Published online Aug 12, 2015. doi: 10.5501/wjv.v4.i3.255
Peer-review started: October 31, 2014
First decision: December 12, 2014
Revised: April 21, 2015
Accepted: May 7, 2015
Article in press: May 8, 2015
Published online: August 12, 2015
Processing time: 288 Days and 13.6 Hours
Shared routes of transmission lead to frequent human immunodeficiency virus (HIV)-hepatitis B virus (HBV) co-infection in a host which results in about 10% of HIV positive individuals to have chronic hepatitis B infection worldwide. In post-antiretroviral therapy era, liver diseases have emerged as the leading cause of morbidity and mortality in HIV-infected individuals and HBV co-infection have become the major health issue among this population particularly from the regions with endemic HBV infection. In setting of HIV-HBV co-infection, HIV significantly impacts the natural history of HBV infection, its disease profile and the treatment outcome in negative manner. Moreover, the epidemiological pattern of HBV infection and the diversity in HBV genome (genotypic and phenotypic) are also varied in HIV co-infected subjects as compared to HBV mono-infected individuals. Several reports on the abovementioned issues are available from developed parts of the world as well as from sub-Saharan African countries. In contrast, most of these research areas remained unexplored in India despite having considerable burden of HIV and HBV infections. This review discusses present knowledge from the studies on HIV-HBV co-infection in India and relevant reports from different parts of the world. Issues needed for the future research relevant to HIV-HBV co-infection in India are also highlighted here, including a call for further investigations on this field of study.
Core tip: Various parameters of hepatitis B virus (HBV) infection including molecular epidemiology, disease profile and treatment outcome remains unexplored in human immunodeficiency virus (HIV)-positive individuals from India, a major reservoir for HBV and HIV infection of the globe. Only few reports particularly from eastern Indian HIV-HBV co-infected cohort represented some interesting findings in context to the global reports on this co-infection. Comparing with the available worldwide studies, issues that should be addressed for research in India are identified and a call for further investigations on HIV-HBV co-infection in India is highlighted through this article. This is needed for proper management of HIV-HBV co-infected Indian population.
- Citation: Chakravarty R, Pal A. Insights into human immunodeficiency virus-hepatitis B virus co-infection in India. World J Virology 2015; 4(3): 255-264
- URL: https://www.wjgnet.com/2220-3249/full/v4/i3/255.htm
- DOI: https://dx.doi.org/10.5501/wjv.v4.i3.255
Human immunodeficiency virus (HIV) and hepatitis B virus (HBV), the two important blood-borne human pathogens, are major public health concerns in the current era. Since the introduction of combination antiretroviral therapy (cART) in 1996, acquired immunodeficiency syndrome (AIDS) related deaths among HIV-infected individuals have been reduced significantly worldwide[1]. In this situation of improved life expectancy due to ART, liver disease associated mortality has emerged as the leading cause of deaths in HIV-infected global population. Of all the possible causes of liver-related deaths, HBV co-infection has become one of the important burdens among HIV-positive individuals in post-ART era. Moreover, HBV shares its routes of transmission (sexual contact, percutaneous route and perinatal route) with HIV[2] and thus the incidence of HIV-HBV co-infection becomes a frequent phenomenon in a host[3]. As a consequence, in an estimated 40 million people living with HIV worldwide, approximately 10% (2-4 million) have chronic HBV co-infection [defined by the presence of serum hepatitis B surface antigen (HBsAg) for more than 6 mo][2]. In addition, the biological signs of prior HBV infection [defined by the presence of serum anti-hepatitis B core antibody (HBcAb)] could be observed in 90% of HIV-positive individuals in the regions with high endemic HBV infection such as south-east Asia and sub-Saharan Africa.
During the setting of co-infection, HIV and HBV simultaneously interact in a host complicating pathogenesis and disease progression of these two infections, immune-responses to both the virus and treatment outcome against them. Till date, several studies have been conducted worldwide which have shown significant negative impact of HIV on the natural history of HBV infection[4-8] whereas such confirmed evidences are missing that state the effect of HBV on HIV infection[9]. Co-infection with HIV modifies the natural history of HBV infection by increasing the rate of HBV chronic infection, lowering the rate of HBsAg, hepatitis B e antigen (HBeAg) seroconversion and increasing HBV replication[5,6]. The deleterious effect of HIV leads to more rapid progression towards end-stage liver diseases (liver cirrhosis and hepatocellular carcinoma) and higher risk for liver-disease related mortality in HIV-HBV co-infected individuals as compared to those infected with HBV only[10,11]. Simultaneously in presence of HIV, management of HBV becomes complicated greatly[12].
Most of the developed parts of the world (for, e.g., western Europe, Australia and United States) where HBV is less endemic (HBsAg prevalence < 2%) and some of the developing countries (mostly sub-Saharan African countries) with endemic HBV infection (HBsAg prevalence ≥ 8%) have largely contributed to the studies regarding HIV-HBV co-infection in these parts of the globe. Harbouring the third largest population of HIV infection and the second largest pool of chronic HBV infection (HBsAg prevalence 2%-7%) of the world, reports on HIV-HBV co-infection in India are scarce. Interestingly, few reports available from India indicate that studies on HIV-HBV co-infection are required from national as well as global perspectives. Therefore here we have highlighted HIV-HBV co-infection in India in comparison to the reports from different parts of the world to understand the present scenario of this co-infection in this subcontinent.
