Published online Mar 25, 2025. doi: 10.5501/wjv.v14.i1.100489
Revised: October 1, 2024
Accepted: October 28, 2024
Published online: March 25, 2025
Processing time: 102 Days and 1.1 Hours
Arboviral diseases are viral infections transmitted to humans through the bites of arthropods, such as mosquitoes, often causing a variety of pathologies associated with high levels of morbidity and mortality. Over the past decades, these in
Core Tip: This review delves into the historical characteristics, pathogenesis, and clinical management of the four major arboviruses that have triggered outbreaks worldwide: Dengue, Zika, yellow fever, and chikungunya fever. It aims to elucidate the viral characteristics, cellular tropism, and immune evasion mechanisms, as well as the primary clinical manifestations and their complications, laboratory diagnosis, treatment, prevention, and vaccines either currently available or under development. Thus, with a focus on the medical and scientific fields, this review enables the reader to acquire comprehensive and generalized knowledge about each of these arboviruses.
- Citation: Cenci Dietrich V, Costa JMC, Oliveira MMGL, Aguiar CEO, Silva LGO, Luz MS, Lemos FFB, de Melo FF. Pathogenesis and clinical management of arboviral diseases. World J Virol 2025; 14(1): 100489
- URL: https://www.wjgnet.com/2220-3249/full/v14/i1/100489.htm
- DOI: https://dx.doi.org/10.5501/wjv.v14.i1.100489
Arboviruses are an extensive group of viruses that have arthropods (insects and arachnids) as their primary vectors, transmitting the viruses to vertebrate hosts, such as humans, through their bites along with their saliva[1]. These infections are endemic to tropical and subtropical regions, where approximately 3.9 billion people live today, disproportionately affecting the poorest populations[2]. However, with rising temperatures due to global warming, there is a greater spread of the main urban vectors of arboviruses worldwide, such as mosquitoes of the genus Aedes [Aedes aegypti
Currently, the main arboviruses with the potential to cause worldwide outbreaks are the dengue virus (DENV), with an estimated 96 million symptomatic cases and 40000 annual deaths in more than 129 countries[2], in addition to the Zika virus (ZIKV), the yellow fever (YFV) virus, and the chikungunya (CHIKV) virus (viruses which also have the capacity to generate morbidity and mortality). All these diseases have epidemic potential, as evidenced by their significant global outbreaks in the last decades[7,8].
According to the World Health Organization (WHO), arboviruses are responsible for 17% of all infectious diseases and cause approximately 700000 deaths annually worldwide[2], directly affecting the healthcare systems of developing and even developed countries. These diseases can overcrowd hospitals during outbreak periods and cause chronic complications in infected patients, leading to increased healthcare costs and considerable social damages. These impacts are further exacerbated by the limited knowledge about the viral characteristics and the suitable clinical management. Thus, this article aims to elucidate the pathogenic mechanisms of each of the four main arboviruses, with the intention of understanding aspects related to viral characteristics, tropism, immune response, and viral evasion, as well as the main symptoms, possible complications, treatment, vaccines, and prevention, highlighting the main points of appropriate patients’ clinical management.
DENV is a positive-sense single-stranded RNA virus that belongs to the Flavivirus genus within the Flaviviridae family. There are four known serotypes spread globally: DENV-1, DENV-2, DENV-3, and DENV-4. Its RNA encodes 10 proteins, including three structural: Capsid (C), membrane (prM/M), and envelope (E); and seven non-structural, associated with RNA replication: NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5[9,10].
DENV is primarily transmitted by Ae. aegypti and Ae. albopictus mosquitoes[11]. Initially, Ae. aegypti was thought to be the sole vector capable of causing significant outbreaks. However, recent research indicates that Ae. albopictus also plays a significant role in sustaining large outbreaks and has contributed to the resurgence of DENV, particularly in Southeast Asia[12]. Dengue fever, caused by DENV infection, is considered the most important mosquito-borne viral disease, spread globally[13], with 50-100 million people infected yearly[14]. Dengue incidence has started to notoriously increase since 1990[15], and numerous social and economic factors might explain this phenomenon[9], as well as global warming, seeing that it can contribute to the re-emergence of DENV by expanding the geographical range of mosquitoes and increasing infection rates[16].
Although most dengue cases present as mild or asymptomatic (anorexia, retro-orbital pain, myalgia and rash), approximately 5% progress to a more severe form[17], primarily attributed to immunological factors such as the cytokine storm and antibody-dependent enhancement[18], that cause an exacerbation of the inflammatory process, leading to increased vascular permeability and a risk for hemorrhage and shock[19], usually followed by persisting vomiting and abdominal pain, as well as other various warning signs[20]. Such severe cases are mostly seen in heterotypic secondary infections (infection with a different serotype) but can also occur primarily in children who inherit immunity from their mothers[21] correlating with an impaired adaptative immune response. Even though hard to predict, certain authors argue that elevated viremia is correlated with severity[22], while others refute this idea[23], and instead propose evaluating factors like viral NS1 protein levels or host cytokine expression[24].
This complex host-pathogen interaction occurs within the context of the DENV’s cyclical transmission process, involving human-mosquito and mosquito-human transmission. It is not very easy to predict sporadic outbreaks, as around 80% of DENV transmission occurs via asymptomatic hosts[25]. In this process, healthy mosquitoes acquire the virus by feeding on the blood of an infected host, and once the virus has infiltrated the vector’s tissues, it becomes infected for life, and capable of transmitting the virus[26]. When an infected vector feeds on a naive host’s blood, mos
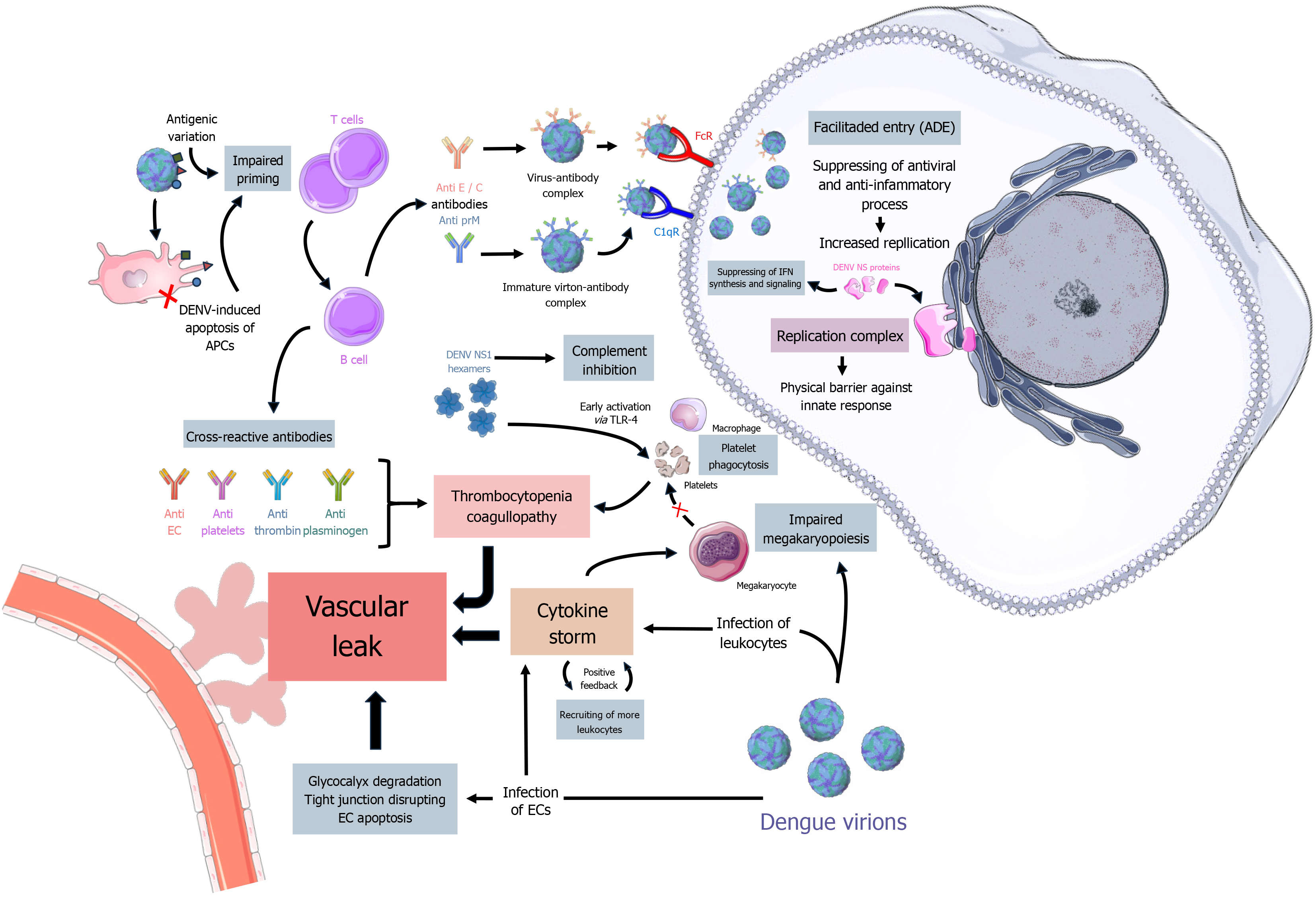
Viral entry, replication, and release: Upon injection into the skin, cells of the monocyte lineage, such as macrophages, Langerhans cells and dendritic cells are among the primary targets[27]. Other potential targets include endothelial and epithelial cells, lymphocytes, hepatocytes, fibroblasts and keratinocytes[28] with reports of viral presence in various tissues, including the dermis (skin), blood, bone marrow, lymph nodes, liver and occasionally, the brain[29]. Cellular internalization of DENV occurs through envelope protein (E) interaction with numerous different receptors, including heparan sulfate proteoglycans[30], DC-Sing[31], heat shock protein (HSP) 70 and HSP90[32], triggering receptor-mediated endocytosis, that can be dependent or independent of clathrin. Fc and C1q receptors can also facilitate entry via antibody-mediated pathway[33]. Additionally, alternative entry pathways such as diffusion and macropinocytosis have been reported. The specific entry route for DENV also varies depending on the virus serotype[34,35].
Subsequently, the virus’s E protein undergoes irreversible conformational changes due to the endosome’s low pH, leading to fusion with the endosomal membrane[36], facilitated by hydrophobic peptides[36,37]. Following its release, the viral genome acts as a mRNA and gets translated into a single polypeptide, later processed and cleaved by host and viral proteases, resulting in the ten viral proteins. The seven non-structural proteins form a replication complex through in
During this process, numerous viral mechanisms have been observed to take place in order to maximize replication, including reprogramming protein synthesis in host cells to favor viral RNA translation[41], regulating cellular apoptosis through interaction between NS5 protein and the mechanistic target of rapamycin complex 2[42], binding between NS1 and Beclin 1[43], and maximizing ATP production by promoting anaerobic glycolysis via the hypoxic response[44] or activating lipophagy to enhance β-oxidation[45].
Once enough proteins are synthesized and the virion is assembled, it is again processed by the Golgi apparatus changing its surface from spiky to smooth[9,46]. Then, mature virions are secreted from the infected cell along with NS1 hexamers, that play a crucial role in the disease pathogenesis and severity[47].
Innate response and evasion: The innate immune response to DENV initiates upon the first cell infection and progresses concurrently with viral replication and release, rather than following these events[48]. Not surprisingly, the virus has developed multiple strategies to evade the host’s innate response, primarily through its non-structural proteins[49].
When a dengue virion infects its initial target host cells, it activates pattern recognition receptors (PRRs) such as retinoic acid-inducible gene 1 (RIG1), melanoma differentiation-associated protein 5 (MDA5), toll-like receptor 3 (TLR-3), and TLR-7[50]. This sets off the signaling cascade of the innate response, leading to the release of cytokines and ultimately resulting in the expression of type I and III interferons (IFNs), recruitment of additional monocytes, and activation of the complement system[49]. Meanwhile, infected dendritic and Langerhans cells migrate to the lymph nodes and present viral antigens, initiating the adaptive response[51]. If successful, the antiviral response will counteract viral replication and infection. However, DENV evades it through its non-structural proteins (NS1 to NS5), which can disrupt receptor signaling, IFN synthesis, and regulation pathways[52-55]. Additionally, DENV’s NS1 protein can inhibit the complement response by interacting with its protein complexes[56].
In a more passive way, DENV evades the immune response by forming the replication complex, that serves as a “barrier”[40], and by regulating cellular apoptosis, a common mechanism used by the immune system to suppress viral replication[57]. Furthermore, DENV’s capability of infecting defense cells is itself a mean of dysregulating the host’s antiviral response.
Despite its notable role in dengue pathogenesis, the innate response is not typically singled out as a factor in dengue severity, whereas the adaptive immune response has been pointed as the primary contributor, due to impaired immune responses against different serotypes[58].
Adaptative response and antibody-dependent enhancement: The adaptive immune response to DENV begins with antigen-presenting cells (APCs) presenting viral antigens to T cells in lymph nodes. This activation leads to differentiation of T lymphocytes into effector and memory T cells, providing long-term immunity against infections with the same serotype, but only short-term immunity against heterotypic infections[59]. DENV has shown capability of interacting with numerous factors regarding antigen-specific immunity, such as priming and activation of T and B cells[60], antibody production and neutralization properties, along with cytokine release[61]. The highlighted factors have a significant impact on the disease pathogenesis and will be further discussed.
DENV is able to impair T lymphocyte priming and activation either by antigenic variation or inducing apoptosis of APCs[62]. This leads to a compromised T cell response, regarding both CD8+ direct targeting of infected cells and CD4+ differentiation into helper T cells, the ladder being related to activation of B cells and antibody production. Anti-DENV neutralizing antibodies primarily target specific regions of the E and C proteins[63,64], and can also target the NS pro
Antibody-dependent enhancement occurs when antibodies bind to the virus but fail to neutralize it, forming an antibody-virus complex[67]. This phenomenon is exacerbated by the original antigenic sin, where antibodies from the primary infection are more prevalent than those from the secondary infection. The antibody-virus complex can more efficiently enter monocytes by interacting with Fc and C1q receptors and triggering endocytosis, enhancing viral re
Recent studies have also demonstrated the potentiality of autoimmunity in dengue pathogenesis. Anti-NS1 antibodies can interact with endothelial cells (ECs), triggering inflammatory cytokine signaling and cellular apoptosis[72], and autoantibodies targeting ECs and platelets have also been observed, correlating to the increased vascular permeability seen in secondary dengue infections[73,74].
Overall, the adaptive immune response is a significant factor in dengue severity during secondary infections, especially regarding the process of antibody-dependent enhancement. These events, related to the evasion of the adaptive response, ultimately result in increased inflammatory process and vascular permeability, which are the main causes of dengue severity.
