COLLAPSING FOCAL SEGMENTAL GLOMERULOSCLEROSIS: CURRENT CONCEPTS
Focal segmental glomerulosclerosis (FSGS) is a common cause of nephrotic syndrome (NS) in both children (20%) and adults (40%), and has an estimated incidence of 7 per million. It is also the most common primary glomerulopathy causing end-stage renal disease (ESRD) in the United States, with a prevalence of 4%[1]. One of its variants, collapsing focal segmental glomerulosclerosis (cFSGS), also known as collapsing glomerulopathy (CG), is of particular interest for a variety of reasons. It is an aggressive form of renal injury that has emerged as an important cause of ESRD throughout the world[2-6]. Its nosological identity is still debatable. Recent studies suggest that it may be distinct from FSGS and sooner or later it may be classified as a separate entity[7-11]. Collapsing FSGS is an interesting renal lesion, which is currently at the forefront of scientific research, because of its increasing incidence, expanding association with diseases other than human immunodeficiency virus (HIV)-1 infection or intravenous drug abuse, propensity to occur in black populations, rapid progression to ESRD, and uniformly poor response to empirical therapy[12-19]. The lesion has been reported more frequently in western countries, but with recent growing awareness of the condition it has been increasingly reported in tropical countries[20-23]. This editorial briefly describes the current thinking about cFSGS in the light of its history, epidemiology, etiology, pathogenesis and pathology, treatment and prognosis in order to help understand the problem in a wider perspective.
HISTORY
The historical background, the nomenclature and the nosological status of cFSGS has stimulated considerable debate in the nephropathology community. There has been a lot of confusion and debate, especially on the nomenclature and the nosological status of this distinctive proliferative renal parenchymal lesion. It was first recognized as a possibly distinct clinicopathological entity in 1986, when Weiss et al[7] described six cases of this condition in young black patients. However, cases with similar pathological findings and clinical behavior were reported in the literature as “malignant FSGS” as early as the 1970s, and provide evidence that this lesion existed well before its formal identification as a distinct entity[4]. Its relationship with other renal glomerular diseases, especially FSGS, is both interesting and controversial[4,5]. The authors of the first report of this condition considered this lesion to be a unique and novel clinicopathological entity, although as mentioned earlier, with a degree of uncertainty. However, during the mid-1990s reports appeared in the literature linking this lesion to the growing spectrum of FSGS and Detwiler et al[8] were the first to consider this lesion as a distinct variant of FSGS, followed by Valeri et al[9], Columbia classification of FSGS officially classified cFSGS as a distinct variant of FSGS[10]. However, more recently, some investigators have argued that this nosological association with FSGS may not be sustainable, and that sooner or later, cFSGS may be designated as a separate entity[11]. Indeed, a recently proposed classification of the podocytopathies has categorized cFSGS separately from FSGS and further subdivided it into three major subtypes: idiopathic, genetic, and secondary or reactive[12]. Our experience with a number of secondary cFSGS lesions also supports the later proposal[2]. Further studies are needed to clarify this nosological conundrum.
EPIDEMIOLOGY
The epidemiological picture of cFSGS is becoming clearer with the passage of time. Although cFSGS was originally reported in North America, the disease is truly global in distribution[2-5]. However, until recently, the majority of cases were reported from North America with few reports from Europe and other western countries[15-19], and only occasional cases reported from developing countries[20-23]. This geographical disparity in the reporting of cFSGS was predominantly due to detection bias rather than true differences in the prevalence of the disorder. More recently, tropical countries have reported frequencies of cFSGS detection in both native and transplanted kidney biopsies approaching those in western countries[20-23]. The growing reporting of cFSGS in the literature from both western and tropical countries reflects both a true increase in the incidence of the lesion and diagnostic bias. The upward surge in the incidence of this lesion has been clearly shown in studies from North America and Europe. However, so far no similar upward trend in cFSGS incidence has not been formally reported from any developing country. The exact cause of the increase in the incidence of cFSGS is not known, and may be reflective of a possible change in exposure to certain infections, chemical agents, or other environmental factors.