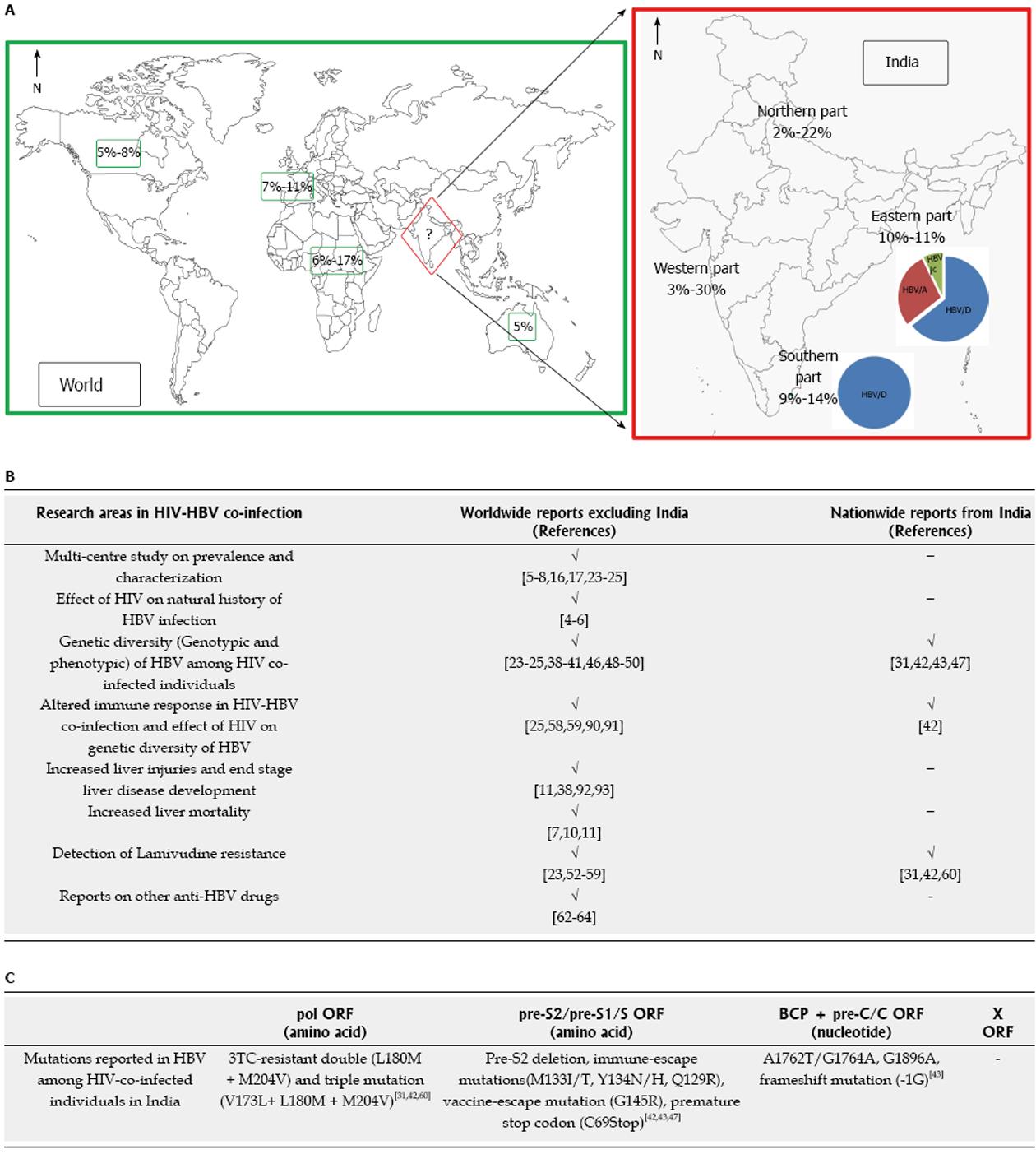
HIV-HBV co-infection showed global heterogeneity in epidemiological pattern. Two major determinants of this variation are geographical origin and risk groups of infected patients[2]. In regions of low endemicity of HBV infection (prevalence of HBsAg < 2%) such as United States, Western Europe and Australia, prevalence of chronic hepatitis B was reported to be 5%-14% among HIV-positive individuals[7,13-17]. In the countries of developed parts of the world, acute HBV infection occurs in adolescents and young adults through primarily sexual transmission (both heterosexual and homosexual), followed by percutaneous transmission. HIV-infected men who have sex with men (MSM) showed the highest frequency of chronic HBV infection (9%-17%)[3]. In contrast, perinatal transmission (south and south-east Asia) and horizontal transmission (Africa) are the major threats in the intermediate (HBsAg prevalence 2%-7%) and high endemicity (HBsAg prevalence ≥ 8%) zones of HBV infection where persons obtain HBV infection in childhood[18]. Adults could acquire HIV-HBV co-infection through sexual contact and unsafe blood transfusion process in the resource limited settings of low-income countries[18,19]. Most studies reported 10%-20% prevalence of HIV-HBV co-infection in these countries[2]. Moreover reports from different parts of sub-Saharan Africa suggests that HBsAg prevalence could vary considerably (from Kenya; 6% to Nigeria; 16.7%)[20,21]. Evidences of variations in the prevalence of HBsAg among different risk groups were also found across the countries of this continent[22]. Regarding the epidemiological scenario several studies have been performed worldwide on multi-centre cohort of HIV-HBV co-infection which revealed the overview of prevalence, clinical and virological profile of these patients from a country[7,8,10,15-17,23-25]. Recently, a study including a multi-national cohort from 11 countries showed the concordant prevalence of HIV-HBV co-infection in Africa, America and Asia similar to the previous reports[25].
In contrast, sporadic reports from India[26-37] has addressed the issue of prevalence of HBsAg among HIV-infected patients majority being from the northern part of the country[32-37]. Taking together these reports, HBsAg prevalence among HIV-infected Indian population could be estimated as 2%-14%. These reports mostly included HIV-positive patients either from one ART centre[31,37] or from single risk group for, e.g., injecting drug users[27], female sex workers[30]. However in another two studies quite high frequency of HBsAg were found - approximately 22% (6/27)[32] and approximately 30% (34/110)[26]. These variations in results were observed possibly due to small sample size data, lack of multi-centre studies and unavailability of multi-risk group data. Thus, overall epidemiological trend of HIV-HBV co-infection in India still remains obscure (Figure 1). Nevertheless, two findings from these sporadic studies are concordant with the worldwide reports[21,22,25], i.e., (1) the male gender is predominant over the females; and (2) sexual contact is the chief transmission route of HIV-HBV co-infection in India[26-31].
To date, significant adverse effects of HIV co-infection on the natural history of HBV infection have been demonstrated in several studies from the perspectives of increased chronicity, accelerated rate of advance liver disease development and heightened mortality rates[4-6,10,11]. In a retrospective study, Bodsworth et al[4] showed increased rate of HBV chronicity development in HIV seropositive homosexual men than those without HIV (23% vs 4%)[4]. In several studies, HIV-HBV co-infected individuals showed decreased rate of HBeAg seroclearance along with increased HBe antigenemia[5,6,8]. Incidence rate of HBeAg seroclearance was decreased five times in HIV-positive patients compared to HIV-negative ones during a mean follow up for 5 years in a study from France[8]. Moreover in accordance to high HBeAg positivity, high serum HBV DNA load is associated with HIV-HBV co-infected patients[5,6]. HBeAg positive HIV-infected patients mostly had higher HBV viraemia as compared to HBeAg negative individuals. Thio et al[25] showed that 66% of HBeAg negative subjects had HBV DNA < 2000 IU/mL in a multi-national treatment-naïve cohort suggesting HBeAg as a predictive factor for HBV treatment in absence of HBV DNA quantification data[25]. Remarkably, studies describing the impact of HIV on HBV related mortality showed that HIV-HBV co-infected individuals had increased rate of liver associated deaths as compared to those with HBV mono-infection[7,10]. HIV-HBV co-infected men had 17 times higher incidence of liver-disease related deaths than HBV mono-infected ones[10].
Besides the worldwide reports, studies regarding the abovementioned parameters are lacking in India. However in one study from eastern India showed high HBV DNA load among HBeAg negative HIV infected individuals where 61% had HBV DNA ≥ 2000 IU/mL and required HBV treatment[31]. According to the study by Thio et al[25], detection of HBeAg may be useful to assess the need for treatment in a setting where HBV DNA quantification facility is unavailable. Saha et al[31] demonstrated that HBeAg could be helpful to indicate the need for treatment among HBeAg positive HIV-HBV co-infected patients from eastern India however DNA quantification is necessary to consider HBeAg negative patients for treatment or not. Thus eastern Indian HIV-HBV co-infected individuals need serious attention for their clinical management and the conformation of this finding from the different parts of the country is an urgent necessity.
