life. Current pediatric practice structures are apparently poorly suited for the increasing demands of chronic disease in children. Kidney diseases account for around 8%-10% of total outpatients and 12% of admissions to the pediatric ward in hospitals. The major causes of pediatric CKD in registries are congenital anomalies of the kidney and urinary tract (around 50%), followed by inherited nephropathies and glomerulonephritis. The nephrologist’s role is important for specialized investigations and treatment. Urologist’s services are essential for the wide variety of conditions from birth to early adult age for complete cure and complementing medical management. Children have a right to treatments and to resources that are as sophisticated and advanced as those available to adults. Simple and sophisticated care for all children with ailments of the kidneys and related structures is important for ensuring ‘health for all’. The availability of ‘Child Kidney Care Centers’ will go a long way in improving the lives of affected children.
Core Tip: Current pediatric practice structures are apparently poorly suited to meet the growing demands of chronic disease. Serious childhood morbidity and mortality can result from chronic disorders. Specialized centers provide the opportunity for systematic and focused delivery of high-quality clinical care in a sophisticated manner. An understanding of the etiology of chronic renal failure in children guides efforts and excellence goals. The availability of specialized investigations in a center is required, and will ensure prompt care, avoiding unnecessary referrals, which causes delays. ‘Children Kidney Care Centers’ will ensure correct treatments, both nephrology and urology, with sophistication for success.
- Citation: Jain S. ‘Children Kidney Care Centers’: Rationale, requirements and recommendations for best facilities and better future. World J Nephrol 2023; 12(1): 10-20
- URL: https://www.wjgnet.com/2220-6124/full/v12/i1/10.htm
- DOI: https://dx.doi.org/10.5527/wjn.v12.i1.10
Kidneys are important for maintaining the interior milieu and hence in children for ensuring growth, for preventing morbidity, and for obviating mortality all diseases of the kidneys need focused attention. The urinary system disorders and diseases spectrum is diverse and distinct, incidence is a cause for concern, investigations are specialized, and treatment specific and timely. Hence, the need for centers for nephrology and urology care for children and the necessity for review and recommendations. Also, it has been commented that current pediatric practice structures are not suitable in meeting the growing demands of chronic disease in children. All this points to the need for major reform in organization, financing, and training[1]. Specialized centers provide the opportunity for systematic and sophisticated focused delivery of high-quality clinical care[2]. Specialized care is often challenging but universal access to treatment services is possible with commitment[3]. ‘Children Kidney Care Centers’ will be an important step in the right direction.
The International Pediatric Nephrology Association (www.ipnaonline.org) role in enhancing knowledge and communication among pediatric nephrologists, aligned practitioners, and other health professionals has been critical on progress and praiseworthy[4]. Establishment of ‘Children Kidney Care Centers’ will go a long way in specialized practices with sophisticated protocols. All the latest research developments need to be put into proper perspective for practice and future developments.
The leading causes of global under-five year of age mortality rate are preterm birth complications (15.9%) and pneumonia (15.5%). The third largest is other causes (13.5%)[5,6]. It is high time that we also focus on these other causes. The evolving trend, as suggested by analysis in the past several decades, is that chronic disease prevalence has risen, and serious acute illness incidence in children has fallen. This has resulted in an increasing concentration of serious childhood morbidity and mortality due to chronic disorders[1]. Children with chronic kidney disease (CKD) are at risk of increased lifelong morbidity, mortality, and decreased quality of life[7].
The magnitude of CKD varies in different geographical areas. This is due to genetic and environmental factors. The major causes of pediatric CKD in registries are congenital anomalies of the kidney and urinary tract (UT) (approximately 50%), followed by the inherited nephropathies and glomerulonephritis[8]. In India, the true incidence and burden of CKD in children is not known, due to the lack of a national registry. In one of the Command Hospitals in India the work load of kidney disease in children is approximately 8%-10% of total outpatient attendance. This has accounted for 12% of admissions to the pediatric ward[9]. Alarmingly, it has been reported that 58% of children with renal failure presented with end stage renal disease (ESRD)[10]. Thus, prompt diagnosis and proper management is essential. Evidence shows that children fare better than adults if they receive kidney replacement therapy including dialysis and transplantation. Disparities in access to care exist. All this justifies effective efforts for children with kidney disease. These should be for all regions and including all economic strata[11]. These distinctive features justify dedicated facilities.
Similarly, statistics for the newborn period reveal that 0.8% of cases have abdominal masses, and renal origin masses account for the majority of these. The most common etiologies are: (1) Polycystic kidneys; (2) multicystic dysplastic kidney; (3) hydronephrosis; and (4) renal vein thrombosis. There is a need for specific and specialized management[12]. Thus, there is a need for focused committed comprehensive centers.
High quality systems of healthcare delivery require health leaders and managers with adaptable and relevant capabilities. This has been thought of as critical[13]. Our justifications given above based on epidemiological evidence and the importance of timely intervention point to the need for adaptation to establish ‘Children Kidney Care Centers’. Furthermore, relevant capabilities’ building requires understanding of the spectrum of illness, the specialized investigations, and the specific treatments. These are elaborated for sophisticated establishment and scientific execution.
