Published online Mar 25, 2022. doi: 10.5527/wjn.v11.i2.58
Peer-review started: October 29, 2021
First decision: December 27, 2021
Revised: January 9, 2022
Accepted: March 23, 2022
Article in press: March 23, 2022
Published online: March 25, 2022
Processing time: 147 Days and 1.9 Hours
Coronavirus disease 2019 (COVID-19) is still a menacing pandemic, especially in vulnerable patients. Morbidity and mortality from COVID-19 in maintenance hemodialysis (MHD) patients are considered worse than those in the general population, but vary across continents and countries in Europe.
To describe the clinical course and outcomes of hospitalized MHD patients with COVID-19 in a retrospective observational single center study in Greece.
We correlated clinical, laboratory, and radiological data with the clinical outcomes of MHD patients hospitalized with COVID-19 during the pandemic. The diagnosis was confirmed by real-time polymerase chain reaction. Outcome was determined as survivors vs non-survivors and “progressors” (those requiring oxygen supplementation because of COVID-19 pneumonia worsening) vs “non-progressors”.
We studied 32 patients (17 males), with a median age of 75.5 (IQR: 58.5-82) years old. Of those, 12 were diagnosed upon screening and 20 with related symptoms. According to the World Health Organization (WHO) score, the severity on admission was mild disease in 16, moderate in 13, and severe in 3 cases. Chest computed tomography (CT) showed 1-10% infiltrates in 24 patients. Thirteen “progressors” were recorded among included patients. The case fatality rate was 5/32 (15.6%). Three deaths occurred among “progressors” and two in “non-progressors”, irrespective of co-morbidities and gender. Predictors of mortality on admission included frailty index, chest CT findings, WHO severity score, and thereafter the increasing values of serum LDH and D-dimers and decreasing serum albumin. Predictors of becoming a “progressor” included increasing number of neutrophils and neutrophils/lymphocytes ratio.
Patients on MHD seem to be at higher risk of COVID-19 mortality, distinct from the general population. Certain laboratory parameters on admission and during follow-up may be helpful in risk stratification and management of patients.
Core Tip: Maintenance hemodialysis patients, a group of patients with presumed high mortality, have been reported to experience worse outcomes of coronavirus disease 2019 (COVID-19), compared to the general population internationally. However, there is a considerable variation in the reported rates of disease remission and death between different continents and countries. In this article, we present the outcomes of 32 patients on chronic dialysis who became positive for COVID-19 in the era before vaccines became available.
- Citation: Bacharaki D, Karagiannis M, Sardeli A, Giannakopoulos P, Tziolos NR, Zoi V, Piliouras N, Arkoudis NA, Oikonomopoulos N, Tzannis K, Kavatha D, Antoniadou A, Vlahakos D, Lionaki S. Clinical presentation and outcomes of chronic dialysis patients with COVID-19: A single center experience from Greece . World J Nephrol 2022; 11(2): 58-72
- URL: https://www.wjgnet.com/2220-6124/full/v11/i2/58.htm
- DOI: https://dx.doi.org/10.5527/wjn.v11.i2.58
Nearly two years have elapsed after the pronouncement of the novel coronavirus disease 2019 (COVID-19) on March 11, 2020 by the World Health Organization (WHO) as a global pandemic, following its first recognition in Wuhan, China in December 2019[1]. The disease is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and is manifested in the majority of cases with symptoms related to the upper respiratory system or with development of mild pneumonia in 81% of cases[2]. Only 15% of infected patients develop severe lung disease, requiring oxygen support, while 5% of them progress to critical disease with complications, such as respiratory failure, acute respiratory distress syndrome, sepsis and septic shock, thromboembolism, and multiorgan failure[3-4]. A dysfunctional as opposed to healthy host immune response is supposed to play an important role for the final outcome[5]. Patients prone to the severe form of the disease are considered to be elderly, and those with co-morbidities including diabetes mellitus, chronic obstructive pulmonary disease, coronary artery disease, obesity[6-7], and chronic kidney disease, although at first not included[8]. Regarding patients with end-stage kidney disease (ESKD) who are maintained with hemodialysis or peritoneal dialysis, results from the ERACODA collaboration (the European database collecting clinical information of patients on kidney replacement therapy with COVID-19) revealed some peculiarities compared to the general population, i.e., prevalent co-morbidities like hypertension, diabetes mellitus, coronary artery disease, heart failure, and chronic lung disease did not emerge as independent risk factors for mortality[6]. Notably, the aforementioned co-morbidities are highly prevalent in patients with chronic kidney disease, which is itself considered by default an independent risk factor for increased cardiovascular and all-cause mortality[9-10]. Yet, some studies have reported increased mortality in ESKD patients with COVID-19[11-12], where others have concluded that these patients are somehow being “protected” from the severe form of COVID-19[13-14]. The reported death rates vary substantially across countries[15] and thus, genetic factors have been implicated to play a role in the development of the severe form of the disease[16].
A cohort of patients with COVID-19 and ESKD on dialysis, who were admitted in our hospital during the pandemic, were studied, attempting to identify potential differences in terms of the clinical presentation and outcome of COVID-19 compared to the general population. We also searched for distinctive features (clinical, radiological, or laboratory) that could serve as predictors in order to recognize patients at high risk for COVID-19 adverse outcome.
This is an observational, analytical, retrospective cohort study which took place in a single center from Greece. It was approved by the Scientific Committee of the Hospital.
The study included maintenance hemodialysis (MHD) patients, who were admitted in our hospital from April 23, 2020 till February 3, 2021 and were followed until death or release from hospital. All data were retrospectively collected from patients’ electronic records and medical charts and included demographics, clinical features, laboratory and radiological data, treatment schemes, clinical course, and outcome.
All included patients provided signed informed consent, were ≥ 18 years old, had COVID-19 confirmed by polymerase chain reaction (PCR) test within the last 5 d prior to admission, and were on MHD for more than 3 mo. The exclusion criteria were patients with COVID-19 with acute kidney injury undergoing temporary hemodialysis, and MHD patients who were hospitalized with other types of pneumonia (non-related to SARS-CoV-2), active cancer, or autoimmunity. The PCR test was performed either because of symptoms, which might be attributed to COVID-19, or in case of a history of exposure to an infected patient or working personnel, or as a regular routine screening test.
Diagnosis of COVID-19 was confirmed by positive throat-swab specimens for SARS-CoV-2 using the PCR methodology, as has been described[17]. Symptoms, if present, were recorded.
Regarding clinical presentation, each patient was classified at the time of admission, according to the classification of WHO for COVID-19 severity (mild, moderate, severe, and critical disease) as described previously[4]. Accordingly, the disease was characterized as mild if there was absence of pneumonia or hypoxia, moderate if there were clinical signs of pneumonia with oxygen saturation (SatO2) > 90%, and as severe if the patient had one or more of the following: Respiratory rate > 30/min, respiratory distress, or SatO2 < 90%. The disease was determined as critical in case of acute respiratory distress syndrome, sepsis, or septic shock (Supplementary Table 1). In addition, at the time of admission, all patients were scored for their status of frailty, using the 9-point frailty scale, as previously described[18].
Regarding the clinical course, patients were grouped based on worsening or not of COVID-19 pneumonia, as follows: Those who required oxygen supplementation (for the first time, or amplification of previous) because of worsening of COVID-19 pneumonia at the time of admission, at discharge, or before death, were categorized as “progressors”, while those who remained in stable clinical condition were categorized as “non-progressors” or “stable”.
Regarding the final outcome (death or release from hospital), patients were grouped into a survival group and a non-survival (deceased) group. In case of death, the precise cause was recorded and characterized as COVID-19 related or not. The case fatality rate (CFR) was calculated according to previous reports[19]: The number of deaths attributed to the disease were divided by the number of diagnosed cases and multiplied by 100. Since causes of death in COVID-19 patients have been reported to differ between MHD patients and the general population[12], we recorded the CFR as the total number of deaths in COVID-19 patients but also distinguished COVID-19 related deaths attributed to respiratory failure from SARS-CoV-2 pneumonia vs non-related to COVID-19, i.e., attributed to other causes, in patients with no respiratory worsening.
Information regarding the past medical history of patients was recorded from their medical charts including the presence of all comorbidities such as hypertension, diabetes mellitus, coronary artery disease, heart failure, and chronic lung disease.