The aforementioned negative impacts on natural history of HBV might be the consequences of influence of HIV on the diversity of HBV genome, modification of host immune response and ART related complications. Some reports could be found to address the genetic diversity of HBV among HIV co-infected individuals[23,38-43]. HBV genome diversity can be described from two aspects - genotypic and phenotypic.
Genotypic diversity is related to the natural history and the genotypes of HBV infection occurring during the gradual evolution of HBV in a host without selective pressures. Having a high mutation rate (10-5/replication cycle), HBV results in the generation of different genotypes and each genotype can further be divided into several sub-genotypes. So far ten HBV genotypes (A-J) have been described depending upon their > 8% nucleotide divergence in complete genome sequences, whereas subgenotypes have that divergence of > 4% - < 8%[44]. HBV genotypes and subgenotypes showed varied distribution according to the geographical regions. Moreover, HBV genotypes/sub-genotypes differ considerably in the mutational patterns, ethnicity and their clinical as well as treatment outcomes[44].
In HIV-HBV co-infection, distribution of HBV genotypes was found to vary with geographical origin which is similar to HBV mono-infection[45]. In a recent collaborative study from 19 French university hospitals, 223 HIV-HBV co-infected patients were evaluated[24], where primarily prevalence of HBV/A were found in European and HBV/D in African patients. While, HBV/E was found mainly in patients with sub-saharan African origin, as this genotype is reported to be confined mostly to that region. Interestingly, a report from Mexico observed differential predominance of genotype between HBV mono-infected (HBV/H) and HIV-HBV co-infected patients (HBV/G)[46]. Moreover in a recent report on a multi-national HIV infected cohort (n = 113), Thio et al[25] reported the predominance of HBV/A (72%) and HBV/D (16%) in HIV-HBV co-infection worldwide and the divergence of HBV genotype with geographical regions[25]. Till date only one study could be found to report an association of HBV genotype (HBV/G) with liver severity, i.e., the degree of liver fiborsis in HIV-HBV co-infected patients[38].
In comparison to worldwide data, only four studies could be found from India that analyzed the genetic diversity of HBV among HIV co-infected patients; three from eastern India[31,42,43] and one from north-eastern India[47]. Reports from other parts of the country are still lacking. Interestingly, a recent multi-national study[25] that included patients from India (n = 13), showed 100% prevalence of HBV/D. In contrast, three studies from eastern India, that included a larger number of patients (n = 73[42], 119[31] and 85[43]), reported predominance of HBV/D, followed by HBV/A and found a few HBV/C infected patients. Pal et al[42], showed that the HBV genotypes/subgenotypes found among co-infected patients from eastern India are consistent with the previous data on HBV mono-infection, but the proportion differs between the HIV-HBV co-infected and the HBV mono-infected patients. Significantly higher prevalence of HBV genotype D (HBV/D - 67%) and HBV sub-genotype D2 (HBV/D2 - 68%) was observed among HBsAg positive HIV co-infected patients from this region[42]. Moreover, the predominance of HBV/C among the HBsAg negative HIV co-infected IDUs from Manipur, a state of northeastern India, has also been reported[47]. The presence of HBV/C has been thought to be correlated with drug-trafficking routes and epidemic use of injection drug in that geographical region. Additionally in this study, HBV recombinant strains (HBVA/D, HBVA/C) were found from two IDUs. Few studies could be found worldwide to report recombination in HBV DNA among HIV co-infected patients[48-50]. However, clinical consequences of these recombinants are unknown.
Phenotypic diversity results from the attempts to escape from host immune pressure or selective pressure of drugs. In HIV-infected individuals known HBV phenotypic diversity as well as novel viral variants have been reported which arise from several mutations in the four open-reading frames (ORFs) of HBV genome (pol, pre-S1/pre-S2/S, pre-C/C and X).
The basal core promoter (BCP) mutations reported to be associated with fulminant hepatitis in HBV-mono-infection namely T1753C, A1762T, G1764A occur in X ORF leading to down-regulation of HBeAg by decreasing its mRNA synthesis. The BCP double mutations (A1762T/G1764A) and triple mutation (T1753C/A1762T/G1764A) could also be found in HIV infected patients from United States, Australia and Thailand[23,41]. But the frequency of A1762T and G1764A was lower in HIV-HBV co-infected individuals as compared to those with HBV mono-infection (39.8% vs 59.3% and 39.8% vs 61% respectively)[41]. Moreover, presence of HBV precore stop codon mutation was reported in the co-infected cohort from different parts of the world though prevalence of G1896A (W28Stop) varied among these studies[23,38,39]. Among HIV-HBV co-infected patients, a novel -1G deletion mutation in precore/core region of HBV was reported[40]. This mutation was found to be associated with genotype A and high HBV load. This mutation was suggested to be associated with altered pathogenesis in this population by two mechanisms - firstly, development of a premature stop codon and truncated pre-core/core protein might be responsible for increased viral load and secondly, stop codon in the MHC-class II restricted epitope might lead to immune escape. In the same study, in addition to -1G mutation, substitutions in x gene, polymerase gene, precore/core gene and regulatory regions were also found[40]. Study by Audsley et al[41] supported the earlier result showing -1G frameshift mutation to be unique for HIV-HBV co-infected patients (10.8%). The only report[43] demonstrating the molecular epidemiology of HIV-HBV co-infected individuals from eastern India also found these BCP, precore/core mutations however prevalence of these mutations varies from the worldwide reports. In this Indian cohort lower frequency of A1762T/G1764A (13.6%) and-1G mutation (1.75%) were found, but the prevalence of G1896A is high (22%) as compared to the reports available from different parts of the globe[43]. This discrepancy could be explained by the high prevalence of HBV/D than HBV/A and HBV/C in India.
In a recent study analyzing complete HBV genome from HIV co-infected patients, pre-S2 deletion was more frequently found in pre-S1/pre-S2/S ORF among HIV-HBV co-infected individuals as compared to those infected with HBV only (14.6% vs 3.3%)[41].The majority of Pre-S2 deletions were located close to the N-terminus of the Pre-S2 protein. In contrast this deletion mutation is uncommon in eastern Indian cohort with HIV-HBV co-infection (5.41%)[43]. Some immune escape mutations (P120T/S and G145R/K/A) could also be found in context to HIV-HBV co-infection[38]. In the study from eastern India, low frequency of some immune escape mutations (Q129R, M133I/T, Y134N/H and G145RS) has been reported from the surface gene region of HBV genome[42,43]. Interestingly, in the upstream of “a” determinant region, a stop codon at C69 was found mainly in HBV/D2 isolates from these HBsAg positive HIV co-infected patients. This mutation was previously reported in Iranian HBV mono-infected patients with cirrhosis[51]. Though, the effect of this nonsense mutation remains unknown among HIV-HBV co-infected patients.