Conceptual frameworks illuminate and magnify illustratively. These simplify ways of representing how complex things work. A conceptual framework pictorial representation for need and necessary facilities of ‘Children Kidney Care Centers’ is given at Figure 1.
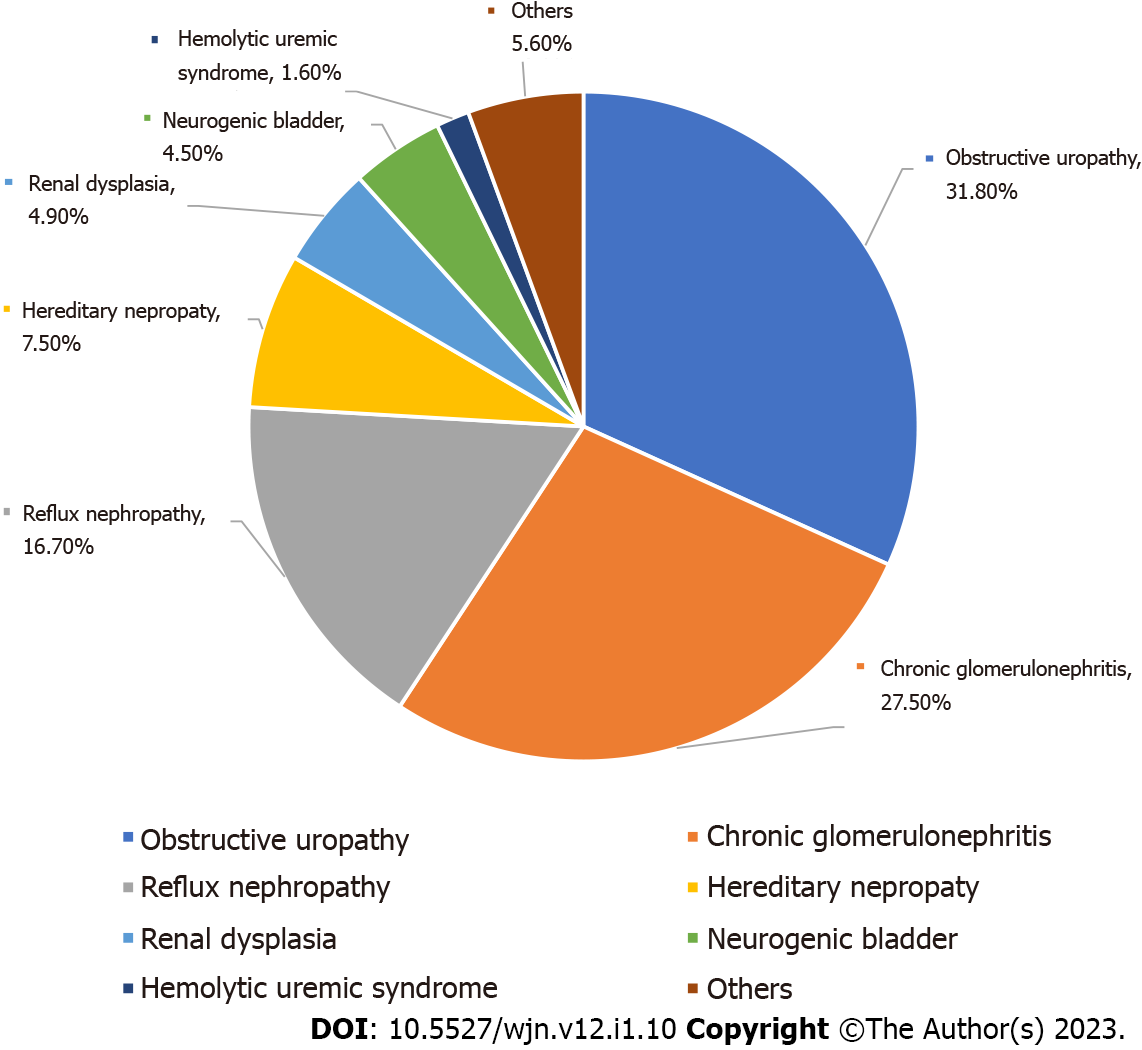
In a study of the etiology of pediatric chronic renal failure (ages 0-18 years) the common causes reported are obstructive nephropathy (31.8%), chronic glomerulonephritis (27.5%), and reflux nephropathy (16.7%). The less common causes are hereditary nephropathy (7.5%), renal dysplasia (4.9%), neurogenic bladder (4.5%), and hemolytic uremic syndrome (1.6%)[14]. Pictorial depiction of various etiologies is given at Figure 2. All this guides efforts and excellence goals at ‘Children Kidney Care Centers’.
Most childhood obstructive lesions are congenital. In a study of the etiology and burden of comor
A common renal disorder and a leading cause of ESRD is glomerulonephritis[16,17]. Its presentations are protean. The general features include proteinuria, hematuria, renal failure, and hypertension. Early therapeutic intervention is warranted and leads to renal function improvements and long-term preservation of renal function. All this is required to prevent the progression to end-stage renal failure. Hence, early evaluation is important in appropriate centers.