Laboratory data: Routine blood examinations included complete blood count, coagulation profile, inflammatory markers [i.e., C-reactive protein (CRP) and ferritin], and serum biochemistry (renal and liver function and albumin). The data were recorded from the day of admission till death or release from hospital. Thus, we had the opportunity to study the kinetics of certain laboratory parameters that have emerged as prognostic markers in the general population[20] including neutrophils to lymphocytes ratio (NLR), lymphocytes, lactate dehydrogenase (LDH), CRP, ferritin, Il-6, D-dimers, troponin, albumin, and white blood cells (WBC). Specifically, we recorded the maximal value (or lowest in parameters such as albumin) in the time interval between admission and the 10th day and calculated the increase as a percentage from admission to the highest (or lowest) value of 10 d by dividing this difference with the value at admission.
Radiology data: All patients with COVID-19 underwent a computed tomography (CT) scan of the chest on admission, as per hospital protocol for COVID-19. All CT scans performed in COVID-19 patients were conducted using a Philips Brilliance 64 CT scanner with a 1 mm slice thickness and a high-resolution CT algorithm. Typically, a non-contrast chest CT scan was performed, with images being obtained during end-inspiration breath hold. Imaging disease extent/severity was estimated according to the COVID visual assessment scale (CoVASc), which is a visual assessment scale that roughly estimates the percentage of pulmonary parenchyma affected by COVID-19, as seen on chest CT, when both lungs are evaluated as a whole (0%, 1%-10%, 11%-25%, 26%-50%, 51%-75%, and > 75%)[21].
Since this a single center study, there was no bias regarding management. Since COVID-19 presents with stages of evolution[20], in order to overcome potential bias of delayed admission, we recorded and present mean time to admission when indicated.
By February 2021, Greece had experienced three waves of COVID-19 pandemic, March to April, September, and December 2020. Admitted patients were evaluated from the infectious disease department who decided about the therapeutic protocol based on the clinical picture and the available international therapeutic data. Five patients, who were admitted during the 1st wave, were mildly symptomatic, without severe pneumonia. They received hydroxychloroquine plus azithromycin as per infectious department protocol[22]: A loading dose of 200 mg of hydroxychloroquine at day 1, followed by 100 mg twice per day for 5 d and azithromycin 500 mg daily for 5 d.
During the 2nd and 3rd waves, the aforementioned protocol for mild disease was abandoned, as data questioned its efficacy[23]. Admitted patients requiring supplementary oxygen due to COVID-19 pneumonia to maintain SaO2 > 93%, received 6 mg intravenous dexamethasone for up to 10 d or until discharge, if sooner. Based on clinical judgment for concurrent microbial pneumonia, patients receiving dexamethasone were also prescribed azithromycin at a dose of 500 mg on day 1, and 250 mg on the following 4 d. An electrocardiograph to exclude long QT was performed in advance for both hydroxychloroquine and azithromycin prescription. Low molecular weight heparin was prescribed at a prophylactic dose in all admitted patients at a dose of 3500 benzaparin (body weight > 60 kg) and 2500 IU (body weight < 60 kg). On dialysis day, it was given during the dialysis session. Patients who experienced an incident thromboembolic event or those who were highly suspected to have thromboembolic disease were managed with therapeutic doses of anticoagulant therapy.
Hemodialysis was performed in an isolated room, regularly three times per week, according to the related practice guidelines as described by others[24]. Blood access status was regularly recorded, as well as events necessitating intervention (hypokalemia, hypotension, and thrombosis).
Patients’ data were analyzed on an exploratory basis. Continuous variables are summarized with the use of descriptive statistical measures [median and interquartile range (IQR; 25th, 75th percentile)], and categorical variables are displayed as frequency tables (n, %). Statistical tests used to check univariate associations between categorical or continuous variables and outcomes were Pearson’s chi-squared test, Fisher’s exact test, t-test, or Wilcoxon rank-sum test as appropriate. Box plots are used to visualize the laboratory data at admission and at their highest/lowest value. The level of 5% was used for statistical significance. All statistical analyses were performed using STATA/SE 16.1 software (Copyright 1985–2019; Stata Corp LP, College Station, TX, United States).
Of 40 patients who were eligible to be included in the study, 32 were finally included, since two patients were discharged from hospital in less than 5 d, one had been diagnosed with COVID-19 for more than a week, one had active cancer, one had active autoimmune disease, one had been on hemodialysis for less than 3 mo, and two had acute on chronic kidney disease, necessitating hemodialysis only temporally.
The study included 32 patients on MHD, who were infected with SARS-CoV-2, were diagnosed by nasopharyngeal PCR, and were hospitalized for more than 10 d until discharge or death. Five of them were diagnosed during the first wave and the rest presented during the second and third waves. As shown in Table 1, they had a median age of 75.5 (IQR: 58.5-82) and 17 of them were males (53.1%). The prevalent co-morbidity was arterial hypertension found in 20 (62.5%) patients, followed by diabetes mellitus in 10 (31.3%). The median number of comorbidities was 3 (IQR: 2-3.5). The median frailty index was 3 (IQR: 2-5). Diagnosis was made by routine screening in 12 (37.5%) cases or because of symptoms suggestive of COVID-19 (62.5%). The symptoms included fever in 13 (65%) patients, upper respiratory symptoms (dry cough and dyspnea) in 6 (30%), and diarrhea in 1 (5%). None of the patients reported anosmia, while one (3.125%) reported ageusia. In order to exclude potential confounders of delayed admission to the hospital, we recorded the median time to admission. It was 2 d (IQR = 1-3, min = 0, max = 5) for symptomatic patients and 1 d (IQR = 0.5-1) for those diagnosed after routine screening.
| Total patients, n (%) | Survivors, n (%) | Non-survivors, n (%) | P value | Non-progressors, n (%) | Progressors, n (%) | P value | |
| Characteristic | 32 (100) | 27 (84.4) | 5 (15.6) | 19 (59.3) | 13 (40.6) | ||
| Male | 17 (53.1) | 16 (59.3) | 1 (20) | NS | 9 (47.4) | 8 (61.5) | NS |
| Female | 15 (46.9) | 11 (40.7) | 4 (80) | 10 (52.6) | 5 (38.5) | ||
| 1Age | 75.5 (58.5-82) | 75 (56-82) | 76 (75-80) | NS | 70 (53-82) | 78 (75-82) | NS |
| 1Frailty index | 3 (2-5) | 3 (2-5) | 7 (3-8) | < 0.05 | 3 (2-5) | 3 (2-5) | NS |
| CT (%) | < 0.01 | NS | |||||
| 0-10% | 24 (77.4) | 23 (88.5) | 1 (20) | 16 (84.2) | 8 (66.7) | ||
| > 10% | 7 (22.6) | 3 (11.5) | 4 (80) | 3 (15.8) | 4 (33.3) | ||
| WHΟ | 0.05 | NS | |||||
| 0 | 16 (50) | 15 (55.6) | 1 (20) | 11 (57.8) | 5 (38.5) | ||
| 1 | 13 (40.6) | 11 (40.7) | 2 (40) | 8 (42.1) | 5 (38.5) | ||
| 2-3 | 3 (9.4) | 1 (3.7) | 2 (40) | 0 (0) | 3 (23) | ||
| Diabetes | 10 (31.3) | 7 (25.9) | 3 (60) | NS | 7 (36.8) | 3 (23.1) | NS |
| Hypertension | 20 (62.5) | 18 (66.7) | 2 (40) | NS | 11 (57.8) | 9 (69.2) | NS |
| 1Number of comorbidities | 3 (2-3.5) | 3 (2-4) | 3 (2-3) | NS | 3 (1-4) | 3 (2-3) | NS |
| Symptoms | NS | NS | |||||
| Fever | 13 (65) | 10 (62.5) | 3 (75) | 8 (80) | 5 (50) | ||
| Respiratory | 6 (30) | 5 (31.2) | 1 (25) | 1 (10) | 5 (50) | ||
| Diarrhea | 1 (5) | 1 (6.3) | 0 (0) | 1 (10) | 0 (0) | ||
| COVID diagnosis | NS | NS | |||||
| With symptoms | 20 (62.5) | 16 (59.3) | 4 (80) | 10 (52.6) | 10 (76.9) | ||
| Screening | 12 (37.5) | 11 (40.7) | 1 (20) | 9 (47.4) | 3 (23.1) |
According to the WHO severity score on admission, 50% of patients[16] presented with mild and 40.6% with moderate disease[13], while severe disease was observed only in three (9.4%) patients. No patient presented with critical disease.