Besides spontaneous genetic variability, several diversities could be found in HBV genome, mainly in polymerase gene, under the selective pressure of nucles(t)ide analogues having anti-HBV activity. As a first line ART, lamivudine has been extensively used among HIV co-infected individuals. Benhamou et al[52] first estimated that after 4 years of lamivudine (3TC) therapy, 90% of a HIV-HBV co-infected cohort developed drug-resistant HBV which was higher compared to HBV mono-infected patients (67%). Furthermore, a later study showed increased frequency of double mutation (rt L180M + rt M204V) and triple mutation (rtV173L + rt L180M + rt M204V) during longer duration of 3TC therapy and they found 3TC resistance in 94% of the HIV-HBV co-infected patients experiencing 3TC for > 4 years[23]. The high frequency of lamivudine-resistance associated with HIV-HBV co-infected patients could also be supported in several studies from different parts of the world so far[23,39,53-59]. Another adverse consequence of the 3TC-resistant triple mutation is that it generates vaccine escape mutation (E164D + I195M) in the overlapping surface gene region. Therefore, possibility to infect the unvaccinated as well as the HBV vaccinated persons makes this a serious health issue. In the earlier study of Pal et al[42], the presence of 3TC-resistant triple mutation was observed among the HIV-HBV co-infected patients from eastern India. In a recent study from same part of the India demonstrated the high incidence of 3TC-resistant double and triple mutation among HIV-HBV co-infected patients who had exposure of 3TC as a sole HBV-active agent during prolonged ART[60]. Studies on 3TC-resistance among HIV-HBV co-infected patients from different parts of the world found higher frequency of 3TC-resistant double mutation compared to triple mutation[23,39,53-57]. It is noteworthy to mention in this context that HIV-HBV co-infected patients from eastern India receiving long-term ART showed predominance of 3TC-resistant triple mutation over double mutation and the former prevailed in significantly higher frequency among HBV viraemic patients experiencing 3TC for ≥ 4 years [frequency of 3TC-resistant triple mutation (vaccine escape mutation): 60% vs double mutation: 10%][60]. Moreover these 3TC-resistance associated vaccine-escape HBV mutants showed the presence of liver damages in these HIV-HBV co-infected patients. This finding by Pal et al[60] underscored the urgent need to study the overall burden of 3TC-resistant mutations in HIV-HBV co-infected Indian pool for proper management of 3TC-resistant mutants of HBV from clinical and public health perspectives in this country. Considering the adverse effects of 3TC-monotherapy, use of combination therapy using tenofovir has been introduced by World health organisation worldwide and National AIDS Control Organization in India for the management of HBV among HIV co-infected individuals[61]. Among other drugs used for treatment of HBV, development of drug resistance have been reported for adefovir and entacavir among HIV-HBV co-infected patients[62,63], however tenofovir resistance could not be detected among this population. So far, Tenofovir has been reported to suppress HBV DNA even in presence of lamivudine resistance and thus is recommended for the treatment of HBV infection in setting of HIV co-infection[64]. Use of tenofovir has been started in India among HIV-HBV co-infected patients from 2012[61], however the evaluation on treatment response for HBV during tenofovir treatment is still missing.
Besides the genotypic and phenotypic diversity, modulation in HIV-associated immune status could not be overlooked. HIV-HBV co-infected subjects were mostly associated with lower CD4+ T-cell count as compared to HIV mono-infected ones[25]. This observation indicates towards the potential effect of HIV related immune dysfunction on HBV diversity as well as in the clinical outcome of HBV infection among HIV-positive patients. Few available reports showed interesting findings. The study by Pal et al[42] highlighted the influence of HIV induced immune modulation on the genetic heterogeneity of HBV among HIV-HBV co-infected patients from eastern India. Here a trend of negative association between the frequency of the HBV/D2, the predominant HBV subgenotype, isolates and CD4+ T cell counts was found. The HBV/D2 isolates showed decreased genetic diversity in low CD4+ T cell count group which in turn was attributed to increased HBV viremia and favourable selection of HBV/D2 isolates in HIV induced low immune pressure. Moreover, increased non-synonymous substitutions with increase in CD4+ T cell count in this study underscored the possibility that ART induced immune reconstitution might lead to the development of vaccine/immune escape and lamivudine resistant mutations among HBV/D2 infected patients. In contrast to HBV/D2, interestingly in HBV/A1 genetic variability was modified differently in presence of HIV. This contrasting substitution pattern with varying immune suppression between HBV/A1 and HBV/D2 was proposed to be related to the differences in host immune response against these two subgenotypes. An earlier study from Argentina showed that as a consequence of lower CD4+ T cell count, HBV subjects from HIV co-infected patients had low quasispecies diversity as well as evolutionary rate when compared to that from HBV mono-infected patients[65].
Another study reported the association of HBV serological outcome with CD4+ T-cell count. Landrum et al[66] showed increased proportion of chronic HBV infection in patients with CD4+ T-cell count < 200 cells/mm3 (19%) compared to those with ≥ 500 cells/mm3 (11%) and 200-499 cells/mm3 (16%). Individuals with HBV infection occurring after HIV diagnosis had high risk of chronic HBV infection and also this risk reduced after initiation of highly active antiretroviral therapy. However, conformation of these findings is missing due to limited studies in the respective fields.
In India, HIV and HBV mono-infection have been studied thoroughly from different parts of the country[67-89]. These reports represent the subcontinent as a region epidemic of HIV infection and intermediately endemic to HBV infection. But, “HIV-HBV co-infection in India” remains unexplored even after knowing the epidemiological trends of these virus and adverse effects of HIV on outcome of HBV infection in setting of co-infection. In comparison to the global scenario of researches on HIV-HBV co-infection, information from India is scanty and thus need investigations in this field (Figure 1). Few studies from eastern India have shown some interesting findings highlighting the need for the studies from the different parts of this country to get the national scenario on the whole. The foremost requirement is to elucidate the overall burden of HIV-HBV co-infection in India, not only the prevalence of chronic HBV infection but the rate of prior infection should be studied to know its threat level among HIV infected population of India. To fulfill this aim, multi-centre study with different risk groups across the country should be included. Besides epidemiological studies, characterization of virological parameters (HBV genotype/subgenotype distribution, their association with degrees of immunosuppression, HBeAg status) and clinical aspects (ALT, fibrosis stage) also needs attention. Moreover, full genome sequencing of HBV from HIV co-infected Indian population is required to get results whether genetic diversity from this cohort shows concordance with worldwide reports and to screen for the possible presence of any unusual mutations. Data obtained from HIV-HBV co-infected patients from eastern India, i.e., effect of HIV on genetic heterogeneity of HBV also needs conformation from the other parts of this country. Another important aspect for future research includes the study on response of anti-HBV treatment among HIV-HBV co-infected patients of India in this ART era. This requires the evaluation on the incidence of drug resistant mutations, follow-up studies to elucidate its clinical and treatment outcome on combination therapy. Taken together, research on HIV-HBV co-infection in India could lead to better understanding of this global health problem which would explore the scenario to the rest of the world. Finally this will help to develop the strategy for proper management of HIV-HBV co-infection in Indian population.