Cytokine storm and vascular permeability: Cytokine release is a common physiological event in the immunological system[75]; however, the exacerbated release of cytokines is one of the main events in severe cases of dengue. It is mostly observed in secondary infections[76], correlating with the impairment of the immune response. The molecular mecha
Events of the cytokine storm can be triggered by viral infection of both leukocytes and ECs[77], but also by the interaction of the NS1 protein with receptors such as TLR-4[78,79]. High levels of cytokines such as interleukin 1 (IL-1), IL-4, IL-8, IL-10, IL-13, and IL-17, as well as C-X-C motif chemokine ligand 10 (CXCL10), tumor necrosis factor-alpha (TNF-α), vascular endothelium growth factor A (VEGFA), macrophage migration inhibiting factor (MIF), IFN-β, and IFN-γ have largely associated with disease severity[61,80]. These cytokines are closely related to the increase of vascular permeability and subsequent plasma leak, and also to disrupting tight junctions[81] and inducing autophagy[82], with in vitro studies suggesting more specific roles of different cytokines, such as MIF’s role in EC glycocalyx degradation[83,84] and the roles of CXCL10, VEGFA, and TNF-α in increased permeability and EC apoptosis[80,85,86]. For instance, a recent longitudinal study conducted by Bhatt et al[87] suggests that the analysis of cytokine levels in patients can be useful in predicting dengue severity.
Interestingly, anti-inflammatory cytokines such as IL-4 and IL-10 have been shown to paradoxically contribute to more inflammation, as they can suppress the immune response and impair viral clearance, increasing viral dissemination[88]. Moreover, cytokines that recruit more leukocytes to the site of inflammation, such as IL-8, IFN-γ, MIF, TNF-α, and CXCL10, can contribute to more infection and viral replication, since DENV has a tropism for these types of cells[89].
In summary, the cytokine storm is amplified during DENV infection due to a process of positive feedback, in which the release of cytokines and recruiting of leukocytes leads to even more inflammation. This ultimately results in events of vascular leak, which are maintained and amplified by DENV-induced coagulopathy and thrombocytopenia[90].
Coagulopathy and thrombocytopenia: Besides inducing vascular leak through endothelial damage and a cytokine storm, DENV employs numerous mechanisms to disrupt coagulation, which is meant to contain plasma leakage. Such events can occur via activating and dysregulating coagulation pathways, and also impairing various steps of the coagulation process[91].
In vitro and in vivo studies have demonstrated multiple ways DENV induces coagulopathy and thrombocytopenia. In bone marrow, the virus has been shown to suppress its activity and interfere with megakaryocyte maturation, either directly through infection and interaction with E protein[92] or indirectly via cytokines[93]. Apart from affecting megakaryopoiesis, early activation through DENV NS1 binding to TLR-4 on platelets can potentially cause apoptosis[79], platelet phagocytosis by macrophages and dysregulated clot formation[94], leading to platelet ‘waste’ and thrombocytopenia. DENV infection can also promote the production of cross-reactive antibodies that target platelets[95] and coagulation factors such as thrombin and plasminogen[73], resulting in imbalanced coagulation and fibrinolysis, ultimately leading to amplified vascular permeability. A simplified schematic representation of the discussed immunological aspects of dengue pathogenesis can be seen in Figure 1.
All of the highlighted aspects act dynamically and synergetically, ultimately resulting in increased vascular leak and risk for hemorrhage, correlating with dengue’s clinical manifestations and major complications.
Clinical manifestations: Most cases of dengue infection are asymptomatic, but some patients can manifest symptoms after an incubation period of 3-15 days. Traditionally, dengue was classified into the stages of dengue fever (DF), dengue hemorrhagic fever, and dengue shock syndrome (DSS)[96], with plasma leakage identified as the main factor to disease severity[97]. However, in 2009, the WHO published an update, categorizing the disease as “dengue without warning signs” (DWS-), “dengue with warning signs” (DWS+), and “severe dengue” (SD), aiming to improve on the limitations of the previous classification and broaden the assessment and management of warning signs. Unusual manifestations and involvement of various body systems beyond typical dengue symptoms are categorized under “extended dengue” (ED)[98]. Due to its importance and recent resurgence, the specific guidelines for dengue identifying and management have been reinforced worldwide, with the publishing of management manuals by important health organs.
Dengue presents dynamically with an abrupt onset of symptoms. It starts with a febrile phase, usually exceeding 38 °C[99] and lasting for 2-7 days, followed by symptoms such as anorexia, retro-orbital pain, myalgia, arthralgia, diarrhea, nausea, vomiting[100] and a maculopapular rash in 50% of cases, spreading from the face to the limbs[99]. The early phase of the disease can be nonspecific and challenging to differentiate from other febrile illnesses, especially arboviral diseases. In such cases, if the epidemiological factors are compatible, dengue management measures are recommended even if laboratory diagnosis has not been obtained, due to its potential for severe complications[99,101].
Most patients experience defervescence and recover within 3-7 days[100]. However, some patients may progress to a critical phase following fever reduction, characterized by increased plasma leakage. Symptoms can include abdominal pain, persistent vomiting, lethargy, bleeding tendencies, and fluid accumulation in the lungs (pleural effusion) or abdominal cavity (ascites) as well as disseminated intravascular coagulation[100]. Laboratory findings during this stage typically reveal an increase in hematocrit, thrombocytopenia, and leukopenia[102]. The critical phase usually lasts about 48 hours, with peak vascular leakage occurring approximately 24 hours after onset. Said vascular leak can lead to a hypovolemic shock, causing metabolic acidosis and multisystemic impairment, potentially fatal within only 24 hours or less, if left untreated[98,99].
Patients in use of non-steroidal anti-inflammatory drugs (NSAIDs) such as ibuprofen or aspirin may present instances of hemorrhage that are not directly related to thrombocytopenia[99], but to reduced thromboxane A2 synthesis via cyclooxygenase-1 inhibition[103]. Furthermore, ED presentations may include neurological, cardiac, and renal inflammation and dysfunction, that may present as encephalitis[104] myocarditis[105,106] and acute kidney failure[107]. Such complications have been documented in literature and require careful analysis and more specific interventions, although less common[108,109].
As of the listed symptoms, abdominal pain, persistent vomiting (three occurrences per hour or four occurrences in six hours), and mucosal bleeding are categorized as warning signs for DWS+, along with lethargy or restlessness, hepatomegaly (greater than 2 cm below the costal margin), increased hematocrit (observed in two consecutive measurements), and a decrease in platelet count (below 100000 cells/mm³)[98,110]. These signs indicate a higher risk of developing severe dengue and necessitate careful monitoring.
If the patient survives the critical phase, the recovery is characterized by gradual improvement in well-being, return of appetite, fluid reabsorption, and hemodynamic stabilization. Additionally, hematocrit levels decrease and white blood cell count will increase, in a returning to a normal state. However, it is important to note that DSS can also promote these hematological manifestations as a stress response[98], therefore, a careful analysis of these variables, alongside other clinical presentations, is crucial. Nonetheless, it is not unusual for recovering patients to present bradycardia, pruritus, polyuria, and islands of pallid areas in between the rash, indicating vascular recovery[98,99].
Diagnosis: Dengue diagnosis is crucial for decision-making in the management of suspected patients. It should be conducted carefully, considering the particularities of each specific case and context. In endemic areas, diagnosis can be based solely on clinical findings and the epidemiological context, not always requiring laboratory testing[99]. However, specific testing is also a very important tool in diagnosis, especially in cases with warning signs.
If a patient is suspected of dengue infection, tracking potential exposure by evaluating the presence of infected family members and recent travelling to endemic areas is crucial[98,99]. Vital signs, level of consciousness, and hemodynamic state must be assessed, as well as noting the presence of the most common dengue symptoms, discussed earlier (see “Clinical Manifestations”). A positive tourniquet test, along with other symptoms, suggests dengue infection, although it can yield negative results in obese patients or those already in shock, and should not be the only diagnostic tool[99,111]. All these factors should be paired with analysis of complementary laboratory findings, paying special attention to leukopenia, which has been shown to accurately indicate dengue infection[112]. It is extremely important to monitor for warning signs in newly admitted patients to promptly manage complications.
When specific testing is needed, the current stage of the disease and available resources should be considered. Up to 5 days from symptom onset, reverse transcription-polymerase chain reaction (RT-PCR) can be used, but as of requiring specialized work, it is usually not the first option[113]. Given that the acute viremic phase is sometimes missed because patients may seek medical assistance only after symptoms worsen[114], detection of NS1 antigen and IgM antibodies using enzyme-linked immunosorbent assay (ELISA), immunochromatographic assay or rapid diagnostic test kits can be useful. NS1 antigen levels are present from the beginning and typically peak around 6-10 days after disease onset[115], while IgM antibodies begin to rise after 6-14 days. These tests are simpler and provide quicker results, although they may cross-react with other flaviviruses[116]. In secondary infections, NS1 detection shows limited sensitivity[117], and IgM testing is complicated due to rapid IgG rise[114]. Distinguishing between primary and secondary infections is crucial not only for diagnostic purposes but also due to the risk of developing a more severe disease.
Therefore, detection of viral genome by RT-PCR is mostly recommended up until the first 5 days of symptoms, whereas detecting NS1 antigen and IgM antibodies with ELISA can be more effective for patients that have exceeded this mark, with higher serological levels at around 6-10 and 6-14 days, respectively[115-117]. Additionally, if a secondary infection is suspected, IgM and IgG detection can be conducted concomitantly for more specific results[116]. For every case, it is always important to pair laboratory findings with clinical and epidemiological factors in order to reassure the diagnosis.
Besides the most commonly used in clinical practice, the plaque reduction neutralization test (PRNT) is considered the gold standard for identifying DENV antibodies, seeing it is highly specific and can distinguish serotypes. However, it is not routinely used for clinical purposes, as it requires samples from both the febrile and convalescent phases with a minimum interval of 14 days, as well as specialized materials and professionals[99,118]. It is primarily used for research and surveillance purposes.
Treatment: Currently, there is no antiviral drug available for dengue, so disease treatment focuses on symptomatic control and managing complications. Patients can be categorized into groups for more specific management decisions based on their disease status, although specific guidelines for categorization may vary. The WHO categorizes patients as follows: DWS- (Group A); DWS+ and DF with co-existing risk factors or important social circumstances (Group B); and severe dengue (Group C)[98]. In contrast, the Brazilian dengue management manual categorizes patients as: Absence of warning signs (Group A); absence of warning signs but with spontaneous bleeding (Group B); presence of warning signs and/or risk factors (Group C); and presence of signs of shock, severe bleeding or organ disfunction (Group D)[99]. The Brazilian manual also emphasizes that a suspected case is enough for taking the according measurements, even if laboratory testing has not yet been done[99]. Although not always equal, the division into groups is important in order to optimize the use of hospital beds, especially in the context of large regional outbreaks, as well as how to specifically manage the disease complications. Even then, an individualized analysis to each context is important.
Patients without warning signs can be sent home, prescribed oral hydration, and advised to seek the nearest hospital if any warning signs appear. Paracetamol (acetaminophen) is usually the preferred drug for pain and fever control[98], though metamizole is also widely used in Latin American countries[99,119]. NSAIDs are not recommended, as they can increase the risk of internal bleeding and Reye’s syndrome[100].
When warning signs are present and patients are hospitalized, intravenous fluid replacement is important to prevent further complications. If shock occurs, fluid resuscitation is necessary. Crystalloid solutions are primarily used, but if shock persists and progresses to a hypotensive state, a colloid solution may be required[98,99]. Careful attention to fluid administration is crucial, as fluid overload can cause or exacerbate complications. For that, laboratory indicators should be closely monitored, and IV fluid therapy should not exceed 48 hours[99,120].
There is a lack of consensus and clear evidence about some therapeutic measurements for dengue disease. Prophylactic platelet transfusion, for example, is sometimes conducted, but some procedures seem to be inappropriate[121]. Randomized trials and observational studies have shown that there is no evidence of prophylactic transfusion in bleeding prevention[122], and that unnecessary transfusions can be harmful[122]. Still in regard to bleeding, the WHO emphasizes that patients in use of anticoagulant therapy have a higher risk of severe hemorrhage[98], but no clear instructions are given to manage this situation. Temporary suspending of these medications by specific evaluation of clinicians appears to be safe, but more studies are needed to further specify this action[123].
Due to the immunological aspects of dengue, the idea of using corticosteroids to prevent severe cases pops to mind. These drugs could supposedly be useful in the intermediate phase of the disease, seeing that is when the immune system plays the bigger role in the pathogenesis[124]. However, there is no indication for the use of corticosteroids, as most studies conducted present inconclusive results or low-quality evidence[125]. All the discussed aspects highlight the importance of a careful and individualized analysis of each patient and case, even when following specific guidelines, as it is very hard to make general affirmations[124].
The development of drugs that target dengue structural and non-structural proteins emerges as a possibility for controlling viral entry and replication in hosts. Several in vitro and in silico studies have shown potentiality in this activity[126] but it is still hard to further correlate with clinical practice, as the available animal models for testing lack a mirroring to disease severity as seen in humans[127,128]. Targeting host cellular receptors that facilitate viral entry is also a possibility, but cytotoxicity should be taken into consideration, as these receptors also serve a purpose to the host[129].
Prevention: Various prevention methods have been implied as an attempt of controlling DENV vectors, encompassing biological, chemical and environmental techniques[130]. However, they present some serious issues that impair their capacity of controlling the disease transmission.