Regarding radiological characteristics on admission, all except one patient, had a chest CT scan on admission. The patient without chest CT was asymptomatic and had normal chest X-rays on admission. The majority of patients [24 (77.4%)] had a CoVASc score of 0%-10%, i.e., low grade pulmonary infiltrates, corresponding to mild and moderate WHO. Of the remaining seven patients with a CoVASc score > 10%, four had a score of 11%-25%, corresponding to moderate disease, two had a score of 26%-50% and one had a score of 51%-75%, corresponding to severe WHO disease group.
Comparison of patients who were admitted with mild vs those with moderate/severe disease (16 patients in each group) (Table 2) revealed that they differed only regarding the presence of symptoms. Asymptomatic patients were mostly in the mild group[11,16] vs 1/32 in the moderate group with statistical significance (P = 0.001). Age, frailty index, sex, number of comorbidities, and CoVaSc CT score were not statistically different.
| Disease severity | Mild (16/32) | Moderate/severe (16/32) | P value |
| Median (IQR) | Median (IQR) | ||
| Age (yr) | 77.5 (54.5-84.5) | 75.5 (67.5-78.5) | NS |
| Frailty index | 3.5 (2-5) | 3 (2-4.5) | NS |
| Co-morbidities | 3 (1-3) | 3 (2-4) | NS |
| Men, n (%) | 7 (43.8) | 10 (62.5) | NS |
| Women, n (%) | 9 (56.2) | 6 (37.5) | |
| Screening, n (%) | 11 (68.8) | 1 (6.3) | < 0.01 |
| Symptomatic, n (%) | 5 (31.2) | 15 (93.7) | |
| CT infiltrates | NS | ||
| 0-10% | 13 (86.7) | 11 (68.8) | |
| > 10% | 2 (13.3) | 5 (31.2) | |
| COVID death, n (%) | 0 (0) | 3 (21.4) | NS |
| Non-COVID death, n (%) | 1 (7.1) | 1 (9.1) | NS |
| COVID progression, n (%) | 5 (31.3) | 8 (50) | NS |
Sixteen (50%) patients received therapy for COVID-19, including hydroxychloroquine plus azithromycin. Thirteen (40.6%) patients received dexamethasone plus azithromycin. One patient developed severe COVID-19 pneumonia, despite dexamethasone treatment, and was further deteriorated to severe acute respiratory distress syndrome. He was treated with tocilizumab (8 mg/kg once), and he was gradually improved and was discharged with no need for oxygen support. Broad spectrum antibiotics were prescribed in case of suspected superimposed bacterial pneumonia, or other in-hospital infections in 17 (53.1%) cases.
The mean time in dialysis prior to COVID-19 was 4 years. The most prevalent primary disease was arterial hypertension. Arteriovenous access was arm fistula in 15 (46.8%) patients, graft in 2 (6.2%), and ventral venous catheters in the rest. Potassium supplementation during dialysis was required in 12 (37.5%) patients. Hypotensive episodes were recorded on 17 (53.1%) patients. Thromboembolic events associated with access were recorded in 5 (15.6%) patients.
“Progressors” vs “non-progressors”: Thirteen (40.6%) patients experienced progression of COVID-19, manifesting as respiratory deterioration, which occurred 7-10 d after documentation of the infection (Table 1). “Progressors” (eight males and five females) had a median age of 78 (IQR: 75-82) years and a median frailty index 3 (IQR: 2-5). Eight of them (66.7%) had very limited findings on CT of the chest on admission (< 10%) and four patients had moderated findings (> 10%). Five (38.5%) patients presented with mild disease on admission, five (38.5%) had moderate disease, and three (23.1%) were asymptomatic. The median time to admission was similar between “progressors” [median: 1 (IQR: 1-3) d] and “non-progressors” [median: 1 d (IQR: 1-2) (P = 0.68)]. Ten (76.9%) of “progressors” were diagnosed with symptoms (76.9%) while three by screening.
Comparison between “progressors” vs “non-progressors” did not reveal any difference in terms of age, gender, or frailty. Those patients who did not progress tended to have a higher percentage of mild disease, but it did not differ statistically form that of “progressors” (P = 0.095). Compared to stable patients, “progressors” tended to be older (median age: 78 vs 70, P = 0.087), and experienced more respiratory symptoms on initial presentation (50% vs 10%, P = 0.14).
Survivors vs non-survivors: Overall (Table 1), 27 (75.8%) patients were discharged from hospital, after a median hospitalization time of 22 d (IQR = 15-35). Five patients died (Table 2) (CFR 15.6%) within a median time to death of 35 d (IQR: 24-35). The deceased vs survivors differed in being more frail (median: 7 vs 3, P = 0.016), with worse WHO severity (P = 0.05) and worse CT findings on admission (P = 0.005).
There were three cases of COVID-19 related death (respiratory failure), all among “progressors” (23%). Two of them died after they had been intubated and transferred to the intensive care unit. Two of them were female and one was male, aged 75-80 years old, with a frailty index on admission of 2.8 and 3, respectively. All three dying from COVID-19 related death had a CoVASc score > 10% on chest CT and they had moderate (2 cases) or severe (1 case) disease on admission.
Two deaths, non-related to COVID-19, were recorded in female patients, aged 70 and 85 years with recorded time to death being in 24 and 35 d, respectively, from admission. The frailty index was 7 in both cases and the cause of death was sudden cardiovascular event and aspiration, respectively.
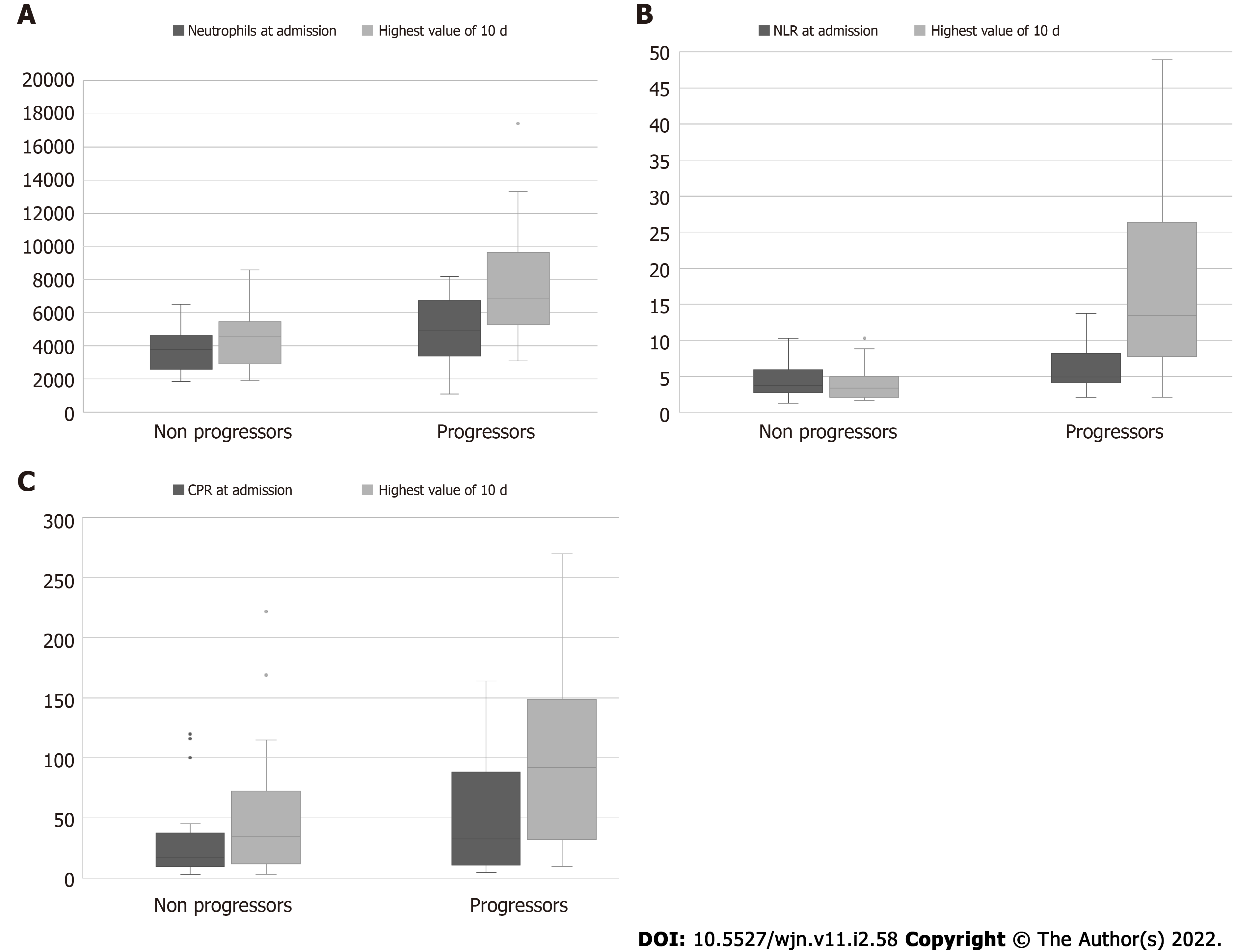
Laboratory analysis: Laboratory parameters on admission did not show any statistically significant association with outcome, either death or progression of COVID-19 (Table 3). There was a trend, though, for “progressors” and non-survivors to present with lower levels of lymphocytes, and higher CRP and NLR values, compared to patients who remained stable thereafter, and the survivors. “Progressors” had also a trend for higher numbers of neutrophils and level of serum ferritin values on admission. (Table 3, Figures 1 and 2).