ETIOLOGY
The exact cause of primary cFSGS is still not known. It is apparent, however, that cFSGS is not a single disease entity but rather a heterogeneous group of disorders unified by a distinctive morphologic pattern of renal parenchymal injury[2-6]. There are increasing reports of secondary and genetic causes of this lesion and these provide an insight into the widening etiopathogenetic pathways of the condition[2-6]. Among the known causes, HIV-1 infection is the most frequent, the lesion being labeled as HIV-associated nephropathy (HIVAN) in these patients[24]. Other infectious agents and immunological disturbances are the next most commonly reported associations[25-27]. One important predisposing factor is various forms of ischemic injury to the kidney[28]. The later mechanism has been most commonly, although not exclusively, implicated in the development of cFSGS in transplanted kidneys[29-32]. We have observed cFSGS lesions involving the glomeruli in the vicinity of acute cortical necrosis (ACN) of obstetric origin on native renal biopsies from young females with no underlying vasculopathy[13,14]. We have also found the typical lesions of cFSGS in surgically removed non-functioning kidneys from cases of vesico-ureteric reflux and occasionally other causes (unpublished observations). The list of conditions/illnesses associated with this morphology is growing day by day. The lesion is also being increasingly recognized in transplanted kidneys, not only from developed countries but also from developing countries[29-32]. As alluded to earlier, this growing heterogeneity of the underlying causes/associations clearly demonstrates that cFSGS is not a single disease entity, but rather a common final pathway resulting from widely divergent insults, unified by a similar histological picture.
PATHOGENESIS
As with the etiology of the disease, its pathogenesis in humans is still not completely understood. However, significant advances have been made recently in identifying many of the crucial steps leading to the final common manifestation of the disease. As discussed previously in relation to the etiology of the condition, the discovery of secondary and genetic causes of cFSGS was instrumental in providing an understanding the pathophysiology of the disorder. The study of HIVAN in humans, animal models and in vitro laboratory results have also provided important insights into the pathomechanisms of both HIVAN and idiopathic cFSGS and it is, therefore, useful to briefly review the pathogenesis of HIVAN to help better understand the mechanism of idiopathic cFSGS. HIVAN is a leading cause of ESRD in HIV-1-seropositive patients. Clinically and morphologically, HIVAN closely resembles idiopathic cFSGS. Similar to idiopathic cFSGS, HIVAN is characterized by heavy proteinuria and a rapid decline in renal function with pathological findings of cFSGS[33-35]. Unlike the idiopathic variety of cFSGS, for which the cause is still elusive, HIVAN is caused by the direct HIV-1 infection and subsequent expression of viral genes in renal epithelial cells in genetically susceptible patients. The exact mechanism of viral entry into the renal epithelial cell compartment is unknown. HIV-1 typically uses CD4 and a co-receptor such as CCR5 or CXCR4 to enter cells. So far, there is no evidence of the expression of these receptors in the podocytes. These negative findings, however, do not exclude the possibility that another, CD4 independent, co-receptor may be used for viral entry into epithelial cells in the kidney. Recent demonstration of an extensive network of dendritic cells in the kidney parenchyma has raised further questions on the role of these cells in the intrarenal spread of HIV-1 infection. The genetic factors responsible for the susceptibility to HIVAN among black patients include a noncoding variant in the podocyte-expressed gene nonmuscle myosin heavy chain 9 as well as other genes yet to be identified[33-37]. Research using animal models and in vitro studies has shown that podocyte and tubular dysfunction results from the expression of viral genes, in particular nef and vpr, and the subsequent dysregulation of several host factors, including critical signaling pathways, mediators of inflammation, apoptosis, proliferation, transcription, and cell-cell interactions. Further studies are required to define the mechanisms of HIVAN pathogenesis further so that more effective interventions can be implemented to prevent and treat this disease and the related cFSGS[38-46].