The releasing of mosquitoes infected with Wolbachia, as well as the sterile insect technique was believed to be an effective way of vector control for Ae. aegypti and Ae. albopictus, but further analysis revealed that by eliminating larvae competition, they could increase rates of surviving adults[131]. Trying to eliminate reproduction sites and using pe
Vaccines: The development of vaccines for dengue is specially difficulted due to the phenomenon of antibody-dependent enhancement[58]. Several different types, such as live attenuated, inactive virus, viral vector and DNA vaccines are now in development[134], but only live-attenuated vaccines have made into phase III trials, as registered in the Cli
| Vaccine | Intervention/treatment | ClinicalTrials.gov ID | Status | Sponsor |
| DengVaxia© | CYD tetravalent dengue vaccine/human papillomavirus quadrivalent vaccine | NCT02993757 | Completed | Sanofi Pasteur |
| CYD tetravalent dengue vaccine/human papillomavirus bivalent vaccine | NCT02979535 | Completed | Sanofi Pasteur | |
| CYD tetravalent dengue vaccine/yellow fever vaccine | NCT01436396 | Completed | Sanofi Pasteur | |
| CYD tetravalent dengue vaccine/pentaxim™ vaccine | NCT01411241 | Completed | Sanofi Pasteur | |
| Placebo/CYD tetravalent dengue vaccine | NCT01374516 | Completed | Sanofi Pasteur | |
| Placebo/CYD tetravalent dengue vaccine | NCT01373281 | Completed | Sanofi Pasteur | |
| Placebo/CYD tetravalent dengue vaccine | NCT01254422 | Completed | Sanofi Pasteur | |
| Placebo/CYD tetravalent dengue vaccine | NCT01134263 | Completed | Sanofi Pasteur | |
| QDenga© | Placebo/TAK-003 tetravalent dengue vaccine | NCT06060067 | Recruiting | Takeda |
| 9vHPV vaccine/TAK-003 tetravalent dengue vaccine | NCT04313244 | Completed | Takeda | |
| Placebo/TAK-003 tetravalent dengue vaccine | NCT03999996 | Completed | Takeda | |
| TAK-003 tetravalent dengue vaccine | NCT03771963 | Completed | Takeda | |
| Placebo/TAK-003 tetravalent dengue vaccine/HAV vaccine | NCT03525119 | Completed | Takeda | |
| Placebo/TAK-003 tetravalent dengue vaccine | NCT03423173 | Completed | Takeda | |
| Placebo/TAK-003 tetravalent dengue vaccine/yellow fever vaccine | NCT03342898 | Completed | Takeda | |
| Placebo/TAK-003 tetravalent dengue vaccine | NCT03341637 | Completed | Takeda | |
| Placebo/TAK-003 tetravalent dengue vaccine | NCT02747927 | Active, not recruiting | Takeda | |
| Dengue Vaccine by Butantan | Placebo/butantan tetravalent dengue vaccine | NCT02406729 | Active, not recruiting | Butantan Institute |
DengVaxia© was the first dengue vaccine to be licensed. It is based on a YFV strain (YF17D) but with the prM and E regions substituted with those from DENV serotypes 1-4[135]. Conducted clinical trials have demonstrated that Deng
QDenga©, on the other hand, is based on a DENV-2 strain, with recombinant strains of the other 3 serotypes[139]. A phase III trial with patients across 8 endemic countries has demonstrated, after 4.5 years, that QDenga© is effective and safe for all 4 DENV serotypes in patients that have been previously infected, but only shows effectiveness against DENV-1 and DENV-2 for dengue naive patients[140]. However, there is no evidence of Qdenga© vaccinees having a higher risk of developing a severe illness, in contrast to what is observed to DengVaxia©. Considering that DengVaxia© is only capable of inducing anti-DENV antibodies against E and prM proteins, that might explain why QDenga© has a higher effectiveness, and also why DengVaxia© vaccinees can develop a higher risk of severity, seen that E and prM proteins are the ones involved in antibody-dependent enhancement.
ZIKV is an enveloped virus that upon infecting humans, primarily through the human-mosquito-human route, con
This virus possesses a single-stranded, positive-sense RNA genome. It encodes proteins associated with the capsid (C), envelope (E), precursor of membrane protein (prM), and non-structural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5). Initially, these proteins are translated into a single polyprotein (5’-C-prM-E-NS1-NS2A-NS2B-NS3-NS4A-NS4B-NS5-3’), which is subsequently cleaved[142,143].
The envelope protein (E) is a surface protein essential for the adsorption and fusion of the viral envelope with the host cell’s plasma membrane[144]. Consequently, it is the primary target for the majority of vaccines in development, as it elicits an innate immune response, triggering the neutralizing antibodies production (nAbs). These vaccines commonly utilize an alignment of the prM and E sequences (prM-E), which has already demonstrated favorable results, generating high levels of nAbs in both immunocompetent and immunocompromised mice[145-147].
The precursor of membrane protein (prM) plays a role in viral maturation and release from cells[148]. Therefore, its inhibition also yields a beneficial response by reducing viral multiplication in tissues. Additionally, the non-structural proteins are involved in the replication of the ZIKV and inhibit the expression of IFN I. As a result, they ensure a less effective immune response[149].
The ZIKV was first isolated in 1947 from a febrile Rhesus monkey in Uganda’s Zika Forest. This was followed by its isolation from Ae. africanus mosquitoes the next year[150]. Human infection was first documented in 1954 in Nigeria[6], with only 14 reported cases over the subsequent fifty years. After this long period, a significant outbreak occurred in 2007 on Yap Island, Micronesia, involving 45 confirmed cases associated with Ae. hensilli mosquitoes, where patients presented with fever, rash, and arthralgia[151] and subsequent outbreaks in French Polynesia revealed severe complications such as Guillain-Barré syndrome and microcephaly resulting from maternal-fetal transmission[147]. The virus rapidly spread across the Pacific[141] and into the Americas, culminating in a major outbreak in Brazil in 2015, with an estimated 440000 to 1.3 million suspected cases[151].
As of May 2024, the WHO reported through its epidemiological update that ninety-two countries in Africa, Oceania, America, Europe, and Asia presented current or previous ZIKV transmission. Sixty other countries across the aforementioned continents have established Ae. aegypti vectors but without known cases of viral transmission[152].
Main vectors of transmission: The vectors of ZIKV transmission, as with all other arboviruses, are arthropods, primarily mosquitoes of the family Culicidae and genus Ae. These mosquitoes inject the virus with their saliva when biting vertebrates such as humans, thereby infecting the host (horizontal transmission)[141,153,154]. These mosquitoes have both sylvatic and urban transmission cycles, with Ae. aegypti being the main cause of outbreaks in urban environments around the world[153]. Furthermore, other species within the family are also responsible for epidemics: Ae. albopictus is associated with the 2007 outbreak in Gabon[4]; Ae. hensilli was identified as the primary cause of the Zika outbreak on the island of Yap in the same year[5,155]. The ZIKV has also been detected in Ae. polynesiensis, Ae. africanus, and even in the common household mosquito Cx. pipiens, among others[150,153,156].
Non-vector means of transmission: The viral transmission is not only limited to horizontal transmission through mos
There are several reports reinforcing the possibility of sexual transmission. It is likely that the viral presence in semen components and tissues associated with the reproductive organs contributes to this infection form[165], since some research has demonstrated that the viral presence in semen components can be substantial even weeks after infection onset[157,166]. The aforementioned reports provide data on confirmed symptomatic infections through positive se
Therefore, considering the possibility of sexual transmission of ZIKV, the use of condoms during sexual intercourse, especially when pregnant, is extremely relevant. This not only helps prevent other sexually transmitted diseases but also reduces the possibility of sexually transmitting ZIKV, thus inhibiting the probability of developing another type of trans
Vertical transmission of pathogens is typically not facilitated, as the placenta acts as a protective barrier for the fetus against invading pathogens, preventing infection from crossing the placental barrier in most cases. The placenta is formed by cellular layers of syncytiotrophoblasts, originating from trophoblasts that merge to form syncytia. These initially penetrate the endometrium to secure the blastocyst and subsequently act as a barrier between maternal and fetal blood. Blastocysts are highly resistant to infections from various viruses and confer viral resistance to non-trophoblast paracrine cells through the release of effectors such as type III IFN[170,171].
However, one of the viruses capable of crossing the placenta is the ZIKV, and its invasion mechanisms remain unclear. The complexity of simulating vertical transmission, all stages of pregnancy, and the fact that the placentas of animals used for analysis usually have significant anatomical differences compared to the human placenta, make it challenging to conduct high-quality research[163,172,173].
Viral tropism, entry, replication and exocytosis: ZIKV has a capsule with surface glycoproteins that facilitate its ad
The viral presence in rodents, humans, and other primates tissue analyses has been detected in placental cells, trophoblasts, endothelium, epithelium, immune cells, mature and progenitor neuronal cells, ocular tissues (cornea, retina, optic nerve, and aqueous humor), and bodily fluids such as tears, saliva, semen, cervical mucus, and urine. There is also infection evidence in the male reproductive system, including testicular cells (Sertoli cells, Leydig cells, and spermatogenic cells), as well as in the female reproductive system in vaginal epithelial cells and uterine fibroblasts[165,178].
Upon entering the cell, the virus begins its replication using the host’s machinery to generate new viral copies. In the ER of the cell, the virus undergoes RNA replication and capsid assembly[179]. The cell then uses reticulophagy to degrade the ER and prevent the maturation of ZIKV. The reticulophagy receptor FAM134B is essential for this process. However, the viral protein NS2B3 cleaves and inactivates FAM134B, preventing reticulophagy and enhancing ZIKV replication[180].
After the virus is assembled in the ER, it is transported to the Golgi complex, undergoing maturation processes and conformational changes, facilitating the subsequent fusion of the mature virus with the plasma membrane and cellular exocytosis[181].
Host intrinsic defenses and IFN inhibition: Upon being infected, a significant portion of the host’s cells have the ca
However, the virus has the ability to inhibit the host’s immune response through various mechanisms. The ZIKV has non-structural proteins responsible for several actions, including the inhibition of IFN production. The NS3 protein can bind to the 14-3-3 protein of RIG-I-like receptors, blocking the translocation of RIG-I and MDA5 to the mitochondria and thereby preventing the pathway for IFN transcription factors from occurring[185]. NS4A acts directly on the mito
Adaptative response and the viral strains: The adaptive response against ZIKV is primarily mediated by CD4+ and CD8+ T cells, playing an important role in inhibiting viral replication, especially when there is inhibition of type I IFN[188]. CD4+ T cells predominantly differentiate into T helper 1 cells during Zika infection, increasing levels of cytokines such as IFN-γ, IL-2, TNF-α, and the transcription factor T-bet, given their crucial role in orchestrating the immune response through cytokine production[189]. CD8+ T cells, in turn, also aid in the release of cytokines like IFN-γ and TNF-α, along with higher expression of granzyme B[189]. B lymphocytes also participate in the adaptive immune response, eventually transforming into plasma cells, ensuring humoral immunity by releasing antibodies against ZIKV. This action is also driven by CD4+ T cells, and one of the main antibodies released are EDIII-specific neutralizing antibodies that neutralize epitopes present in the virus’s EDIII protein[190,191].
The inhibition of CD8+ T cells during infection has been correlated with increased viral infection in the central nervous system (CNS), but with higher survival rates and lower incidence of paralysis[192]. On the other hand, the decrease in CD4+ T cells resulted in paralysis in all studied mice, in addition to increasing the viral load in the CNS and reducing survival[193]. These findings highlight the significant role of CD8+ T cells in neuropathology, as it has been shown that these cells mediate the lysis of virus-infected neurons[189]. Conversely, CD4+ T cells have demonstrated a regulatory function by reducing the immunopathological effects triggered by CD8+ T cells.
Therefore, our immune system, which is responsible for defending the body against pathogens such as ZIKV, has its efficacy dependent on the virus’s ability to evade immune signaling pathways and inhibit IFN production. The different strains of the ZIKV, which emerged after various genetic mutations, also differ in their capacity to manipulate this immune response and are essentially divided into two main lineages with considerably different pathogenic characteristics: The African and Asian lineages.
African strains (such as the East African MR766 and West African lineages) have been shown to induce more potent inflammatory reactions and possess greater virulence[182]. However, the Asian strains (which include contemporary strains from Asia, Oceania, and the Americas) are more strongly associated with neurological disorders and microcephaly present in congenital Zika syndrome[163,194]. The Asian lineage emerged following the viral migration from Africa to Southeast Asia, being detected in Malaysia (1966; P6-740), the Pacific Islands, and later spreading to the Americas[195].
In addition to inhibiting the production and signaling of type I IFN, which is also present in African strains, studies indicate that the Asian strains can prevent the transcription and translocation of INF to the cell nucleus through the actions of NS1, NS4B, and NS5 proteins. This can lead to much more prolonged infections, potentially lasting for months, in contrast to the African strains that typically result in self-limited febrile infections[193,196,197].
Cutaneous pruritus: Pruritus is a frequent symptom in ZIKV -infected patients potentially linked to mast cell degranulation via the IgE-dependent histaminergic itch pathway[164,198]. Mast cells, a type of innate immune system cell, are among the main culprits for the symptomatic manifestation of various inflammatory and allergic reactions, and their G protein-coupled receptors (mainly H1R and H4R) play an important role in the development of cutaneous itching[164,198].
The ZIKV has already shown tropism for the HMC-1 Lineage of mast cells isolated from the human placenta, triggering histamine degranulation and releasing various cytokines, thus being identified as a potential cause of itching during infection[164]. This itching can be alleviated by administering antihistamine medications, thereby reducing this uncomfortable symptom.
Congenital Zika virus syndrome and neuronal tropism: The correlation between Zika infection in pregnant women and microcephaly in their offspring is widely known[199]. However, all the associated mechanisms are not yet completely elucidated. Although, ZIKV effectively replicates in immature neurons in vivo, as shown in studies infecting embryonic mouse brains with the Asian strain SZ01. This replication triggers apoptosis, disrupts the cell cycle, inhibits neural progenitor cell differentiation, and ultimately causes cerebral cortex thinning and microcephaly, a critical factor in the development of human microcephaly[194].
Wu et al[163] also demonstrated vertical transmission in immunocompetent pregnant mice through intraperitoneal injection containing the contemporary Asian strain (ZIKV SZ01) isolated in serum, infecting radial glial cells of the dorsal ventricular zone in offspring with decreased proliferation of cortical neuronal progenitor cells, which are their main target. The infection was shown to affect brain development, reducing the lateral ventricle cavity and cortical surface area. A considerable number increase of IL-17a receptors in brain samples was also observed, likely due to maternal immune activation in response to the virus.
The ZIKV can activate T cells (Th1, Th2, Th9, and Th17), increasing cytokine levels in the acute phase with significant elevation of interleukins IL-1b, IL-2, IL-4, IL-6, IL-9, IL-10, IL-13, and IL-17, decreasing their levels in the subsequent phase (subacute)[178]. However, the action of interleukins can extend beyond their antiviral role, as IL-17a is likely involved in the development of microcephaly in infected humans.
In 2016, a study subjected pregnant rodents to controlled immune response activation by the IL-17a pathway. The results demonstrated impairments in offspring cortical development alongside behavioral abnormalities similar to autism spectrum disorder, with impaired social interactions and considerable phenotypic changes. Blocking IL-17a activity through specific antibodies protected the offspring against brain damage, demonstrating the significant role of this cytokine in the observed alterations, such as fetal brain malformation. Therefore, in addition to the virus’s direct action on CNS cells, cytokine reactions released due to maternal immune activation may also influence the brain damage caused by congenital Zika virus syndrome[200].
Guillain-Barré Syndrome and molecular mimicry: Adults can also develop neurological complications due to the virus’s strong tropism for nervous tissues[165]. One of the most common complications of the infection is Guillain-Barré Syndrome (GBS), an immune-mediated polyradiculoneuropathy that results in axonal neuropathy and is the leading cause of flaccid paralysis worldwide[201].