| Variable | Survival status | Respiratory progression due to COVID-19 | |||||
| Total (n = 32) | Survivors | Non-survivors | P value | No (n = 19) | Yes (n = 13) | P value | |
| Median (IQR) | Median (IQR) | ||||||
| Lymphocytes (k/μL) | |||||||
| On admission | 0.9 (0.8-1.4) | 1 (0.8-1.4) | 0.6 (5.3-1.3) | NS | 1 (0.8-1.5) | 0.8 (0.5-1.3) | NS |
| Highest value of 10 d | 1.4 (1-1.7) | 1.3 (1-1.7) | 2.5 (1.4-3.4) | NS | 1.4 (1.2-1.8) | 0.9 (0.5-1.5) | NS |
| Increase (%) | 10.4 (-2.3-51.6) | 10.3 (-2.6-42.7) | 60.8 (6.8-365.1) | NS | 37.6 (5.4-83.2) | 6.8 (-9.4-10.6) | NS |
| CRP (mg/L) | |||||||
| On admission | 19.3 (9.6-47.7) | 17.2 (8.1-88.2) | 22 (19.3-41.8) | NS | 17.2 (8.1-41.2) | 32.8 (10.6-88.2) | NS |
| Highest value of 10 d | 55.6 (15.5-111.5) | 55.2 (15.1-108) | 83.5 (31.9-220) | NS | 34.8 (10.6-79) | 92 (31.9-149) | NS |
| Increase (%) | 61.6 (-8.2-312.6) | 54.3 (-0.9-308.8) | 426.3 (-36.3-435.3) | NS | 45 (-15.5-160.4) | 300.9 (0-513.2) | NS |
| WBC (mg/L) | |||||||
| On admission | 5.9 (4.7-7.9) | 5.9 (4.5-8) | 6.2 (5.3-7.7) | NS | 5.9 (4.8-7.7) | 5.9 (4.2-8.8) | NS |
| Highest value of 10 d | 7 (5.4-10.4) | 7 (5.3-10) | 9.4 (8-10.8) | NS | 6.9 (5.3-9.4) | 8 (5.9-12.6) | NS |
| Increase (%) | 16.9 (-2.5-73.2) | 15.4 (-2.9-44.5) | 88.1 (21.4-103.8) | NS | 15.4 (0-36.4) | 74.9 (-10.1-103.8) | NS |
| Neutrophils (k/μL) | |||||||
| On admission | 4 (2.8-5.8) | 4 (2.8-5.8) | 3.8 (3.7-4.4) | NS | 3.8 (2.5-4.7) | 4.9 (3.4-6.7) | NS |
| Highest value of 10 d | 5.3 (3.2-7.3) | 4.8 (3.1-7.3) | 5.6 (5.5-7.3) | NS | 4.6 (2.7-5.6) | 6.8 (5.3-9.6) | < 0.05 |
| Increase (%) | 19.7 (-1.8-82.9) | 16.7 (-1.8-73.3) | 47.6 (19.8-154.8) | NS | 18.7 (3.3-39.8) | 102.4 (-6.8-162.8) | NS |
| NLR | |||||||
| On admission | 4.4 (2.9-6.5) | 4.1 (2.9-6.4) | 5.6 (2.8-7.1) | NS | 3.7 (2.6-6) | 4.9 (4.1-8.2) | NS |
| Highest value of 10 d | 5 (2.7-10.6) | 4.7 (2.7-10.2) | 10 (3.3-14.6) | NS | 3.3 (2-5.3) | 13.4 (7.7-26.3) | < 0.01 |
| Increase (%) | 17.8 (-12.8-116.1) | 18.4 (-14-65.6) | 6.4 (3.9-263.6) | NS | 2.5 (-31.8-25.9) | 235.9 (18.4-394.4) | < 0.01 |
| Albumin (g/dL) | |||||||
| On admission | 3.8 (3.5-4.1) | 3.8 (3.5-4.1) | 3.9 (3.7-4) | NS | 3.8 (3.5-4.1) | 3.9 (3.5-4) | |
| Lowest value of 10 d | 3.3 (2.9-3.7) | 3.5 (2.9-3.7) | 2.9 (2.7-3.1) | < 0.05 | 3.3 (2.8-3.7) | 3.2 (2.9-3.5) | NS |
| Decrease (%) | 12.1 (3.6-20.5) | 10 (3.6-18.8) | 25.6 (16.2-26.7) | NS | 10 (3.6-18.8) | 17.1 (7.7-20.5) | NS |
| Ferritin (ng/mL) | |||||||
| On admission | 448 (241.5-911) | 459 (249-940) | 408 (224-745) | NS | 341 (202-940) | 745 (369-904) | NS |
| Highest value of 10 d | 1018 (445.5-1507) | 1038 (428-1559) | 605 (520-666) | NS | 548 (295-1455) | 1102 (666-1837) | NS |
| Increase (%) | 49.3 (24.5-129.5) | 54.8 (26.3-129.2) | 27.5 (-21.9-146.6) | NS | 30.2 (26.3.4-97.7) | 129.7 (12.4-197.3) | NS |
| LDH (U/L) | |||||||
| On admission | 216 (174-285) | 222 (175-276) | 207 (174-298) | NS | 216 (158-297) | 217.5 (193-232.5) | NS |
| Highest value of 10 d | 227 (183-273) | 225.5 (183-256) | 313 (272-330) | < 0.05 | 224 (184-256) | 261 (177.5-321.5) | NS |
| Increase (%) | 5.7 (-13.8-60.6) | 5.6 (-13.8-25.2) | 89.7 (5-95.7) | < 0.05 | 5.8 (-14.7-25.2) | 5 (-11.6-89.7) | NS |
| Ddimers (ng/mL) | |||||||
| On admission | 1325 (772-2841) | 1080 (772-21562349 | 3089 (1244-5205) | NS | 1080 (732-3136) | 1640 (996-2349) | NS |
| Highest value of 10 d | 1861.5 (1215-3503) | 1624 (1073-2526) | 3503 (3447-5032) | <0.05 | 1624 (1259-3191) | 2526 (1073-4134) | NS |
| Increase (%) | 13 (-1.6-61.2) | 7.3 (-1.6-41.2) | 82.6 (19.1-195.8) | NS | 18.5 (0-52) | 1.4 (-21.3-104.3) | NS |
| Troponin | |||||||
| On admission | 72.3 (33.6-99.6) | 72.9 (26.9-102) | 71.4 (53-86.7) | NS | 53 (25.8-84.4) | 86.7 (49.8-102) | NS |
| Highest value of 10 d | 84.6 (46.7-116) | 84.4 (38.3-118) | 92.6 (62-114) | NS | 66.5 (29.4-108) | 103 (83.2-118) | NS |
| Increase (%) | 17.7 (2-39.6) | 17.6 (1-45) | 29.7 (17.3-31.5) | NS | 17.6 (1-50.4) | 29.7 (2.9-34.1) | NS |
We found a statistically significant difference between “progressors” and stable patients, regarding the highest 10-d value of neutrophils [6800 (IQR: 5300-9600) vs 4600 (IQR: 2700-5600), P = 0.018], the highest value of NLR [13.4 (IQR: 7.7-26.3) vs 3.3 (IQR: 2-5.3) P = 0.001], and the related percentage increase [235.9 (IQR: 18.4-394.4) vs 2.5 (IQR: -31.5-25.9), P = 0.005].
