Published online Aug 6, 2012. doi: 10.5527/wjn.v1.i4.100
Revised: July 23, 2012
Accepted: July 29, 2012
Published online: August 6, 2012
Improving material biocompatibility has been a continuous effort and remains a major goal of dialysis therapy. In this respect, vitamin E-modified copolymers have been used to produce a generation of biomaterials that has offered new clinical challenges and the chance of further improving the quality of synthetic hemodialyser membranes. This mini review article describes the evolution of these copolymers that only recently have been adopted to develop new vitamin E-modified polysulfone hemodialysers. Biomaterial characteristics and clinical aspects of these membranes are discussed, starting from the most recent contributions that have appeared in the literature that are of interest for the community of nephrology and dialysis specialists, as well as biomaterial scientists.
- Citation: Galli F. Vitamin E-derived copolymers continue the challenge to hemodialysis biomaterials. World J Nephrol 2012; 1(4): 100-105
- URL: https://www.wjgnet.com/2220-6124/full/v1/i4/100.htm
- DOI: https://dx.doi.org/10.5527/wjn.v1.i4.100
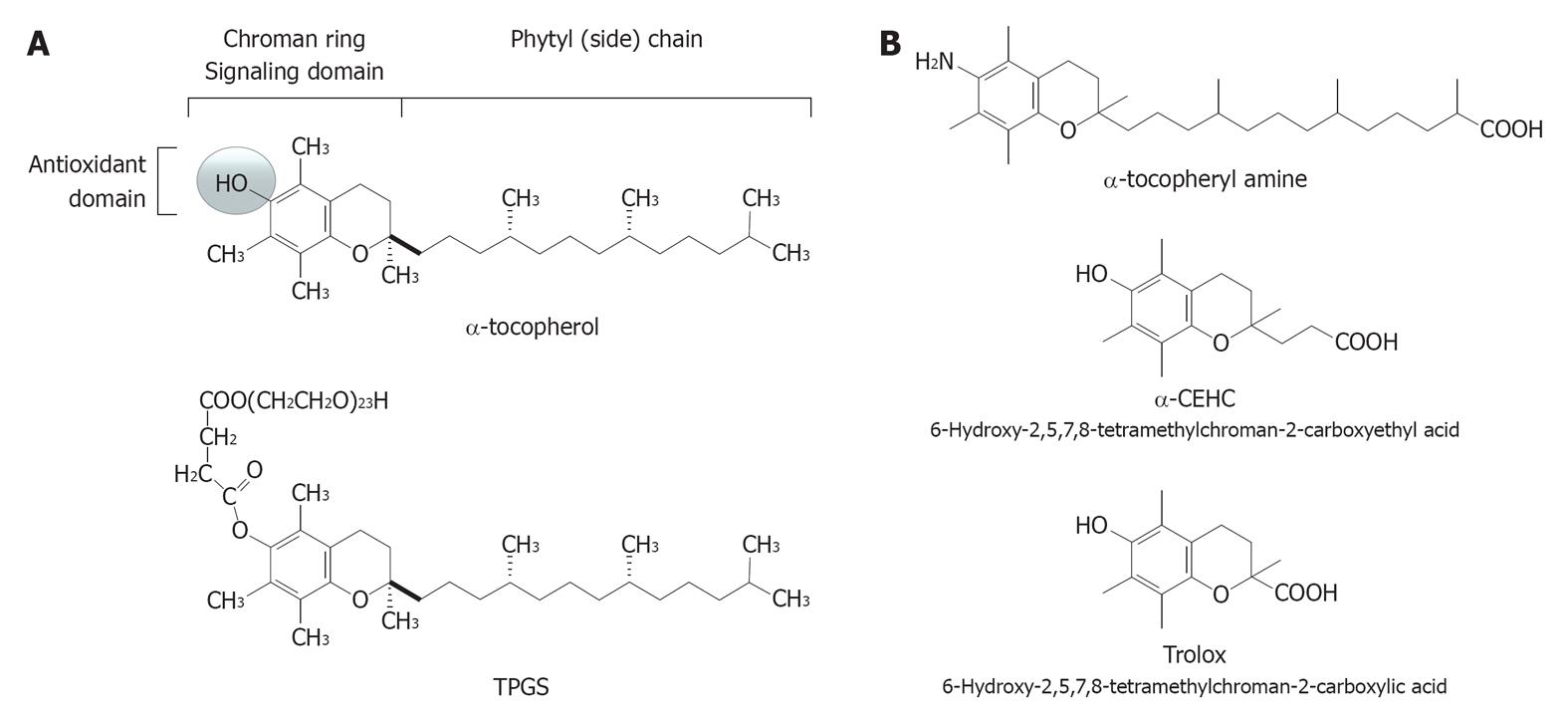
By definition, a vitamin E-derived copolymer is a macromolecular complex of natural or synthetic origin with repeated subunits connected by covalent chemical bonds that contain vitamin E as a stable modifier. This is a fat-soluble antioxidant vitamin (Figure 1A) with 8 natural vitamers and several synthetic analogues so far produced for nutritional and pharmacological purposes[1-3].