Data from seven countries in Central and South America showed a significant 2.0 to 9.8-fold increase in GBS cases during ZIKV outbreaks, with subsequent decreases observed once outbreaks were controlled[202]. The presence of antiglycolipid antibodies, mainly against GA1, has been reported by Cao-Lormeau et al[203] in one-third of the analyzed patients diagnosed with GBS having anti-ZIKV IgG or IgM. Other analyses have demonstrated IgM and IgG antiganglioside antibodies in patients under similar conditions[204,205]. This immune response is likely triggered by molecular mimicry between viral structures and neuronal proteins, a situation where the patient’s body begins to recognize parts of its own body as structures of the invading pathogen, causing self-damage.
Among the probable structures involved in molecular mimicry, the neuronal proteins associated with GBS (Heat Shock 70 kDa protein 12A and voltage-dependent L type Calcium channel subunit α-1C) and the glycan loop region of the viral envelope protein E may be responsible for the immune recognition error due to the conservation of an IVNDT motif present in both proteins[206]. This situation ultimately triggers the production of antibodies and an immune response against the myelin sheath of peripheral nerves, thus causing the axonal neuropathy associated with GBS.
Clinical manifestations and major complications: After being transmitted through arthropod bites, the ZIKV enters a period of incubation, which typically lasts between 3- and 12-day following transmission. Notably, only a minority of those infected (20% to 25%) develop symptoms after the incubation period[153,207].
Symptoms are generally mild, nonspecific, and often self-limiting[199], making them easily confusable with other infections, especially those caused by other arboviruses[153]. Therefore, diagnosis should not rely solely on symptoms but also on epidemiological factors, taking into account current endemic and epidemic conditions in the patient’s residence and places they may have traveled.
Concurrent infections are not uncommon[208], especially in regions where common arbovirus vectors are endemic, such as parts of Africa, Asia, and Latin America[152]. Consequently, many symptoms may be erroneously attributed to another pathogen causing simultaneous infection in the host. Laboratory tests are essential allies in diagnosis, particularly for concurrent infections, allowing for the prediction and potential prevention of various complications associated with the involved pathogens, including gestational complications related to ZIKV[209].
Among the signs and symptoms, ZIKV infection can present with a maculopapular rash typically associated with pruritus. This rash usually has a centrifugal distribution, developing in proximal regions and later affecting distal limbs, lasting between one to four days. Fever tends to be mild (between 37.4-38.0 degrees Celsius), unlike the higher fevers associated with the DENV infection[153,199].
Other symptoms such as arthralgia, extremity edema, myalgia, fatigue, mild headache, dizziness, loss of appetite, digestive disturbances, auditory issues, and hypotension may also occur, rarely persisting for more than two weeks[153,199,207]. Retro-orbital pain, commonly linked with dengue, can also arise in ZIKV infections, and conjunctivitis or conjunctival hyperemia is more frequent in ZIKV infections compared to other arboviral infections[153]. Elevated intraocular pressure can also be a complication, potentially leading to glaucoma[210].
Genitourinary symptoms, including hematospermia, have been reported[167]. The virus’s tropism for tissues in this region, as well as its presence in urine, semen, and secretions, is well-documented[157,165,166,199].
One of the most concerning complications of ZIKV infection is microcephaly. Reports from the 2013 outbreak in French Polynesia[211] to the 2015 outbreak in Brazil[212] have highlighted the detrimental impact of ZIKV on ongoing pregnancies. An increase from 20 cases of microcephaly per 10000 live births during the ZIKV outbreak in Brazil, compared to 0.5 per 10000 live births before the outbreak[213], underscores the virus’s aggressive potential in disrupting fetal CNS development.
The virus’s strong neurotropism also poses risks for adults, often underestimated. Besides potentially causing autoimmune complications such as Guillain-Barré Syndrome due to molecular mimicry[201,204,206,214], ZIKV infection has been associated with complications like transverse myelitis, encephalitis, chronic inflammatory demyelinating polyneuropathy, meningitis, seizures, optic neuropathy, and acute demyelinating polyneuropathy[215]. A case-control neuroimaging study demonstrated changes in gray matter volume in certain brain regions post-infection, thereby altering the functional organization and structure of the adult brain[216].
Deaths related to ZIKV infection are rare and typically linked to microcephaly[162]. In 2015, three deaths were reported in Brazil: An adult man with lupus erythematosus, rheumatoid arthritis, chronic corticosteroid use, and a history of alcoholism, a 16-year-old girl, and a newborn[217]. In Colombia, a 15-year-old girl with sickle cell anemia also died following ZIKV infection[218], indicating that comorbidities can increase mortality risk. When ZIKV infection is suspected, the WHO emphasizes the importance of asking the patient about the onset of symptoms. This information guides the selection of appropriate laboratory tests based on the duration of symptoms. A thorough travel history, especially if travel occurred within the past two weeks, should be obtained, including dates, locations, and duration of travel, as well as possible sexual contact with confirmed Zika cases, breastfeeding status, and recent vaccinations, particularly against other flaviviruses such as YFV, Japanese Encephalitis, and dengue[219].
Laboratory diagnosis: The laboratory diagnosis of ZIKV is typically performed using ELISAs to detect the presence of anti-ZIKV IgM and IgG antibodies. IgM is detectable from 5 days to 12 weeks after the onset of symptoms, whereas IgG antibodies appear a few days after IgM detection and usually remain detectable for over a year[220-222].
ELISA is generally a less complex, quicker, and cheaper method compared to other tests still used worldwide, such as the PRNT, fluorescent antibody test, hemagglutination-inhibition test, and complement fixation test[223]. However, false-positive and false-negative (particularly in immunocompromised patients with inadequate adaptive immune responses or when tests are conducted before the onset of the antibody-associated adaptive immune response) results for IgM and IgG tests are still significantly reported. Cross-reactivity, especially with viruses from the same family (Flaviviridae)[221], remains a common issue in these tests, potentially leading to incorrect diagnoses.
Therefore, PRNT can be used to confirm laboratory diagnoses by quantifying neutralizing antibodies in serum or cerebrospinal fluid (CSF) samples more specifically, serving as the gold standard for diagnosis[223]. For viral RNA detection, RT-PCR can be utilized during the infection, offering high sensitivity and specificity[141,155,223,224].
However, these tests are not very compatible with common outpatient settings, as they require appropriate in
According to the WHO[219], laboratory diagnosis can be conducted using serum, whole blood, or urine samples. Other samples may be considered when neurological complications related to ZIKV infection are suspected, including in cases of a sexual transmission suspected, as semen samples, since the virus tends to persist longer in urine and semen tests compared to blood analyses[166,227].
For patients within 7 days of symptom onset, nucleic acid tests (NAT), such as RT-PCR, are recommended to diagnose viral presence. For those with more than 7 days of symptoms, serological detection tests for IgM antibodies (ELISA), present in the acute phase of infection, can be used. NAT can also be employed, although negative results do not rule out infection since viremia may be low after one week of symptom onset[219]. IgG detection just indicates a past infection that elicited an immune memory response.
During outbreak periods, the WHO recommends prioritizing testing for a select group of patients[219] as there is normally a limited number of tests available to be used (Table 2).
| No. | Priority recommendation for viral testing during periods of ZIKV outbreak-WHO |
| 1 | Symptomatic patients who have had sexual relations with a partner with probable or confirmed infection |
| 2 | Suspected patients with neurological complications |
| 3 | Pregnant women with a travel history to endemic areas, residents in endemic areas or those in current outbreak regions |
| 4 | Pregnant women who have had sexual relations with a confirmed or probably infected patient |
| 5 | Pregnant women with suspected or confirmed fetal brain anomalies who have a travel history to endemic areas or reside in endemic areas or current outbreak regions |
| 6 | Women who have had miscarriages or stillbirths and traveled or resided in Zika virus-affected areas during pregnancy |
| 7 | Infants born with microcephaly or neurological complications whose mothers traveled or resided in endemic areas or current outbreak regions |
| 8 | Breastfeeding infants with mothers diagnosed with the viral infection |
Findings in complementary exams: During ZIKV infection, additional exams are commonly requested to assist in the clinical management of the patient. However, reports contain limited information about other laboratory exams performed. The results of a complete blood count and leukogram are usually normal, without leukopenia or thrombocytopenia, which are common in CHIKV and DENV infections[153]. The patient may show slight elevations in C-reactive protein, ferritin, and fibrinogen, as well as increased serum lactate dehydrogenase and liver enzymes, findings commonly seen in other viral infections[153,199]. These clinical data have little impact on the management of ZIKV infection, as the disease lacks research indicating a likelihood of a worse outcome even when the patient presents such altered exams.
Treatment: There is no specific treatment for ZIKV. However, appropriate management focuses on alleviating symptoms. The WHO recommends using antipyretics for patients with fever, antihistamines for those with itching, and analgesics for pain relief. Adequate hydration and rest can also aid in the patient’s recovery[228].
The use of NSAIDs is contraindicated without completely ruling out DENV infection, as there is a risk of hemorrhagic complications[228]. Additionally, NSAIDs are contraindicated in pregnant women after the 32nd week of gestation regardless of the infecting virus, due to the risk of premature closure of the ductus arteriosus[229]. Administration of acetylsalicylic acid is also contraindicated, as it increases the risk of developing Reye’s syndrome[229].
However, even if the current treatment is still just medication to alleviate the symptoms of the infection, various compounds have been studied in recent years as anti-flavivirus substances and may be available on the market for infection treatment in the coming years. An ideal drug should possess the ability to penetrate the blood-brain barrier, characterized by small and/or lipophilic molecules. Additionally, it should target the infection’s host cells, such as fetal neural progenitor cells, exhibit placental penetrance to prevent vertical transmission, and be highly safe for administration to pregnant women[230].
The coinfection and cocirculation of flaviviruses like DENV, YFV, Mayaro, and Oropouche virus in various parts of the world infect hosts and cause immune responses with cross-reactivity. This has led researchers to conclude that the best option for developing an anti-ZIKV drug would also be one that protects against other flaviviruses. This is because the structures of several studied target proteins are similar, and recently developed preclinical compounds have shown activity against multiple flaviviruses, making them promising candidates for medication[230].
Consequently, considering the most promising proteins for the development of this medicine when examining host factors associated with infection, the TIM1 binding protein proves to be a significant factor for the binding of the viral phospholipid during ZIKV entry. However, it is also involved in the infection of DENV-2 and West Nile virus, making it an interesting target for a multi-flavivirus drug[230,231]. Inhibitors of the E protein binding to host receptors also show potential as drug targets, thus preventing viral adsorption. Nevertheless, one of the most promising targets for drug creation is certainly viral RNA-dependent RNA polymerases (RdRp), as they are extensively studied targets with substantial literature supporting their efficacy and safety[232]. By inhibiting RdRp, viral replication and the production of infectious particles are also inhibited.
To understand the importance of RdRp in the virus replication cycle, research shows that Zika, being part of a genus of single-stranded positive-sense RNA viruses, has RdRp proteins that synthesize a complementary (negative-sense) strand used to synthesize new positive-sense strands, which will later be used as mRNA in virus replication. In ZIKV, the RdRp protein responsible for creating new strands is NS5, which also suppresses type I IFN and is the focus of various drugs currently being developed.
The RdRp inhibitors have been successful in treating other viruses such as hepatitis C virus, HIV, and herpes simplex virus. A ProTide technology nucleoside analog for HCV, already approved by the FDA (Sofosbuvir), has shown significant effects and high safety as an anti-ZIKV agent both in vivo in mice (demonstrating prevention of vertical transmission and reduced morbidity) and in vitro in neuronal cells. Sofosbuvir is classified as category B for administration in pregnant women, meaning that while there are no controlled studies in pregnant women, animal studies have not shown risk to the fetus.
Conversely, analogs of Sofosbuvir (such as 2’-C-ethynyluridine aryoxyl phosphoramidate and 2’-C-methyluridine aryoxyl phosphoramidate) have demonstrated superior anti-ZIKV effects compared to the medication. However, clinical trials are also necessary to prove their safety in pregnant women and efficacy against sexual and vertical transmission.
Finally, other potential preclinical compounds under investigation include protease inhibitors, viral assembly inhibitors, inhibitors of ZIKV fusion to the cell membrane, nucleoside biosynthesis inhibitors, and ZIKV antivirals targeting the host. These drugs are still being evaluated for safety and efficacy.
Prevention: Preventive measures range from personal care, such as using effective repellents containing DEET, IR3535, or icaridin, and wearing long clothing that covers as much skin as possible, especially in areas known to have ZIKV vectors, to collective measures, like eliminating potential breeding sites where water can accumulate. Special caution is advised during the peak activity times of the major urban vector, Ae. aegypti (early morning and late afternoon), using mosquito nets, window and door screens, and electric repellents at home to aid in prevention[228].
During pregnancy, women are advised not to travel to endemic or outbreak areas. Ultrasound scans to monitor fetal development are recommended every 3 to 4 weeks for patients with confirmed or suspected ZIKV infection, and newborns should be tested at birth[209].
Furthermore, regarding men, the WHO also advises that those who have traveled to endemic or outbreak areas should avoid sexual relations with their pregnant partners or use condoms for up to three months after exposure[228].
Vaccines in development: Successful flavivirus vaccines, such as the current dengue vaccine (Qdenga) from Takeda, along with knowledge of ZIKV pathogenesis and general characteristics, are key factors directly influencing the advancements in the development of various ZIKV vaccines. Nucleic acid vaccines (DNA and mRNA), inactivated virus vaccines, live attenuated virus vaccines, viral-vectored vaccines, virus-like particle (VLP) vaccines, protein antigen-based vaccines, and mosquito saliva antigen-based vaccines are all in preclinical or clinical stages of human testing[145,146].
