INTRODUCTION
Acute post-streptococcal glomerulonephritis (APSGN) is the most common cause of acute glomerulonephritis among children which is mostly caused by group A beta-hemolytic streptococci (GABHS)[1]. APSGN primarily affects children aged between 3 and 12 years and is uncommon among children below age 3[2,3]. The most common presenting features of APSGN are hematuria, azotemia, hypertension, and peripheral edema[2]. The clinical spectrum of APSGN can vary as acute nephritic syndrome, nephrotic syndrome, and rapidly progressive glomerulonephritis (RPGN), or it may be subclinical[1]. Therefore, the severity of APSGN can vary among patients, and they can present with subclinical disease to RPGN requiring dialysis[4]. APSGN is generally self-limiting and has a good long-term prognosis[5].
The estimated global incidence of APSGN is 472000 cases per year with 77% of the cases from the low- and middle-income countries[6]. The rate of APSGN has decreased over the last few decades in high-income countries due to the use of antibiotics, improved socio-economic status, and improved hygiene[7]. However, APSGN remains one of the important causes of acute kidney injury among the pediatric populations and the leading cause of hospital admission in developing countries[5]. The reported estimated annual incidence of APSGN is 9.3 cases per 100000 persons in developing countries[8].
Most cases of APSGN occur following pharyngitis with streptococci rather than skin infection[9]. However, the nature of the preceding infectious disease is not associated with the clinical course and severity of APSGN[2]. The two main antigens contributing to the pathogenesis of APSGN are nephritis-associated plasmin receptor (NAPlr) and streptococcal pyrogenic exotoxin B (SPeB)[7]. The infection activates the antibodies and complement proteins against NAPlr and SPEB, through the immune complex-mediated mechanism causing aggregation of blood vessels in the glomeruli[2]. C3 is generally low in blood tests due to the activation of the alternate complete pathway[10]. However, 15%-30% of patients may have reduced C1 and C3 levels and 10% have normal complement levels[11]. This review discusses the management, prognosis, and outcomes of APSGN.
MANAGEMENT OF ACUTE GLOMERULONEPHRITIS
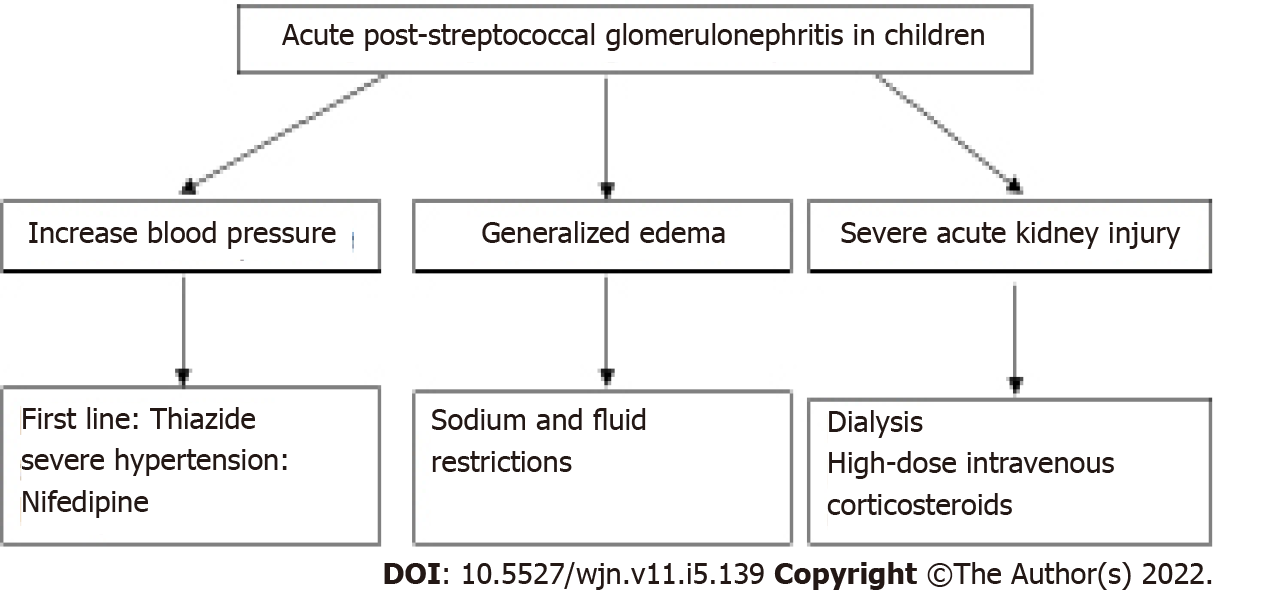
The management of APSGN is mainly supportive in nature as the disease is self-limiting[12]. Children who present with hypertension, generalized edema, or impaired renal function should be hospitalized to monitor the blood pressure and renal function[12]. APSGN should be managed with fluid restriction, anti-hypertensives, diuretics, and renal replacement therapy with dialysis when necessary[7] (Figure 1).