In the early 1990s, vitamin E copolymers were developed and used for the first time to produce hollow-fiber hemodialyser membranes that soon after were introduced in clinical practice, first in Japan and then in Europe. The aim of that pioneering biomaterial technology was to coat the blood surface of both cellulosic and polysulfone (PS)-based hemodialysis membranes (Figure 2) with a physiological molecule present in cell membranes and circulating lipoproteins. The ultimate goal was that of achieving higher biocompatibility and antioxidant protection in the extracorporeal circulation. Available knowledge cannot disclose if these two features are separated by a functional dichotomy or are just two faces of the same vitamin E-derived function. Actually, vitamin E is a physiological fat-soluble micronutrient with ubiquitous distribution in solid tissues and body fluids and so it is biocompatible by definition. Clinical studies examined in this review paper have provided conclusive recognition of the good level of biocompatibility reached by the last generation of PS-based vitamin E-modified dialysers. At the same time, vitamin E has well-known antioxidant properties[4], functioning as a hydroperoxyl radical scavenger and chain breaker (i.e., inhibitor of the chain reaction of lipid peroxidation) and as a H-atom or electron donator. Antioxidant effects are due to the hydroxyl group in position 6 of the chroman ring in the vitamin E structure (Figure 1A) and have been conclusively confirmed in vitro for the coating of vitamin E of the hollow-fiber dialysers[5]. Clinical studies have demonstrated effects of prevention against oxidative stress markers and particularly on lipid oxidation and low-density lipoprotein (LDL) damage[6-8]. These pieces of evidence pave the way to the definition of “interactive or functional membrane”, which extends the concept of biocompatibility to include that of (antioxidant) bioactivity. Besides filtration and biocompatibility, antioxidant bioactivity represents a third and original dimension in the functional chart of hemodialyser membranes recently described in[9].
As recently shown by Dahe et al[10], other analogues of this vitamin, such as D-α-tocopheryl polyethylene glycol 1000 succinate (TPGS; Figure 1A), could be used to produce biocompatible copolymers that may have future application in hollow-fiber membrane production technology. Several of the synthetic derivatives of vitamin E are, however, “redox-silent” and this is the case of TPGS. Therefore, TPGS-derived copolymers cannot claim to express the “antioxidant bioactivity” of vitamin E. Synthetic derivatives of tocopherols and tocotrienols include promising antioxidant agents, such as amine derivatives, recently characterized in our laboratories[11].
These innovations and a brief historical overview of the literature of vitamin E-modified hemodialysers are presented in this mini review paper.