Currently, more than 50 ZIKV vaccines are under development and a large portion is detailed on the ClinicalTrials.gov website, a global database maintained by the National Library of Medicine[233]. However, the WHO and the National Institutes of Health (NIH) report that Phase III field efficacy trials become unfeasible in the absence of a new outbreak, thus hindering effective human trials and subsequent approvals[234](Table 3). Finally, the lack of substantial investment from both private and governmental initiatives also delays the development process.
| Vaccine technology platforms | Intervention/treatment | ClinicalTrials.gov ID | Phase | Status | Sponsor |
| DNA vaccine | Biological: VRC-ZKADNA090-00-VP; Other: VRC-PBSPLA043-00-VP | NCT03110770 | 2 | Completed | NIAID |
| Biological: VRC-ZKADNA090-00-VP | NCT02996461 | 1 | Completed | NIAID | |
| Biological: VRC-ZKADNA085-00-VP | NCT02840487 | 1 | Completed | NIAID | |
| mRNA vaccine | Placebo/biological: mRNA-1325 | NCT03014089 | 1 | Completed | ModernaTX, Inc. |
| Placebo/biological: mRNA-1893 | NCT04064905 | 1 | Completed | ModernaTX, Inc. | |
| Placebo/biological: mRNA-1893 | NCT04917861 | 2 | Active, Not Recruiting | ModernaTX, Inc. | |
| Viral vectored vaccine | Placebo/biological: MV-ZIKA-RSP vaccinations (high or low doses) | NCT04033068 | 1 | Completed | Themis Bioscience GmbH |
| Biological: ChAdOx1 Zika | NCT04015648 | 1 | Completed | University of Oxford | |
| Placebo/biological: MV-ZIKA | NCT02996890 | 1 | Completed | Themis Bioscience GmbH | |
| Live attenuated vaccine | Placebo/biological: rZIKV/D4Δ30-713 | NCT03611946 | 1 | Completed | NIAID |
| Purified inactivated vaccine | Placebo/biological: VLA1601 | NCT03425149 | 1 | Completed | Valneva Austria GmbH |
| Placebo/biological: Zika virus purified inactivated vaccine | NCT02937233 | 1 | Completed | Kathryn Stephenson | |
| Biological: VLA1601/CpG 1018®/3M-052-AF | NCT06334393 | 1 | Recruiting | Valneva Austria GmbH | |
| Placebo/biological: PIZV | NCT03343626 | 1 | Completed | Takeda | |
| Placebo/biological: Zika virus purified inactivated vaccine | NCT03008122 | 1 | Completed | NIAID | |
| Placebo/biological: IXIARO; YF Vax 17D strain and Zika virus purified inactivated vaccine | NCT02963909 | 1 | Completed | NIAID | |
| Drug: Saline/biological: Zika virus purified inactivated vaccine | NCT02952833 | 1 | Completed | NIAID |
According to the WHO and the United Nations International Children’s Emergency Fund (UNICEF), the target product against ZIKV should provide adequate protection against congenital Zika syndrome, particularly focusing on the immunization of women of childbearing age and pregnant women[235]. Innovative technologies recently developed, such as mRNA vaccines authorized to treat COVID-19[236], can be a promising alternative for ZIKV immunization, especially after the successful performance observed during the pandemic[237,238].
Inactivated virus vaccines are generally safe, even for pregnant women and immunocompromised individuals, making them an ideal candidate for preventing congenital Zika syndrome. However, this type of vaccine typically requires higher doses to ensure an adequate and long-lasting immune response. On the other hand, live attenuated virus vaccines usually elicit efficient immune responses from the first dose and tend to provide a much more durable immune response. However, given the pathogenesis of ZIKV, this type of vaccine may not be ideal for pregnant women due to the potential for maternal-fetal transmission and associated complications[239].
Probably, the combination of various types of vaccines that may become available on the market in the coming years will offer greater benefits to the population, as they have diverse indications, contraindications, and benefits.
Yellow fever (YF) is caused by the YFV and has historically been one of the world’s most lethal and feared diseases. YFV belongs to the Flaviviridae family and is one of over 70 members of the Flavivirus genus, with “flavus” being the Latin word for yellow. The virion has a spherical shape with a diameter of 40-50 nm[143]. Its genome consists of a single-stranded positive-sense RNA of approximately 10.8 kb, containing an open reading frame that encodes a single polyprotein of 3411 amino acids[240], which is post-translationally cleaved to produce mature viral proteins. These viral proteins are classified as structural and non-structural. Despite having different genotypes, the YFV has a single serotype[241].
The structural proteins crucial for the formation and structure of the virion include the capsid protein C, the pre-membrane protein prM, and the envelope protein E, which together constitute the viral particle[242]. The transmembrane domains of the prM and E proteins act as localization signals to the ER, containing specific sequences that direct these proteins to the ER[243]. Notably, the E protein plays a crucial role in recognizing and binding to host cell receptors, facilitating viral entry[244], and is an important target of the immune system during YFV infection, triggering the pro
YF is a zoonotic viral disease endemic to various tropical regions in Africa and the Americas, characterized by a transmission dynamic involving non-human primates (NHP) reservoir and humans, mediated by mosquito vectors. The YFV is primarily transmitted to humans by primates through the bite of infected mosquitoes, posteriorly establishing a human-mosquito-human transmission cycle[247]. This interaction can result in a wide range of symptoms, from mild fever to severe liver disease and jaundice, the latter giving the disease its name[241]. Effective transmission control and a thorough understanding of the virus-host interaction are crucial for the management of the disease.
Although YF cannot be eradicated, epidemics can be prevented through mass immunization of the population and the maintenance of routine childhood vaccinations. Low vaccination coverage has led to significant outbreaks in Angola (2015-2016), the Democratic Republic of Congo (2016), and Brazil (2017-2018)[248], highlighting the urgent need to address this gap. Consequently, in 2016, to combat and eliminate the growing urban outbreaks of YFV and prevent its international spread, the WHO, in partnership with UNICEF and Gavi, launched the Eliminate Yellow Fever Epidemics initiative. This initiative has strategic objectives to protect at-risk populations, prevent the international spread of YFV, and quickly contain outbreaks. One of its key actions is mass vaccination campaigns, with an estimate to have over one billion people properly vaccinated by 2026[249].
Phylogenetic analyses suggest that the virus, which gave rise to the currently circulating strains, originated in Africa within the last 1500 years and was introduced to the Americas around 300-400 years ago during the transatlantic slave trade[250]. The first recorded epidemic occurred in Yucatan in 1648[251]. The Spanish-American War highlighted the disease’s impact, with more soldiers dying from YF than combat in Cuba invasions. Walter Reed’s research between 1899 and 1901 confirmed mosquito transmission of the virus[251,252]. The isolation of the virus from a patient named Asibi in 1927 led to the development of the 17D vaccine by Max Theiler, who received a Nobel Prize for this achievement in 1936[253].
As of 2023, the WHO reports that YF remains endemic in 34 African countries and 13 countries in Central and South America[254]. Ongoing vaccination efforts and surveillance are vital to prevent outbreaks and control the spread of the disease. The 17D vaccine continues to be a cornerstone in the fight against YF, highlighting the importance of immunization programs in endemic regions.
Transmission cycle: YF is a zoonotic infection transmitted from primates to humans through the bite of infected mosquitoes[241]. The virus is inoculated into the host via the mosquito’s saliva. After an incubation period of 3 to 7 days, viremic hosts can infect other mosquitoes that feed on their blood, thus continuing the transmission cycle. Mosquitoes remain infected for life and can pass the virus to their eggs through transovarial transmission[255,256].
YFV has different transmission cycles: Sylvatic, urban, and intermediate. In the sylvatic cycle, transmission occurs in forested areas between blood-feeding mosquitoes and NHP. In the Americas, the primary vectors are mosquitoes of the Haemagogus and Sabethes genera, while in Africa, Aedes mosquitoes predominate. NHPs are the natural hosts of the virus. Following urbanization, YF entered the urban cycle, where transmission occurs among humans in urban and peri-urban areas, with Ae. aegypti being the primary vector[257]. There is a high risk of outbreaks when infected humans from forest areas travel to densely populated areas with low immunity to the virus, where vector mosquitoes are present[258].
In addition to the sylvatic cycle, there is an intermediate transmission cycle for YF. Small epidemics can occur at the edges of African savanna forests, in humid and semi-humid zones, known as emergent zones, where humans come into contact with the wild cycle. In this cycle, transmission occurs among NHPs, humans, and mosquitoes, such as Ae. africanus[257].
The recent detection of Ae. albopictus mosquitoes contaminated with YFV in urban and rural areas around the world is concerning and needs attention[259]. Additionally, transmission can occur through other routes, such as exposure to infected blood and aerosols in laboratories[260]. Cases of perinatal transmission to newborns have also been reported[261,262]. Furthermore, the attenuated 17D vaccine strain of the virus has been reported to be transmissible through blood transfusion[263], organ transplantation[264] and, in rare cases, breastfeeding[265,266]. An investigation in the United States in 2021 revealed that transmission of the YF vaccine virus virus via organ transplantation and blood transfusion caused severe neurological disease and fatalities in four recipients. These findings suggest that after receiving a vaccine dose, blood donation should be delayed for at least two weeks[264]. Continuous surveillance and adequate vaccination are essential to prevent outbreaks.
Binding and entry: After the inoculation of the virus, the process of viral replication and dissemination to other tissues begins. In the Flaviviridae family, viral replication occurs through the synthesis of an antigenome template used for the production of genomic RNA and the synthesis of viral proteins[267]. The entry of flaviviruses into their target cells is mediated by the interaction of the E glycoprotein with receptor molecules on the cell surface, promoting fusion with the cell membrane for viral entry[268]. However, the host cell receptors that bind to the E glycoprotein have not yet been identified[269]. The DIII domain of the E protein is considered the receptor-binding domain of flaviviruses[244,270-273].
YFV binds non-specifically to heparan sulfate on the surface of host cells, such as hepatocytes and dendritic cells[274,275]. Despite using different pathways, both wild-type and attenuated strains of YFV employ a pH-dependent entry mechanism[276]. The wild-type Asibi strain enters host cells through clathrin-mediated endocytosis, while the attenuated YFV-17D strain utilizes a clathrin-independent pathway[277]. The conformational rearrangement of the E glycoprotein occurs in the lower pH environment of the endosome, facilitating the fusion of the viral lipid envelope with the endosomal membrane[278].
It is important to note that the valosin-containing protein (VCP/p97), a cellular ATPase that unfolds and extracts ubiquitinated client proteins from large complexes, has been reported as a factor with various functions in flavivirus replication, such as viral uncoating[279]. Consequently, the viral genome is released into the host cell cytoplasm.
After the flavivirus RNA enters the host cell, the viral genome acts as messenger RNA (mRNA) and is translated at the rough ER into a polyprotein, anchored in the ER itself[280]. This polyprotein is cleaved by cellular peptidases and viral proteases NS2B/3[281-283].
The NS5 protein is particularly notable as it houses the methyltransferase and RdRp domains, which play a crucial role in viral RNA synthesis and regulation of replication processes[284], providing insights into targets for the development of therapeutic strategies[241]. Additionally, it is suggested that G protein-coupled receptor kinase 2 contributes to various stages of the virus life cycle by enhancing both viral entry and RNA synthesis, being a regulator of flavivirus infection[285].
Host factors play essential roles in this process: The signal peptidase complex associated with the ER (SPCS) is responsible for processing the Pr-E junction and secreting viral particles[286]; the DNAJC14 protein, a Hsp40 cocha
Following this processing, the viral RNA replication complex is assembled by non-structural proteins such as NS1, NS2B, NS3, NS4A, NS4B, and NS5. The NS4A protein induces rearrangements in the ER membrane, promoting active viral RNA replication, which is then packaged forming the virion[290]. These immature virions are secreted in vesicles and transported to the Golgi apparatus, where they traverse chambers with increasingly lower pH. The enzyme furin cleaves the viral envelope proteins, resulting in mature virions that are then released by exocytosis[291,292].
Immune response: The understanding of the pathogenic mechanisms of the YFV remains limited, primarily due to the lack of animal models that accurately reproduce the disease observed in humans[269]. Current knowledge is largely based on human tissue biopsies from fatal YF cases and indirect observations made in animal models[293], which do not fully capture the characteristics of human infection.
Upon being bitten, the infected mosquito injects the YFV into the skin. The virus is then recognized by APCs, such as dendritic cells, which activate PRRs, including RIG-I-like receptor (RLR) family[294] and TLRs such as TLR-2, 7, 8, and 9. This activation leads to the production of pro-inflammatory mediators and type I IFNs (such as IFN-α and IFN-β). These IFNs play a fundamental role in the antiviral response by promoting an antiviral state in adjacent cells and activating immune cells, which trigger multifaceted immune responses to present the antigen to CD4+ T lymphocytes in lymphoid tissues like the spleen and lymph nodes[295]. Plasmacytoid dendritic cells (pDCs) produce IFNs in response to YFV in a TLR-7-dependent manner, with this stimulation being more effective with immature viral particles[296]. In addition to this, antiviral response is mediated by RNA-sensing proteins like RIG-I and protein kinase R, which induce pro-inflammatory cytokines upon detection of viral genetic material independently of stress granules[297].
Studies have demonstrated that the viral non-structural protein NS5 interacts with the human transcription factor hSTAT2, induced by IFN-1, playing a crucial role in modulating the host’s immune response, viral replication, and determining the tropism of YFV. This interaction allows the virus to evade type I IFN-mediated antiviral defenses[298,299]. YFV uses its NS5 protein to inhibit IFN-I signaling by interacting with STAT2, a process dependent on IFN-I-induced modifications, specifically STAT1 phosphorylation and NS5 polyubiquitination by TRIM23[300,301].
APCs like macrophages and dendritic cells are crucial in activating Th1-type CD4+ T lymphocytes, where antigens are presented on the surface of APCs via class II major histocompatibility complex molecules, recognized by T cell receptors[302]. The immune response against YFV begins with innate immunity, the body’s first line of defense. Dendritic cells (S100+), present in tissues such as the skin and liver, are among the first to detect YFV. They phagocytize the virus and present its antigens to the adaptive immune system. Macrophages (CD68+), in addition to phagocytizing the virus and infected cells, secrete cytokines and inflammatory mediators, including reactive oxygen species, nitric oxide (NO), and TNF-α, which promote inflammation and recruit other immune cells to the infection site[303]. This release of inflammatory substances and cytokines, causes a “cytokine storm” that triggers disturbances. Consequently, there is midzonal apoptosis of hepatocytes, along with pathological changes like heart apoptosis and acute tubular necrosis in renal tissues[302]. Regarding serum markers, elevated levels of IL-6, MCP-1, IP-10, TNF-α, and IL-1RA were found in the sera of YFV-infected patients who had fatal outcomes compared to those with non-fatal outcomes[304].
Adaptive immunity is triggered when dendritic cells present viral antigens to CD4+ T cells. These helper T cells proliferate and release cytokines that activate other immune cells. In fatal YF cases, CD4+ T cells are predominant and crucial for coordinating the immune response[303]. CD8+ T lymphocytes target and lyse virus-infected liver cells, releasing viral particles that expose antigens to B lymphocyte-produced antibodies. B lymphocytes (CD20+), activated by helper T cells, produce specific antibodies that neutralize YFV, prevent viral entry into new cells, and mark the virus for destruction by macrophages[305].
Hepatic damage: The liver is particularly affected in YF. Immunohistochemical studies on the livers of patients who succumbed to the disease have revealed damage characterized by macro/microvesicular steatosis, apoptosis, and necrosis, especially in the intermediate zone of the liver, where viral antigens were most frequently observed[306].
Studies in animal models[293,307-310] and human tissue biopsies from fatal cases[303,306,311,312] highlight apoptosis as a central mechanism in the pathogenesis of YF, attributed not only to the cytopathic effect (CPE) induced by the virus but also to an imbalanced cytokine response.