Figure 1 Management strategy for acute post-streptococcal glomerulonephritis in children.
ANTIBIOTICS PROPHYLAXIS
Two randomized controlled trials showed no significant difference in the risk of developing APSGN between cefuroxime for 5 d and penicillin V for 10 d as antibiotics prophylaxis[13,14]. Furthermore, a Cochrane review of 27 trials showed that the efficacy of antibiotic treatment in preventing the development of APSGN after a throat infection is statistically insignificant[15]. Antibiotic therapy during the initial GABHS infection may help prevent the spread of infection and thereby prevent the development of APSGN[8]. However, antibiotic prophylaxis is generally not necessary in APSGN as the resolution of APSGN can occur without eradication of GABHS, and recurrence of APSGN is uncommon[7].
ANTI-HYPERTENSIVE AGENTS
Thiazide diuretics are effective as a first-line medication in APSGN; however, loop diuretics may be considered in patients with renal impairment, especially those with an estimated glomerular filtration rate (eGFR) < 30 mL/min per 1.73 m2 and significant edema[8]. Thiazide diuretics are associated with electrolyte abnormalities such as hypokalemia, hyperglycemia, and hypercalcemia[16]. Therefore, serum potassium and calcium levels should be monitored when thiazides are used[16]. Hypertension in APSGN can be managed with diuretics alone or a combination of a diuretic and a vasodilator such as a calcium channel blocker to treat the hypervolemia from sodium and water retention[12]. Edematous or hypertensive patients should also be instructed on a reduced-sodium diet and may require fluid restriction[8]. Calcium channel blockers or beta-blockers may be considered in patients with the need for greater hypertension control[8]. Several studies showed that short-acting nifedipine is safe in children with severe hypertension or hypertensive emergencies and requiring a rapid reduction of blood pressure[17-19]. The minor adverse effects of short-acting nifedipine include flushing, tachycardia, edema, headache, dizziness, nausea and vomiting, pruritus, and gastrointestinal pain[18]. The occurrence of major adverse effects such as reduction in blood pressure by more than 40%, oxygen desaturation, and change in neurologic status is rare among pediatric populations[18,19]. Furthermore, several studies have shown that angiotensin-converting enzyme (ACE) inhibitors have better control of blood pressure and edema in APSGN compared to diuretics[7]. However, ACE inhibitors or angiotensin receptor blockers are usually avoided in the acute phase because they may exacerbate any reduction in glomerular ultrafiltration and hyperkalemia[12].
SODIUM AND FLUID RESTRICTION AND PULMONARY EDEMA
Patients who present with generalized edema due to acute kidney injury or acute glomerulonephritis due to APSGN may benefit from sodium restriction[20]. A sodium-restricted diet between 1 and 2 mEq/kg·d is recommended for the reduction of edema and positive natriuresis[21]. Patients who are compliant with Na+ restriction will have self-limiting fluid restriction[21]. However, patients with severe edema may be treated with fluid restriction to two-thirds of maintenance or half or less of urine output once a brisk diuresis is achieved[21,22]. Patients who are on fluid restriction should have close monitoring of fluid input and output, serum electrolytes, and vital signs[21].