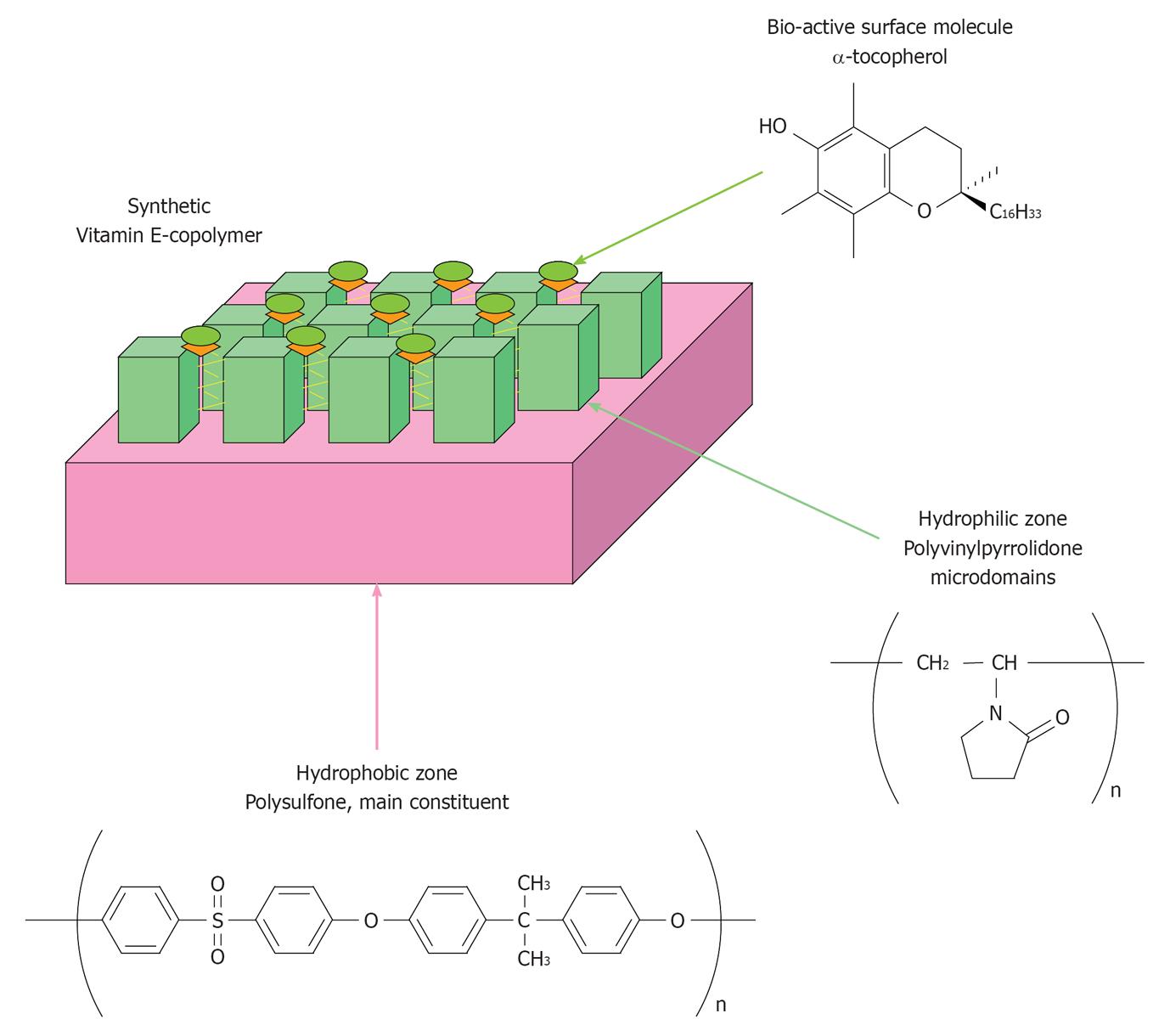
Vitamin E-derived copolymers for hemodialysis therapy were originally produced by Terumo Co., Japan, and introduced in clinical practice in the early 1990s as cellulosic membranes coated with α-tocopherol (Figure 1A)[6,7]. Synthetic dialyser membranes modified with vitamin E have been developed starting from these prototypal membranes and at the beginning of 2000, Ashai Kasei Medical Co., Japan, launched a composite PS-polyvinylpyrrolidone (PVP) copolymer embedded with α-tocopherol (Figure 2) that is marketed with the commercial name of VitabranE™.
Extensive investigation of these dialyser membranes over more than two decades of clinical practice and in vitro studies[12], demonstrated that coating cellulosic membranes with vitamin E helps to prevent lipid oxidation and LDL damage as cardiovascular risk factors for chronic kidney disease (CKD) patients treated with cellulosic and even synthetic dialyser membranes[6,7]. This effect was confirmed in subjects treated with VitabranE™, i.e., PS-PVP membranes modified with vitamin E[8], and appears to be the consequence of an antioxidant effect of these membranes. Such an antioxidant activity was conclusively identified and quantified in this laboratory[5] using a recirculation model system in which the actual antioxidant power of VitabranE minimodule dialysers was measured together with the quota of vitamin E that is active at the blood-biomembrane interface.