The inflammatory infiltrate in the liver of fatal YF cases predominantly consists of CD4+ T lymphocytes, with smaller quantities of CD8+ T lymphocytes, macrophages CD68+, CD20+ B lymphocytes, NKT+ cells, and S100+ dendritic cells[303,311]. Cytokine expression is also notable, with significant numbers of cells expressing TGF-β, and to a lesser extent, TNF-α and IFN-γ[311]. Apoptosis, rather than necrosis, has been identified as the primary mechanism of cell death during infection, likely influenced by viral antigens and the presence of TGF-β, an apoptosis-inducing cytokine responsible for the downregulation of inflammatory infiltrates observed in the liver of fatal cases[303,306,311]. The interaction between the Fas receptor (CD95) and its ligand FasL, induced by TGF-β, is one of the mechanisms by which CD8+ T cells promote the apoptosis of infected cells, thereby limiting viral spread[312]. Other apoptotic markers are also found in the hepatic parenchyma, including CASPASE 3, CASPASE 8, BAX, GRANZYME B, and SURVIVIN[313]. These mechanisms contribute to the pathology observed in fatal cases and various symptoms associated such vascular leak syndrome, thrombocytopenia, changes in aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels, elevated blood urea nitrogen and creatinine, jaundice, vomiting, and hemorrhagic diathesis[302,311].
In situ studies suggest that viral infection activates the endothelium, exacerbating the inflammatory response in the liver. This occurs through the increased expression of adhesion molecules such as E-selectin, P-selectin, ICAM-1, VCAM-1, and VLA-4, which facilitate the adhesion and migration of inflammatory cells into the hepatic parenchyma, con
Moreover, histopathological and immunohistochemical analyses of fatal YF patients revealed a predominant ex
Research indicates that inositol trisphosphate receptor type 3 (ITPR3), a calcium channel isoform, expressed in hepatocytes infected by the virus, can stimulate hepatocyte proliferation and reduce both steatosis and cell death, thereby protecting the organ against insufficiency. This protective mechanism implies that enhancing ITPR3 expression could be an effective therapeutic intervention to prevent the progression of liver failure and reduce mortality, potentially eli
Clinical manifestation: The YFV is predominantly viscerotropic, primarily affecting the liver, as well as organs such as the kidneys, spleen, lymph nodes, and heart[241]. The disease classically manifests in three stages: Infection, remission, and intoxication. These stages are often not well demarcated[320].
In the first stage, the acute infection phase occurs approximately 3 to 6 days after the mosquito bite, presenting clinical characteristics such as high fever (approximately 39 °C), headache, malaise, photophobia, back pain, myalgia, irritability, restlessness, nausea, and vomiting, as well as conjunctival infection, white tongue with a red tip, and bradycardia (Faget’s sign)[302,321]. During this phase, viremia occurs, where the virus replicates and disseminates through the bloodstream, making the host infectious to vectors. Laboratory findings may demonstrate leukopenia, neutropenia, and low C-reactive protein; elevated transaminases and proteinuria may also occur. Only about 45% of infected individuals exhibit symptoms[322].
Next, the remission stage begins, characterized by a temporary improvement in symptoms, lasting between 12 hours and 2 days. The final stage is the intoxication stage, marked by an exacerbated inflammatory response associated with hemodynamic collapse, where there is a sudden deterioration of the patient’s clinical condition, with typical signs of liver and kidney failure, hemorrhagic fever, and multiple organ dysfunction. Liver dysfunction results from apoptosis with limited inflammation and manifests with elevated transaminases and bilirubin, as well as decreased synthesis of coagulation factors produced by the liver[241,303]. Patients may present with jaundice, oliguria or anuria, cardiovascular instability, and hemorrhagic diathesis[302,321]. Notably, about 12% of infected individuals reach this phase, of which approximately 47% succumb to the disease[322].
In the intoxication stage of YF, various laboratory and pathophysiological changes occur that reflect the severity of the disease, including thrombocytopenia with platelet counts below 50000 per milliliter; prolonged coagulation time and prothrombin time; decreased hepatic coagulation factors; decreased fibrinogen and factor VIII; and elevated fibrin degradation products, characteristic of disseminated intravascular coagulation[323,324].
The increase in AST/SGOT can exceed that of ALT/SGPT, which differs from other forms of viral hepatitis and may be related to skeletal or cardiac muscle injuries[241,325].
Laboratory diagnosis: Due to the numerous differential diagnoses stemming from the similarity of symptoms with a wide range of diseases, such as dengue, leptospirosis, viral hepatitis, malaria, and other hemorrhagic diseases, the clinical diagnosis of YF is challenging. Therefore, laboratory confirmation plays an essential role[320]. Laboratory diagnostic methods for YF are crucial to accurately identify the infection in suspected cases. According to the WHO, a suspected case is defined as any person with an acute onset of fever, accompanied by jaundice within 14 days of the onset of initial symptoms[326]. However, this categorization remains restrictive as the disease progresses to jaundice only in its more advanced stages (Table 4).
| Classification | Criteria |
| Probable case | A suspected case and at least one of the following |
| Presence of YF IgM antibody in the absence of YF immunization within 30 days of illness onset | |
| Epidemiological link to a confirmed case or an outbreak (e.g., household members or persons in close proximity through work, residence in past month) | |
| Confirmed case | A probable case and at least one of the following |
| Negative results of differential neutralization testing with flaviviruses endemic in the area of exposure | |
| Seroconversion in appropriately paired samples tested by YF neutralization testing | |
| And absence of YF immunization within 30 days before onset of illness | |
| Or a suspected case and at least one of the following | |
| Detection of YFV genome in blood or other organs by real-time reverse transcriptase polymerase chain reaction | |
| Detection of YF antigen in liver or other organs by immunohistochemistry | |
| Isolation of YF virus | |
| And absence of YF immunization within 14 days before onset of illness | |
| Discarded case | A person who tests negative for YF antibody testing (with specimen collected > 7 days post onset) |
| Or negative immunohistochemistry on tissue samples |
Laboratory tests for YF diagnosis, performed on blood, serum, CSF, urine, or other tissue samples, can be conducted through serological methods, viral isolation, viral genome detection, and viral antigen detection[259].
The most commonly used serological procedure is the detection of anti-YFV antibodies by IgM-ELISA, which offers a presumptive diagnosis of YF within a few hours[241]. The accuracy of this test for detecting YF is generally regarded as high, with a minimum precision of 90%[259]. However, case confirmation requires evaluation of the epidemiological context, detailed vaccination history of the individual, timing of sample collection, and potential co-circulation of other flaviviruses in the region[259]. It is important to consider that this type of test may present limitations, including cross-reactions with other flaviviruses and the need for additional confirmation[327].
NS1 antigen capture ELISA assays have proven efficient for early diagnosis of YF, with high sensitivity and no cross-reactions with other flaviviruses[328]. In serological tests, the PRNT, also known as the virus neutralization test, is more specific for the detection of anti-YFV antibodies compared to other assays and is considered the “gold standard” for differential diagnosis of flaviviruses[329]. However, the requirement for specific cell culture facilities, standardized controls, and well-trained personnel for reproducible results, as well as the 4-7 days needed for result analysis, limit the use of this method during outbreaks[320]. Another technique commonly used to identify IgM and IgG antibodies against the YFV is the indirect immunofluorescence[330].
For viral genome detection, the RT-PCR technique is highly sensitive, capable of detecting YFV in the early stages of infection, even before clinical symptoms manifest. Additionally, RT-PCR can distinguish between wild and vaccine strains of YFV, making it useful for monitoring viral spread and evaluating vaccination strategies[331,332]. Furthermore, other molecular approaches, such as loop-mediated isothermal amplification (LAMP)[333] and reverse transcription-mediated amplification (RT-LAMP)[334,335], offer the possibility of rapid and sensitive YFV diagnosis in field settings without requiring complex equipment.
Treatment, care and prophylaxis in the management of infection: YF presents a significant challenge for healthcare professionals, as it is a severe viral hemorrhagic disease requiring early diagnosis despite often having nonspecific symptoms. There is no specific therapeutic treatment for YF, and its management relies on supportive care. The Pan American Health Organization categorizes YF patients into three groups, corresponding to the classic phases of the disease[241] and reflecting its severity: Group A for mild cases, Group B for patients in remission, and Group C for severe forms with hepatic and renal complications[336].
Due to the potential for rapid disease progression, it is crucial to monitor the warning signs and risk factors associated with YF. For confirmed cases in the infection phase (Group A), attention should be paid to alarm signs such as dehydration, vomiting, diarrhea, abdominal pain, and mild bleeding. Clinical findings such as AST levels more than five times the upper limit of normal, platelet count below 50000/mm³, and proteinuria are also significant[336]. These findings are associated with hepatic damage, hemorrhage risk, and renal impairment[337], respectively. Clinical management in this group includes oral and/or intravenous hydration, pain and fever control using dipyrone (maximum 8 g per day) and acetaminophen (maximum 2 g per day), taking into account the patient’s hepatic condition and avoiding the use of NSAIDs. It is noteworthy that these medication recommendations may not be applicable worldwide, as the use of dipyrone is restricted in several countries.
For Groups B and C, the required level of attention increases due to the worsening of the patient’s overall condition. Severe signs and symptoms at this stage include clinical findings such as jaundice, oliguria, mental confusion, seizures, hemorrhagic phenomena, tachypnea, hypotension, and signs of poor blood perfusion. Group B requires hospitalization, as patients in this group experience dehydration, multiple episodes of vomiting, nausea, diarrhea, altered urinary output, and hemodynamic instability, which may progress to shock. Finally, Group C patients exhibit characteristic signs of hepatic failure, acute renal failure, and hepatic encephalopathy, necessitating intensive care unit (ICU) support with advanced care[336].
Antiviral therapies have not yet been formally recommended for the treatment of YF. However, there is evidence supporting the efficacy of such therapies, including the use of sofosbuvir (an NS5 RNA-dependent RNA polymerase inhibitor), an antiviral drug used against hepatitis C, which has demonstrated antiviral activity against YFV in vitro and in vivo[338,339]. An off-label cohort study using sofosbuvir in YF patients suggested that this medication reduces the viral load of the virus in these patients[340].
Currently, there is no predictive model for the severity and mortality caused by the YFV[336]. However, during the YF epidemic in Brazil, several studies evaluated these parameters. A study conducted on patients admitted to a Brazilian ICU during the 2017 and 2018 outbreaks indicated that factors such as PT-INR, APACHE II, and grade IV hepatic encephalopathy were significant prognostic indicators of mortality risk, independent of other factors[341]. Another 2018 study in São Paulo found that advanced age, high neutrophil count, elevated creatinine, increased AST, and higher viral load were independently associated with higher mortality in YF cases[342]. Additionally, another study demonstrated a higher mortality rate for patients with a history of diabetes mellitus compared to those without this condition. This study also showed that prophylactic use of anticonvulsants for patients with hepatic encephalopathy or arterial ammonia levels above 70 μmol/L reduced the frequency of seizures from 28% to 17%[343]. Furthermore, early aggressive hemodialysis, routine use of intravenous proton pump inhibitors, and plasma exchange were found to be beneficial[343].
Vaccines: Currently, all YF vaccines are derived from the 17D vaccine strain. Vaccination is the most important measure for preventing YF, being safe, accessible, and capable of providing lifelong immunity with a single dose. The YFV has seven genotypes, but only one serotype[241], ensuring that a single vaccine can protect individuals against all genotypes.
The immune response to the attenuated 17D virus (YF-17D) is mediated by both innate and adaptive immune mechanisms. The virus induces a strong innate immune response, triggered by the TLR-2, 7, 8, and 9 receptors on dendritic cells, leading to the production of pro-inflammatory mediators and type 1 IFNs[295]. The stimulation of these receptors induces a balanced Th1 and Th2 immune response[344]. Additionally, pDCs activate downstream signaling pathways that contribute to the antiviral state[252]. Notably, the complement system also plays a role in the innate immune response[345].
The adaptive immune response is characterized by the rapid and specific production of antibodies. The neutralizing IgM antibody is the first to be produced in the humoral response, peaking two weeks after vaccination and persisting for at least 18 months[344]. IgG antibodies are produced subsequently and can last up to 40 years in the body[252]. The vaccine provides effective immunity for 80%-100% of those vaccinated within 10 days and for over 99% within 30 days[254].
A randomized study conducted in Uganda demonstrated that a fractional dose (1/5 of the standard dose) was non-inferior to the standard dose in inducing seroconversion 28 days after vaccination[346]. These findings support the use of fractional doses during outbreaks when vaccine demand surges and in endemic regions where there is a shortage of YF vaccines.
According to WHO, people who are usually excluded from vaccination include[254]: (1) Infants aged less than 9 months; (2) Pregnant women-except during a YF outbreak when the risk of infection is high; (3) People with severe allergies to egg protein; and (4) People with severe immunodeficiency due to symptomatic HIV/AIDS or other causes, or who have a thymus disorder.
Despite the high reliability of the YF-17D vaccine, the occurrence of serious adverse events has been reported. These include vaccine-associated neurotropic disease, with a case fatality rate of 63%, and vaccine-associated viscerotropic disease, with a case fatality rate of less than 1.5%[241,344]. Additionally, anaphylaxis caused by the egg protein present in YF-17D vaccines is also a significant adverse effect[347].