P- Reviewer: Datta S, Tetsuya T S- Editor: Ji FF L- Editor: A E- Editor: Yan JL
| 1. | Price JC, Thio CL. Liver disease in the HIV-infected individual. Clin Gastroenterol Hepatol. 2010;8:1002-1012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 148] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 2. | Alter MJ. Epidemiology of viral hepatitis and HIV co-infection. J Hepatol. 2006;44:S6-S9. [PubMed] |
| 3. | Thio CL. Hepatitis B and human immunodeficiency virus coinfection. Hepatology. 2009;49:S138-S145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 233] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 4. | Bodsworth NJ, Cooper DA, Donovan B. The influence of human immunodeficiency virus type 1 infection on the development of the hepatitis B virus carrier state. J Infect Dis. 1991;163:1138-1140. [PubMed] |
| 5. | Gilson RJ, Hawkins AE, Beecham MR, Ross E, Waite J, Briggs M, McNally T, Kelly GE, Tedder RS, Weller IV. Interactions between HIV and hepatitis B virus in homosexual men: effects on the natural history of infection. AIDS. 1997;11:597-606. [PubMed] |
| 6. | Colin JF, Cazals-Hatem D, Loriot MA, Martinot-Peignoux M, Pham BN, Auperin A, Degott C, Benhamou JP, Erlinger S, Valla D. Influence of human immunodeficiency virus infection on chronic hepatitis B in homosexual men. Hepatology. 1999;29:1306-1310. [PubMed] |
| 7. | Konopnicki D, Mocroft A, de Wit S, Antunes F, Ledergerber B, Katlama C, Zilmer K, Vella S, Kirk O, Lundgren JD. Hepatitis B and HIV: prevalence, AIDS progression, response to highly active antiretroviral therapy and increased mortality in the EuroSIDA cohort. AIDS. 2005;19:593-601. [PubMed] |
| 8. | Piroth L, Sène D, Pol S, Goderel I, Lacombe K, Martha B, Rey D, Loustau-Ratti V, Bergmann JF, Pialoux G. Epidemiology, diagnosis and treatment of chronic hepatitis B in HIV-infected patients (EPIB 2005 STUDY). AIDS. 2007;21:1323-1331. [PubMed] |
| 9. | Chun HM, Roediger MP, Hullsiek KH, Thio CL, Agan BK, Bradley WP, Peel SA, Jagodzinski LL, Weintrob AC, Ganesan A. Hepatitis B virus coinfection negatively impacts HIV outcomes in HIV seroconverters. J Infect Dis. 2012;205:185-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 96] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 10. | Thio CL, Seaberg EC, Skolasky R, Phair J, Visscher B, Muñoz A, Thomas DL. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS). Lancet. 2002;360:1921-1926. [PubMed] |
| 11. | Sellier P, Schnepf N, Jarrin I, Mazeron MC, Simoneau G, Parrinello M, Evans J, Lafuente-Lafuente C. Description of liver disease in a cohort of HIV/HBV coinfected patients. J Clin Virol. 2010;47:13-17. [PubMed] |
| 12. | Núñez M, Soriano V. Management of patients co-infected with hepatitis B virus and HIV. Lancet Infect Dis. 2005;5:374-382. [PubMed] |
| 13. | Kellerman SE, Hanson DL, McNaghten AD, Fleming PL. Prevalence of chronic hepatitis B and incidence of acute hepatitis B infection in human immunodeficiency virus-infected subjects. J Infect Dis. 2003;188:571-577. [PubMed] |
| 14. | Cooley L, Sasadeusz J. Clinical and virological aspects of hepatitis B co-infection in individuals infected with human immunodeficiency virus type-1. J Clin Virol. 2003;26:185-193. [PubMed] |
| 15. | Chun HM, Fieberg AM, Hullsiek KH, Lifson AR, Crum-Cianflone NF, Weintrob AC, Ganesan A, Barthel RV, Bradley WP, Agan BK. Epidemiology of Hepatitis B virus infection in a US cohort of HIV-infected individuals during the past 20 years. Clin Infect Dis. 2010;50:426-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | Soriano V, Mocroft A, Peters L, Rockstroh J, Antunes F, Kirkby N, de Wit S, Monforte Ad, Flisiak R, Lundgren J. Predictors of hepatitis B virus genotype and viraemia in HIV-infected patients with chronic hepatitis B in Europe. J Antimicrob Chemother. 2010;65:548-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Pérez Cachafeiro S, Caro-Murillo AM, Berenguer J, Segura F, Gutierrez F, Vidal F, Martinez-Perez MA, Sola J, Muga R, Moreno S. Association of Patients’ Geographic Origins with Viral Hepatitis Co-infection Patterns, Spain. Emerg Infect Dis. 2011;17:1116-1119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Hoffmann CJ, Thio CL. Clinical implications of HIV and hepatitis B co-infection in Asia and Africa. Lancet Infect Dis. 2007;7:402-409. [PubMed] |
| 20. | Harania RS, Karuru J, Nelson M, Stebbing J. HIV, hepatitis B and hepatitis C coinfection in Kenya. AIDS. 2008;22:1221-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Idoko J, Meloni S, Muazu M, Nimzing L, Badung B, Hawkins C, Sankalé JL, Ekong E, Murphy R, Kanki P. Impact of hepatitis B virus infection on human immunodeficiency virus response to antiretroviral therapy in Nigeria. Clin Infect Dis. 2009;49:1268-1273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 22. | Burnett RJ, François G, Kew MC, Leroux-Roels G, Meheus A, Hoosen AA, Mphahlele MJ. Hepatitis B virus and human immunodeficiency virus co-infection in sub-Saharan Africa: a call for further investigation. Liver Int. 2005;25:201-213. [PubMed] |
| 23. | Matthews GV, Bartholomeusz A, Locarnini S, Ayres A, Sasaduesz J, Seaberg E, Cooper DA, Lewin S, Dore GJ, Thio CL. Characteristics of drug resistant HBV in an international collaborative study of HIV-HBV-infected individuals on extended lamivudine therapy. AIDS. 2006;20:863-870. [PubMed] |
| 24. | Thibault V, Gaudy-Graffin C, Colson P, Gozlan J, Schnepf N, Trimoulet P, Pallier C, Saune K, Branger M, Coste M. Epidemiological, virological and clinical characteristics of HBV infection in 223 HIV co-infected patients: a French multi-centre collaborative study. Virol J. 2013;10:87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Thio CL, Smeaton L, Saulynas M, Hwang H, Saravanan S, Kulkarni S, Hakim J, Nyirenda M, Iqbal HS, Lalloo UG. Characterization of HIV-HBV coinfection in a multinational HIV-infected cohort. AIDS. 2013;27:191-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (1)] |
| 26. | Tankhiwale SS, Khadase RK, Jalgoankar SV. Seroprevalence of anti-HCV and hepatitis B surface antigen in HIV infected patients. Indian J Med Microbiol. 2003;21:268-270. [PubMed] |