Similarly, the development of more than a dozen independent murine models of cFSGS in different laboratories of the world is a landmark achievement in the study of the pathophysiology of the disease at the fundamental level[2-6]. In marked contrast to human disease, the pathogenic triggers in each of the four categories of murine models of cFSGS are known. These include HIV-1 gene products, immunoglobulins (Ig), oxidative stress, and the disturbance of podocyte paracrine/autocrine regulatory mechanisms[4]. A “best-fit” model has been proposed to explain the final common expression of the disease caused by such seemingly divergent stimuli[4]. According to this model, the initiating event consists of focused epithelial cell injury caused by either intrinsic or extrinsic agents involving different compartments of renal parenchyma, i.e., both the podocytes and the renal tubular epithelium. The surrounding uninjured epithelial cells subsequently respond to the injury, not by the normal repair or regeneration, but in an irregular manner involving the processes of dedifferentiation, proliferation and transdifferentiation of, for example, podocytes to the macrophage-like phenotype in the glomerular compartment. These phenotypic changes are accompanied by changes in the behavior of these cells and in their immunophenotypic profile. The immunophenotypic markers of maturity are lost, and the markers of immature phenotype, proliferation and macrophage lineage are expressed, the latter phenomenon being known as transdifferentiation. The podocytes become cuboidal or epithelioid cell-like, lose primary foot processes, and detach from the glomerular basement membrane (GBM) and proliferate to form pseudo-crescents. These phenotypic changes result in marked alterations in the normal structure-function relationships of the different compartments of the nephron with consequent drastic changes in the function, so characteristic of this disease. The luminal factors and the preexisting disturbance in the immune system, coupled with genetic susceptibility, probably contribute to the above sequence of events[2-5].
Two major common pathways have been proposed to explain the stereotyped response of the renal parenchyma to a variety of triggering/injurious agents in cFSGS: activation of the immune system and the dysregulation of mitochondrial function[3]. Many of the diseases associated with cFSGS involve an alteration in immune homeostasis, suggesting at least some role for immune system activation in the development of cFSGS. The precise immunological mechanisms involved are still not completely clear, but studies show that many involve T helper type 1 lymphocyte responses, an immune dysregulation that already is known to aggravate and accelerate other proliferative parenchymal renal diseases, particularly crescentic glomerulonephritis (CresGN), the disease that cFSGS most resembles on renal biopsies.
The second major common pathway involves some form of acute ischemic insult to the kidney such as that resulting from thrombotic microangiopathy, cyclosporine A toxicity or severe hyaline arteriopathy[4]. This phenomenon has been particularly observed in transplanted kidneys. Recently, three cases of cFSGS have been reported in renal allograft biopsies in association with areas of frank segmental infarction[32]. We have also observed a similar phenomenon in native kidney biopsies from patients with ACN with no associated vasculopathy[13,14]. Thus, ischemic injury to the glomeruli does have a role in the development of cFSGS. This may be mediated by dysregulation of mitochondrial function and/or altered autocrine/paracrine interaction of podocytes, endothelial cells, and/or parietal epithelial cells (PECs)[4].