Non-cardiogenic pulmonary edema can occur due to renal failure in patients with APSGN causing acute respiratory distress syndrome[22]. The management should focus on maintaining adequate oxygenation to the lung and treat the underlying cause[23]. Non-invasive positive pressure ventilation can be used in mild cases for respiratory support while conventional mechanical ventilation and high-frequency oscillatory ventilation can be used in more severe cases while the underlying cause is being treated[24]. The pharmacological management of non-cardiogenic pulmonary edema is limited[25]. Inhaled nitrate oxide (INO) can be used in patients with pulmonary hypertension and right ventricular dysfunction to reduce the ventilation/perfusion mismatch[24]. However, corticosteroids and surfactants are not recommended as routine therapy[26].
IMMUNOSUPPRESSANTS AND DIALYSIS
Patients may require kidney biopsy if they present with undifferentiated and rapidly progressive severe acute kidney injury to exclude other causes of kidney disease which may have specific managements[27]. High-dose intravenous corticosteroids may be used in patients who have severe clinical presentations requiring renal biopsy; however, the use of corticosteroid is based on anecdotal evidence only[28]. Immunosuppression with corticosteroids with or without an alkylating agent can be used in patients with severe crescentic glomerulonephritis (> 75% crescents) to reduce the extra-capillary inflammation[4]. However, several studies also show that immune suppressive therapy does not have a clear benefit on the long-term outcome[4]. Finally, dialysis is recommended in children with severe renal impairment causing volume excess and electrolyte abnormalities such as hyperkalemia or acidosis[12,29]. Renal replacement therapy (RRT) should be initiated in patients with overt fluid overload with cumulative fluid overload of more than 20% or more than 10% of the body weight and not responsive to diuretics[30,31]. The available modalities for RRT are intermittent hemodialysis (IH), continuous renal replacement therapy (CRRT), and peritoneal dialysis (PD) in patients with acute kidney injury due to APSGN[32]. IHD is suitable for patients who are hemodynamically stable while CRRT is more suitable for patients who are hemodynamically less stable, especially in the ICU settings[29]. PD is less suitable in critically ill patients because the dialysis depends on peritoneal circulation and there are increased risks of catheter-related infections and peritoneal fluid leakage[29].
COMPLICATIONS
The complications that might occur during the acute phase of APSGN include congestive heart failure, pulmonary edema, and severe hypertension-induced encephalopathy due to hypervolemia[10]. Serious complications such as hypertensive emergency, congestive heart failure, encephalopathy, and retinopathy were reported in 21.5%, 12.3%, 4.6%, and 1.5% of all cases of APSGN, respectively[33]. A study in French Polynesia demonstrated that 22%of the patients had severe presentations which include cardiac failure and severe hypertension with or without encephalopathy[34]. A study by Kasahara et al[35] showed that hypertension is the most common initial complication of APSGN with 64% of the children presenting with hypertension. Around 30%-35% of children with APSGN have been reported to have cerebral complications of hypertension[9,33]. Children with severe hypertension may present with abnormal neurological symptoms such as generalized seizures[27]. A study by Gunasekaran et al[33] reported that 21.5 % of children required the treatment of intravenous infusion of sodium nitroprusside in an intensive care setting due to hypertensive emergency. Anemia is the most common laboratory abnormality in patients with APSGN due to intravascular fluid overload and/or suppressed erythropoietin secretion and is significantly associated with the degree of azotemia[2].
PROGNOSIS AND OUTCOMES
Studies showed that around 34%–44% of proteinuria cases in APSGN are in the nephrotic range at APSGN onset; however, it is not associated with disease severity or renal failure[27,34]. A study done in Turkey by Demircioglu Kılıc et al[1] showed that hypoalbuminemia, high CRP, neutrophil count, and neutrophil/lymphocyte ratio (NLR) were associated with decreased eGFR in APSGN. Besides that, the study also showed that 75% of the 16 children with low C4 with nephrotic range proteinuria at APSGN showed decreased eGFR[1]. However, another study from New Zealand showed that none of the patients had reduced C4 among 27 patients with APSGN with severe kidney involvement[4]. On the other hand, a study by Becquet et al showed that patients with severe-onset APSGN had decreased C3 levels[34]. Furthermore, another study by Dagan et al[2] also showed that decreased C3 levels were associated with the presence of azotemia and/or full-blow nephritic syndrome. In addition to that, the study by Han et al[5] showed that a decrease in serum C3 level was associated with an increased rate of acute nephritic features such as edema. Decreased serum in C3 levels are found in 90% of children with APSGN and are associated with an increase in severity due to deposition of C3 glomerular sub-epithelial through complement activation via the alternate pathway[4,36]. Therefore, increased CRP, hypoalbuminemia, and hypocomplementemia are associated with disease severity and more severe clinical presentations[2].