| 27. | Solomon SS, Srikrishnan AK, Mehta SH, Vasudevan CK, Murugavel KG, Thamburaj E, Anand S, Kumar MS, Latkin C, Solomon S. High prevalence of HIV, HIV/hepatitis C virus coinfection, and risk behaviors among injection drug users in Chennai, India: a cause for concern. J Acquir Immune Defic Syndr. 2008;49:327-332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 78] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 28. | Sekar R, Amudhan M, Sivashankar M, Mythreyee M. Higher prevalence of sexually transmissible co-infections among the human immunodeficiency virus-infected population of South India. J Med Microbiol. 2011;60:394-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 29. | Saravanan S, Velu V, Kumarasamy N, Nandakumar S, Murugavel KG, Balakrishnan P, Suniti S, Thyagarajan SP. Coinfection of hepatitis B and hepatitis C virus in HIV-infected patients in south India. World J Gastroenterol. 2007;13:5015-5020. [PubMed] |
| 30. | Praseeda S D, Anuradha D, Jayanthi S S. A Study on the HBV and the HCV Infections in Female Sex Workers and their Co-Infection with HIV. J Clin Diagn Res. 2013;7:234-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Saha D, Pal A, Biswas A, Panigrahi R, Sarkar N, Sarkar J, Pal M, Guha SK, Saha B, Chakrabarti S. Characterization of treatment-naive HIV/HBV co-infected patients attending ART clinic of a tertiary healthcare centre in eastern India. PLoS One. 2013;8:e73613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 32. | Sud A, Singh J, Dhiman RK, Wanchu A, Singh S, Chawla Y. Hepatitis B virus co-infection in HIV infected patients. Trop Gastroenterol. 2001;22:90-92. [PubMed] |
| 33. | Gupta S, Singh S. Hepatitis B and C virus co-infections in human immunodeficiency virus positive North Indian patients. World J Gastroenterol. 2006;12:6879-6883. [PubMed] |
| 34. | Hussain T, Kulshreshtha KK, Sinha S, Yadav VS, Katoch VM. HIV, HBV, HCV, and syphilis co-infections among patients attending the STD clinics of district hospitals in Northern India. Int J Infect Dis. 2006;10:358-363. [PubMed] |
| 35. | Tripathi AK, Khanna M, Gupta N, Chandra M. Low prevalence of hepatitis B virus and hepatitis C virus co-infection in patients with human immunodeficiency virus in Northern India. J Assoc Physicians India. 2007;55:429-431. [PubMed] |
| 36. | Jindal N, Arora U, Singh K. Prevalence of human immunodeficiency virus (HIV), hepatitis B virus, and hepatitis C virus in three groups of populations at high risk of HIV infection in Amritsar (Punjab), Northern India. Jpn J Infect Dis. 2008;61:79-81. [PubMed] |
| 37. | Jain M, Chakravarti A, Verma V, Bhalla P. Seroprevalence of hepatitis viruses in patients infected with the human immunodeficiency virus. Indian J Pathol Microbiol. 2009;52:17-19. [PubMed] |
| 38. | Lacombe K, Massari V, Girard PM, Serfaty L, Gozlan J, Pialoux G, Mialhes P, Molina JM, Lascoux-Combe C, Wendum D. Major role of hepatitis B genotypes in liver fibrosis during coinfection with HIV. AIDS. 2006;20:419-427. [PubMed] |
| 39. | Ramos B, Núñez M, Martín-Carbonero L, Sheldon J, Rios P, Labarga P, Romero M, Barreiro P, García-Samaniego J, Soriano V. Hepatitis B virus genotypes and lamivudine resistance mutations in HIV/hepatitis B virus-coinfected patients. J Acquir Immune Defic Syndr. 2007;44:557-561. [PubMed] |
| 40. | Revill PA, Littlejohn M, Ayres A, Yuen L, Colledge D, Bartholomeusz A, Sasaduesz J, Lewin SR, Dore GJ, Matthews GV. Identification of a novel hepatitis B virus precore/core deletion mutant in HIV/hepatitis B virus co-infected individuals. AIDS. 2007;21:1701-1710. [PubMed] |
| 41. | Audsley J, Littlejohn M, Yuen L, Sasadeusz J, Ayres A, Desmond C, Spelman T, Lau G, Matthews GV, Avihingsanon A. HBV mutations in untreated HIV-HBV co-infection using genomic length sequencing. Virology. 2010;405:539-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 42. | Pal A, Panigrahi R, Biswas A, Datta S, Sarkar N, Guha SK, Saha B, Banerjee A, Chakrabarti S, Chakravarty R. Influence of HIV-associated degree of immune suppression on molecular heterogeneity of hepatitis B virus among HIV co-infected patients. Virology. 2013;436:134-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 43. | Saha D, Pal A, Biswas A, Panigrahi R, Sarkar N, Das D, Sarkar J, Guha SK, Saha B, Chakrabarti S. Molecular characterization of HBV strains circulating among the treatment-naive HIV/HBV co-infected patients of eastern India. PLoS One. 2014;9:e90432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 44. | Lin CL, Kao JH. The clinical implications of hepatitis B virus genotype: Recent advances. J Gastroenterol Hepatol. 2011;26 Suppl 1:123-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 279] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 45. | Lacombe K, Bottero J, Lemoine M, Boyd A, Girard PM. HIV/hepatitis B virus co-infection: current challenges and new strategies. J Antimicrob Chemother. 2010;65:10-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 46. | Mata Marín JA, Arroyo Anduiza CI, Calderón GM, Cazares Rodríguez S, Fuentes Allen JL, Arias Flores R, Gaytán Martínez J. Prevalence and resistance pattern of genotype G and H in chronic hepatitis B and HIV co-infected patients in Mexico. Ann Hepatol. 2012;11:47-51. [PubMed] |
| 47. | Datta S, Banerjee A, Chandra PK, Mahapatra PK, Chakrabarti S, Chakravarty R. Drug trafficking routes and hepatitis B in injection drug users, Manipur, India. Emerg Infect Dis. 2006;12:1954-1957. [PubMed] |
| 48. | Martin CM, Welge JA, Blackard JT. Hepatitis B virus (HBV) X gene diversity and evidence of recombination in HBV/HIV co-infected persons. J Med Virol. 2011;83:1142-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 49. | Fallot G, Halgand B, Garnier E, Branger M, Gervais A, Roque-Afonso AM, Thiers V, Billaud E, Matheron S, Samuel D. Recombination of hepatitis B virus DNA in patients with HIV. Gut. 2012;61:1197-1208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 50. | Araujo NM, Araujo OC, Silva EM, Villela-Nogueira CA, Nabuco LC, Parana R, Bessone F, Gomes SA, Trepo C, Kay A. Identification of novel recombinants of hepatitis B virus genotypes F and G in human immunodeficiency virus-positive patients from Argentina and Brazil. J Gen Virol. 2013;94:150-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 51. | Veazjalali M, Norder H, Magnius L, Jazayeri SM, Alavian SM, Mokhtari-Azad T. A new core promoter mutation and premature stop codon in the S gene in HBV strains from Iranian patients with cirrhosis. J Viral Hepat. 2009;16:259-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 52. | Benhamou Y, Bochet M, Thibault V, Di Martino V, Caumes E, Bricaire F, Opolon P, Katlama C, Poynard T. Long-term incidence of hepatitis B virus resistance to lamivudine in human immunodeficiency virus-infected patients. Hepatology. 1999;30:1302-1306. [PubMed] |