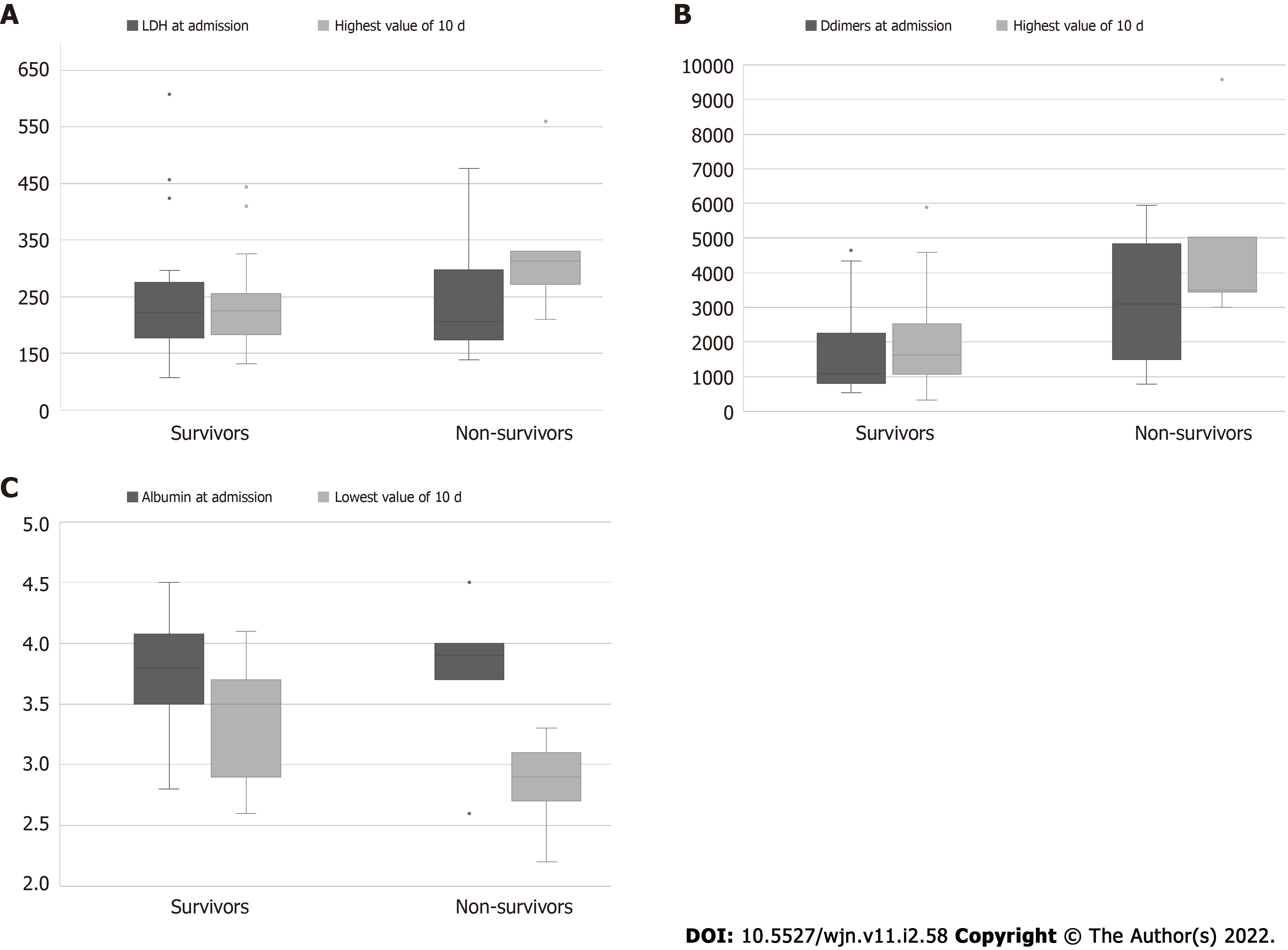
Comparison between non-survivors vs survivors, revealed that they differed significantly regarding the highest value of LDH [median: 313 (IQR: 272-330) vs 225.5 (IQR: 183-256), P = 0.028] and its percentage increase [89.7% (IQR 5-97.5) vs 5.6% (-13.8-25.2) increase, P = 0.039]. Additionally, non-survivors had the lowest 10-d value of albumin [median: 2.9 g/dL (IQR: 2.7-3.1] vs [3.5 (IQR: 2.9-3.7), P = 0.028], and the highest 10-d value of D-dimers [median 3503 ng/mL (3447-5032) vs 1624 (1073-2526), P = 0.011]. Troponin levels did not show any statistically significant difference neither in deceased patients nor in progressors.
This article analyzes our experience with COVID-19 in a cohort of 32 patients on MHD during an 11-m period before COVID-19 vaccination was available. The aim of the study was to describe the clinical characteristics of the disease at presentation and its outcomes in this group of patients, and look for distinctive features predicting outcome. According to our findings, age, gender, and the presence of co-morbidities did not show any statistical difference between survivors and non- survivors and between “progressors” and “non- progressors”. On the contrary, the frailty index, the WHO severity score, and the CoVASc score on admission seemed to matter, since they differed statistically between survivors and non-survivors. In terms of laboratory parameters at the time of admission, a more “inflamed” laboratory profile (CRP and NLR) and lower lymphocytes were shown to be a potential alarm for adverse clinical evolution (“progressors and deceased patients”). However, the kinetics of inflammation markers (NLR and neutrophils) over 10 d of hospitalization were able to distinguish with statistical significance “progressors” vs “non-progressors”. In addition, the kinetics of LDH and D-dimers (increase) and albumin (decrease) were able to distinguish with statistical significance non-survivors from survivors.
The vast majority of MHD patients in our study (90. 6%) presented with mild (50%) or moderate (40.6%) severity of COVID-19, according to the WHO classification system. Apart from symptoms, being statistically more prevalent in moderate disease, the severity groups did not differ statistically regarding age, gender, number of co- morbidities, or CoVASc radiology data. In relation to this, a recent study which compared patients on chronic dialysis with a propensity matched cohort found that dialysis patients had a less severe COVID-19 phenotype[25]. In the present study, 12 patients were diagnosed by screening (37.5%) and 20 (62.5%) with symptoms, mainly fever (65%), respiratory symptoms (30%), and diarrhea (5%). Interestingly, no patient complained of anosmia or ageusia, in contrast to the general population, as reported by others as well[26]. Anosmia and ageusia have been attributed to the fact that angiotensin-converting enzyme II has been identified as the cellular receptor for SARS-CoV-2, which is found in the oral cavity and nasal mucosa[27,28]. However, dialysis patients have been shown to have reduced angiotensin-converting enzyme II plasma cell activity[29].
Despite the relatively mild initial presentation, 40.6% of patients experienced progressive disease of the respiratory system. The CFR in our cohort was 15.5%. Four of the deaths occurred among “progressors” (30.7%), with three of them being related to COVID-19 (9.3%). Non-COVID-19 related death (sudden death and aspiration) occurred in 6.2%, one in “progressors” and one in “non-progressors”. In a dialysis population of similar size from Spain[11], the CFR was reported in 30.5%. However, the Spanish cohort had worse disease status at presentation, with poor oxygen saturation (< 95%) in breathing room air observed in 22 out of 36 patients[11]. Accordingly, in a cohort study of ICU patients, the rate of death related to COVID-19 differed in dialysis patients compared to the general population, with a higher prevalence of sudden death/arrhythmia and septic shock in the dialysis population[12].
Patients on chronic dialysis have been reported to be either more vulnerable[11-12] or rather protected[13-14,25]. An international study including dialysis patients concluded that these patients were both more susceptible to severe COVID-19 disease and experienced increased mortality, although with great disparity in mortality rates[30].
In clinical practice, the most challenging question is the identification of prognostic factors, which might help clinicians to recognize those patients at high risk for disease progression and/or death. We did not find any specific clinical characteristics or radiology indexes that could discriminate “progressors” from stable patients on admission. The clinical implication, in the setting of chronic dialysis, is that even almost asymptomatic patients were candidates for disease aggravation. In the general population, the CT severity score, inflammatory markers, and older age on admission have been described as independent risk factors for short-term progression[31-32].
From the laboratory perspective, on admission there was a trend, in the “progressors” group, of lower lymphocyte count and higher NLR, CRP, and ferritin values, i.e., a more inflammatory profile, as previously shown[25]. These laboratory parameters have been associated with severe COVID-19 in the general population[32-36] as well.
However, follow-up of laboratory measurements revealed that there was a statistically significant increase of neutrophils and NLR during the first 10 d, between “progressors” and stable patients. Similar findings have been reported for laboratory data on the 7th day after admission for dialysis patients with COVID-19[11]. Also, CRP has been used in hospitalized patients with COVID-19 for disease stratification and prognostication[36]. However, in our cohort there was only a trend for the value of day 10 for the “progressors”.
In terms of survival, the WHO severity score on admission, the frailty index, and the CoVAsc radiology data were shown to differ between survivors and non-survivors. Interestingly, no difference was found in clinical and radiological data on admission between “progressors” and “non-progressors”. Yet, death occurred also from non-COVID-19 respiratory failure, i.e., non-COVID19 related. Zeng et al[37] compared the annual all-cause mortality in dialysis patients during the pandemic and found that it was significantly higher in 2020 (4.89%) than in 2018 (2.55%) or 2019 (1.97%). During the COVID-19 outbreak, the mortality rate from all causes excluding COVID-19 was 2.73%, which was slightly higher than that from COVID-19 (2.16%). In our cohort, we recorded a rate of 5.9% non-COVID-19 related deaths. As has been reported[2], patients with severe underlying diseases often die with COVID-19, i.e., they die of their original co-morbidities. In our cohort, as in the large ERA-CODA[6], the frailty index in contrast to co-morbidities, discriminated survivors from non-survivors patients in chronic dialysis.
None of the laboratory parameter on admission could discriminate survivors from non-survivors, except a tendency for lower lymphocytes, and higher CRP, NLR, and D-dimer values on admission, i.e., a more inflammatory profile. Importantly, follow-up of the laboratory values over 10 d revealed that non-survivors differed significantly from survivors only regarding the 10th-d value of LDH and D-dimers (higher values) and the lowest 10-d value of albumin. The sequential increase of LDH has been described as a prognostic laboratory marker for severe COVID-19 in the general population[38] and dialysis patients[11,39], indicating cytokine-induced lung tissue damage[38]. Increased levels of D-dimers have also associated with adverse outcomes in COVID-19 patients both in the general population[40] and in patients on MHD[39]. Interestingly, troponin levels did not show any significant difference either in deceased patients or in “progressors”. Troponin levels have been described as a predictive marker of COVID-19 mortality in the general population[33], a finding which was not confirmed in dialysis patients[39]. This is probably related to the fact that troponin levels in patients with chronic kidney disease may be related to chronic structural heart disease rather than acute ischemia[41].
Due to the small number of patients, we cannot draw any conclusions on the effect of treatment. During the 1st wave, the combination of hydroxychloroquine and azithromycin was given only in three symptomatic patients, all of whom survived. However, they had all presented with very mild disease and low CoVASc score (< 10%) although they were quite old and moderately frail. This type of treatment has not been shown to be efficient for mild and moderate COVID-19[42]. During the 2nd wave, there was no specific treatment, except the use of dexamethasone, in patients who required administration of oxygen, according to the recovery trial[43]. Azithromycin was given based on its antiviral and immunomodulatory activity[44]. No adverse effects were recorded[45]. A patient who did not respond to dexamethasone during the 3rd wave received tocilizumab for severe pneumonia and showed remarkable improvement[46].
In general, ESKD is associated with increased mortality rates compared to age-matched controls[47], especially death from cardiovascular events[48] and in the intensive care unit[49]. Since cardiovascular complications are rapidly emerging as a key threat in COVID-19 in addition to respiratory disease[50], it would be expected that this “fragile” population would be devastated by the pandemic. Patients with ESKD were shown to have the paradox of immune-activation and immune-depression[51] at the same time. For the general population, a unique immune response to SARS-CoV-2 has been described[52]. It has been proposed that ESKD patients may be rather protected for severe COVID-19, as unable to mount a cytokine hyper-active response, a cardinal feature of severe COVID-19[14]. Thus, being in chronic dialysis may not always an independent risk factor for COVID-19 adverse outcome[39].
In conclusion, herein we describe a cohort of patients on chronic dialysis who were admitted with COVID-19. A proportion of patients were diagnosed following routine testing and presented with mild disease. Absence of pneumonia or mild pneumonia was documented clinically on admission in 90.6% of patients, while CT tomography revealed infiltrates > 10% only in 13.3% of admitted patients. A CFR of 15.6%[5,32] was recorded in the whole cohort and 30.7% among “progressors”. On admission a more “inflamed” profile reflected by CRP, WBC, NLR, and lower lymphocytes indicated a “hint” for upcoming progression to respiratory failure, although with no statistical significance. Clinically, statistical significance for disease progression was shown by the highest 10-d value of NLR, and its percentage increase from admission, and the highest 10-d value of neutrophils. As for survival, the frailty index, the severity stage by WHO classification, and the CoVASc score were shown statistically different on admission. Likewise, the highest 10 -d value of LDH and D-dimers and the lowest of albumin were shown to be important. Further studies are needed to unravel the immune response to COVID-19 in chronic dialysis patients and stratify the best management algorithm.
Coronavirus disease 2019 (COVID-19) pandemic runs as mild upper respiratory infection or being asymptomatic in 80% of infected patients, 15% develop severe lung disease, and 5% progress to respiratory failure or septic shock. Mortality ranges from 2%-50%.
Τo analyze our experience with patients with end-stage kidney disease (ESKD) on maintenance hemodialysis (MHD) with COVID-19 before the era of vaccination.
To identify predictors of worst outcome in patients with ESKD on MHD with COVID-19 in the era prior to vaccination, and to study all the range of clinical pictures of COVID-19 in this group of patients, including asymptomatic to severe cases all from a single center.
This was a retrospective cohort study from a single referral center from April to February 2021. We examined the kinetics of laboratory evolution of certain parameters linked to COVID-19 pathophysiology, as potential prognostication markers of adverse outcome. Patients were scored according to the WHO severity system for COVID-19 and frailty index, besides classic demographics, and co-morbidities. A new simplified scoring system of severity (Covid Visual Assessment score, CoVAsc) was used.
Thirty-two hospitalized MHD patients with COVID-19 were studied, from admission to outcome. Although initial presentation was mild on admission regarding WHO severity (16 with mild disease, 13 with moderate, and 3 with severe) and CoVAsc score (24 patients had 0-10% lung infiltrates), the outcome was quite adverse. Approximately 40.6% of patients progressed to severe disease and 15.5% died. “Progressors” tended to have a more “inflamed” laboratory profile at the time of admission and statistically significant higher neutrophils to lymphocytes ratio during the first 10 d of hospitalization. The deceased differed from “survivors” with statistical significance as having a worse WHO severity score, frailty index, and CoVASc score and regarding the first 10-d kinetics of lactate dehydrogenase (increase), D-dimers (increase), and albumin (decrease).
Traditional risk factors for adverse COVID-19 outcome including male gender and comorbidities do not seem to apply in MHD patients. Potential new clinical indicators of adverse outcome, according to our findings, include the WHO severity score, frailty index, CoVASc score, and the 10-d kinetics of certain laboratory parameters.
A larger number of dialysis patients might be studied especially after vaccination and the evolving various mutations of SARS-CoV-2.
We acknowledge the support and contribution in the management of the coronavirus disease 2019 patients from nephrologists; Kalogeropoulou S, Katsoudas S, Gounari P, Nikolopoulos P, Tsotsorou O, and nurses; Zorba I, Polymerou Z, Xoxakou L, Maniati A, Flevotomou M, Siopi D, Karasideri M, and Kyriakidis V. We acknowledge the support of Dionisios Ouzounis for the revision of tables and figures.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Infectious diseases
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Islam MM, Turkey; Markic D, Croatia; Shoenfeld Y, Israel S-Editor: Xing YX L-Editor: Wang TQ P-Editor: Xing YX
| 1. | Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11409] [Cited by in RCA: 11508] [Article Influence: 2301.6] [Reference Citation Analysis (0)] |
| 2. | Cucinotta D, Vanelli M. WHO Declares COVID-19 a Pandemic. Acta Biomed. 2020;91:157-160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2625] [Reference Citation Analysis (0)] |
| 3. | World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard. Available from: https://covid19.who.int/. |
| 4. | World Health Organization. Clinical management of COVID-19 interim guidance. [cited 27 May 2020] Available from: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---27-may-2020.. |
| 5. | Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20:363-374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3174] [Cited by in RCA: 2918] [Article Influence: 583.6] [Reference Citation Analysis (0)] |
| 6. | Hilbrands LB, Duivenvoorden R, Vart P, Franssen CFM, Hemmelder MH, Jager KJ, Kieneker LM, Noordzij M, Pena MJ, Vries H, Arroyo D, Covic A, Crespo M, Goffin E, Islam M, Massy ZA, Montero N, Oliveira JP, Roca Muñoz A, Sanchez JE, Sridharan S, Winzeler R, Gansevoort RT; ERACODA Collaborators. COVID-19-related mortality in kidney transplant and dialysis patients: results of the ERACODA collaboration. Nephrol Dial Transplant. 2020;35:1973-1983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 321] [Cited by in RCA: 296] [Article Influence: 59.2] [Reference Citation Analysis (0)] |
| 7. | Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, Curtis HJ, Mehrkar A, Evans D, Inglesby P, Cockburn J, McDonald HI, MacKenna B, Tomlinson L, Douglas IJ, Rentsch CT, Mathur R, Wong AYS, Grieve R, Harrison D, Forbes H, Schultze A, Croker R, Parry J, Hester F, Harper S, Perera R, Evans SJW, Smeeth L, Goldacre B. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430-436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4343] [Cited by in RCA: 4211] [Article Influence: 842.2] [Reference Citation Analysis (0)] |
| 8. | Khullar D, Bond AM, Schpero WL. COVID-19 and the Financial Health of US Hospitals. JAMA. 2020;323:2127-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 165] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 9. | Couser WG, Remuzzi G, Mendis S, Tonelli M. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int. 2011;80:1258-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 862] [Cited by in RCA: 981] [Article Influence: 70.1] [Reference Citation Analysis (0)] |
| 10. | Stern LD, Waikar S. Time to Expand Access and Utilization of Home Dialysis: Lessons From the COVID-19 Pandemic. Mayo Clin Proc. 2020;95:1323-1324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Goicoechea M, Sánchez Cámara LA, Macías N, Muñoz de Morales A, Rojas ÁG, Bascuñana A, Arroyo D, Vega A, Abad S, Verde E, García Prieto AM, Verdalles Ú, Barbieri D, Delgado AF, Carbayo J, Mijaylova A, Acosta A, Melero R, Tejedor A, Benitez PR, Pérez de José A, Rodriguez Ferrero ML, Anaya F, Rengel M, Barraca D, Luño J, Aragoncillo I. COVID-19: clinical course and outcomes of 36 hemodialysis patients in Spain. Kidney Int. 2020;98:27-34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 233] [Cited by in RCA: 224] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 12. | Flythe JE, Assimon MM, Tugman MJ, Chang EH, Gupta S, Shah J, Sosa MA, Renaghan AD, Melamed ML, Wilson FP, Neyra JA, Rashidi A, Boyle SM, Anand S, Christov M, Thomas LF, Edmonston D, Leaf DE; STOP-COVID Investigators. Characteristics and Outcomes of Individuals With Pre-existing Kidney Disease and COVID-19 Admitted to Intensive Care Units in the United States. Am J Kidney Dis. 2021;77:190-203.e1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 155] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 13. | Naaraayan A, Nimkar A, Hasan A, Pant S, Durdevic M, Elenius H, Nava Suarez C, Basak P, Lakshmi K, Mandel M, Jesmajian S. End-Stage Renal Disease Patients on Chronic Hemodialysis Fare Better With COVID-19: A Retrospective Cohort Study From the New York Metropolitan Region. Cureus. 2020;12:e10373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Ma Y, Diao B, Lv X, Zhu J, Chen C, Liu L, Zhang S, Shen B, Wang H. Epidemiological, Clinical, and Immunological Features of a Cluster of COVID-19-Contracted Hemodialysis Patients. Kidney Int Rep. 2020;5:1333-1341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 15. | Sorci G, Faivre B, Morand S. Explaining among-country variation in COVID-19 case fatality rate. Sci Rep. 2020;10:18909. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 204] [Cited by in RCA: 157] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 16. | Pairo-Castineira E, Clohisey S, Klaric L, Bretherick AD, Rawlik K, Pasko D, Walker S, Parkinson N, Fourman MH, Russell CD, Furniss J, Richmond A, Gountouna E, Wrobel N, Harrison D, Wang B, Wu Y, Meynert A, Griffiths F, Oosthuyzen W, Kousathanas A, Moutsianas L, Yang Z, Zhai R, Zheng C, Grimes G, Beale R, Millar J, Shih B, Keating S, Zechner M, Haley C, Porteous DJ, Hayward C, Yang J, Knight J, Summers C, Shankar-Hari M, Klenerman P, Turtle L, Ho A, Moore SC, Hinds C, Horby P, Nichol A, Maslove D, Ling L, McAuley D, Montgomery H, Walsh T, Pereira AC, Renieri A; GenOMICC Investigators; ISARIC4C Investigators; COVID-19 Human Genetics Initiative; 23andMe Investigators; BRACOVID Investigators; Gen-COVID Investigators, Shen X, Ponting CP, Fawkes A, Tenesa A, Caulfield M, Scott R, Rowan K, Murphy L, Openshaw PJM, Semple MG, Law A, Vitart V, Wilson JF, Baillie JK. Genetic mechanisms of critical illness in COVID-19. Nature. 2021;591:92-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1045] [Cited by in RCA: 934] [Article Influence: 233.5] [Reference Citation Analysis (0)] |
| 17. | Pascarella G, Strumia A, Piliego C, Bruno F, Del Buono R, Costa F, Scarlata S, Agrò FE. COVID-19 diagnosis and management: a comprehensive review. J Intern Med. 2020;288:192-206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 702] [Cited by in RCA: 733] [Article Influence: 146.6] [Reference Citation Analysis (0)] |
| 18. | Mendiratta P, Latif R. Clinical Frailty Scale. 2021 Jun 20. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. [PubMed] |
| 19. | Our World in Data. Mortality Risk of COVID-19. Available from: https://ourworldindata.org/mortality-risk-covid. |
| 20. | Gautret P, Million M, Jarrot PA, Camoin-Jau L, Colson P, Fenollar F, Leone M, La Scola B, Devaux C, Gaubert JY, Mege JL, Vitte J, Melenotte C, Rolain JM, Parola P, Lagier JC, Brouqui P, Raoult D. Natural history of COVID-19 and therapeutic options. Expert Rev Clin Immunol. 2020;16:1159-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 80] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 21. | Arkoudis NA, Tsochatzis A, Argentos S, Kontopoulou C, Mademli M, Spiliopoulos S, Oikonomopoulos N. CT in patients with COVID-19: Imaging patterns, disease extent and evolution; our experience in a Greek reference University Hospital. Hell J Radiol. 2021;6:2-12. |
| 22. | Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M, Doudier B, Courjon J, Giordanengo V, Vieira VE, Tissot Dupont H, Honoré S, Colson P, Chabrière E, La Scola B, Rolain JM, Brouqui P, Raoult D. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;56:105949. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3279] [Cited by in RCA: 3272] [Article Influence: 654.4] [Reference Citation Analysis (0)] |