The WHO recommends vaccination against YFV for travelers over nine months of age who are visiting areas at risk of YFV transmission. Additionally, with certain exceptions, vaccination is advised for all individuals residing in these regions, where it should be part of routine immunization programs. In the event of outbreaks, the WHO advocates for mass vaccination campaigns to achieve the necessary coverage to interrupt virus transmission[259] (Table 5).
| Intervention/treatment | ClinicalTrials.gov ID | Status | Phase | Sponsor |
| Biological: 17D Yellow fever vaccine | NCT05332197 | Unknown status | Phase 3 | London School of Hygiene and Tropical Medicine |
| Biological: STAMARIL®/biological: Yellow fever vaccine, bio-manguinhos/biological: yellow fever vaccine, institut pasteur/biological: Yellow fever vaccine, chumakov institute (fractional doses) | NCT02991495 | Completed | Phase 4 | Epicentre |
| Biological: Yellow fever vaccine, institut pasteur (fractional doses) | NCT04059471 | Completed | Phase 4 | University of Oxford |
| Biological: 17DD yellow fever vaccine | NCT02555072 | Completed | Phase 4 | The Immunobiological Technology Institute (Bio-Manguinhos)/Oswaldo Cruz Foundation (Fiocruz) |
| Biological: SII yellow fever vaccine/biological: STAMARIL® | NCT05421611 | Recruiting | Phase 3 | Serum Institute of India Pvt. Ltd. |
| Biological: 17D yellow fever vaccine/other: Deuterad water | NCT01290055 | Recruiting | Phase 4 | Sri Edupuganti |
| Biological: SII yellow fever vaccine/biological: STAMARIL® | NCT05447377 | Recruiting | Phase 3 | Serum Institute of India Pvt. Ltd. |
| Biological: 17DD yellow fever vaccine (fractional doses) | NCT03725618 | Unknown status | Phase 4 | Centers for Disease Control and Prevention |
| Biological: YF-VAX® | NCT00694655 | Recruiting | Phase 4 | Emory University |
| Placebo/biological: CYD tetravalent dengue vaccine/biological: STAMARIL® | NCT01436396 | Completed | Phase 3 | Sanofi Pasteur |
| Biological: YF-VAX® | NCT05374317 | Completed | Phase 4 | United States Army Medical Research Institute of Infectious Diseases |
| Biological: STAMARIL® | NCT01426243 | Completed | Phase 3 | French National Agency for Research on AIDS and Viral Hepatitis |
| Biological: Yellow fever vaccine/biological: MMR vaccine | NCT03368495 | Completed | Phase 4 | Alba Maria Ropero |
| Dietary supplement: vitamin A/biological: candidate plasmodium falciparum malaria vaccine/biological: MR-Vac/biological: STAMARIL® | NCT02699099 | Completed | Phase 3 | GlaxoSmithKline |
| Biological: 17 DD yellow fever vaccine, biomanguinhos | NCT03132311 | Recruiting | Phase 4 | Oswaldo Cruz Foundation |
| Biological: Typhoid Vi polysaccharide vaccine/biological: Yellow fever vaccine/biological: Japanese encephalitis vaccine/biological: Rabies Vaccine/biological: MenACWY-CRM vaccine | NCT01466387 | Completed | Phase 3 | Novartis |
| Placebo/TAK-003 tetravalent dengue vaccine/YF-17D yellow fever vaccine | NCT03342898 | Completed | Phase 3 | Takeda |
| Placebo/biological: BCG vaccine/biological: Yellow fever vaccine/drug: Vancomycin/drug: neomycin | NCT06148025 | Recruiting | Phase 4 | South Australian Health and Medical Research Institute |
CHIKV is a mosquito-borne virus responsible for periodic and explosive outbreaks of a febrile disease that is characterized by severe and sometimes prolonged polyarthritis[348]. CHIKV is in the Togaviridae family, genus Alphavirus. It is a small spherical enveloped virus, with a 60-70 nm diameter and its genome is a single strand RNA molecule of positive polarity, encoding four nonstructural (nsP1-4) and three structural proteins (C, E1, and E2)[349].
CHIKV is maintained in a complex sylvatic and rural cycle. The sylvatic cycles of CHIKV exist primarily in Africa between NHPs, rodents and possibly in bats and forest dwelling Ae. species (Ae. albopictus, Ae. furcifer, Ae. africanus, and Ae. taylori)[350]. Regarding rural and urban cycles, CHIKV transmission is primarily sustained within urban envi
Recent outbreaks of CHIKV in the Indian Ocean basin and Southeast Asia have been attributed to strains from the Indian Ocean lineage, a newly emerged subgroup within the ECSA clade[353]. This subgroup includes strains with an adaptive mutation (E1-A226V) that enhances viral fitness in Ae. albopictus mosquitoes, while maintaining replication efficiency in Ae. aegypti[354-357].
Thus, the intensification and expansion of vector-borne diseases are likely to become significant threats due to climate change. Although complicating factors such as mosquito range limits and viral evolution exist, climate change is expected to cause a substantial increase in exposure to Aedes-borne viruses. Moreover, several modeling studies predict that climate change will result in the expansion of vectors into temperate zones[358,359].
Despite the low mortality rates associated with CHIKV, it results in significant illness, severely affecting the quality of life for those affected and causing substantial economic losses, particularly in developing nations[360]. Recent outbreaks have heightened concerns about the potential public health impact of CHIKV in temperate regions. Factors such as urbanization, increased human mobility, viral adaptation, inadequate control measures, and the spread of new vectors are likely driving the resurgence of CHIKV infections[361].
CHIKV infection typically begins suddenly with a high fever, often accompanied by joint pain. Additional symptoms, though less common, can include severe polyarthralgia and arthritis, as well as rash, muscle pain, and headaches[348]. Acute symptomatic CHIKV disease often resembles other well-known arbovirus infections, such as DENV and ZIKV disease[362]. The simultaneous presence of these viruses in the same area complicates accurate diagnosis, as their symptoms can overlap and lead to frequent misdiagnoses.
Viral entry, replication and release: After the skin bite, the CHIKV enters subcutaneous capillaries and replicates in variety of susceptible cells, including dendritic cells, macrophages, synovial fibroblasts, ECs, and myocytes[362,363]. Additionally, it infects osteoblasts, contributing to the joint pathology and erosive disease observed in chronic arthritis patients[364]. To mediate the processes of entry and viral cell-cell spread, the fusion-related envelope glycoproteins of CHIKV, especially E1 and E2, expressed on the surface of the virion, are essential[365].
Thereby, multiple pathways and mechanisms are employed for CHIKV entry in a cell-type specific manner[366], such as then cell-adhesion molecule, matrix-remodeling-associated protein 8 (MXRA8), a multiple arthritogenic alphavirus receptor, widely expressed in epithelial and mesenchymal cells. Through the creation of various deletion variants, it has been demonstrated that the stalk region of MXRA8 is crucial for facilitating CHIKV entry as it binds in the ‘‘canyon’’ between two protomers of the E spike on the surface of the virion[365,367-369]. Besides, another host protein also identified as an entry factor for CHIKV is the CD147 complex, involved in its replication cycle. CD147 is widely expressed in various human cell types, including fibroblast and ECs. Interestingly, CD147 contains similar protein domains and high structural homology as the previously mentioned alphavirus entry factor MXRA8[370].
Glycosaminoglycans were also shown to be host molecules involved in the binding of CHIKV, particularly heparin/heparan sulfate[371,372]. Some of the other already know CHIKV cell receptors are prohibitin[373], the phospha
Furthermore, during the entry process, most reports indicate that CHIKV enters cells via clathrin-mediated endocytosis[377], although clathrin-independent pathways have been reported to mediate CHIKV entry to the target cells[378]. In addition, other pathways such as macropinocytosis[379,380], and the engulfment of apoptotic blebs[381]. Following entry, the virus’s incubation period ranges from three to seven days[362].
Upon the early endosome’s formation, clathrin molecules detach from the endocytic vesicle. This detachment, along with the endosome’s pH acidification, prompts the fusion of the endosomal membrane with the viral membranes (via the E1 protein), leading to the release of genomic RNA. Immediately following this release, the ribosome translates the non-structural polyproteins (P1234 precursor). Then, the P1234 polyprotein is cleaved by nsP2, liberating the individual non-structural proteins, which forms the viral replicase complex. Consequently, this complex mediates the synthesis of negative-strand RNA, which serves as templates for both new positive-strand RNA and 26S subgenomic RNA. Next, the synthesis of these RNA forms takes place in specialized replication compartments known as spherules. The subgenomic RNA is then translated into the structural polyprotein precursor C-pE2-6K-E1 in the rough ER. The C protein, containing a protease domain, self-cleaves, dissociates from the polyprotein, and assembles with the genomic RNA to form the icosahedral nucleocapsid core in the cytoplasm. The pE2-6K-E1 precursor is directed to the RER lumen for maturation, culminating in the formation of E1-E2 heterodimers. These heterodimers are inserted into the cell membrane, creating the “virus budding microdomain”. Finally, the assembled icosahedral nucleocapsid core migrates to this domain, where new viral particles are released extracellularly via the budding process[382-386].
Currently, the precise cellular mechanisms of the disease are still in need of elucidation. Although the structural details of the coat glycoproteins essential for viral entry are well understood, the potential target cell receptors and the exact mechanism of cell entry remain less well-known[387]. Lastly, numerous cell types, many of which are found at disease sites, are susceptible to CHIKV. As previously mentioned, these include chondrocytes, ECs, fibroblasts, hepatocytes, macrophages, monocytes, muscle satellite cells, myocytes, and osteoblasts[388-391].
Innate antiviral IFN response: Foremost, CHIKV infection induces systemic innate responses, primarily involving antiviral IFN-α, pro-inflammatory cytokines, and chemokines. This process is subsequently followed by the activation of adaptive immune responses[392].
When a chikungunya virion infects its initial target cells, it activates specific PRRs for RNA viruses, such as RLRs (RIG-I and MDA5) in the cytoplasm and TLRs, including TLR-3, TLR-7, and TLR-8 in the endosomal compartment[386,393]. This viral recognition initiates the signaling cascade of the innate immune response, activating IFN regulatory factors[394,395] and leading to the induction of type I-IFNs and various proinflammatory chemokines and cytokines. CHIKV infection has been demonstrated to induce the increased production of type I IFNs (IFN-α and IFN-β) as well as IFN-γ[394,396], that ultimately result in the induction of hundreds of ISGs that cooperatively establish an antiviral state within the infected and adjacent cells, including inhibition of viral replication and virion maturation[397-399].
Consequently, to persist within infected host cells, CHIKV has developed various strategies to evade IFN responses, much like many other viruses, such as CHIKV-encoded proteins strongly inhibiting the activation of the IFN-β and NF-κB promoters. Thus, CHIKV-encoded proteins may evade viral detection, resulting in a reduction of IFN responses[400-402].
Analysis of chemokines in CHIKV-infected patients shows a significant increase in IFN-γ-induced chemokines, such as CXCL9/MIG and CXCL10/IP-10, during the early days of infection[396]. IFN-γ, a primary cytokine for type 1 T helper cells, promotes the production of these chemokines, which are associated with the severity of CHIKV disease[403]. Additionally, elevated levels of CCL2/MCP-1, a known monocyte attractant, were detected, suggesting its involvement in disease progression and bone loss during chronic stages[404,405]. Furthermore, high levels of C5a anaphylatoxin were found early in the infection, a new observation. C5a’s role in inflammation, similar to its effects in rheumatoid arthritis and dengue fever, indicates its potential as a therapeutic target for managing rheumatic complications associated with CHIKV[406-408].
Adaptative immune response: There is relatively more knowledge about innate immune responses to infection, which primarily react to the acute phase of the disease[409]. Research indicates that CD4 T cells are essential for mediating humoral immunity against CHIKV[410]. In patients who have chronic chikungunya infection or have recovered from it, IFN-γ-producing CD4+ T and CD8+ T cells were detectable in the majority (85%) of the patients 12 to 24 months post-infection[392]. These T cells were primarily directed against the nsP1 and E2 peptides[411]. However, the pathogenic roles of CD4+ T cells have also been demonstrated, as findings indicate that CD4+ T, and not CD8+ T cells, are responsible for the joint inflammation induced by CHIKV infection[412].
In patients with acute CHIKV infection, CD8+ T cells were shown to be activated, evidenced by increased expression of activation markers such as CD69, CD107a, perforin, and granzyme, which suggests that CD8+ T cells are active during the acute phase and mediate cytotoxic activities[413]. Results such as the increased activation of CD8+ T cells in patients with acute CHIKV infection, the accumulation of CHIKV-specific CD8+ T cells in mouse spleen and joint-associated tissues, and their ability to produce IFN-γ upon ex vivo stimulation, suggest that CD8+ T cells are functionally active during the acute phase[414]. However, the fact that these effector T cells did not reduce viremia levels, combined with the observation that pre-existing functional effector CD8+ T cells led to CHIKV clearance mainly in the spleen, suggests that CHIKV has developed strategies to evade CD8+ T cell recognition, allowing it to establish chronic infection in the joints[414].
Antibodies play a crucial role in controlling CHIKV infection[410]. Anti-CHIKV IgM levels gradually increase from as early as day 2-4 after symptom onset[415,416] and then remain stable for up to 4 months[417]. Similarly, anti-CHIKV IgG can be detected in the early convalescent stage, appearing around 10 days post-symptom onset in some patients, and persists for 2-3 months[418]. Early formation of IgG3 antibodies at day 10 correlates with reduced viremia levels and mitigates chronic and severe disease[418]. Moreover, early production of IgM antibodies, which possess neutralizing capabilities, contributes to lowering viremia levels, and the neutralizing activity of IgM complements early IgG antibodies and plays a critical role from days 4 to 10 post-symptom onset[416]. Additionally, the presence of both IgM and IgG is associated with modulation of cytokine and chemokine levels, suggesting their role in regulating immune responses in CHIKV-infected patients[418,419].
Antibodies directed against the E2 glycoprotein are critical in the immune response to CHIKV infection[410,420-422]. Furthermore, studies in CHIKV-infected patients have identified several monoclonal antibodies that neutralize the virus by targeting epitopes in the E1 and E2 glycoproteins, with E2-specific antibodies proving effective in protecting against lethal chikungunya infection[423]. These antibodies block viral fusion and release from infected cells[420]. Moreover, analysis of antibody responses in patients has revealed that CHIKV-specific IgG avidity increases over time, correlating with improved neutralizing capacity[414]. Additionally, patients in the acute phase show higher avidity against E1 and E2 proteins compared to those with chronic infections[424], suggesting that robust antibody responses may play a crucial role in preventing chronic disease progression.
Chronic chikungunya arthritis: It is roughly estimated that 30% to 40% of infected individuals experience some long-term sequelae. These sequelae include persistent arthralgia and/or arthritis, with severe pain present in about 37% of individuals suffering from persistent arthralgia[425]. Factors associated with the persistence of arthralgia in CHIKV-infected patients have not been fully explored. However, the few available studies indicate that patients over 40 years old, females, and those with higher levels of CXCL8 detected during the acute phase of the disease are more likely to experience persistent arthralgia[426,427].
Recent studies have identified human synovial tissues as sanctuaries for CHIKV, where viral RNA persists in joint fluid[428]. This persistence is thought to contribute to CHIKV -associated arthritis by infecting fibroblast-like synoviocytes and promoting the migration of primary human monocytes[429]. These monocytes/macrophages can then transform into osteoclast-like cells, producing high levels of TNF-α and IL-6 proinflammatory cytokines.
Despite advancements in our understanding of CHIKV infection, the exact immunopathogenic mechanisms that lead to CHIKV-induced arthralgia remain unclear. Cytokines and chemokines are key players in CHIKV immunopathology, considering that during the early acute phase, serum proinflammatory cytokines such as IFN-α, IFN-β, IFN-γ, CXCL10/IP-10, and IL-1β show a strong upregulation. IFN-α is detected early during infection, often on the first day, and its concentration correlates with viral load, which is significantly higher in elderly patients[430]. Additionally, the elevation of MCP-1, IL-6, IL-8, MIP-1α, and MIP-1β is most prominent in the chronic phase[431].