| 53. | Aghasadeghi MR, Bahramali G, Sadat SM, Farahani A, Mohraz M, Davar Siadat S, Mostafavi E, Memarnejadian A, Ardestani MS, Vahabpour R. Detection of hepatitis B virus variants in HBV monoinfected and HBV/HIV coinfected Iranian patients under lamivudine treatment. Curr HIV Res. 2011;9:263-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 54. | Kouanfack C, Aghokeng AF, Mondain AM, Bourgeois A, Kenfack A, Mpoudi-Ngolé E, Ducos J, Delaporte E, Laurent C. Lamivudine-resistant HBV infection in HIV-positive patients receiving antiretroviral therapy in a public routine clinic in Cameroon. Antivir Ther. 2012;17:321-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 55. | Bottecchia M, Souto FJ, O KM, Amendola M, Brandão CE, Niel C, Gomes SA. Hepatitis B virus genotypes and resistance mutations in patients under long term lamivudine therapy: characterization of genotype G in Brazil. BMC Microbiol. 2008;8:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 56. | Mendes-Correa MC, Pinho JR, Locarnini S, Yuen L, Sitnik R, Santana RA, Gomes-Gouvêa MS, Leite OM, Martins LG, Silva MH. High frequency of lamivudine resistance mutations in Brazilian patients co-infected with HIV and hepatitis B. J Med Virol. 2010;82:1481-1488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 57. | Sheldon J, Ramos B, Garcia-Samaniego J, Rios P, Bartholomeusz A, Romero M, Locarnini S, Zoulim F, Soriano V. Selection of hepatitis B virus (HBV) vaccine escape mutants in HBV-infected and HBV/HIV-coinfected patients failing antiretroviral drugs with anti-HBV activity. J Acquir Immune Defic Syndr. 2007;46:279-282. [PubMed] |
| 58. | Iacomi F, Vincenti D, Vairo F, Solmone M, Mariano A, Piselli P, Puro V, Capobianchi MR, Antonucci G. Effect of HIV co-infection on mutation patterns of HBV in patients with lamivudine-resistant chronic hepatitis B. J Med Virol. 2009;81:1151-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 59. | Taramasso L, Caligiuri P, Di Biagio A, Bruzzone B, Rosso R, Icardi G, Viscoli C. Lamivudine resistance mutations in European patients with hepatitis B and patients co-infected with HIV and hepatitis B. J Med Virol. 2011;83:1905-1908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 60. | Pal A, Sarkar N, Saha D, Guha SK, Saha B, Chakrabarti S, Chakravarty R. High incidence of lamivudine-resistance associated vaccine-escape HBV mutant among HIV-coinfected patients on prolonged antiretroviral therapy. Antivir Ther. 2015;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 61. | Antiretroviral Therapy Guidelines for HIV-infected Adults and Adolescents May 2013. Department of AIDS Control National AIDS Control Organisation Ministry of Helath & Family Welfare Government of India. [accessed. 2014;Oct 17] Available from: http://www.naco.gov.in/upload/Policies & Guidelines/Antiretroviral Therapy Guidelines for HIV-Infected Adults and Adolescents.pdf. |
| 62. | Benhamou Y, Thibault V, Vig P, Calvez V, Marcelin AG, Fievet MH, Currie G, Chang CG, Biao L, Xiong S. Safety and efficacy of adefovir dipivoxil in patients infected with lamivudine-resistant hepatitis B and HIV-1. J Hepatol. 2006;44:62-67. [PubMed] |
| 63. | Pessôa MG, Gazzard B, Huang AK, Brandão-Mello CE, Cassetti I, Mendes-Corrêa MC, Soriano V, Phiri P, Hall A, Brett-Smith H. Efficacy and safety of entecavir for chronic HBV in HIV/HBV coinfected patients receiving lamivudine as part of antiretroviral therapy. AIDS. 2008;22:1779-1787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 64. | Price H, Dunn D, Pillay D, Bani-Sadr F, de Vries-Sluijs T, Jain MK, Kuzushita N, Mauss S, Núñez M, Nüesch R. Suppression of HBV by tenofovir in HBV/HIV coinfected patients: a systematic review and meta-analysis. PLoS One. 2013;8:e68152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 65. | Cassino L, Torres C, Mbayed V, Laufer N, Campos RH, Quarleri J. Comparative analysis of hepatitis B virus genotype a molecular evolution in patients infected with HBV and in patients co-infected with HBV and HIV. J Med Virol. 2012;84:562-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 66. | Landrum ML, Fieberg AM, Chun HM, Crum-Cianflone NF, Marconi VC, Weintrob AC, Ganesan A, Barthel RV, Wortmann G, Agan BK. The effect of human immunodeficiency virus on hepatitis B virus serologic status in co-infected adults. PLoS One. 2010;5:e8687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 67. | Banerjee A, Banerjee S, Chowdhury A, Santra A, Chowdhury S, Roychowdhury S, Panda CK, Bhattacharya SK, Chakravarty R. Nucleic acid sequence analysis of basal core promoter/precore/core region of hepatitis B virus isolated from chronic carriers of the virus from Kolkata, eastern India: low frequency of mutation in the precore region. Intervirology. 2005;48:389-399. [PubMed] |
| 68. | Banerjee A, Datta S, Chandra PK, Roychowdhury S, Panda CK, Chakravarty R. Distribution of hepatitis B virus genotypes: phylogenetic analysis and virological characteristics of genotype C circulating among HBV carriers in Kolkata, Eastern India. World J Gastroenterol. 2006;12:5964-5971. [PubMed] |
| 69. | Banerjee A, Kurbanov F, Datta S, Chandra PK, Tanaka Y, Mizokami M, Chakravarty R. Phylogenetic relatedness and genetic diversity of hepatitis B virus isolates in Eastern India. J Med Virol. 2006;78:1164-1174. [PubMed] |
| 70. | Gandhe SS, Chadha MS, Arankalle VA. Hepatitis B virus genotypes and serotypes in western India: lack of clinical significance. J Med Virol. 2003;69:324-330. [PubMed] |
| 71. | Kumar A, Kumar SI, Pandey R, Naik S, Aggarwal R. Hepatitis B virus genotype A is more often associated with severe liver disease in northern India than is genotype D. Indian J Gastroenterol. 2005;24:19-22. [PubMed] |