Glomerulonephritis types presenting with recurrent hematuria are immunoglobulin A nephropathy (Berger nephropathy) and Alport syndrome. Membranoproliferative glomerulonephritis (also termed mesangiocapillary glomerulonephritis) most commonly occurs in children or young adults. The presentation is varied and includes: (1) Nephrotic syndrome; (2) acute nephritic syndrome (hematuria, hypertension, and some level of renal insufficiency); and (3) persistent asymptomatic microscopic hematuria and proteinuria. The presentation of rapidly progressive glomerulonephritis and crescentic glomerulonephritis in most children is acute nephritis (hematuria, some degree of renal insufficiency, and hypertension). These children usually have concomitant proteinuria, often with nephrotic syndrome. Other important presentations are systemic lupus erythematosus (SLE)-associated glomerulonephritis, Henoch-Schonlein purpura nephritis, and Goodpasture disease. A common cause of community acquired acute kidney injury in young children is hemolytic-uremic syndrome (HUS). The characteristic triad of HUS is: (1) Microangiopathic hemolytic anemia; (2) thrombocytopenia; and (3) renal insufficiency. Expert diagnosis and early treatment is especially suited in specialized centers.
Vesicoureteral reflux (VUR), the reverse flow of urine from the bladder to the ureter and kidney, is a risk for kidney infection (pyelonephritis). The reaction due to inflammation caused by pyelonephritis results in renal injury or scarring. Renal function is impaired with extensive renal scarring. This also leads to hypertension (renin-mediated), renal insufficiency/ESRD, and somatic growth impairment.
Kidney injury occurs due to a variety of different mechanisms. Investigations into the type of injury, the degree of injury and assessment of progression involve laboratory tests, diagnostic imaging, and often tissue sample studies. All investigations available in a specialized center are required, and ‘Children Kidney Care Centers’ can guarantee this. This will ensure prompt care and avoid unnecessary referrals, which causes delays. The best evidence-based practices are as follows.
Glomerular filtration rate (GFR) is a measure of kidney function. The GFR can be measured optimally by the clearance of inulin. However, in clinical practice it is cumbersome. Hence, GFR estimation is commonly performed using the endogenous creatinine clearance test. The Schwartz formula is the most widely used bedside pediatric formula[18,19].
More recently, GFR estimation is assessed by cystatin C, an endogenous marker of renal function. This non-glycosylated protein is produced in all cells in relative constancy. The advantage of measuring cystatin C is that it is not influenced by age, gender, body habitus or composition[20].
Proteinuria assessment for diagnosis, prognosis, and monitoring therapy response is carried out routinely. Proteinuria on urinalysis is often the first clue to renal injury. Subtle and early glomerular injury is identified by microalbuminuria measurements. Twenty-four-hour urinary protein is the gold standard for defining proteinuria. It has been largely replaced by the spot protein/creatinine ratio measurement in a random urine sample. This correlates to 24 h urine protein reasonably well[21].
Hematuria is a nonspecific finding. It indicates some injury, and does not provide more information. However, dysmorphic red blood cells (RBCs) study with phase contrast microscopy is useful for identifying the injury site in the UT. If 75% or more of the observed RBCs are dysmorphic, the site of injury is the kidney, and most likely the glomerulus. If less than 25% of the observed RBCs are dysmorphic, the injury site is the UT from the renal pelvis downwards[22].
The commonly used dynamic tests of tubular function are fractional calcium, phosphate or sodium excretion calculations. The general formula for calculations is: (urine concentration of analyte × serum creatinine)/(serum concentration of analyte × urinary creatinine). A very low fractional excretion of calcium indicates familial benign hypercalcemia. Increased fractional excretion of phosphate indicates hypophosphatemic rickets. Calculation of fractional excretion of sodium (FENa) is useful for differentiating volume depletion from acute tubular necrosis. In volume depletion, the tubules avidly conserve sodium and FENa is typically less than 1.0. In acute tubular necrosis, the tubules are damaged and are less able to conserve sodium, and FENa is typically more than 1.0.
Facilities for measurement of 24 h excretion of solutes (calcium, phosphate, oxalate, uric acid, and dibasic amino acids) are useful in the diagnosis of renal stone disease. Twenty-four-hour collection is best, but random measurement of the urine calcium (mg/dL): Urine creatinine (mg/dL) ratio can be used in the diagnosis of hypercalciuria[23].
The diagnosis of renal disease secondary to SLE is performed by anti-nuclear antibodies tests. These include antibodies to extractable nuclear antigens and the anti-double-stranded DNA antibodies. In glomerulonephritis secondary to systemic vasculitis, antineutrophil cytoplasmic antibodies detection aids diagnosis. In Goodpasture syndrome, antibodies to glomerular basement membrane are seen. Low levels of complement are seen in SLE, systemic vasculitis and HUS.
Complement level measurements are required for fixed proteinuria and glomerular hematuria initial evaluation.
Ultrasound (US) for visualization of the UT is the principal imaging modality. For children US is advantageous. The use of transducers with high-frequency (7-11 MHz) and lower-frequency (3.5-5 MHz) is recommended. High frequency sound waves have less penetration, but provide greater resolution. Ideally one must use the maximum frequency that penetrates to the depth where study is required. As compared to large or obese individuals, high frequency US can be readily used in infants and small children producing excellent resolution.
Doppler US should be used for blood flow evaluation. It is useful in study of: (1) Renal artery disease; (2) renal vein thrombosis; (3) tumor thrombosis in the renal vein and inferior vena cava; and (4) arteriovenous fistula thrombosis. Power Doppler mode allows blood flow detection with increased sensitivity. It is better than color Doppler US. Power Doppler is useful in detecting intrarenal blood flow, and in identifying areas of decreased perfusion within the kidney. It should be utilized for detecting acute pyelonephritis[24].
US contrast imaging has many potential applications. These include: (1) Characterization of complex renal cysts; (2) assessment of renal vascular disorders, infection, and transplant kidneys; and (3) differentiation of complex renal cysts and solid lesions, and between renal pseudomasses and tumors[25].
Voiding cystourethrography (VCUG) is the gold standard for the diagnosis of VUR. It is the only modality that detects VUR and gives detailed information on the bladder and urethra[26]. The use of pulsed fluoroscopy importantly reduces VCUG-radiation exposure side effects[27].
The availability of US and magnetic resonance urography has dramatically decreased the need for and use of intravenous pyelography (IVP)[28]. Bowel gas and immaturity of renal function in children often results in suboptimal IVP images. Also, IVP has risks of radiation and contrast exposure.
This is carried out with percutaneous nephrostomy tube insertion and contrast agent injection. The indications are nephrostomy tube placement to drain an obstructed infected kidney or to provide percutaneous nephrolithotomy access. In cases in which retrograde studies are prevented by obstruction at the extreme lower end of the ureter, antegrade pyelography is useful.
Excellent anatomic resolution of the UT is provided by computed tomography (CT). Thin-section helical imaging (spiral CT scan) gives multiplanar reformatted images quickly. Its uses include: (1) Initial evaluation of possible symptomatic nephrolithiasis, as a preferred modality; and (2) diagnosis of suspected trauma of the UT, as the optimal modality. It is useful for renal tumor staging. Assessment of renal artery stenosis is carried using CT angiography.
The advantages of magnetic resonance imaging (MRI) are: (1) Multiple plane images; (2) excellent resolution; and (3) good distinction between different tissue types. All this is without radiation exposure. As compared to iodinated contrast studies, gadolinium-based contrast studies at standard doses are significantly less nephrotoxic. However, when gadolinium-based contrast is used at radiographic doses for angiographic procedures it is nephrotoxic[29,30]. MRI is valuable in assessing congenital abnormalities.
Nuclear medicine imaging provides accurate evaluation of renal function and useful renal imaging in many clinical situations. The requirements are radiotracers (radio-pharmaceuticals) and a scintillation detector/gamma camera. Exposure to radiation occurs, but is less compared to other modalities such as VCUG, IVP, or CT.
Dynamic renal scintigraphy is performed for functional information. This is carried out using mercaptoacetyltriglycine labelled with technetium (99mTc-MAG3). For most indications, 99mTc-MAG3 is better than 99mTc-DTPA. This is due to rapid excretion of 99mTc-MAG3 hence providing a superior renal/background ratio. A 99mTc-MAG3 scan provides information on the perfusion of each kidney, which is valuable in various clinical settings. It is useful in diagnosis when collecting system dilatation is caused by obstruction. In cases with significant obstruction of the outflow tract, 99mTc-MAG3 persists in the renal pelvis and a loop diuretic fails to accelerate its descent (diuresis scintigraphy). The functional significance of a ‘baggy’ or equivocally obstructed collecting system is distinguished, without undertaking pyelography.
Static renal scintigraphy is used for structural information. This uses dimercaptosuccinic acid labelled with technetium (99mTc-DMSA). It has the property of being taken up by proximal tubular cells. The intravenous injection is followed by the renal cortex images captured. These show the shape, size and relative function of each kidney. The method is sensitive in demonstrating cortical scarring in reflux nephropathy and is thus a way of assessing the function of each kidney individually. It usefully quantifies the amount of renal cortex in patients with renal dysplasia and hypoplasia.
Small caliber flexible fiberscopic cystoscopes are useful in diagnostic cystourethroscopy, and retrograde ureterography. Posterior urethral valves are definitively diagnosed using endoscopy.
Investigations for urinary incontinence or other urinary symptoms are required to make a definitive, objective diagnosis. A Cochrane review had concluded that “While urodynamic tests have been found to change clinical decision making, there is also some evidence that this does not result in better outcomes, as urinary incontinence rates difference after treatment”[31]. For further research to achieve better outcomes, special facilities can play an important role.
Urodynamic studies evaluate parameters of filling and emptying of the lower urinary tract. The bladder is filled with either water, or carbon dioxide while monitoring the pressure in the bladder (via bladder catheter) and in the abdomen (via rectal balloon). Simultaneously, the pelvic floor electromyography activity is measured by needle or surface electrodes placed in the perineum. The study is often carried out with fluoroscopy-“the video urodynamics”. This is done to obtain information on the appearance of the bladder wall, presence/absence of VUR, emptying efficiency, and most importantly, the bladder neck and urethra appearance, along with their respective pressure recording for evaluation. The anatomy and physiology of the lower urinary tract requires careful performance with proper patient cooperation. The latter, being the limiting factor in its use in the evaluation of children.