PHENOTYPIC CHANGES IN PODOCYTES AND THE CELLULAR ORIGIN OF THE PSEUDO-CRESCENTS
In normal adult kidneys, podocytes are terminally differentiated, postmitotic cells whose function is largely based on their complex cytoskeletal architecture[47-51]. In cFSGS, severe podocyte damage results in profound changes in the phenotype of podocytes, reflected in morphological and immunohistochemical alterations of dedifferentiation, dysregulation and transdifferentiation. Dedifferentiation refers to the loss of expression of maturity markers, such as synaptopodin, podocin, podocalyxin, glomerular epithelial protein (GLEPP1), CD10 (common acute lymphoblastic leukemia antigen), and C3b receptor, as well as re-expression of proliferation markers, such as, Ki-67. Morphologically, dedifferentiation is manifested as change in cell shape with rounding of the cell outlines to cuboidal shape, loss of primary and foot processes, loss of actin-based cytoskeleton and slit diaphragms. The amount of cytoplasm is increased with numerous protein-resorption droplets. Microvillous transformation of the surface of podocytes is also seen in late or advanced stages of the disease. Concurrent with these changes, podocytes lose contact with GBM and detach from the glomerular tufts and fill the Bowman’s space. The dedifferentiated podocytes also enter the cell cycle and proliferate at an increased rate as shown by immunohistochemistry for Ki-67. Dysregulation of the phenotype involves the loss of expression of Wilms’ tumor antigen 1. It should be noted that the dysregulation is not complete, as some other markers, such as nestin and dystroglycan, are preserved[4,5]. Transdifferentiation refers to the acquisition of macrophage marker, CD68, by the podocytes[36-38].
Historically, the proliferating cells of the pseudo-crescents were thought to be of podocyte origin, but more recent studies have also shown an important contribution of PECs to the viscerally located proliferative lesion in both idiopathic cFSGS and HIVAN. PECs participate in the proliferative lesion as direct contact points of parietal cell bridges or migration along the podocyte-PEC interface at the vascular pole of the glomerulus[52-55].
DIAGNOSIS
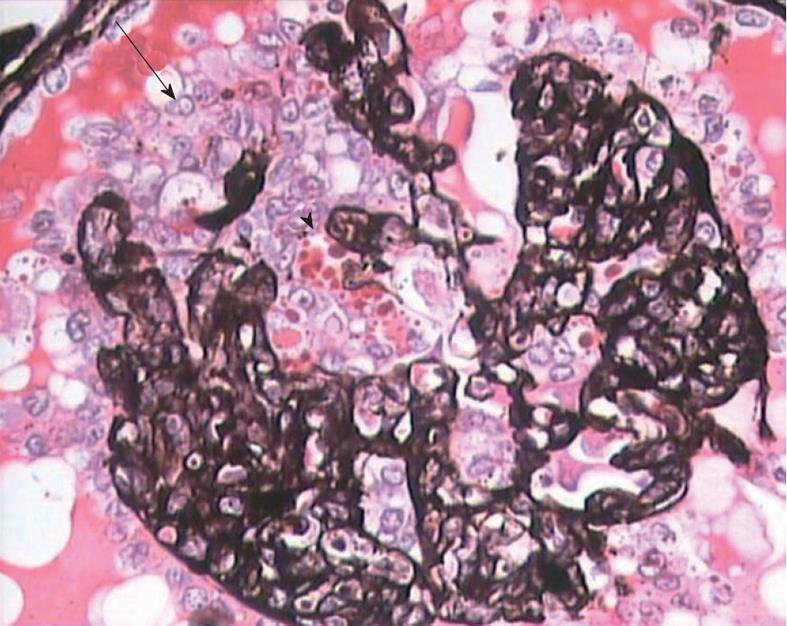
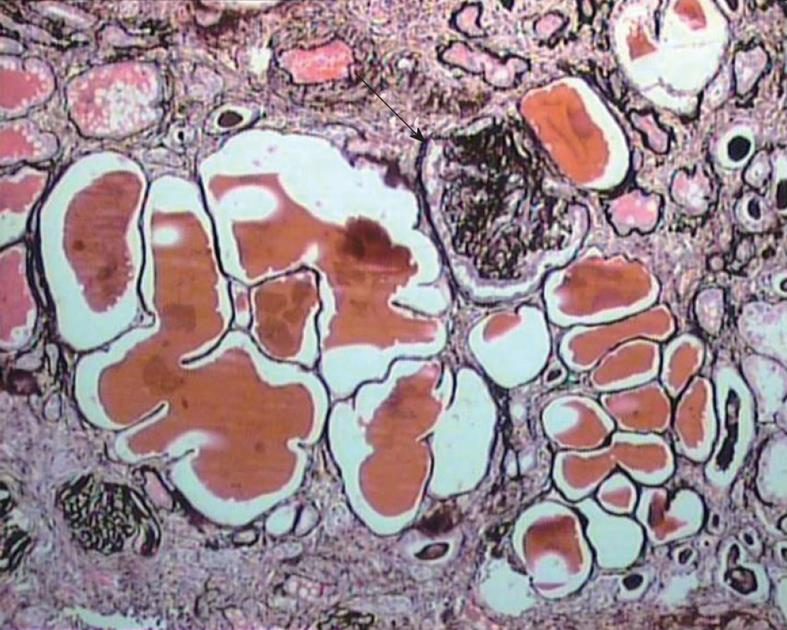
The diagnosis of cFSGS can only be made by histological evaluation of renal biopsies. It is diagnosed pathologically by characteristic morphological changes in the glomeruli on light microscopy (LM) of renal biopsies[2-6]. These consist of focal-to-diffuse, segmental-to-global, implosive collapse of the glomerular capillary tufts associated with hyperplasia and hypertrophy of overlying podocytes, a defining feature of this entity. These proliferating podocytes fill the Bowman’s space caused by collapsed tufts and result in the formation of so called pseudo-crescents, which differ from the true crescents of CresGN by their centripetal location, the presence of a cleft-like space between the pseudo-crescents and the parietal epithelium, the epithelioid appearance of cells forming the pseudo-crescents, and the absence of fibrin in the pseudo-crescents (Figure 1). These extracapillary cells lack the spindle cell appearance or pericellular matrix with fibers typically noted in true crescents. The proliferating podocytes exhibit drastic changes in the expression of immunophenotypic markers coupled with similar changes in cellular behavior. These features can also be exploited to distinguish cFSGS from CresGN. Another distinguishing feature is that Bowman’s capsule itself is intact, without the ruptures typical of cellular crescents of the inflammatory type. The proliferating podocytes also exhibit marked cytoplasmic vacuolization and prominent, hyaline, protein resorption droplets. Usually there is also concurrent segmental and/or global glomerulosclerosis at the time of pathologic diagnosis of cFSGS. This feature is the basis for the current classification of the lesion under the rubric of FSGS. The distribution of lesions may be segmental or diffuse and may involve any number of the glomeruli. Due primarily to its poor prognosis among all the other variants of FSGS, even a single glomerulus with the characteristic collapsing lesion is sufficient for the diagnosis of cFSGS on the biopsy. Although, cFSGS is defined on the basis of glomerular lesions, the tubulointerstitial involvement is an equally, and in view of some authors, even more important component of the condition and often appears out of proportion to the degree of glomerular sclerosis[12]. It has been suggested that the poor prognosis of the condition results from the severity of tubulointerstitial pathology rather than the glomerular lesions[12]. The tubulointerstitial component shows both acute and chronic changes. These include variable degrees of tubular atrophy, interstitial fibrosis, edema, and inflammation, associated with widespread degenerative and regenerative changes of the tubular epithelium, including microcyst formation. The later are filled with hyaline casts with scalloped margins, as shown in Figure 2. It has been proposed that the extent of tubulointerstitial involvement may help in the differential diagnosis of different etiologies of cFSGS[3-5]. As is evident from the above discussion of pathology, the biopsy diagnosis of cFSGS is based almost solely on LM study. Immunofluorescence (IF) microscopy for renal panel immunoglobulins and complement components is usually negative or in some cases shows only focal segmental positivity of IgM, C3, and occasionally C1q, in collapsed lobules of the glomeruli. The intensity of staining is usually mild, ranging from trace positivity to 1+, but may be up to 2+ on a scale of 0 to 3+. The main utility of IF microscopy is to rule out the secondary causes of cFSGS. Similar to IF, electron microscopic (EM) features are also non-specific and consist of wrinkling and collapse of GBM with little or no thickening of GBM. The overlying podocytes are greatly enlarged with diffuse foot process effacement with focal areas of separation from the underlying GBM. No electron dense deposits are observed. EM study also helps in excluding secondary causes of cFSGS, such as HIVAN, which reveals characteristic tubuloreticular inclusions[3-6].