In recent years, the introduction of vitamin E-modified PS-PVP in clinical practice has renewed interest and stimulated further clinical research on these membranes. Several aspects of CKD comorbidity have been investigated and early randomized trials have described that these PS-PVP modified membranes may help to alleviate the resistance to erythropoiesis stimulating agents and to reduce, to some extent, sub-clinical markers of inflammation[13-15], intradialytic hypotension[16] and anti-coagulation therapy[17].
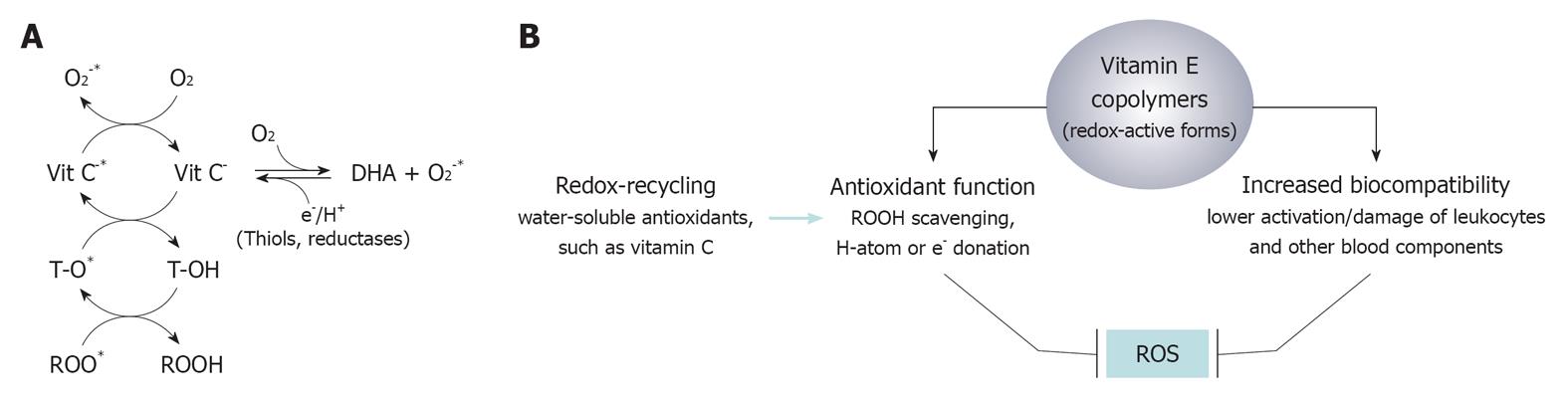
If this burden of clinical evidence is confirmed in further trials of larger proportions, this copolymer will represent one of the highest examples of biocompatibility in dialysis therapy. As introduced above, vitamin E copolymers can offer a functional extension of the concept of biocompatibility, which deals mainly with a better control of blood cell activation, towards the inclusion of antioxidant bioactivity that may provide protection against lipid peroxidation and other oxidative reactions occurring in the extracorporeal circulation. The functional dichotomy between biocompatibility and antioxidant function could be trivial and available data are not sufficient to define whether these are distinct functions of the same molecule or just two faces of the same vitamin E-derived function. Actually, this fat-soluble vitamin is a physiological, and so biocompatible, component of blood cell membranes and lipoprotein particles, which operates to maintain the physical and chemical characteristics of these lipid micro-environments. If, on one hand, the main function for the vitamin E used as a coating agent is that of masking the structure of other components of the copolymer, thus avoiding their contact with blood components, then on the other hand, vitamin E is a lipophilic antioxidant[5] with the importance in the scavenging of hydroperoxyl radicals (Figure 3A) in the plasmalemma and in the body of lipoprotein particles[4]. Vitamin E also stabilizes the physical structure of lipid bilayers, showing strategic localization (penetration) and movements that produce key lipid-lipid interaction in actual cell membranes. This function is facilitated by the phytyl chain and is strategic to explaining the dynamic activity of vitamin E as an anti-peroxidative molecule in complex lipid structures. Actually, during lipid peroxidation reactions, the antioxidant function of vitamin E produces a tocopheryl radical intermediate that has higher stability than hydroperoxyl radicals formed on polyunsaturated lipids. This relatively stable radical intermediate of vitamin E delocalizes from the oxidation sites to the water interface on the outer part of the cell membrane to undergo physiological recycling by cytosolic antioxidants such as vitamin C (ascorbic acid). These steps ultimately avoid the progression of lipid peroxidation chain reactions.
Moreover, vitamin E has other and so far poorly understood biological properties that appear to arise from antioxidant-independent mechanisms[18,19]. For instance, besides physiological lipid-lipid interactions, the chroman ring and the hydrophobic tail (Figure 1) represent functional domains that may lead vitamin E molecules to interact with the hydrophobic moieties of surface proteins of blood cells, and may provide the physiological environment for the hydrophobic interaction with main plasma proteins and particularly with albumin, i.e., a circulating ligandin operating a low-affinity binding of vitamin E as well as free fatty acids and several other fat-soluble molecules. Worthy of note, this interaction may ultimately affect the antioxidant activity of vitamin E[20] and could influence that antioxidant status of serum albumin, a main sacrificial target of oxidative reactions in the uremic blood[21].
Antioxidant activity of natural substances often coexists with anti-inflammatory properties that have been described both in vitro and in vivo for this vitamin, as well as for some of the physiological products of its metabolism, such as carboxyethyl-hydroxychromans, long chain metabolites and tocopheryl-phosphate[22].
Although some of these (non-antioxidant) biological effects of vitamin E could be hindered to some extent by the immobilization on the copolymer structure, their existence suggests different biological mechanisms and clinical applications of vitamin E copolymers. Accordingly, clinical and biological evidence of the antioxidant effects of vitamin E copolymers could be also ascribed to the effect that the improved biocompatibility of this type of biomaterials may exert on blood cells (mainly leukocytes), mitigating their activation during the contact with the dialyser membrane. And antioxidant effects of the other vitamin E copolymers could be erroneously ascribed to the indirect effect that an improved biocompatibility exerts on the production of reactive oxygen species (ROS), by a lowered leukocyte activation (Figure 3B). In this respect, NADPH-oxidase, myeloperoxidase and the inducible isoform of nitric oxide synthase, i.e., iNOS, can be involved as the main ROS generating enzymes, and both tocopherols and tocotrienols have been described to produce inhibitory effects on enzymatic and transcriptional events of these inflammatory and ROS generating pathways[2,3,23]. Accordingly, cellulosic membranes coated with vitamin E produce lower leukocyte activation and thus lower generation of ROS than uncoated membranes[24]. This is expected also for VitabranE™ dialyser membranes that have been recently suggested to lower inflammatory indices in HD patients[15].
Further investigation of the anti-inflammatory contribution that vitamin E can provide to the biocompatibility of hemodialysis copolymers is awaited. In this respect, future trials should take into account that antioxidant and non-antioxidant responses to vitamin E in humans varies on an individual (genetic) basis[3]. This was also recently documented for the anti-inflammatory response to oral vitamin E in individuals assessed for the polymorphic expression of main inflammatory genes[25].
A recent study by Dahe et al[10] reported on a new PS-based hollow fiber membrane incorporating from 5% to 20% (w/w) TPGS (Figure 1A). This study demonstrates that synthetic derivatives of vitamin E obtained starting from the backbone of natural vitamers can be used to develop other vitamin E copolymers, thus contributing new generations of biocompatible and possibly functional biomaterials for hemodialysis therapy. Several of these synthetic forms of vitamin E have been prepared and investigated by us and others[1-3] as pharmacological agents of possible relevance in cancer and other immuno-inflammatory diseases.
Biocompatibility and separation performance of these new TPGS-PS fibers were assessed with different in vitro methods and the reported results suggest the achievement of an “enhanced biocompatibility”. The authors, however, incorrectly claimed this biomaterial as “antioxidative composite PS” and this definition was supported by the definition of TPGS as “biologically active vitamin E”.
TPGS is not an “antioxidative” molecule but rather a redox-inert derivative of vitamin E. Indeed, the succinylation of the chroman ring that serves to produce TPGS, as in the case of α-tocopheryl succinate that is the prototypal succinyl ester of α−tocopherol with well known anti-cancer activity[1], is operated in position 6 by esterification on the hydroxyl moiety that provides the classical antioxidant function to the vitamin E molecule (for further structural and biological details on synthetic derivatives of vitamin E see[1,2] and the references therein). This esterification prejudices the radical scavenging and electron donating properties of α-tocopherol. It is worthy of note that TPGSs (there are ester derivatives of α-tocopherol with PEG chains from 4 to 136 monomeric units and molecular masses from 200 to 6000 kDa, respectively) have completely different properties when compared with authentic α-tocopherol (see structural features in Figure 1A). TPGSs are redox-silent forms of vitamin E comprised of a hydrophilic polar (water-soluble) head and a lipophilic (water-insoluble) alkyl tails. These behave as non-ionic surfactants and TPGS100 is the most widely used, from applications in cosmetics and the pharmaceutical industry. Actually, this is a solubilizer and emulsifier that is used as a vehicle for lipid-based drug delivery formulations[26], being recognized as a biocompatible compound with defined molecular stability and pharmacological properties[27]. This means that the most likely biological consequence of incorporating TPGS or other synthetic redox-silent derivatives of vitamin E into PS-like copolymers, is that of producing an inert (eventually less bioactive) biomaterial, which may represent an advantage in terms of biocompatibility, but not any direct antioxidant or radical scavenging effect. Structural investigation by Dahe et al[10] suggested that TPGS is stabilised in the fiber structure, which is key to exclude that the modifier would be released under normal operative conditions in the patient’s blood. In vitro data[4] have demonstrated that this is the case of the vitamin E embedded in the copolymer of VitabranE™ dialyser membranes and covalently bound to the PS-PVP copolymer. Accordingly, the treatment with these membranes does not influence blood levels of vitamin E of the patient[14].
The molecular stability of TPGS copolymers should be assumed as a positive feature. The possibility that TPGS would be exposed or released from the composite PS may offer a chance for untoward biological consequences due to the pharmacological effects of this molecule that include, for instance, the inhibition of metabolic enzymes as the ATP-dependent pump p-glycoprotein[27]. Alternatively, TPGS could liberate free vitamin E by enzymatic de-esterification through the activity of endogenous esterases. These enzymes could be released after blood cell damage on the surface of the membrane dialyser. Neither the release of TPGS nor that free (and thus bioactive) vitamin E from TPGS have been investigated in detail by Dahe et al[10], which are aspects of possible interest deserving further consideration in the future. Recirculation experiments carried out as described in[5] or cell uptake tests could be used to investigate these points dealing with stability and pharmacological activity of vitamin E biomaterials.
This aspect was also preliminarily investigated by means of in vitro biocompatibility tests for the TPGS-PS copolymer[10]. The production of ROS by cells maintained in culture with TPGS-PS hollow fibers was lower than in unmodified PS but available information cannot conclusively demonstrate if this is due to TPGS activity and bioavailability. At the same time, in vitro biocompatibility tests carried out with the approach of fibers maintained in a static cell culture used in the study by Dahe et al[10] can produce artifactual results, being the cells in static contact with the external surface of the fibers which may have different composition and morphology with respect to the inner surface. Circulation experiments are more appropriate to simulate the type of interaction (or contact) occurring in vivo between the plasmalemma of blood cells and the inner surface of the hollow fibers. This aspect suggests the need for further investigation by suitable circulation model systems and appropriate controls to conclusively define biocompatibility characteristics described for TPGS-PS hollow fiber membranes.
Other synthetic derivatives of vitamin E recently investigated in this laboratory may provide functionalised copolymers of interest to dialyser membrane technology. Amine derivatives of tocopherols and tocotrienols, such as α-tocopheryl amine (Figure 1B), have been demonstrated to be effective antioxidants in different model systems that include radical scavenging and electron transferring reactions[11]. Importantly, preliminary data suggest that tocopheramines may provide higher radical scavenging activity than α-tocopherol in polar solvents (i.e., under reaction conditions mimicking, for instance, the lipid-water interface of plasma lipoproteins), while α-tocopherol was found to be a superior scavenger in organic solvents (i.e., the conditions of reaction found within the lipid structure of a lipoprotein particle) that is in agreement with other studies comparing this natural form with other vitamin E forms[4].
Short chain metabolites and the pharmacological analogue Trolox (Figure 1B) are also redox-active forms of possible interest in the development of copolymers. These types of derivatives lose most of the phytyl tail of tocopherols and tocotrienols, which ultimately lowers their hydrophobic strength. Prototypal copolymers have been produced in our laboratories using activated cellulose beads as support backbone and preliminary characterization demonstrated that the hydroxylic groups of the cellulose backbone were blocked by the modifiers and these maintained their antioxidant activity (Galli F, unpublished observation).
In conclusion, vitamin E-derived copolymers, already introduced in clinical practice, have clearly shown the potential for providing one of the highest standards of biocompatibility, remaining a unique example of biomaterial with application in the antioxidant therapy of CKD patients on regular HD. In this context, the reference co-polymer used to produce hollow-fiber membranes for clinical use is vitamin E-modified PS-PVP. New generations of vitamin E copolymers such as the redox-silent TPGS-PS are now approaching the field of biomaterials for HD therapy with promising biocompatibility and filtration performances that need to be confirmed with further pre-clinical and clinical investigation. Many redox-silent and -active derivatives are available to design other functionalized copolymers with anticipated antioxidant and/or biocompatibility properties.
Peer reviewer: Theodoros Kelesidis, MD, Department of Medicine, Division of Infectious Diseases, David Geffen School of Medicine at UCLA, 10833 Le Conte Ave., Los Angeles, CA 90095, United States
S- Editor Zhang DN L- Editor Roemmele A E- Editor Zheng XM
| 1. | Mazzini F, Betti M, Canonico B, Netscher T, Luchetti F, Papa S, Galli F. Anticancer activity of vitamin E-derived compounds in murine C6 glioma cells. ChemMedChem. 2010;5:540-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Zingg JM. Molecular and cellular activities of vitamin E analogues. Mini Rev Med Chem. 2007;7:543-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Galli F, Azzi A. Present trends in vitamin E research. Biofactors. 2010;36:33-42. [PubMed] |
| 4. | Traber MG, Atkinson J. Vitamin E, antioxidant and nothing more. Free Radic Biol Med. 2007;43:4-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1074] [Cited by in RCA: 891] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 5. | Floridi A, Piroddi M, Pilolli F, Matsumoto Y, Aritomi M, Galli F. Analysis method and characterization of the antioxidant capacity of vitamin E-interactive polysulfone hemodialyzers. Acta Biomater. 2009;5:2974-2982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Sosa MA, Balk EM, Lau J, Liangos O, Balakrishnan VS, Madias NE, Pereira BJ, Jaber BL. A systematic review of the effect of the Excebrane dialyser on biomarkers of lipid peroxidation. Nephrol Dial Transplant. 2006;21:2825-2833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Galli F. Vitamin E-modified dialyzers. Contrib Nephrol. 2002;137:95-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Morimoto H, Nakao K, Fukuoka K, Sarai A, Yano A, Kihara T, Fukuda S, Wada J, Makino H. Long-term use of vitamin E-coated polysulfone membrane reduces oxidative stress markers in haemodialysis patients. Nephrol Dial Transplant. 2005;20:2775-2782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Piroddi M, Pilolli F, Aritomi M, Galli F. Vitamin E as a functional and biocompatibility modifier of synthetic hemodialyzer membranes: an overview of the literature on vitamin E-modified hemodialyzer membranes. Am J Nephrol. 2012;35:559-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Dahe GJ, Teotia RS, Kadam SS, Bellare JR. The biocompatibility and separation performance of antioxidative polysulfone/vitamin E TPGS composite hollow fiber membranes. Biomaterials. 2011;32:352-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 11. | Galli F, Mazzini F, Bamonti L, Gille L, Böhmdorfer S, Piroddi M, Netscher T, Kelly FJ, Rosenau T. Tocotrienamines and tocopheramines: reactions with radicals and metal ions. Bioorg Med Chem. 2011;19:6483-6491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Aritomi M, Galli F. Review of the Effectiveness of Cellulose-and Polysulfone-based Vitamin E-bonded Dialysis Membranes. Hemodialysis. Rijeka: InTech-Open Access Publisher 2011; . |
| 13. | Mandolfo S, Corradi B, Bucci R, Farina M, Pilolli F, Galli F. Evaluation of the impact of a new synthetic vitamin E-bonded membrane on anemia and rHuEPO requirement in ESRD patients with central venous catheters: a pilot study. Int Urol Nephrol. 2012;44:1493-1500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Andrulli S, Di Filippo S, Manzoni C, Stefanelli L, Floridi A, Galli F, Locatelli F. Effect of synthetic vitamin E-bonded membrane on responsiveness to erythropoiesis-stimulating agents in hemodialysis patients: a pilot study. Nephron Clin Pract. 2010;115:c82-c89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Panichi V, Rosati A, Paoletti S, Ferrandello P, Migliori M, Beati S, Bernabini G, Daini R, Casani A, Angelini D. A vitamin E-coated polysulfone membrane reduces serum levels of inflammatory markers and resistance to erythropoietin-stimulating agents in hemodialysis patients: results of a randomized cross-over multicenter trial. Blood Purif. 2011;32:7-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Matsumura M, Sasaki H, Sekizuka K, Sano H, Ogawa K, Shimizu C, Yoshida H, Kobayashi S, Koremoto M, Aritomi M. Improved management of intradialytic hypotension (IDH) using vitamin E-bonded polysulfone membrane dialyzer. Int J Artif Organs. 2010;33:147-153. [PubMed] |
| 17. | Aoun B, Janssen-Lozinska Y, Ulinski T. Effect of vitamin E coated dialyzers on anticoagulation requirement in hemodialyzed children. Saudi J Kidney Dis Transpl. 2010;21:466-470. [PubMed] |
| 18. | Azzi A. Molecular mechanism of alpha-tocopherol action. Free Radic Biol Med. 2007;43:16-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 251] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 19. | Brigelius-Flohé R, Galli F. Vitamin E: a vitamin still awaiting the detection of its biological function. Mol Nutr Food Res. 2010;54:583-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Barclay LR, Bailey AM, Kong D. The antioxidant activity of alpha-tocopherol-bovine serum albumin complex in micellar and liposome autoxidations. J Biol Chem. 1985;260:15809-15814. [PubMed] |
| 21. | Mera K, Anraku M, Kitamura K, Nakajou K, Maruyama T, Otagiri M. The structure and function of oxidized albumin in hemodialysis patients: Its role in elevated oxidative stress via neutrophil burst. Biochem Biophys Res Commun. 2005;334:1322-1328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 109] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 22. | Zingg JM, Meydani M, Azzi A. alpha-Tocopheryl phosphate--an active lipid mediator. Mol Nutr Food Res. 2010;54:679-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Viola V, Pilolli F, Piroddi M, Pierpaoli E, Orlando F, Provinciali M, Betti M, Mazzini F, Galli F. Why tocotrienols work better: insights into the in vitro anti-cancer mechanism of vitamin E. Genes Nutr. 2012;7:29-41. [PubMed] |
| 24. | Shimazu T, Ominato M, Toyama K, Yasuda T, Sato T, Maeba T, Owada S, Ishida M. Effects of a vitamin E-modified dialysis membrane on neutrophil superoxide anion radical production. Kidney Int Suppl. 2001;78:S137-S143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | England A, Valdes AM, Slater-Jefferies JL, Gill R, Howell WM, Calder PC, Grimble RF. Variants in the genes encoding TNF-α, IL-10, and GSTP1 influence the effect of α-tocopherol on inflammatory cell responses in healthy men. Am J Clin Nutr. 2012;95:1461-1467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Collnot EM, Baldes C, Wempe MF, Hyatt J, Navarro L, Edgar KJ, Schaefer UF, Lehr CM. Influence of vitamin E TPGS poly(ethylene glycol) chain length on apical efflux transporters in Caco-2 cell monolayers. J Control Release. 2006;111:35-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 134] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 27. | Collnot EM, Baldes C, Schaefer UF, Edgar KJ, Wempe MF, Lehr CM. Vitamin E TPGS P-glycoprotein inhibition mechanism: influence on conformational flexibility, intracellular ATP levels, and role of time and site of access. Mol Pharm. 2010;7:642-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 177] [Article Influence: 11.8] [Reference Citation Analysis (0)] |