A noninvasive urodynamics study involves: (1) Voided volume measurement; and (2) flow pattern assessment with a flowmeter. Essential information gathered is displayed graphically, and includes: (1) The measured urine flow rate; (2) urine volume voided along the shape of the voiding curve; and (3) the maximum flow rate. US is used for determination of post-void residual urine volume.
The facilities for kidney biopsy are desirable. The most commonly used method is percutaneous renal biopsy. Automatic spring-loaded biopsy systems are now used, as the technique is simple and easy to use[32,33]. Biopsy examination requires special light microscopy, immunofluorescence techniques, and electron microscopy providing the most accurate diagnosis.
In the transplant setting, fine-needle aspiration biopsy is most often used for the analysis of immunologically activated cells. As this is less invasive, it is useful for possible serial monitoring of interstitial cellular infiltrates in transplanted kidneys[34].
Children should have access to treatments and resources that are as sophisticated and advanced as those available to adults. Pediatricians, pediatric nephrologists, and pediatric urologists are integral in ‘Children Kidney Care Centers’. The multi-disciplinary teams required for comprehensive care provision in children with kidney disease and their families are: geneticists, genetic counselors, nurse specialists, dialysis personnel, nutritionists, social workers, and mental health professionals. All these professionals under one roof is desirable. A regular supply of all consumables needed should be ensured. All medicines including immunomodulatory drugs should be readily available. Relevant clinical practice guidelines are an important component of specialized centers. The facilities, features, and functioning in ‘Child Kidney Care Centers’ are discussed below.
The choice of dialysis modality to manage a specific patient is influenced by several factors. These are: (1) The goals of dialysis; (2) the unique advantages and disadvantages of each modality; and (3) institutional resources[35]. The last factor should not limit the management of these important conditions.
In the United States, peritoneal dialysis (PD) continues to be the most utilized dialysis modality (~55%) as compared with hemodialysis (~44%). However, hemodialysis (HD) as the initial maintenance dialysis therapy is being increasingly utilized. In the selection of dialysis modality age is a defining factor. In the age group from birth to 5 years of age maintenance dialysis treatment using PD is preferred (85%). In children ≥ 13 years of age initiation of maintenance dialysis treatment with HD is common (50%)[36].
There are some universal rules for the choice of dialysis modality: (1) HD avoidance in infants due to difficulties with vascular access; and (2) HD use when PD cannot be used due to technique failure, intra-abdominal pathology, or social difficulties.
PD is preferred and is a convenient treatment modality for acute kidney injury (AKI) and patients with hemodynamically instability[37]. There is a recent trend towards increased continuous renal repl
The cornerstone of successful PD is a reliable peritoneal catheter. The PD catheters made of soft material (silicon rubber or polyurethrane) are suitable for long-term placement. A number of dialysate transfer sets and associated devices have been developed to reduce the risk of bacterial contamination during either the catheter-to-transfer set or the transfer set-to-dialysate bag connections. This has contributed to simplifying PD connecting maneuvers[39].
Second generation PD solutions are more biocompatible. For standard nighttime automated PD, the neutral pH bicarbonate/lactate-buffered solution is used. For a long daytime dwell, the solution used is icodextrin. For malnourished patients, an amino acid-based solution is used. These solutions are safe and effective[40].
For patients with ESRD, PD can provide continuous ambulatory peritoneal dialysis and automated therapy using a cycler (continuous cyclic peritoneal dialysis/intermittent peritoneal dialysis/nocturnal intermittent peritoneal dialysis).
Intermittent HD is useful in children with a relatively stable hemodynamic status. Pediatric HD machines with specific features for children need to be provided. These are useful as they have low blood flow speed capability and can function with lines of varying blood volumes. With the capability to measure and remove very small amounts of fluid these are suitable even for infants. The volumetric fluid removal system allows accurate fluid removal. New machines have advanced systems for continuous online monitoring and automatically adjust parameters using a biofeedback system.
Incorporation of the online hemodiafiltration (OL-HDF) module into the dialysis proportioning machine hardware makes the handling procedure simple. It secures the process by maintaining the safety regulation of the monitor. This has the advantage of virtually unlimited amounts of sterile and nonpyrogenic substitutive solution[41]. Incorporating OL-HDF in the RRT of children is beneficial, and improves most of the clinical and laboratory parameters measured[42].
The crucial factor for success of dialysis is good vascular access. The best form of access is an arterio-venous (a-v) fistula. Otherwise a line that is tunneled subcutaneously is used, or shunts/grafts may rarely be required[43]. Tunneled subcutaneous lines are used in: (1) Children too young for an a-v fistula; and (2) children not expected to be on dialysis long-term e.g. children awaiting a living related transplant.
CRRT is advantageous in patients with: (1) Unstable hemodynamic status; (2) concomitant sepsis; and (3) multiorgan failure in the intensive care setting. CRRT can be performed as: (1) Continuous venovenous hemofiltration; (2) continuous venovenous hemofiltration dialysis; and (3) continuous hemodiafiltration. Modern CRRT machines are very user-friendly, and with computer modules from which physicians can choose the CRRT modality[44].
Wearable and implantable artificial kidneys are the future of hemodialysis, and should be designed specifically for children.
The interventions assuming importance and impacting advantageously are insertion of tunneled hemodialysis and peritoneal dialysis catheters, endovascular procedures, percutaneous nephrostomy, ureteral stent placement, etc.
Urologist’s services are essential for a wide variety of conditions from birth to early adult age for complete cure and complementing medical management. The specialized management that should be available in the ‘Child Kidney Care Centers’ is discussed below.
Ureteropelvic junction obstruction: Correction of this anomaly requires pyeloplasty. The success rate ranges from 91% to 98%. Pyeloplasty can be carried out using laparoscopic techniques, and is often robot-assisted using the da Vinci robot. Surgery conducted by surgical robots has the advantages of: (1) Small incision; (2) very minimal blood loss; (3) quick recovery; (4) shorter hospital stay; and (5) faster return to normal life. The advantages for the surgeon are: (1) A magnified, high-definition, three-dimensional view; and (2) tiny surgical instruments for manipulation, that have better flexibility than human hands.
Posterior urethral valves: Definitive treatment is performed by destruction of the valves endoscopically. Continuing supportive treatment is required for: (1) Dilated urinary tract; (2) recurrent urinary infections; and (3) uremia.
Other conditions for which surgery may be required are an ectopic ureter, ureterocele, megaureter, etc.
Calculus removal is necessary if: (1) The calculus does not pass; (2) seems unlikely to pass; and (3) if there is associated urinary tract infection. Lithotripsy of bladder, ureteral, and small renal pelvic calculi can be done using the holmium laser through a flexible or rigid ureteroscope. This is quite effective. Extracorporeal shock wave lithotripsy is the other option, and can be used in children with renal and ureteral stones. This has a success rate of more than 75%. Percutaneous nephrostolithotomy is another alternative. In this where the renal collecting system is accessed percutaneously, and breaking of the calculi is carried out using ultrasonic lithotripsy. If these modalities are unsuccessful, laparoscopic removal is an alternative. The da Vinci robot can be utilized for this procedure.
Surgery is needed to minimize the risks of ongoing VUR. Nonsurgical therapy is required for infection prophylaxis and follow-up testing. VUR correction options include: (1) Lower abdominal or inguinal incision (open); (2) laparoscopically (with or without robotic assistance); and (3) endoscopically with sub-ureteral injection.
Reconstructive urinary tract surgery is needed in cases of incontinence persisting despite medical therapy. This can almost always provide complete or satisfactory continence.
The two indications and comprehensive treatments are as follows.
Low urethral resistance: bladder neck reconstructive procedures (such as a periurethral sling) are often successful. Alternatively, an artificial sphincter implantation is usually successful. The components used in this technique are: (1) An inflatable cuff placed around the bladder neck; (2) a pressure-regulating balloon implanted in the extraperitoneal space; and (3) pumping mechanism implanted in the scrotum of boys and in the labia majora of girls.
Low bladder capacity or compliance, or persistent uninhibited contractions despite anticholinergic therapy: enlargement of the bladder with a patch of small or large intestine, termed augmentation cystoplasty or enterocystoplasty, is effective. Following this there is still a need to perform clean intermittent catheterization. If there is difficulty in urethral catheterization, a continent urinary stoma may be incorporated into the urinary tract reconstruction. The Mitrofanoff procedure is a useful and commonly performed technique. During this procedure, the appendix is isolated from the cecum on its vascular pedicle and is interposed between the bladder and abdominal wall. This is done to allow intermittent catheterization through a dry stoma.
The indications for surgery are: (1) To improve sexual function; (2) to correct problems with the urinary stream; and (3) for cosmetic reasons. The plastic surgical procedures used correct the chordee and re-site the urethral opening. The available procedures are: (1) Meatal advancement and glanuloplasty repair; (2) transverse island flap repair; and (3) island tube repair.
Except for proximal hypospadias, all cases are repaired in a single operation on an ambulatory basis. Proximal hypospadias may require a 2-stage repair.
This is the optimal therapy for children with ESRD. Survival rates with kidney transplantation are better than hemodialysis or peritoneal dialysis. Children and adolescents with ESRD require a renal transplant more than adults. This is justified in order to achieve normal growth and cognitive development. Successful transplantation leads to: (1) Improvement in linear growth; (2) possible school attendance; and (3) freedom from dietary restrictions.
Pediatric Centers of Excellence in Nephrology are needed and the National Institutes of Health is promoting these centers. Grants are provided for accelerating basic, translational and clinical research in pediatric kidney disease. Important research being funded for pediatric patients include: (1) CKD in Children study; (2) Nephrotic Syndrome Study Network; (3) Cure Glomerulonephropathy; and (4) Polycystic Kidney Disease Research Resource Consortium.
Basic science research projects include: (1) The (Re) Building a Kidney Consortium; (2) the GenitoUrinary Development Molecular Anatomy Project; and (3) the Kidney Precision Medicine Project. All this is inspiring the pediatric nephrology research community. Progress has been made in molecular and genetic analyses. Specific gene products have been linked to normal and abnormal kidney growth and development causation in a few human pediatric kidney diseases. It has been commented that much remains to be explored. The future is exciting.
Health for all should include all children with disorders of the kidneys and related structures. The availability of ‘Child Kidney Care Centers’ will go a long way in improving the lives of affected children. Pertinent and professional care, both simple and sophisticated is likely to reap rewards. Strategies suggested for focused attention and favorable actions can lead to success, happiness and health.
The author is thankful to authors of all the references quoted, for all the insights for advancing the care of children.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Health care sciences and services
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Cury CM, Brazil; Dasuqi SA, Saudi Arabia; Gadelkareem RA, Egypt; Gupta L, Indonesia S-Editor: Zhang H L-Editor: Webster JR P-Editor: Zhang H
| 1. | Wise PH. The future pediatrician: the challenge of chronic illness. J Pediatr. 2007;151:S6-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 2. | Jain S, Dewey RS. The Role of "Special Clinics" in Imparting Clinical Skills: Medical Education for Competence and Sophistication. Adv Med Educ Pract. 2021;12:513-518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Allison SJ. The globalization of nephrology. Nat Rev Nephrol. 2015;11:125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | Cochat P, Salusky IB. IPNA: Global Pediatric Nephrology, Introduction and Overview. In: Avner ED, Harmon WE, Niaudet P, Yoshikawa N, Emma F, Goldstein SL. Pediatric Nephrology. 7th ed. Heidelberg: Springer-Verlag, 2016: 2607-2612. |
| 5. | Pachter LM. Overview of Pediatrics. In: Kliegman RM, St. Geme III JW, Blum NJ, Shah SS, Tasker RC, Wilson KM, Behrman RE. Nelson Textbook of Pediatrics. 21st ed. Philadelphia: Elsevier, 2019: 654-680. |
| 6. | Liu L, Oza S, Hogan D, Chu Y, Perin J, Zhu J, Lawn JE, Cousens S, Mathers C, Black RE. Global, regional, and national causes of under-5 mortality in 2000-15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet. 2016;388:3027-3035. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2109] [Cited by in RCA: 2203] [Article Influence: 244.8] [Reference Citation Analysis (1)] |
| 7. | Kaspar CD, Bholah R, Bunchman TE. A Review of Pediatric Chronic Kidney Disease. Blood Purif. 2016;41:211-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 8. | Harambat J, van Stralen KJ, Kim JJ, Tizard EJ. Epidemiology of chronic kidney disease in children. Pediatr Nephrol. 2012;27:363-373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 617] [Cited by in RCA: 581] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 9. | Kanitkar M. Chronic Kidney Disease in Children: An Indian Perspective. Med J Armed Forces India. 2009;65:45-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Gulati S, Mittal S, Sharma RK, Gupta A. Etiology and outcome of chronic renal failure in Indian children. Pediatr Nephrol. 1999;13:594-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 39] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Ingelfinger JR, Kalantar-Zadeh K, Schaefer F; World Kidney Day Steering Committee. World Kidney Day 2016: Averting the legacy of kidney disease-focus on childhood. Pediatr Nephrol. 2016;31:343-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 12. | Samuels JA, Muñoz H, Swinford RD. Neonatal Kidney Conditions. In: Eicnenwald EC, Hansen AR, Martin CR, Stark AR, Jain N. Cloherty & Stark’s Manual of Neonatal Care. 8th edition. Gurgaon: Wolters Kluwer, 2021: 380-409. |
| 13. | Figueroa CA, Harrison R, Chauhan A, Meyer L. Priorities and challenges for health leadership and workforce management globally: a rapid review. BMC Health Serv Res. 2019;19:239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 127] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 14. | Hari P, Singla IK, Mantan M, Kanitkar M, Batra B, Bagga A. Chronic renal failure in children. Indian Pediatr. 2003;40:1035-1042. [PubMed] |
| 15. | Kamath N, Iyengar AA. Chronic Kidney Disease (CKD): An Observational Study of Etiology, Severity and Burden of Comorbidities. Indian J Pediatr. 2017;84:822-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 16. | Mookerje BK, Lohr JW, Jenis EH, Heffner HM. Glomerulonephritis for the generalist. J Med. 2001;32:115-134. [PubMed] |
| 17. | Chadban SJ, Atkins RC. Glomerulonephritis. Lancet. 2005;365:1797-1806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 114] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 18. | Schwartz GJ, Haycock GB, Edelmann CM Jr, Spitzer A. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics. 1976;58:259-263. [PubMed] |