Figure 1 High magnification view showing global collapse of capillary tufts associated with marked hypertrophy and hyperplasia of podocytes (arrow), forming a pseudo-crescent.
Marked cytoplasmic vacuolization and protein resorption droplets (arrowhead) are seen in podocytes. Jones’ methenamine silver, × 400.
Figure 2 Medium-power view showing marked tubular atrophy associated with interstitial fibrosis and mononuclear inflammatory cell infiltrate in the interstitium.
Many dilated tubules contain eosinophilic casts. One glomerulus shows ischemic solidification and another one, global collapse with podocyter hypertrophy but little podocyte proliferation (arrow). Jones’ methenamine silver, × 200.
DIFFERENTIAL DIAGNOSIS
There are a few morphological lesions on renal biopsies which mimic cFSGS and contribute to differential diagnosis. These include other variants of FSGS as well as other forms of glomerular disease, most notably CresGN. Among the variants of FSGS, the closest differential diagnosis to cFSGS is cellular FSGS. Both the collapsing and cellular variants are characterized by marked hyperplasia and hypertrophy of podocytes. The differentiating features that separate the two variants are the implosive wrinkling and retraction of the GBM seen in cFSGS associated with endocapillary hypocellularity, as opposed to the expansile lesions of endocapillary hypercellularity seen in cellular FSGS. In many cases of cFSGS, the podocyte proliferation may be so extensive as to form a cellular crescent-like structure, known as pseudo-crescent, to differentiate it from true crescents. In such cases, the biopsy findings may be misinterpreted as CresGN. A closer scrutiny of the lesions with special attention to the features described in the Diagnosis section, along with appropriate integration of the clinical history and LM, IF, and EM findings will aid in this differential diagnosis[2,3].
CLINICAL FEATURES
The clinical spectrum is also developing rapidly through the expanding list of associated conditions and reflects to some extent the underlying causes of cFSGS. In general, the demographic, clinical and laboratory features of the most common type of lesion (the primary or idiopathic type) resemble those observed in non-collapsing FSGS, although the disease is usually more severe. Idiopathic cFSGS is typically a disease of young adults, with a median age of 30 to 40 years. However, a wide range of ages have been reported, with patients as young as 1.5 years and as old as 82 years, although exclusive studies of cFSGS in children are rare[2,3,56-60]. Most studies have reported a male predominance, although in one study, females outnumbered males. Collapsing FSGS has also well described racial predisposition. Since the original report of Weiss et al[7], in which all six patients with cFSGS were black, a predominance of black patients in the USA has been noted[4,5,61]. A review of all published series on cFSGS has shown that, around 50% of the reported cases in the literature are in black populations[4].
Clinically, a majority (> 80%) of patients with cFSGS present with nephrotic range proteinuria, and studies have documented a significantly greater frequency of NS and higher levels of proteinuria in patients with cFSGS than in patients with non-collapsing FSGS. There is also significantly greater renal insufficiency in patients with cFSGS at presentation than in patients with non-collapsing FSGS[3]. Other severe manifestations of NS are frequent, including hypoalbuminaemia, hypercholesterolemia, and edema, but these manifestations are not significantly different from those seen in patients with non-collapsing FSGS.
The clinical presentation of secondary causes of cFSGS is dominated by the underlying disorder, as is their pathology and the prognosis[13,62-73].