| 72. | Thakur V, Guptan RC, Kazim SN, Malhotra V, Sarin SK. Profile, spectrum and significance of HBV genotypes in chronic liver disease patients in the Indian subcontinent. J Gastroenterol Hepatol. 2002;17:165-170. [PubMed] |
| 73. | Vivekanandan P, Abraham P, Sridharan G, Chandy G, Shaji RV, Daniel D, Raghuraman S, Daniel HD, Subramaniam T. High frequency of the 1896 precore mutation in patients and blood donors with hepatitis B virus infection from the Indian subcontinent. Mol Diagn. 2004;8:51-56. [PubMed] |
| 74. | Vivekanandan P, Abraham P, Sridharan G, Chandy G, Daniel D, Raghuraman S, Daniel HD, Subramaniam T. Distribution of hepatitis B virus genotypes in blood donors and chronically infected patients in a tertiary care hospital in southern India. Clin Infect Dis. 2004;38:e81-e86. [PubMed] |
| 75. | Chandra PK, Biswas A, Datta S, Banerjee A, Panigrahi R, Chakrabarti S, De BK, Chakravarty R. Subgenotypes of hepatitis B virus genotype D (D1, D2, D3 and D5) in India: differential pattern of mutations, liver injury and occult HBV infection. J Viral Hepat. 2009;16:749-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 76. | Chauhan R, Kazim SN, Bhattacharjee J, Sakhuja P, Sarin SK. Basal core promoter, precore region mutations of HBV and their association with e antigen, genotype, and severity of liver disease in patients with chronic hepatitis B in India. J Med Virol. 2006;78:1047-1054. [PubMed] |
| 77. | Jameel S, Zafrullah M, Ahmad M, Kapoor GS, Sehgal S. A genetic analysis of HIV-1 from Punjab, India reveals the presence of multiple variants. AIDS. 1995;9:685-690. [PubMed] |
| 78. | Lole KS, Bollinger RC, Paranjape RS, Gadkari D, Kulkarni SS, Novak NG, Ingersoll R, Sheppard HW, Ray SC. Full-length human immunodeficiency virus type 1 genomes from subtype C-infected seroconverters in India, with evidence of intersubtype recombination. J Virol. 1999;73:152-160. [PubMed] |
| 79. | Maitra A, Singh B, Banu S, Deshpande A, Robbins K, Kalish ML, Broor S, Seth P. Subtypes of HIV type 1 circulating in India: partial envelope sequences. AIDS Res Hum Retroviruses. 1999;15:941-944. [PubMed] |
| 80. | Siddappa NB, Dash PK, Mahadevan A, Jayasuryan N, Hu F, Dice B, Keefe R, Satish KS, Satish B, Sreekanthan K. Identification of subtype C human immunodeficiency virus type 1 by subtype-specific PCR and its use in the characterization of viruses circulating in the southern parts of India. J Clin Microbiol. 2004;42:2742-2751. [PubMed] |
| 81. | Deshpande A, Recordon-Pinson P, Deshmukh R, Faure M, Jauvin V, Garrigue I, Lafon ME, Fleury HJ. Molecular characterization of HIV type 1 isolates from untreated patients of Mumbai (Bombay), India, and detection of rare resistance mutations. AIDS Res Hum Retroviruses. 2004;20:1032-1035. [PubMed] |
| 82. | Mandal D, Jana S, Bhattacharya SK, Chakrabarti S. HIV type 1 subtypes circulating in eastern and northeastern regions of India. AIDS Res Hum Retroviruses. 2002;18:1219-1227. [PubMed] |
| 83. | Bhanja P, Sengupta S, Banerjee D, Sarkar K, Jana S, Chakrabarti S. Detection of intersubtype recombinants with respect to env and nef genes of HIV-1 among female sex workers in Calcutta, India. Virus Res. 2007;130:310-314. [PubMed] |
| 84. | Sarkar R, Pal R, Bal B, Mullick R, Sengupta S, Sarkar K, Chakrabarti S. Genetic Characterization of HIV-1 Strains Among the Injecting Drug Users in Nagaland, India. Open Virol J. 2011;5:96-102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 85. | Sarkar R, Sarkar K, Singh NB, Singh YM, Chakrabarti S. Near full-length genomic characterization of a HIV type 1 BC recombinant strain from Manipur, India. Virus Genes. 2012;45:201-206. [PubMed] |
| 86. | Sarkar R, Sarkar K, Brajachand Singh N, Manihar Singh Y, Mitra D, Chakrabarti S. Emergence of a unique recombinant form of HIV-1 from Manipur (India). J Clin Virol. 2012;55:274-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 87. | Sarkar R, Sengupta S, Mullick R, Singh NB, Sarkar K, Chakrabarti S. Implementation of a multiregion hybridization assay to characterize HIV-1 strains detected among injecting drug users in Manipur, India. Intervirology. 2009;52:175-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 88. | Biswas A, Panigrahi R, Banerjee A, Pal M, De BK, Chakrabarti S, Chakravarty R. Differential pattern of pre-S mutations/deletions and its association with hepatitis B virus genotypes in Eastern India. Infect Genet Evol. 2012;12:384-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 89. | Panigrahi R, Biswas A, De BK, Chakrabarti S, Chakravarty R. Characterization of antiviral resistance mutations among the Eastern Indian Hepatitis B virus infected population. Virol J. 2013;10:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 90. | Chang JJ, Wightman F, Bartholomeusz A, Ayres A, Kent SJ, Sasadeusz J, Lewin SR. Reduced hepatitis B virus (HBV)-specific CD4+ T-cell responses in human immunodeficiency virus type 1-HBV-coinfected individuals receiving HBV-active antiretroviral therapy. J Virol. 2005;79:3038-3051. [PubMed] |
| 91. | Chang JJ, Sirivichayakul S, Avihingsanon A, Thompson AJ, Revill P, Iser D, Slavin J, Buranapraditkun S, Marks P, Matthews G. Impaired quality of the hepatitis B virus (HBV)-specific T-cell response in human immunodeficiency virus type 1-HBV coinfection. J Virol. 2009;83:7649-7658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 92. | Salmon-Ceron D, Rosenthal E, Lewden C, Bouteloup V, May T, Burty C, Bonnet F, Costagliola D, Jougla E, Semaille C. Emerging role of hepatocellular carcinoma among liver-related causes of deaths in HIV-infected patients: The French national Mortalité 2005 study. J Hepatol. 2009;50:736-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 80] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 93. | Iser DM, Avihingsanon A, Wisedopas N, Thompson AJ, Boyd A, Matthews GV, Locarnini SA, Slavin J, Desmond PV, Lewin SR. Increased intrahepatic apoptosis but reduced immune activation in HIV-HBV co-infected patients with advanced immunosuppression. AIDS. 2011;25:197-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |