Peer-review started: March 31, 2021
First decision: July 31, 2021
Revised: August 10, 2021
Accepted: December 2, 2021
Article in press: December 2, 2021
Published online: January 25, 2022
Processing time: 294 Days and 22 Hours
Virus-related cancers in humans are widely recognized, but in the case of renal cancer, the link with the world of viruses is not clearly established in humans, despite being known in animal biology. In the present review, we aimed to explore the literature on renal cell carcinoma (RCC) for a possible role of viruses in human RCC tumorigenesis and immune homeostasis, hypothesizing the contribution of viruses to the immunogenicity of this tumor. A scientific literature search was conducted using the PubMed, Web of Science, and Google Scholar databases with the keywords “virus” or “viruses” or “viral infection” matched with (“AND”) “renal cell carcinoma” or “kidney cancer” or “renal cancer” or “renal carcinoma” or “renal tumor” or “RCC”. The retrieved findings evidenced two main aspects testifying to the relationship between RCC and viruses: The presence of viruses within the tumor, especially in non-clear cell RCC cases, and RCC occurrence in cases with pre-existing chronic viral infections. Some retrieved translational and clinical data suggest the possible contribution of viruses, particularly Epstein-Barr virus, to the marked immunogenicity of sarcomatoid RCC. In addition, it was revealed the possible role of endogenous retrovirus reactivation in RCC oncogenesis, introducing new fascinating hypotheses about this tumor’s immunogenicity and likeliness of response to immune checkpoint inhibitors.
Core Tip: An overview of the complex interplay between viral agents and renal carcinogenesis, possibly influencing the course of the disease, the tumor immune microenvironment, the production of new antigens, the host’s and the tumor’s immunogenicity, and, even more, the response to immune checkpoint blockade.
- Citation: Bersanelli M, Casartelli C, Buti S, Porta C. Renal cell carcinoma and viral infections: A dangerous relationship? World J Nephrol 2022; 11(1): 1-12
- URL: https://www.wjgnet.com/2220-6124/full/v11/i1/1.htm
- DOI: https://dx.doi.org/10.5527/wjn.v11.i1.1
Virus-related cancers in humans are widely recognized and listed by the American Cancer Society[1]. The growing knowledge about the role of viruses as a cause of tumors has led to vaccines' development to prevent specific types of human cancers, which effectiveness is often prevented by prior exposure to the wild virus. Viruses known to be directly related to cancer are the human papillomaviruses (HPVs), leading mainly to cervix cancer and other genital or oral cancers; the Epstein-Barr virus (EBV), related to nasopharyngeal and gastric cancers, but also Burkitt and Hodgkin lymphomas; the human herpesvirus 8 (HHV8), associated with Kaposi sarcoma; the hepatitis B and C virus (HBV and HCV), provoking hepatocellular carcinoma, and even the human immunodeficiency virus (HIV) and HHV8, sometimes directly and often indirectly (through immunosuppression) related to a higher risk of developing Kaposi sarcoma, cervical cancer, tumors of the central nervous system and Hodgkin lymphoma[1].
In the case of renal cancer, the link with the world of viruses is established in animal biology, given the cause-effect relationship between the renal carcinoma of leopard frogs (Rana pipiens) and the Lucké tumor herpesvirus (LTHV). In 1974, the Koch-Henle postulates between LTHV and frogs’ renal cancer were fulfilled, demonstrating that (1) The agent was associated with the disease; (2) The agent induced the same disease in a susceptible host; (3) The agent was isolated from the induced disease; and (4) The isolated agent was the same agent originally associated with the disease[2]. Then, in 1982, LTHV was found in the primary tumor and metastatic tumor cells in the liver, fat body, and bladder, revealing both by histopathology and electron microscopy that the virus was retained from the primary tumor to its metastatic cells[3].
Given this ancestral link, we aimed to explore the literature on human renal cancer, namely renal cell carcinoma (RCC), to verify the possible role of viruses in human RCC tumorigenesis and immune homeostasis with the host.
In addition, considering the recent advances in the field of systemic immunotherapies, with the evidence of the efficacy of anti-programmed death 1 (PD-1)/programmed cell death ligand 1 (PD-L1) and anti-CTLA-4 immune checkpoint inhibitors (ICIs) in the treatment of metastatic RCC (mRCC)[4-7], we postulated the possible contribution of viruses to the immunogenicity of this tumor. While an “inflamed” phenotype characterizes other immunogenic tumors, RCC has been defined as a tumor with a prominent dysfunctional immune cell infiltrate[8]. Its immunogenicity is not entirely attributable to an inflamed status or a high tumor mutational burden, another recently identified element responsible for immune responsiveness[9]. Indeed, enigmatic genomic clusters of RCC have been identified as good responders to ICI-based treatment regimens despite very low mutational burden and apparently non-immunogenic features. This is the case of the so-called “Cluster 7”, characterized by increased expression of small nucleolar RNAs (snoRNAs), guiding chemical RNA modifications, especially SNORDs[10,11].
The possibility that the presence of viruses in cancer cells could contribute to tumor immunogenicity is already suggested by the outstanding efficacy of immune checkpoint blockade in a tumor well-known to be highly resistant to standard anticancer therapies, namely Merkel cell carcinoma (MCC)[12]. MCC is a rare and aggressive skin cancer belonging to the family of neuroendocrine tumors, characterized by small cell features non-dissimilar to that of small cell lung cancer (SCLC). Despite the well-known unresponsiveness of neuroendocrine tumors to ICIs, recently non-effortlessly introduced in SCLC's treatment algorithm, MCC is counted among the solid tumors which the new immunotherapy has changed history. Unlike other neuroendocrine tumors, MCC is associated, in 80% of cases, with the Merkel cell polyomavirus (MCPyV). The MCPyV small T oncoprotein can inactivate p53 and contributes to metastatic progression.
Interestingly, similarly to Cluster 7 described for RCC, MCPyV-positive cases bear a much lower mutational load, notwithstanding their immunogenicity[13]. Other than an increased neoantigens' production, this immune responsiveness might be due to the viral agent's contribution to shaping a peculiar tumor immune microenvironment (TIME), likely crucial to defining tumor immunogenicity. Recent findings support this hypothesis, showing that MCPyV presence, found in 101/176 analyzed cases of MCC, was related to changes in the tumor morphology, the density of the inflammatory infiltrate, the phenotype of the neoplastic cells, and the cell composition of the tumor stroma[14]. This evidence suggests that the presence of a virus can enhance inflammation within a tumor.
Given the emerging link between inflammation and ICI responsiveness, we hypothesized that a viral agent could contribute to rendering a tumor inflamed, on the one hand shaping the TIME, and on the other hand providing a more considerable amount of non-self-antigens, finally triggering a more potent immune response. In this view, the possible involvement of viruses in RCC oncogenesis and progression becomes an issue of interest, animating our aim to collect and describe evidence supporting this hypothesis.
A scientific literature search was conducted using the PubMed, Web of Science, and Google Scholar databases with the keywords “virus” or “viruses” or “viral infection” matched with (“AND”) “renal cell carcinoma” or “kidney cancer” or “renal cancer” or “renal carcinoma” or “renal tumor” or “RCC”. The topics included in the literature selection were viruses in RCC, and RCC in patients with chronic viral infections. The use of oncolytic viruses for therapeutic purposes in RCC was an excluded topic. Other relevant issues close to the topics of interest that emerged from the literature screening were furtherly retrieved, and relevant publications were discussed.
Our search for possible links between viruses and RCC brought out two main aspects of their relationship: The presence of viruses within the tumor, and RCC occurrence in cases with pre-existing viral infections, both events documenting a potential causality effect. In addition, our multidirectional review unrevealed the possible role of endogenous retrovirus (ERV) reactivation in RCC oncogenesis, introducing new fascinating hypotheses about this tumor’s immunogenicity.
The research of histopathological findings testifying the presence of viruses in RCC allowed the retrieval of five retrospective publications[15-19]. These studies identified the virus within the tumor tissue through heterogeneous assays, demonstrating the viral presence at a rate of tumor specimens ranging from 7% to 30% of the case series analyzed. Contrariwise, the same viruses were present in the respective control specimens (healthy kidney or peritumoral tissue) at a rate ranging from 0% to 4%. Table 1 summarizes the relevant data, showing HPV, EBV, and BKV polyomavirus among the viruses identified.
| Ref. | Study type | Analyzed specimens | Virus investigated | Analysis method | Positive specimens | ||
| No. of cases and tumor type | No. of controls and tissue type | No. of positive cases and tumor type | No. of positive controls and tissue type | ||||
| Kim et al[18], 2005 | Retrospective | 73 RCC (22 clear cell; 18 papillary; 20 chromophobe; 10 sarcomatoid; 3 oncocytoma) | 18 non-neoplastic kidneys | EBV | EBER-ISH and PCRs (for EBNA-1 and EBNA-3C) | 5/73 (all sarcomatoid histology) (EBER-ISH)2; 4/73 (all sarcomatoid histology) showed amplification of EBNA-1 | 0/18 |
| Neirynck et al[17], 2012 | Case report | 1 RCC1 | 1 peritumoral tissue | BKV | IHC (for SV40 T antigen) | 65%-70% neoplastic cells | < 1% non-neoplastic cells |
| Salehipoor et al[19], 2012 | Retrospective | 49 RCC | 16 non-neoplastic kidneys | HPV; EBV; BKV; JCV | Nested PCR (virus DNA) | 7/49 HPV (5 clear cell; 1 chromophobe 1 mixed type) 0 EBV, BKV JCV | 0/16 |
| Bulut et al[16], 2013 | Retrospective | 50 RCC | 45 non-neoplastic kidneys | BKV | Nested PCR (BKV DNA) and RT-PCR (BKV mRNA) | 10/50 (Nested PCR) 8/50 (RT-PCR) | 2/45 non neoplastic kidneys (nested PCR, RT-PCR) |
| Farhadi et al[20], 2014 | Retrospective | 122 RCC (77 conventional; 26 papillary; 14 chromophobe; 1 collecting duct; 4 unclassified) | 96 peritumoral tissues, 19 non-neoplastic kidneys | HR-HPV | Nested PCR (HR-HPV DNA). IHC (for p16INK4a and L1 Capsid Protein); CSAC-ISH | 37/122 (17 clear-cell; 13 papillary; 4 chromophobe; 3 unclassified) (PCR). 24/118 (IHC for p16INK4a3) 0/118 (IHC for L1 capsid protein); 18/122 (CSAC-ISH) | 4/96 peritumoral tissues; 0/19 non-neoplastic kidneys (PCR); 16/94 peritumoral tissue (IHC for p16INK4a); 0/94 peritumoral tissue (IHC for L1 capsid protein); NA (CSAC-ISH) |
The role of BKV polyomavirus was already known in the field of renal transplants. About 75%-90% of healthy adults are BKV seropositive, but the virus is likely to remain non-pathogen in most cases. Immunosuppressive therapies trigger the reactivation of BKV and graft nephropathy (BKVN) in organ transplant recipients. The treatment of biopsy-proven BKVN consists of the reduction of immunosuppressive drugs. Of note, Neirynck et al[17] reported a case of complete remission of metastatic sites from RCC after the allograft surgical removal and immunosuppressive treatment discontinuation, suggesting the key role of BKV in a case of RCC occurred five years after renal transplant[16].
From a different perspective, a critical role could be attributed to immunosuppression. Renal cancer occurs more frequently in renal transplanted patients than in the general population[20]. Considering the non-negligible rate of primary RCC in the allograft and the native kidney of renal transplant recipients, a possible synergy of immunosuppressive treatments and oncogenic viruses could be hypothesized as the basis of renal cancerogenesis in these patients[21]. According to a recent meta-analysis, renal transplant recipients were found to display a higher risk of all cancers, but their standard incidence ratio (SIR) was 10.77 (95%CI: 6.40-18.12; P < 0.001) concerning RCC, compared to an all-cancers SIR of 2.89 (95%CI: 2.13-3.91)[22].
Besides BKV evidence in the allograft, the role of this virus might be more extensive in renal cancer, given the significant association (P = 0.03) found between BKV DNA positivity of specimens and histological diagnosis of RCC (but not with that of urothelial carcinoma) in a cohort including 50 RCC, 40 urothelial cancers, and 65 non-cancer controls[15]. The levels of BKV mRNA were significantly higher in the RCC samples than in the control samples (P < 0.05), and the presence of BKV DNA resulted in a 5-fold increased risk of RCC[15].
The limitations of the studies analyzed, beyond the limited sample size, are represented by the scarce homogeneity of investigational techniques, in the complete lack of validated assays to assess the viral presence within the tumor tissue. In most cases, the viral nucleic acid was detected by real-time polymerase chain reaction (PCR), but immunohistochemical techniques were also explored, with non-consistent results compared to the respective PCR in the same series[19].
Interestingly, a meaningful number of virus-positive cases were found in non-clear cell RCC (nccRCC) specimens, possibly subtending a different contribution in the etiopathogenesis between clear cell RCC (ccRCC) and non-conventional histologies. In the analyzed studies, Farhadi et al[20] found HPV in 13 of 26 (50%) papillary RCC specimens, compared to 17/77 ccRCC (22%) in the same series; similarly, Kim et al[18] found 50% of RCC with sarcomatoid histology positive for EBV. While the VHL-driven oncogenesis is widely recognized in ccRCC[23], less is known about the chain of oncogenic events in the case of nccRCC, a heterogeneous group of tumors with different histopathological, molecular, and clinical features, which are maybe promoted by shared stimuli.
Sarcomatoid RCC (sRCC) is not considered a distinct histotype: Sarcomatoid dedifferentiation is a histological feature found in any RCC subtype, conferring aggressive behavior and a lower likelihood of response to antiangiogenic therapies when compared to ccRCC[24]. sRCC is characterized by the presence of spindle-shaped cells in a varying proportion of the tumor area, accounting for a sarcoma-like aspect, engaged in epithelial-mesenchymal transition and expressing mesenchymal markers. The differential diagnosis from retroperitoneal leiomyosarcoma or liposarcoma can be challenging in locally advanced cases. Nevertheless, opposite to these latter tumors, sRCC has been recently recognized as a highly immunogenic tumor, characterized by enriched immune signatures and high levels of tumor-infiltrating lymphocytes, likely to respond to ICI more than to antiangiogenic therapy[25]. From the molecular standpoint, sRCC exhibits a lower prevalence of PBRM1 mutations and angiogenesis markers, frequent CDKN2A/B alterations, and increased PD-L1 expression[26]. These findings have been applied to molecularly stratify patients, justifying improved outcomes of sarcomatoid tumors to checkpoint blockade vs antiangiogenics alone in first-line trials with ICI-based combinations, recently pooled in a meta-analysis[27].
In one of the previously cited histopathological research works, among 73 RCC specimens, EBV RNA was present in only 5 samples (6.8%)[17]. Curiously, all 5 EBV-positive tumors were sRCC. Considering the sRCC subgroup of samples, EBV-positive sRCC were 5 cases out of 10 (50%). Interestingly, EBV was located exclusively in the tumor-infiltrating B lymphocytes sRCC, clearly characterizing the TIME more than the tumor cells. These findings might suggest a possible contribution of viruses, in particular EBV, to the marked immunogenicity of sRCC, furtherly reiterated by recent subgroup analyses of new ICI-based combinations[28,29].
Approximately 40% of the mammalian genome is constituted by retrotransposons, archaic genic sequences introduced into the eukaryotic genome during the evolution, which can copy and paste themselves into different genomic locations through reverse transcription. Retrotransposons are epigenetically silenced in most somatic tissues and usually reactivated in early embryos. Their silencing is epigenetically provided through DNA methylation, histone methylation/acetylation, and posttranscriptional regulation. Mammalian retrotransposons include non-long term repeats (non-LTR) retrotransposons and LTR retrotransposons, the latter also known as ERVs[30]. Human ERVs (hERVs) are remnants of exogenous retroviruses integrated into the primate genome over evolutionary time. Besides LTRs, hERVs share other genomic similarities to other retroviruses, like gag, pro, pol, and env genes[31]. Their sequences are not transcribed in mRNA, but they can interfere with gene expression by antisense transcription or premature transcription termination, provide new transcription start sites changing gene regulation, contain regulatory elements on target genes, mediate genomic rearrangement through nonallelic homologous recombination[30].
Recent evidence reveals hERV reactivation in RCC, with LTRs exhibiting HIF binding and transcriptional activity in the RCC genome[32]. Some of these HIF-bound LTRs may function as distal enhancers inducing the expression of genes representing potential therapeutic targets in RCC.
ERV expression was shown to correlate with histone methylation and chromatin regulation genes in multiple cancer types, including ccRCC[33]. Eventually, ERVs provide an epigenomic mechanism for recurrent transcriptional signatures observed in RCC, suggesting that this tumor's epigenomic landscape might at least partially come from viruses.
Exaptation of promoters embedded within LTRs is emerging as a recurrent element of genomic dysregulation of oncogenesis, previously demonstrated in other cancers such as Hodgkin lymphoma, melanoma, and large B cell lymphoma. Recent research reported the first description of retroviral LTR exaptation in RCC, with distinct mechanisms from previous reports about this phenomenon[32]. Further evidence was provided on pan-cancer datasets by the Cancer Genome Atlas (TCGA): Using a previously compilated database of 3173 intact, full-length ERV sequences, Smith and co-investigators designed a computational workflow for identifying the expression of specific ERVs from RNA-sequencing and quantified ERVs expression in different tumors[31]. They evidenced that ccRCC contained the most significant number of prognostic ERVs among all cancer types encompassed, with shorter survival in patients with greater mean ERV expression (testifying a negative prognostic value).
As a further crucial step in this field, ERVs in RCC have recently been demonstrated predicting immunotherapy response in ccRCC, as contemporarily reported in 2018 by two independent research groups[31,33].
Smith et al[32] identified a signature marking anti-PD-1 responsiveness associated with hERV expression, while a signature for non-responder tumors was negatively associated with hERV expression[31]. They explored the mechanisms by which hERV expression in tumor cells influenced the TIME in RCC, discovering immune stimulation evidence through RIG-I-like signaling of the hERV-induced adaptive immune response through B cell activation. Also, they showed that hERVs mediated the tumor-specific presentation of targetable viral epitopes, possibly adding a trigger to the antitumor response. On the other hand, ERV proteins were already known to be expressed and immunogenic in ccRCC[34-36].
Similarly, Panda et al[34] identified 20 potentially immunogenic ERV (πERVs) in ccRCC in TCGA dataset, demonstrating that πERV-high ccRCC tumors had an increased immune infiltration checkpoint pathway upregulation and higher CD8+ T cell fraction in infiltrating immune cells compared to πERV-low ccRCC tumors[33]. Moreover, πERV-high ccRCC tumors were enriched in BAP1 mutations. As a further step, they demonstrated that the RNA level of specific ERVs (ERV3-2) was an excellent predictor of response to immune checkpoint blockade, as statistically significantly higher in tumors from responders compared with tumors from non-responders patients with metastatic ccRCC treated with single-agent PD-1/PD-L1 antibody[33]. This evidence is significant in light of the confirmed poor prognostic significance of πERV-high and πERV-intermediate expression, as verified by the same authors. The validation sample was represented by πERV-high and πERV-intermediate ccRCC patients treated with standard therapy, showing significantly shorter overall survival (OS) than patients with πERV-low tumors [OS, hazard ration (HR) 1.44 (95%CI: 1.06-1.97), P = 0.02][33].
These findings suggested ERVs' striking relevance on the immune checkpoint activation in ccRCC, potentially configuring a new biomarker of inflamed tumors, more likely to respond to ICI immunotherapy.
Chronic viral infections are often subtended by a dysfunctional immune response, possibly conferring a persistently inflamed status to the host, likely dominated by T-cells exhaustion. Several authors have reported the increased incidence of malignancies in patients with chronic viral infections, and some consistent literature also emerged in the field of renal cancer (Table 2)[37-43].
| Ref. | Study type | Type of chronic viral infection | Study population | RCC histology | Mean age (yr) | Aim | Main results/conclusions |
| Gaughan et al[43], 2008 | Case series | HIV infection | 9 HIV-associated RCC1 | 2 papillary, 1 collecting duct, 6 clear cell | 48 | To describe the risk factors, clinical findings, pathology, and response to therapy in RCC patients infected with HIV | The clinical presentation and behavior of RCC in patients with HIV infection appeared similar to that of the HIV-negative population and that chronic immunosuppression plays a lesser role than age and exposure to risk factors |
| Gordon et al[38], 2010 | Retrospective study | HCV infection | 67063 HCV-tested patients: 3057 HCV+ and 64006 HCV- | 17 RCC HCV+: 8 clear cell, 6 papillary, 2 mixed clear cell/papillary, 1 undifferentiated/other; 117 HCV-: 92 clear cell, 43 papillary, 9 mixed clear cell/papillary, 26 undifferentiated/other | 54 in HCV+, 63 in HCV- | To determine whether HCV infection confers an increased risk for developing RCC | RCC was diagnosed in 0.6% (17/3057) of HCV+ and 0.3% (117/64006) of HCV- patients. HCV infection confers a risk for the development of RCC: Overall HR for RCC among HCV patients 1.77 (95% confidence interval, 1.05-2.98; P = 0.0313) |
| Wiwanitkit[42], 2011 | Bioinformatics analysis | HCV infection | NA | NA | NA | To assess the cause–outcome relationship between HCV infection and RCC using the bioinformatics network analysis technique | There might be a cause–outcome relationship between HCV infection and RCC via NY-REN-54 (the only one common protein) |
| Gonzalez et al[39], 2015 | Prospective study | HCV infection | 140 RCC and 100 colon cancer patients (control) | NA | 56.7 in RCC patients with viremia, 61.8 in aviremic patients | To determine whether chronic HCV is associated with an increased risk of RCC | 11/140 RCC and 1/100 colon cancer patients were HCAB+. Of the HCAB+ patients, 9/11 RCC and 0/1 controls had detectable HCV RNA. In the multivariable logistic regression analysis, being HCV RNA positive was a significant risk factor for RCC (P = 0.043) |
| Wijarnpreecha et al[40], 2016 | Systematic review and meta-analysis | HCV infection | 196826 patients from 7 observational studies (4 cohort and 3 case-control studies). Individuals without HCV infection were used as comparators in cohort studies, individuals without RCC as comparators in the cross-sectional and case-control studies | NA | NA2 | To assess the risk of RCC in patients with HCV infection | Significantly increased risk of RCC in HCC+ with the pooled risk ratio of 1.86 (95%CI: 1.11-3.11) |
| Ong et al[44], 2016 | Case series | HIV infection | 7 HIV-associated RCC1 | 5 clear cell, 1 papillary, 1 unknown | 56 | To report presentation, management and outcomes of RCC patients with HIV infection | RCC patients with HIV infection should be offered all treatment options in the same manner as the general population |
| Tsimafeyeu et al[41], 2020 | Retrospective study | HCV infection | 44 mRCC patients: 22 HCV+, 22 HCV- | Clear cell | 62 in mRCC HCV+, 63 in mRCC HCV- | To evaluate Nivolumab efficacy and safety in mRCC patients with or without chronic HCV infection (OS primary endpoint, PFS, ORR and rate of grade 3–4 adverse events secondary endpoints) | HCV-infected patients had significantly longer OS (27.5 vs 21.7, P = 0.005) and PFS (7.5 vs 4.9, P = 0.013), no difference in ORR. Grade 3–4 adverse events were observed in 5 (23%) HCV+ patients and in 3 (14%) HCV- patients |
Chronic HCV infection seems to confer a risk for the development of RCC, according to a cohort study of 67063 HCV-tested patients, among whom RCC was diagnosed in 0.6% of HCV-positive vs 0.3% of HCV-negative patients. The univariate HR for RCC among HCV patients was 2.20 (95%CI: 1.32-3.67; P = 0.0025). In a multivariate model that included the risk factors age, race, gender, and chronic kidney disease, the overall HR for RCC among HCV patients was 1.77 (95%CI: 1.05-2.98; P = 0.0313)[37].
In another report, RCC patients were shown to have a higher rate of hepatitis C antibody positivity (11/140, 8%) than colon cancer patients (1/100, 1%, P = 0.01), viremic RCC patients were significantly younger than RCC patients who were HCV RNA negative (P = 0.013)[38].
A meta-analysis of seven observational studies including 196826 patients, the risk of RCC in HCV patients was found to increase with a pooled risk ratio (RR) of 1.86 (95%CI: 1.11-3.11). Nevertheless, the association between RCC and HCV was marginally insignificant after a sensitivity analysis limited only to studies with adjusted analysis, with a pooled RR of 1.50 (95%CI: 0.93-2.42)[39].
In HIV infection, AIDS-related immunosuppression could play the leading role in promoting oncogenic events instead of the viral infection itself. The literature simply included RCC in the expanding array of non-AIDS-defining malignancies that develop during HIV infection[42,43].
On the other hand, subtending viral infections could represent the epiphenomenon of a dysfunctional immune status, maybe more likely to benefit from immune checkpoint blockade[44]. In a matched cohort study, data were collected from 174 patients with metastatic ccRCC, chronic HCV infection (case study group), no evidence of other malignancy or cirrhosis, and had received nivolumab as systemic anticancer treatment[39]. HCV-infected patients had significantly longer OS and progression-free survival (PFS). Median OS was 27.5 (95%CI: 25.3–29.7) and 21.7 (20.3–23.1) in study and control groups, respectively (P = 0.005). Median PFS was 7.5 (5.7–9.3) and 4.9 (4–5.8) (P = 0.013). Despite no differences in objective response rate between groups, patients with HCV had significantly more durable responses (P = 0.01). Such findings are undoubtedly suggestive but still largely insufficient to draw a causality relationship between chronic viral infections and immunogenicity.
The report of acute viral infections triggering an anticancer immune response in patients with solid and hematological malignancies is rather than new. From the first observation by William Coley that non-self-agents can trigger antitumor immune reactivity to the recent findings by our research group about influenza infection in advanced cancer patients treated with ICI immunotherapy, the literature emphasizes the role of extrinsic immune stimulation in modulating the immune reactivity and also the efficacy of inhibitory molecules targeting immune checkpoints[45,46]. Even SARS-CoV-2 was reported as able to exert an abscopal antitumor effect in solid tumors: Cases of partial or complete remission during COVID-19 have been reported in patients with melanoma and lymphomas without any anticancer treatment, in the latter cases likely due to a direct oncolytic effect on tumor cells[47-49].
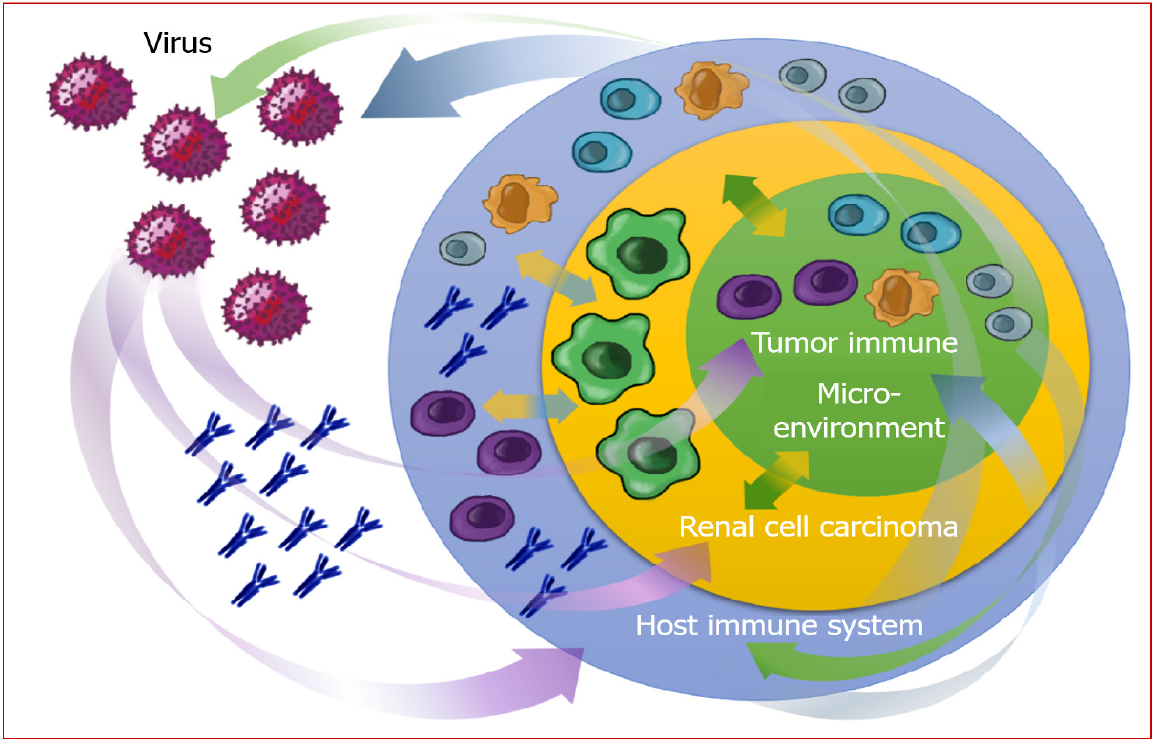
Compared to cancer diagnosis in chronically infected individuals, likely driven by immunosuppression and immune exhaustion[50], the occurrence of viral infections in patients with cancer represents an opposite setting. In this case, the encounter with viral antigens could contribute, as a potent exogenous immunological stimulus, to shift the balance between tolerance and activation, likely favorably influencing the TIME and the complex relationships between the tumor and the host (Figure 1).
For completeness, state-of-the-art about viruses and kidney cancer also included evidence about collecting duct carcinoma (CDC), rare variant histology with poor prognosis, and challenging therapy[51]. Notably, BKV polyomavirus was reported in the literature as linked to CDC in transplant recipients, again highlighting the role of immunosuppression as the playing field for virus-associated carcinogenesis[52,53].
The evidence presented above is a tickling proof-of-concept subtending the possibility to add a dowel for the prediction of cancer patients' outcome to immune checkpoint therapy and even more suggests exploiting the immunogenic potential of viruses for therapeutic purposes in the context of anticancer immunotherapy for RCC. Although manipulating viruses could sound like a dangerous game just in the context of the pandemic currently ongoing, teased by striking findings from this preliminary translational research, the authors of the present opinion review still consider the possibility that dangerous relationships may be the most immunogenic, at least in the context of RCC.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Urology and nephrology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hasan A S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | American Cancer Society. Viruses that can lead to cancer. [cited 10 March 2021]. Available from: https://www.cancer.org/cancer/cancer-causes/infectious-agents/infections-that-can-lead-to-cancer/viruses.html. |
| 2. | Naegele RF, Granoff A, Darlington RW. The presence of the Lucké herpesvirus genome in induced tadpole tumors and its oncogenicity: Koch-Henle postulates fulfilled. Proc Natl Acad Sci U S A. 1974;71:830-834. [PubMed] [DOI] [Full Text] |
| 3. | McKinnell RG, Cunningham WP. Herpesviruses in metastatic Lucké renal adenocarcinoma. Differentiation. 1982;22:41-46. [PubMed] [DOI] [Full Text] |
| 4. | McKinnell RG, Tarin D. Temperature-dependent metastasis of the Lucke renal carcinoma and its significance for studies on mechanisms of metastasis. Cancer Metastasis Rev. 1984;3:373-386. [PubMed] [DOI] [Full Text] |
| 5. | Motzer R, Alekseev B, Rha SY, Porta C, Eto M, Powles T, Grünwald V, Hutson TE, Kopyltsov E, Méndez-Vidal MJ, Kozlov V, Alyasova A, Hong SH, Kapoor A, Alonso Gordoa T, Merchan JR, Winquist E, Maroto P, Goh JC, Kim M, Gurney H, Patel V, Peer A, Procopio G, Takagi T, Melichar B, Rolland F, De Giorgi U, Wong S, Bedke J, Schmidinger M, Dutcus CE, Smith AD, Dutta L, Mody K, Perini RF, Xing D, Choueiri TK; CLEAR Trial Investigators. Lenvatinib plus Pembrolizumab or Everolimus for Advanced Renal Cell Carcinoma. N Engl J Med. 2021;384:1289-1300. [PubMed] [DOI] [Full Text] |
| 6. | Choueiri TK, Powles T, Burotto M, Escudier B, Bourlon MT, Zurawski B, Oyervides Juárez VM, Hsieh JJ, Basso U, Shah AY, Suárez C, Hamzaj A, Goh JC, Barrios C, Richardet M, Porta C, Kowalyszyn R, Feregrino JP, Żołnierek J, Pook D, Kessler ER, Tomita Y, Mizuno R, Bedke J, Zhang J, Maurer MA, Simsek B, Ejzykowicz F, Schwab GM, Apolo AB, Motzer RJ; CheckMate 9ER Investigators. Nivolumab plus Cabozantinib vs Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med. 2021;384:829-841. [PubMed] [DOI] [Full Text] |
| 7. | Powles T, Plimack ER, Soulières D, Waddell T, Stus V, Gafanov R, Nosov D, Pouliot F, Melichar B, Vynnychenko I, Azevedo SJ, Borchiellini D, McDermott RS, Bedke J, Tamada S, Yin L, Chen M, Molife LR, Atkins MB, Rini BI. Pembrolizumab plus axitinib vs sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): extended follow-up from a randomised, open-label, phase 3 trial. Lancet Oncol. 2020;21:1563-1573. [PubMed] [DOI] [Full Text] |
| 8. | Motzer RJ, Tannir NM, McDermott DF, Arén Frontera O, Melichar B, Choueiri TK, Plimack ER, Barthélémy P, Porta C, George S, Powles T, Donskov F, Neiman V, Kollmannsberger CK, Salman P, Gurney H, Hawkins R, Ravaud A, Grimm MO, Bracarda S, Barrios CH, Tomita Y, Castellano D, Rini BI, Chen AC, Mekan S, McHenry MB, Wind-Rotolo M, Doan J, Sharma P, Hammers HJ, Escudier B; CheckMate 214 Investigators. Nivolumab plus Ipilimumab vs Sunitinib in Advanced Renal-Cell Carcinoma. N Engl J Med. 2018;378:1277-1290. [PubMed] [DOI] [Full Text] |
| 9. | Bersanelli M, Gnetti L, Varotti E, Ampollini L, Carbognani P, Leonardi F, Rusca M, Campanini N, Ziglioli F, Dadomo CI, Pilato FP, Cortellini A, Rapacchi E, Caruso G, Silini EM, Maestroni U, Buti S. Immune context characterization and heterogeneity in primary tumors and pulmonary metastases from renal cell carcinoma. Immunotherapy. 2019;11:21-35. [PubMed] [DOI] [Full Text] |
| 10. | Marabelle A, Fakih M, Lopez J, Shah M, Shapira-Frommer R, Nakagawa K, Chung HC, Kindler HL, Lopez-Martin JA, Miller WH Jr, Italiano A, Kao S, Piha-Paul SA, Delord JP, McWilliams RR, Fabrizio DA, Aurora-Garg D, Xu L, Jin F, Norwood K, Bang YJ. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020;21:1353-1365. [PubMed] [DOI] [Full Text] |
| 11. | Motzer RJ, Banchereau R, Hamidi H, Powles T, McDermott D, Atkins MB, Escudier B, Liu LF, Leng N, Abbas AR, Fan J, Koeppen H, Lin J, Carroll S, Hashimoto K, Mariathasan S, Green M, Tayama D, Hegde PS, Schiff C, Huseni MA, Rini B. Molecular Subsets in Renal Cancer Determine Outcome to Checkpoint and Angiogenesis Blockade. Cancer Cell. 2020;38:803-817.e4. [PubMed] [DOI] [Full Text] |
| 12. | Brugarolas J, Rajaram S, Christie A, Kapur P. The Evolution of Angiogenic and Inflamed Tumors: The Renal Cancer Paradigm. Cancer Cell. 2020;38:771-773. [PubMed] [DOI] [Full Text] |
| 13. | Kaufman HL, Russell J, Hamid O, Bhatia S, Terheyden P, D'Angelo SP, Shih KC, Lebbé C, Linette GP, Milella M, Brownell I, Lewis KD, Lorch JH, Chin K, Mahnke L, von Heydebreck A, Cuillerot JM, Nghiem P. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016;17:1374-1385. [PubMed] [DOI] [Full Text] |
| 14. | Samimi M, Kervarrec T, Touze A. Immunobiology of Merkel cell carcinoma. Curr Opin Oncol. 2020;32:114-121. [PubMed] [DOI] [Full Text] |
| 15. | Mendoza MD, Santonja C, Gonzalez-Vela C, Concha A, Iglesias Pena N, Andrés-Esteban EM, Vaque JP, Cereceda L, Pajares R, Kutzner H, Requena L, Piris MA. The presence of Merkel cell carcinoma polyomavirus is associated with a distinct phenotype in neoplastic Merkel cell carcinoma cells and their tissue microenvironment. PLoS One. 2020;15:e0232517. [PubMed] [DOI] [Full Text] |
| 16. | Bulut Y, Ozdemir E, Ozercan HI, Etem EO, Aker F, Toraman ZA, Seyrek A, Firdolas F. Potential relationship between BK virus and renal cell carcinoma. J Med Virol. 2013;85:1085-1089. [PubMed] [DOI] [Full Text] |
| 17. | Neirynck V, Claes K, Naesens M, De Wever L, Pirenne J, Kuypers D, Vanrenterghem Y, Poppel HV, Kabanda A, Lerut E. Renal cell carcinoma in the allograft: what is the role of polyomavirus? Case Rep Nephrol Urol. 2012;2:125-134. [PubMed] [DOI] [Full Text] |
| 18. | Kim KH, Han EM, Lee ES, Park HS, Kim I, Kim YS. Epstein-Barr virus infection in sarcomatoid renal cell carcinoma tissues. BJU Int. 2005;96:547-552. [PubMed] [DOI] [Full Text] |
| 19. | Salehipoor M, Khezri A, Behzad-Behbahani A, Geramizadeh B, Rahsaz M, Aghdaei M, Afrasiabi MA. Role of viruses in renal cell carcinoma. Saudi J Kidney Dis Transpl. 2012;23:53-57. [PubMed] |
| 20. | Farhadi A, Behzad-Behbahani A, Geramizadeh B, Sekawi Z, Rahsaz M, Sharifzadeh S. High-risk human papillomavirus infection in different histological subtypes of renal cell carcinoma. J Med Virol. 2014;86:1134-1144. [PubMed] [DOI] [Full Text] |
| 21. | Frascà GM, Sandrini S, Cosmai L, Porta C, Asch W, Santoni M, Salviani C, D'Errico A, Malvi D, Balestra E, Gallieni M. Renal cancer in kidney transplanted patients. J Nephrol. 2015;28:659-668. [PubMed] [DOI] [Full Text] |
| 22. | Moris D, Kakavia K, Argyrou C, Garmpis N, Bokos J, Vernadakis S, Diles K, Sotirchos G, Boletis J, Zavos G. De Novo Renal Cell Carcinoma of Native Kidneys in Renal Transplant Recipients: A Single-center Experience. Anticancer Res. 2017;37:773-779. [PubMed] [DOI] [Full Text] |
| 23. | Wang Y, Lan GB, Peng FH, Xie XB. Cancer risks in recipients of renal transplants: a meta-analysis of cohort studies. Oncotarget. 2018;9:15375-15385. [PubMed] [DOI] [Full Text] |
| 24. | Gerlinger M, Horswell S, Larkin J, Rowan AJ, Salm MP, Varela I, Fisher R, McGranahan N, Matthews N, Santos CR, Martinez P, Phillimore B, Begum S, Rabinowitz A, Spencer-Dene B, Gulati S, Bates PA, Stamp G, Pickering L, Gore M, Nicol DL, Hazell S, Futreal PA, Stewart A, Swanton C. Genomic architecture and evolution of clear cell renal cell carcinomas defined by multiregion sequencing. Nat Genet. 2014;46:225-233. [PubMed] [DOI] [Full Text] |
| 25. | Debien V, Thouvenin J, Lindner V, Barthélémy P, Lang H, Flippot R, Malouf GG. Sarcomatoid Dedifferentiation in Renal Cell Carcinoma: From Novel Molecular Insights to New Clinical Opportunities. Cancers (Basel). 2019;12. [PubMed] [DOI] [Full Text] |
| 26. | Tannir NM, Signoretti S, Choueiri TK, McDermott DF, Motzer RJ, Flaifel A, Pignon JC, Ficial M, Frontera OA, George S, Powles T, Donskov F, Harrison MR, Barthélémy P, Tykodi SS, Kocsis J, Ravaud A, Rodriguez-Cid JR, Pal SK, Murad AM, Ishii Y, Saggi SS, McHenry MB, Rini BI. Efficacy and Safety of Nivolumab Plus Ipilimumab vs Sunitinib in First-line Treatment of Patients with Advanced Sarcomatoid Renal Cell Carcinoma. Clin Cancer Res. 2021;27:78-86. [PubMed] [DOI] [Full Text] |
| 27. | Banchereau R, Leng N, Zill O, Sokol E, Liu G, Pavlick D, Maund S, Liu LF, Kadel E 3rd, Baldwin N, Jhunjhunwala S, Nickles D, Assaf ZJ, Bower D, Patil N, McCleland M, Shames D, Molinero L, Huseni M, Sanjabi S, Cummings C, Mellman I, Mariathasan S, Hegde P, Powles T. Molecular determinants of response to PD-L1 blockade across tumor types. Nat Commun. 2021;12:3969. [PubMed] [DOI] [Full Text] |
| 28. | Quhal F, Mori K, Fajkovic H, Remzi M, Shariat SF, Schmidinger M. Immunotherapy-based combinations in the first-line treatment of metastatic renal cell carcinoma with sarcomatoid features: a systematic review and network meta-analysis. Curr Opin Urol. 2022;32:61-68. [PubMed] [DOI] [Full Text] |
| 29. | Motzer RJ, Choueiri TK, Powles T, Burotto M, Bourlon MT, Hsieh JJ, Maruzzo M, Shah AY, Suarez C, Barrios CH, Richardet ME, Porta C, Goh JC, Tomita Y, Bedke J, Zhang J, Simsek B, Scheffold C, Gupta S, Apolo AB. Nivolumab + cabozantinib (NIVO+CABO) vs sunitinib (SUN) for advanced renal cell carcinoma (aRCC): Outcomes by sarcomatoid histology and updated trial results with extended follow-up of CheckMate 9ER. J Clin Oncol. 2021;39. [DOI] [Full Text] |
| 30. | Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, Pouliot F, Soulieres D, Melichar B, Vynnychenko I, Azevedo SJ, Borchiellini D, McDermott RS, Bedke J, Tamada S, Wan S, Perini RF, Chen M, Atkins MB, Powles T. Pembrolizumab (pembro) plus axitinib (axi) vs sunitinib as first-line therapy for metastatic renal cell carcinoma (mRCC): Outcomes in the combined IMDC intermediate/poor risk and sarcomatoid subgroups of the phase 3 KEYNOTE-426 study. J Clin Oncol. 2019;37:4500. [DOI] [Full Text] |
| 31. | Yin Y, Zhou L, Yuan S. Enigma of Retrotransposon Biology in Mammalian Early Embryos and Embryonic Stem Cells. Stem Cells Int. 2018;2018:6239245. [PubMed] [DOI] [Full Text] |
| 32. | Smith CC, Beckermann KE, Bortone DS, De Cubas AA, Bixby LM, Lee SJ, Panda A, Ganesan S, Bhanot G, Wallen EM, Milowsky MI, Kim WY, Rathmell WK, Swanstrom R, Parker JS, Serody JS, Selitsky SR, Vincent BG. Endogenous retroviral signatures predict immunotherapy response in clear cell renal cell carcinoma. J Clin Invest. 2018;128:4804-4820. [PubMed] [DOI] [Full Text] |
| 33. | Siebenthall KT, Miller CP, Vierstra JD, Mathieu J, Tretiakova M, Reynolds A, Sandstrom R, Rynes E, Haugen E, Johnson A, Nelson J, Bates D, Diegel M, Dunn D, Frerker M, Buckley M, Kaul R, Zheng Y, Himmelfarb J, Ruohola-Baker H, Akilesh S. Integrated epigenomic profiling reveals endogenous retrovirus reactivation in renal cell carcinoma. EBioMedicine. 2019;41:427-442. [PubMed] [DOI] [Full Text] |
| 34. | Panda A, de Cubas AA, Stein M, Riedlinger G, Kra J, Mayer T, Smith CC, Vincent BG, Serody JS, Beckermann KE, Ganesan S, Bhanot G, Rathmell WK. Endogenous retrovirus expression is associated with response to immune checkpoint blockade in clear cell renal cell carcinoma. JCI Insight. 2018;3. [PubMed] [DOI] [Full Text] |
| 35. | Florl AR, Löwer R, Schmitz-Dräger BJ, Schulz WA. DNA methylation and expression of LINE-1 and HERV-K provirus sequences in urothelial and renal cell carcinomas. Br J Cancer. 1999;80:1312-1321. [PubMed] [DOI] [Full Text] |
| 36. | Cherkasova E, Scrivani C, Doh S, Weisman Q, Takahashi Y, Harashima N, Yokoyama H, Srinivasan R, Linehan WM, Lerman MI, Childs RW. Detection of an Immunogenic HERV-E Envelope with Selective Expression in Clear Cell Kidney Cancer. Cancer Res. 2016;76:2177-2185. [PubMed] [DOI] [Full Text] |
| 37. | Takahashi Y, Harashima N, Kajigaya S, Yokoyama H, Cherkasova E, McCoy JP, Hanada K, Mena O, Kurlander R, Tawab A, Srinivasan R, Lundqvist A, Malinzak E, Geller N, Lerman MI, Childs RW. Regression of human kidney cancer following allogeneic stem cell transplantation is associated with recognition of an HERV-E antigen by T cells. J Clin Invest. 2008;118:1099-1109. [PubMed] [DOI] [Full Text] |
| 38. | Gordon SC, Moonka D, Brown KA, Rogers C, Huang MA, Bhatt N, Lamerato L. Risk for renal cell carcinoma in chronic hepatitis C infection. Cancer Epidemiol Biomarkers Prev. 2010;19:1066-1073. [PubMed] [DOI] [Full Text] |
| 39. | Gonzalez HC, Lamerato L, Rogers CG, Gordon SC. Chronic hepatitis C infection as a risk factor for renal cell carcinoma. Dig Dis Sci. 2015;60:1820-1824. [PubMed] [DOI] [Full Text] |
| 40. | Wijarnpreecha K, Nissaisorakarn P, Sornprom S, Thongprayoon C, Thamcharoen N, Maneenil K, Podboy AJ, Cheungpasitporn W. Hepatitis C infection and renal cell carcinoma: A systematic review and meta-analysis. World J Gastrointest Pathophysiol. 2016;7:314-319. [PubMed] [DOI] [Full Text] |
| 41. | Tsimafeyeu I, Gafanov R, Protsenko S, Semenova A, Oganesyan A, Nurgaliyev N, Krasny S, Bondarenko A, Safina S, Zakurdaeva K. Nivolumab in patients with metastatic renal cell carcinoma and chronic hepatitis C virus infection. Cancer Immunol Immunother. 2020;69:983-988. [PubMed] [DOI] [Full Text] |
| 42. | Wiwanitkit V. Renal cell carcinoma and hepatitis C virus infection: is there any cause-outcome relationship? J Cancer Res Ther. 2011;7:226-227. [PubMed] [DOI] [Full Text] |
| 43. | Gaughan EM, Dezube BJ, Aboulafia D, Bower M, Stebbing J, Powles T, Pantanowitz L. Human immunodeficiency virus--associated renal cell carcinoma: a transatlantic case series. Clin Genitourin Cancer. 2008;6:86-90. [PubMed] [DOI] [Full Text] |
| 44. | Ong WL, King K, Koh TL, Chipman M, Royce P, Hoy J, Millar JL. HIV and renal cell carcinoma: Experience in an Australian statewide HIV center. Asia Pac J Clin Oncol. 2016;12:188-193. [PubMed] [DOI] [Full Text] |
| 45. | Coley WB. The treatment of malignant tumors by repeated inoculations of erysipelas. With a report of ten original cases. 1893. Clin Orthop Relat Res. 1991;3-11. [DOI] [Full Text] |
| 46. | Bersanelli M, Scala S, Affanni P, Veronesi L, Colucci ME, Banna GL, Cortellini A, Liotta F. Immunological insights on influenza infection and vaccination during immune checkpoint blockade in cancer patients. Immunotherapy. 2020;12:105-110. [PubMed] [DOI] [Full Text] |
| 47. | Herrscher H, Sauer B, Truntzer P, Robert C. Abscopal antitumor effect in a patient with melanoma and coronavirus disease 2019. Eur J Cancer. 2021;149:91-93. [PubMed] [DOI] [Full Text] |
| 48. | Challenor S, Tucker D. SARS-CoV-2-induced remission of Hodgkin lymphoma. Br J Haematol. 2021;192:415. [PubMed] [DOI] [Full Text] |
| 49. | Pasin F, Mascalchi Calveri M, Calabrese A, Pizzarelli G, Bongiovanni I, Andreoli M, Cattaneo C, Rignanese G. Oncolytic effect of SARS-CoV2 in a patient with NK lymphoma. Acta Biomed. 2020;91:ahead of print. [PubMed] [DOI] [Full Text] |
| 50. | Baniyash M, Sade-Feldman M, Kanterman J. Chronic inflammation and cancer: suppressing the suppressors. Cancer Immunol Immunother. 2014;63:11-20. [PubMed] [DOI] [Full Text] |
| 51. | Veldhuijzena NMH, Rookmaakerb MB, van Zuilenb¸AD, Goldschmedingc R, Nguyenc TQ, Boerb WH. BK virus nephropathy, collecting duct cell proliferation and malignancy in a renal allograft: Case history and review of the literature. Transpl Immunol. 2017;9:33-37. [DOI] [Full Text] |
| 52. | Hasan A, Abozied H, Youssef A, Fayad S, Ismail A. A rare case of collecting duct carcinoma with first presentation of respiratory symptoms. Urol Case Rep. 2020;33:101367. [PubMed] [DOI] [Full Text] |
| 53. | Dao M, Pécriaux A, Bessede T, Dürrbach A, Mussini C, Guettier C, Ferlicot S. BK virus-associated collecting duct carcinoma of the renal allograft in a kidney-pancreas allograft recipient. Oncotarget. 2018;9:15157-15163. [PubMed] [DOI] [Full Text] |