The disease is also being increasingly reported in renal allografts, both recurrent and de novo in origin. However, there is still limited experience of transplantation in patients with cFSGS. The few studies on this subject show that the incidence of recurrence is rather low, and that most of the cases represent de novo disease. The clinical presentation and course of the disease generally resemble those of the native kidney disease[74].
TREATMENT
Currently, there is no specific treatment for cFSGS, and there are no ongoing prospective treatment trials[2-5]. The therapeutic approaches used at present are anecdotal, and analogous to those used for non-collapsing FSGS, based on the use of steroids or immunosuppressive agents[3,75-80]. The particular therapeutic agents, their dosages, duration, and the definition of response vary among different studies, contributing to the variation in results between series. The overall results are poor, with a complete remission rate of < 10% and a partial remission of 15.2% being reported[4].
In view of the present lack of effective therapeutic agents and the uniformly poor prognosis of the disease, there is an urgent need for the development of highly effective and specific agents, based on the knowledge of the pathogenetic pathways of cFSGS. Indeed, some progress is evident on this front as well. The administration of small molecule inhibitors of cyclin-dependent kinases in small animal models in preclinical trials has been shown to prevent the development, and retard the progression, of the lesions of cFSGS[81]. In addition, the use of differentiating agents such as retinoic acid derivatives, to inhibit the proliferation of podocytes and induce their differentiation to a mature, quiescent phenotype, has also been shown to abrogate experimental cFSGS[82]. Promising results have also been obtained in improving renal function in experimental forms of cFSGS by the use of small molecule inhibitors of inflammatory pathways controlled by nuclear factor-κB and cyclooxygenase-2. These findings underscore the multifactorial basis for the proliferative phenotype of podocytes in cFSGS, and suggest the emergence of rational approaches to therapy for cFSGS based on the understanding of pathogenesis in the near future[4].
NATURAL HISTORY AND PROGNOSIS
Due primarily to the retrospective nature of most studies on cFSGS, only generalizations can be made about the natural history of the disease and its prognosis. Patients with cFSGS are at high risk of progressing to ESRD. Even with the currently available treatment, the incidence of ESRD is 50%-100% in most series[2-6]. However, it is worth emphasizing here that the natural history and prognosis of the lesion is primarily governed by the etiological condition leading to the disease[13,14]. In all comparative studies, the renal survival of patients with cFSGS was significantly worse than patients with non-collapsing FSGS. Multiple studies have explored possible prognostic features in cFSGS. Valeri et al[9] found that progression to ESRD was predicted by serum creatinine concentration at the time of biopsy (P < 0.05) and lack of remission of proteinuria (P < 0.025), but they did not find a correlation between severity of proteinuria or other features of NS and the outcome. The rate of progression of renal failure in the collapsing FSGS group correlated strongly with the severity of tubular degenerative and regenerative changes (P < 0.02) but not with any other parameter of tubulointerstitial or glomerular change. The rate of progression of renal failure correlated strongly with male sex. Laurinavicius et al[18] reviewed retrospectively the data from 42 patients with cFSGS and 18 patients with HIVAN to determine the predictors of serum creatinine level, proteinuria, and progression of renal disease. In their multivariate model, the risk for ESRD was increased significantly by interstitial fibrosis of > 20%, creatinine level > 2.0 mg/dL, proteinuria > 8 g/d, glomeruli with collapsing lesions > 20%, and HIV infection (P < 0.0001).
CONCLUSION
In conclusion, recent years have witnessed significant advances in understanding the ever increasing etiology and equally divergent pathogenesis of morphologically uniform cFSGS. These advances are gradually resolving the nosological confusion surrounding this disease categorization and opening up new opportunities for research leading to the development of rational and targeted therapies for this disease.
Peer reviewer: Peter Bárány, Associate Professor, Clintec, Renal Medicine, Karolinska Institutet, Karolinska University Hospital, Huddinge K56, SE-14186 Stockholm, Sweden
S- Editor Zhang DN L- Editor Hughes D E- Editor Zheng XM