| 23. | Xu J, Cao B. Lessons learnt from hydroxychloroquine/azithromycin in treatment of COVID-19. Eur Respir J. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Akbarialiabad H, Kavousi S, Ghahramani A, Bastani B, Ghahramani N. COVID-19 and maintenance hemodialysis: a systematic scoping review of practice guidelines. BMC Nephrol. 2020;21:470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Chan L, Jaladanki SK, Somani S, Paranjpe I, Kumar A, Zhao S, Kaufman L, Leisman S, Sharma S, He JC, Murphy B, Fayad ZA, Levin MA, Bottinger EP, Charney AW, Glicksberg BS, Coca SG, Nadkarni GN; Mount Sinai COVID Informatics Center (MSCIC). Outcomes of Patients on Maintenance Dialysis Hospitalized with COVID-19. Clin J Am Soc Nephrol. 2021;16:452-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 26. | Creput C, Fumeron C, Toledano D, Diaconita M, Izzedine H. COVID-19 in Patients Undergoing Hemodialysis: Prevalence and Asymptomatic Screening During a Period of High Community Prevalence in a Large Paris Center. Kidney Med. 2020;2:716-723.e1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 27. | Agyeman AA, Chin KL, Landersdorfer CB, Liew D, Ofori-Asenso R. Smell and Taste Dysfunction in Patients With COVID-19: A Systematic Review and Meta-analysis. Mayo Clin Proc. 2020;95:1621-1631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 310] [Cited by in RCA: 279] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 28. | Vaira LA, Salzano G, Fois AG, Piombino P, De Riu G. Potential pathogenesis of ageusia and anosmia in COVID-19 patients. Int Forum Allergy Rhinol. 2020;10:1103-1104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 172] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 29. | Wysocki J, Batlle D. Reduced plasma ACE2 activity in dialysis patients: another piece in the conundrum of factors involved in hypertension and cardiovascular morbidity? Nephrol Dial Transplant. 2013;28:2200-2202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Andhika R, Huang I, Wijaya I. Severity of COVID-19 in end-stage kidney disease patients on chronic dialysis. Ther Apher Dial. 2021;25:706-709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 31. | Our World in data. Mortality Risk of COVID-19. Available from: https://ourworldindata.org/mortality-risk-covid#what-do-we-know-about-the-risk-of-dying-from-covid-19. |
| 32. | Feng Z, Yu Q, Yao S, Luo L, Zhou W, Mao X, Li J, Duan J, Yan Z, Yang M, Tan H, Ma M, Li T, Yi D, Mi Z, Zhao H, Jiang Y, He Z, Li H, Nie W, Liu Y, Zhao J, Luo M, Liu X, Rong P, Wang W. Early prediction of disease progression in COVID-19 pneumonia patients with chest CT and clinical characteristics. Nat Commun. 2020;11:4968. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 156] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 33. | Du RH, Liang LR, Yang CQ, Wang W, Cao TZ, Li M, Guo GY, Du J, Zheng CL, Zhu Q, Hu M, Li XY, Peng P, Shi HZ. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur Respir J. 2020;55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 769] [Cited by in RCA: 913] [Article Influence: 182.6] [Reference Citation Analysis (0)] |
| 34. | Liu Y, Du X, Chen J, Jin Y, Peng L, Wang HHX, Luo M, Chen L, Zhao Y. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J Infect. 2020;81:e6-e12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 386] [Cited by in RCA: 564] [Article Influence: 112.8] [Reference Citation Analysis (0)] |
| 35. | Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054-1062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17476] [Cited by in RCA: 18204] [Article Influence: 3640.8] [Reference Citation Analysis (0)] |
| 36. | Sharifpour M, Rangaraju S, Liu M, Alabyad D, Nahab FB, Creel-Bulos CM, Jabaley CS; Emory COVID-19 Quality & Clinical Research Collaborative. C-Reactive protein as a prognostic indicator in hospitalized patients with COVID-19. PLoS One. 2020;15:e0242400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 88] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 37. | Zeng XR, Huang XM, Xu L, Xiao JW. Clinical outcomes of dialysis patients with COVID-19 in the initial phase of the COVID-19 outbreak in Wuhan, China. Int Urol Nephrol. 2021;53:353-357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 38. | Henry BM, Aggarwal G, Wong J, Benoit S, Vikse J, Plebani M, Lippi G. Lactate dehydrogenase levels predict coronavirus disease 2019 (COVID-19) severity and mortality: A pooled analysis. Am J Emerg Med. 2020;38:1722-1726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 232] [Cited by in RCA: 355] [Article Influence: 71.0] [Reference Citation Analysis (0)] |
| 39. | Bacharaki D, Chrysanthopoulou E, Grigoropoulou S, Giannakopoulos P, Simitsis P, Frantzeskaki F, Flevari A, Karagiannis M, Sardeli A, Kavatha D, Antoniadou A, Vlahakos D. Siblings with coronavirus disease 2019 infection and opposite outcome-the hemodialysis's better outcome paradox: Two case reports. World J Nephrol. 2021;10:21-28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 40. | Zhang L, Yan X, Fan Q, Liu H, Liu X, Liu Z, Zhang Z. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J Thromb Haemost. 2020;18:1324-1329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 837] [Cited by in RCA: 837] [Article Influence: 167.4] [Reference Citation Analysis (0)] |
| 41. | Ellis K, Dreisbach AW, Lertora JL. Plasma elimination of cardiac troponin I in end-stage renal disease. South Med J. 2001;94:993-996. [PubMed] |
| 42. | Cavalcanti AB, Zampieri FG, Rosa RG, Azevedo LCP, Veiga VC, Avezum A, Damiani LP, Marcadenti A, Kawano-Dourado L, Lisboa T, Junqueira DLM, de Barros E Silva PGM, Tramujas L, Abreu-Silva EO, Laranjeira LN, Soares AT, Echenique LS, Pereira AJ, Freitas FGR, Gebara OCE, Dantas VCS, Furtado RHM, Milan EP, Golin NA, Cardoso FF, Maia IS, Hoffmann Filho CR, Kormann APM, Amazonas RB, Bocchi de Oliveira MF, Serpa-Neto A, Falavigna M, Lopes RD, Machado FR, Berwanger O; Coalition Covid-19 Brazil I Investigators. Hydroxychloroquine with or without Azithromycin in Mild-to-Moderate Covid-19. N Engl J Med. 2020;383:2041-2052. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 751] [Cited by in RCA: 768] [Article Influence: 153.6] [Reference Citation Analysis (0)] |
| 43. | RECOVERY Collaborative Group; Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, Prudon B, Green C, Felton T, Chadwick D, Rege K, Fegan C, Chappell LC, Faust SN, Jaki T, Jeffery K, Montgomery A, Rowan K, Juszczak E, Baillie JK, Haynes R, Landray MJ. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. 2021;384:693-704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6762] [Cited by in RCA: 7391] [Article Influence: 1847.8] [Reference Citation Analysis (1)] |
| 44. | Echeverría-Esnal D, Martin-Ontiyuelo C, Navarrete-Rouco ME, De-Antonio Cuscó M, Ferrández O, Horcajada JP, Grau S. Azithromycin in the treatment of COVID-19: a review. Expert Rev Anti Infect Ther. 2021;19:147-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 141] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 45. | Giaime P, Guenoun M, Pedinielli N, Narbonne H, Bergounioux JP, Solas C, Guilhaumou R, Sampol J, Ollier J, Sichez H, Serveaux M, Brunner F, Bataille S. Hydroxychloroquine and azithromycin tolerance in haemodialysis patients during COVID-19 infection. Nephrol Dial Transplant. 2020;35:1346-1353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 46. | Gupta S, Madhyastha R, Hamed F, Balkis M, El Nekidy W, Attallah N. Tocilizumab Use in a Chronic Hemodialysis Patient for the Management of COVID-19-Associated Pneumonia and Acute Respiratory Distress Syndrome. Case Rep Nephrol. 2020;2020:8829309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 47. | Prichard SS. Comorbidities and their impact on outcome in patients with end-stage renal disease. Kidney Int. 2020;57:100-104. |
| 48. | Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7995] [Cited by in RCA: 8531] [Article Influence: 406.2] [Reference Citation Analysis (0)] |
| 49. | Apel M, Maia VP, Zeidan M, Schinkoethe C, Wolf G, Reinhart K, Sakr Y. End-stage renal disease and outcome in a surgical intensive care unit. Crit Care. 2013;17:R298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 50. | Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417-1418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4227] [Cited by in RCA: 4584] [Article Influence: 916.8] [Reference Citation Analysis (0)] |
| 51. | Kato S, Chmielewski M, Honda H, Pecoits-Filho R, Matsuo S, Yuzawa Y, Tranaeus A, Stenvinkel P, Lindholm B. Aspects of immune dysfunction in end-stage renal disease. Clin J Am Soc Nephrol. 2008;3:1526-1533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 622] [Cited by in RCA: 778] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 52. | Giamarellos-Bourboulis EJ, Netea MG, Rovina N, Akinosoglou K, Antoniadou A, Antonakos N, Damoraki G, Gkavogianni T, Adami ME, Katsaounou P, Ntaganou M, Kyriakopoulou M, Dimopoulos G, Koutsodimitropoulos I, Velissaris D, Koufargyris P, Karageorgos A, Katrini K, Lekakis V, Lupse M, Kotsaki A, Renieris G, Theodoulou D, Panou V, Koukaki E, Koulouris N, Gogos C, Koutsoukou A. Complex Immune Dysregulation in COVID-19 Patients with Severe Respiratory Failure. Cell Host Microbe. 2020;27:992-1000.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1306] [Cited by in RCA: 1556] [Article Influence: 311.2] [Reference Citation Analysis (0)] |