| 19. | Schwartz GJ, Brion LP, Spitzer A. The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin North Am. 1987;34:571-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1235] [Cited by in RCA: 1242] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 20. | Finney H, Newman DJ, Thakkar H, Fell JM, Price CP. Reference ranges for plasma cystatin C and creatinine measurements in premature infants, neonates, and older children. Arch Dis Child. 2000;82:71-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 205] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 21. | Houser M. Assessment of proteinuria using random urine samples. J Pediatr. 1984;104:845-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 93] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Fassett RG, Horgan BA, Mathew TH. Detection of glomerular bleeding by phase-contrast microscopy. Lancet. 1982;1:1432-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 82] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Foley KF, Boccuzzi L. Urine Calcium: Laboratory Measurement and Clinical Utility. Lab Med. 2010;41:683-686. [RCA] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Dacher JN, Pfister C, Monroc M, Eurin D, LeDosseur P. Power Doppler sonographic pattern of acute pyelonephritis in children: comparison with CT. AJR Am J Roentgenol. 1996;166:1451-1455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 104] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 25. | McArthur C, Baxter GM. Current and potential renal applications of contrast-enhanced ultrasound. Clin Radiol. 2012;67:909-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 26. | Simoneaux SF, Greenbaum LA. Diagnostic Imaging. In: Avner ED, Harmon WE, Niaudet P, Yoshikawa N. Pediatric Nephrology. Verlag: Springer, 2009: 535-564. |
| 27. | Hernandez RJ, Goodsitt MM. Reduction of radiation dose in pediatric patients using pulsed fluoroscopy. AJR Am J Roentgenol. 1996;167:1247-1253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 71] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | Andresen R, Wegner HE. Intravenous urography revisited in the age of ultrasound and computerized tomography: diagnostic yield in cases of renal colic, suspected pelvic and abdominal malignancies, suspected renal mass, and acute pyelonephritis. Urol Int. 1997;58:221-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Townsend RR, Cohen DL, Katholi R, Swan SK, Davies BE, Bensel K, Lambrecht L, Parker J. Safety of intravenous gadolinium (Gd-BOPTA) infusion in patients with renal insufficiency. Am J Kidney Dis. 2000;36:1207-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 40] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 30. | Thomsen HS, Almèn T, Morcos SK; Contrast Media Safety Committee Of The European Society Of Urogenital Radiology (ESUR). Gadolinium-containing contrast media for radiographic examinations: a position paper. Eur Radiol. 2002;12:2600-2605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 86] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 31. | Clement KD, Lapitan MC, Omar MI, Glazener CM. Urodynamic studies for management of urinary incontinence in children and adults. Cochrane Database Syst Rev. 2013;CD003195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | Wiseman DA, Hawkins R, Numerow LM, Taub KJ. Percutaneous renal biopsy utilizing real time, ultrasonic guidance and a semiautomated biopsy device. Kidney Int. 1990;38:347-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 33. | Donovan KL, Thomas DM, Wheeler DC, Macdougall IC, Williams JD. Experience with a new method for percutaneous renal biopsy. Nephrol Dial Transplant. 1991;6:731-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 34. | Yussim A, Shapira Z, Shmueli D, Lustig S, Braslavsky D, Ben-Bassat M. Use of modified fine needle aspiration for study of glomerular pathology in human kidneys. Kidney Int. 1990;37:812-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 35. | Flynn JT. Choice of dialysis modality for management of pediatric acute renal failure. Pediatr Nephrol. 2002;17:61-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 88] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 36. | Claes DJ, Goldstein SL. End-Stage Renal Disease. In: Kliegman RM, Stanton BF, Schor NF, St. Geme III JW, Behrman RE. Nelson Textbook of Pediatrics. 21th ed. Philadelphia: Elsevier, 2020: 10903-10905. |
| 37. | Mishra OP, Gupta AK, Pooniya V, Prasad R, Tiwary NK, Schaefer F. Peritoneal dialysis in children with acute kidney injury: a developing country experience. Perit Dial Int. 2012;32:431-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 38. | Bonilla-Félix M. Peritoneal dialysis in the pediatric intensive care unit setting: techniques, quantitations and outcomes. Blood Purif. 2013;35:77-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 39. | Verrina E, Perfumo F. Technical aspects of the peritoneal dialysis procedure. In: Warady BA, Schaefer FS, Fine RN, Alexander SR. Pediatric Dialysis. Dordrecht: Kluwer, 2004: 113-134. |
| 40. | Canepa A, Verrina E, Perfumo F. Use of new peritoneal dialysis solutions in children. Kidney Int Suppl. 2008;S137-S144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 41. | Canaud B. Online hemodiafiltration. Technical options and best clinical practices. Contrib Nephrol. 2007;158:110-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 42. | Ibrahim MAA, ElHakim IZ, Soliman D, Mubarak MA, Said RM. Online hemodiafilteration use in children: a single center experience with a twist. BMC Nephrol. 2020;21:306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 43. | Chand DH, Brier M, Strife CF. Comparison of vascular access type in pediatric hemodialysis patients with respect to urea clearance, anemia management, and serum albumin concentration. Am J Kidney Dis. 2005;45:303-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 30] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 44. | Goldstein SL, Zappitelli M. Evaluation and Management of Acute Kidney Injury in Children. In: Avner ED, Harmon WE, Niaudet P, Yoshikawa N, Emma F, Goldstein SL. Pediatric Nephrology. 7th ed. Heidelberg: Springer-Verlag, 2016: 2139-2168. |