APSGN generally has a favorable prognosis with less than 1% of children progressing to end-stage renal failure[37]. A 7-year follow-up of children with acute glomerulonephritis in Iran reported that none of the patients had hypertension or renal impairments, 3.1% had proteinuria, and 6.3% had microscopic hematuria[38]. Furthermore, a 10-year follow-up of the children that developed APSGN in Brazil demonstrated an increase in the frequency of hypertension in APSGN groups compared to control groups but no significant difference in renal function evaluation which includes serum creatinine, cystatin C, eGFR, albuminuria, and hematuria[39]. The study also showed improvement in the stabilization of median eGFR and a decrease in albuminuria in the follow-up of the same patients in 2, 5, and 10 years after the acute episode of APSGN[39]. Nevertheless, as few as 5% up to 20% of children may have persistent abnormalities in the urinary findings, either hematuria or proteinuria[2]. A 9-year follow-up study by Kasahara et al[35] demonstrated that serum complement levels were normalized by 12 wk after the diagnosis of APSGN, no patients had residual proteinuria by 3 years of diagnosis, and hematuria disappeared by 4 years. However, children with APSGN in low and middle-income countries may have a poorer prognosis due to severe presentation with 30% requiring dialysis due to acute kidney injury and < 30% of the patients recovering fully[7,40].
Approximately 3% to 6% of patients with resolved APSGN may have persistent hypertension[37]. A study Vivante et al[41] showed that childhood glomerular disease which includes APSGB and steroid-responsive nephrotic syndrome is a risk factor of developing hypertension in adulthood. The predictors of poor long-term prognosis of APSGN include the presence of nephrotic syndrome, renal insufficiency at onset, and crescent formation on biopsy findings[8]. A retrospective study by Wong et al[4] reviewed 27 patients with APSGN requiring renal biopsies due to anuric renal failure, acute severe glomerulonephritis, mixed nephrotic nephritic syndrome, and delayed recovery from glomerulonephritis. The study reported that 12 patients required acute dialysis and 11 patients showed more than 50% of crescents on renal biopsies[4]. Patients with crescentic glomerulonephritis had a higher frequency of needing acute dialysis and tended to have persistent proteinuria up to 8 years of follow-up[4]. Furthermore, 8 of the 12 patients who required acute dialysis had developed ESRD, chronic renal failure, or persistent proteinuria of 2 to 4+ on urinalysis[4]. Kidney damage may persist or be superimposed years after APSGN due to persisting or secondary inflammation after infection and hyper-perfusion or hypertrophy of the nephron[42].
CONCLUSION
In conclusion, APSGN has a good prognosis and outcome in children. Severe systemic complications can occur due to severe renal inflammation and hypervolemia but are rare. Increased CRP, hypoalbuminemia, and hypocomplementemia are associated with disease severity. The predictors of severity of disease and poor outcome in APSGN in children may include the presence of nephrotic syndrome, crescent formations on renal biopsy, and renal insufficiency on presentation. A small percentage of patients may have persistent hypertension, persistent hematuria or proteinuria, or progression to chronic kidney disease following the acute episode of APSGN. Therefore, yearly follow-up is recommended to screen for any urinary abnormalities, hypertension, or renal impairment. Further prospective, multicenter, long-term studies should be conducted to evaluate the long-term outcomes of children with APSGN.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pediatrics
Country/Territory of origin: Malaysia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Moshref RH, Saudi Arabia; Tasic V, North Macedonia; Wang F, China S-Editor: Ma YJ L-Editor: Wang TQ P-Editor: Ma YJ