Furthermore, another study found that levels of CXCL9/MIG and CXCL10/IP-10, along with high concentrations of IgG, were associated with severe symptoms in CHIKV patients[432]. Moreover, TNF-α and IFN-γ-secreting NK-like T cells were elevated in patients with persistent CHIKV arthralgia[433]. In addition, a systematic meta-analysis of immune signatures in patients with acute CHIKV infection showed a correlation between increased proinflammatory cytokines and arthralgia[426].
CHIKV infection is typically classified into three stages: The acute stage (lasting from day 1 to day 21), the post-acute stage (spanning from day 21 to 3 months), and the chronic stage (lasting beyond 3 months)[434].
Acute phase of infection: During the acute stage, infected patients may undergo a viremic phase lasting 5 to 10 days, followed by a post-viremic phase lasting 6 to 21 days[434]. The acute viremic stage of CHIKV infection is characterized by an abrupt onset of high-grade fever (often > 39 °C), arthralgia, myalgia, headache, fatigue, nausea, vomiting, and arthritis. Additionally, other symptoms such as conjunctivitis, exanthema, and edema may also occur. The exanthema can manifest as a diffuse or focal skin rash. Despite these symptoms, the disease is self-limiting, and in most patients, it resolves within 7-10 days[430,435].
In the post-acute phase, symptoms exhibit diverse manifestations stemming from persistent initial acute symptoms, such as joint issues and fatigue, while fever typically subsides[436]. Additionally, polyarthritis tends to affect both sides of the body symmetrically, involving both small and large joints like knees, ankles, hands, and wrists and there may be periarticular involvement, including enthesitis, tenosynovitis, and bursitis[437,438]. Furthermore, acute CHIKV infection can potentially exacerbate pre-existing autoimmune arthritis[439].
Severe symptoms affecting vital organs can develop during CHIKV infection, including encephalitis, encephalopathy, and neuro-ocular diseases (such as uveitis, retinitis, and optic neuritis), as well as myelopathy and myelitis, Guillain-Barré syndrome, myocarditis, hepatitis, acute interstitial nephritis, severe sepsis, septic shock, and multi-organ failure[440-442]. Furthermore, individuals with comorbidities, the elderly, and infants are at a higher risk for these severe symptoms[442-444]. Additionally, perinatal CHIKV infection can result in sequelae such as microcephaly, cerebral palsy, and neurocognitive impairment[445].
Chronic or persistent phase of infection: The chronic stage of CHIKV infection is marked by symptoms that persist for over three months following the initial diagnosis of acute infection. Specifically, this chronic phase often affects distal joints due to continuous viral replication and inflammation. Moreover, the virus has been detected in several organs, including ECs in the liver, mononuclear cells in the spleen, macrophages in the synovial fluid and surrounding tissues, and satellite cells in the muscles[348].
During the chronic stage, patients can suffer from unpredictable relapses of fever, fatigue, joint pain, and stiffness[446,447]. Furthermore, older individuals and those with a history of rheumatic or traumatic joint disorders are more likely to develop this chronic phase[447]. Age, female sex, and dehydration state during the acute phase are also important factors[448].
Diagnosis: The diagnosis of CHIKV infection can be conducted using various methods, including viral isolation, serological assays, and RNA detection through real-time quantitative reverse transcriptase PCR (qRT-PCR)[449]. RT-PCR and virus isolation are most effective when conducted near the onset of febrile illness, when viremia is at its peak. Serological tests to detect IgM and IgG antibodies are best performed approximately seven days after the onset of symptoms[386].
Diagnosing CHIKV infection solely based on clinical symptoms poses significant challenges, particularly in endemic regions where other arboviruses like DENV and ZIKV are also circulating. Healthcare providers should consider CHIKV infection in patients exhibiting acute febrile illness and polyarthralgia, especially among travelers returning from areas known for CHIKV transmission[386].
Virus isolation through cell culture is considered the gold standard for viral detection due to its high specificity[447]. To isolate CHIKV, various cell lines from humans, monkeys, and mosquitoes have been utilized[450]. CHIKV infection typically results in a noticeable CPE in culture, which can be observed as early as 24 hours after infection[363].
Nucleic acid detection is a rapid and highly sensitive assay used to diagnose CHIKV infection. One common method is qRT-PCR, which detects the CHIKV genome[451-454]. This technique can also be employed in multiplex PCR assays to simultaneously detect other arboviruses, including ZIKV[454]. A conserved region of the envelope E1 and E2 genes is the most common target for qRT-PCR, and other targets include nsP1 and nsP4 genes[451,452,455]. The viral RNA of CHIKV can be detected by the qRT-PCR method from 0 to 7 days of infection, after which qRT-PCR detection becomes unreliable[456].
Serological testing, simpler than qRT-PCR, can detect anti-CHIKV immunoglobulin M (IgM) and IgG. These antibodies are detectable using ELISA, immunofluorescence assays (IFA), and PRNT[457]. Among these, IgM ELISA is the most frequently utilized method for diagnosing CHIKV infection[458]. Serology-based diagnosis is constrained by potential cross-reactivity with other arboviruses[459]. CHIKV shares antigenic similarities with other viruses in the Alphavirus genus, such as Semliki forest virus, Mayaro virus, and o’nyong nyong virus[460].
A systematic review and meta-analysis found that IgM detection tests demonstrated over 90% diagnostic accuracy for ELISA-based tests, IFA, in-house developed tests, and samples collected more than seven days after symptom onset. However, sensitivity was lower for rapid tests (42.3%), commercial tests (78.6%), and samples collected within seven days before symptom onset (26.2%). Therefore, IgM detection tests are particularly recommended for use with samples obtained during the convalescent phase of CHIKV infection. The specificity of IgM detection tests remained above 90% across all test formats and sample collection times[461].
Serological tests for CHIKV E1/E2 antigen detection, including rapid assays[461,462], ELISA-based tests[456], and FLISA-based tests[458] have shown promising performance. However, challenges in developing antigen-based tests include variability in performance across different CHIKV genotypes[463]. Therefore, further evaluation in diverse geographical settings is crucial to validate these tests against all circulating CHIKV genotypes.
Treatment: At present, treatment for chikungunya primarily aims to alleviate symptoms, as there is no dedicated vaccine or specific treatment available. As a result, a range of drugs have been utilized with mixed effectiveness, primarily targeting supportive care and the reduction of joint pain. It should be tailored to the clinical context and targeted at specific risk groups, focusing on managing fever and pain, addressing dehydration, providing organ support, and preventing iatrogenic complications and functional impairment[464]. Key pharmaceutical treatments for chikungunya include NSAIDs, disease-modifying antirheumatic drugs, and antivirals[465].
Analgesia using acetaminophen is the preferred treatment. Avoiding NSAIDs and salicylates within the initial 14 days of disease onset is advised due to the risk of bleeding complications associated with dengue fever, unless dengue has been definitively ruled out[464]. No specific NSAID class has demonstrated superiority in effectively managing post-chikungunya symptoms. In cases where conditions such as tenosynovitis, bursitis, tunnel syndrome, capsulitis, or synovitis are not adequately controlled by oral treatments, local anti-inflammatory therapy (either topical or via infiltration) should be prescribed to minimize excessive systemic medication use[434].
The use of corticosteroids is not recommended due to the risk of severe rebound arthritis and tenosynovitis. Therefore, systemic corticosteroids should only be considered for treating inflammatory polyarticular presentations, particularly when there is concurrent tenosynovitis or active synovitis, or when NSAIDs are ineffective or contraindicated[460].
In animal models of CHIKV infection, the use of CHIKV IgG or CHIKV-specific monoclonal antibodies for prophylaxis has demonstrated protective effects[466]. This highlights the potential of antibody-based therapies as a promising strategy for preventing severe CHIKV infection in at-risk individuals.
In cell-based screenings aimed at combating CHIKV infection, several drugs with antiviral properties have been identified. These drugs target distinct stages of the CHIKV replication cycle: Chloroquine[467] and chlorpromazine[468] act on virus entry, while harringtonine and homoharringtonine[469] affect viral protein translation. Others, such as trigocherriolide A[470], ribavirin[471], IFN-alpha[472], apigenin, and silybin[468], and inhibit virus replication.
Vaccines: Given the rapid spread of CHIKV across many countries, vaccinating the susceptible population remains the most effective method to control infection. CHIKV preclinical candidate vaccines under development encompass a variety of approaches, including a whole-virus inactivated vaccine[473], a VEE/CHIKV chimeric vaccine[474], a re
| Vaccine technology platforms | Intervention/treatment | ClinicalTrials.gov ID | Phase | Status | Sponsor |
| Virus-like particle vaccine | Biological: VRC-CHKVLP059-00-VP | NCT01489358 | 1 | Completed | NIAID |
| Biological: PXVX0317 | NCT03992872 | 2 | Completed | Bavarian Nordic | |
| Biological: CHIKV VLP/adjuvant | NCT05072080 | 3 | Completed | Bavarian Nordic | |
| Biological: VRC-CHKVLP059-00-VP | NCT02562482 | 2 | Completed | NIAID | |
| Viral vectored vaccine | Biological: MV-CHIK/ Biological: MMR-vaccine | NCT03101111 | 2 | Completed | Themis Bioscience GmbH |
| Biological: ChAdOx1 Chik | NCT04440774 | 1 | Completed | University of Oxford | |
| Live attenuated vaccine | Biological: VLA1553 | NCT04650399 | 3 | Completed | Butantan Institute |
| Biological: VLA1553 | NCT03382964 | 1 | Completed | Valneva Austria GmbH | |
| Biological: V184 | NCT03807843 | 2 | Completed | Themis Bioscience GmbH | |
| Inactivated whole virion vaccine | Biological: BBV87 | NCT04566484 | 3 | Completed | International Vaccine Institute |
The leading vaccine candidate, a live-attenuated Δ5nsP3 (Valneva), has successfully completed Phase III trials and is now in the process of obtaining licensure. This vaccine stands out due to its superiority over single-dose regimens, achieving a remarkable 98.9% seroconversion in adults and older adults. It holds potential benefits for travelers planning to visit endemic areas. Nevertheless, further research is needed to determine its effectiveness in providing protection within endemic regions[479-481].
Another vaccine candidate, the adjuvanted VLP (CHIKV VLP) PXVX0317PXV, is non-self-replicating and known for its ability to elicit robust immunogenicity. However, it necessitates two doses, and the ongoing trial does not include participants from endemic areas. Therefore, this vaccine might be a viable option for immunocompromised individuals[482-485].
In summary, DENV, ZIKV, YFV, and CHIKV share several common characteristics, including their protein structure, tropism, evasion of the immune response, and various symptoms such as fever and rash. Moreover, they often have common transmission vectors and can exhibit cross-reactions in laboratory tests. Nonetheless, their individualities can significantly aid in differential diagnosis, necessitating healthcare professionals to adopt a clinical management approach tailored to the patients’ symptoms and to prevent potential complications typically associated with a specific virus. For example, dengue is often correlated with hemorrhages, Zika with microcephaly, YFV with hepatitis, and CHIKV with polyarthralgia.
However, it is essential to highlight that the immune response of each patient and their symptoms are not always clear, and symptoms more commonly associated with one viral infection can also be present in others. Therefore, laboratory and epidemiological diagnoses, considering local endemics and current outbreaks, are crucial in accurately diagnosing the aforementioned arboviruses.
| 1. | Beckham JD, Tyler KL. Arbovirus Infections. Continuum (Minneap Minn). 2015;21:1599-1611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 2. | World Health Organization. Vector-borne diseases. September 26, 2024. [cited 1 October 2024]. Available from: https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases. |
| 3. | Rodrigues-Alves ML, Melo-Júnior OAO, Silveira P, Mariano RMDS, Leite JC, Santos TAP, Soares IS, Lair DF, Melo MM, Resende LA, da Silveira-Lemos D, Dutra WO, Gontijo NF, Araujo RN, Sant’Anna MRV, Andrade LAF, da Fonseca FG, Moreira LA, Giunchetti RC. Historical Perspective and Biotechnological Trends to Block Arboviruses Transmission by Controlling Aedes aegypti Mosquitos Using Different Approaches. Front Med (Lausanne). 2020;7:275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (1)] |
| 4. | Grard G, Caron M, Mombo IM, Nkoghe D, Mboui Ondo S, Jiolle D, Fontenille D, Paupy C, Leroy EM. Zika virus in Gabon (Central Africa)--2007: a new threat from Aedes albopictus? PLoS Negl Trop Dis. 2014;8:e2681. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 471] [Cited by in RCA: 476] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 5. | Duffy MR, Chen TH, Hancock WT, Powers AM, Kool JL, Lanciotti RS, Pretrick M, Marfel M, Holzbauer S, Dubray C, Guillaumot L, Griggs A, Bel M, Lambert AJ, Laven J, Kosoy O, Panella A, Biggerstaff BJ, Fischer M, Hayes EB. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360:2536-2543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2211] [Cited by in RCA: 2114] [Article Influence: 132.1] [Reference Citation Analysis (0)] |
| 6. | Laporta GZ, Potter AM, Oliveira JFA, Bourke BP, Pecor DB, Linton YM. Global Distribution of Aedes aegypti and Aedes albopictus in a Climate Change Scenario of Regional Rivalry. Insects. 2023;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 70] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 7. | Kading RC, Brault AC, Beckham JD. Global Perspectives on Arbovirus Outbreaks: A 2020 Snapshot. Trop Med Infect Dis. 2020;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 8. | Tajudeen YA, Oladunjoye IO, Mustapha MO, Mustapha ST, Ajide-Bamigboye NT. Tackling the global health threat of arboviruses: An appraisal of the three holistic approaches to health. Health Promot Perspect. 2021;11:371-381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Harapan H, Michie A, Sasmono RT, Imrie A. Dengue: A Minireview. Viruses. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 205] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 10. | Sinha S, Singh K, Ravi Kumar YS, Roy R, Phadnis S, Meena V, Bhattacharyya S, Verma B. Dengue virus pathogenesis and host molecular machineries. J Biomed Sci. 2024;31:43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 11. | Mbanzulu KM, Wumba R, Mboera LEG, Kayembe JN, Engbu D, Bojabwa MM, Zanga JK, Misinzo G, Kimera SI. Pattern of Aedes aegypti and Aedes albopictus Associated with Human Exposure to Dengue Virus in Kinshasa, the Democratic Republic of the Congo. Trop Med Infect Dis. 2022;7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Huang YS, Higgs S, Vanlandingham DL. Emergence and re-emergence of mosquito-borne arboviruses. Curr Opin Virol. 2019;34:104-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 107] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 13. | World Health Organization. Global strategy for dengue prevention and control 2012-2020. August 22, 2012. [cited 1 October 2024]. Available from: https://www.who.int/publications/i/item/9789241504034. |
| 14. | Guo C, Zhou Z, Wen Z, Liu Y, Zeng C, Xiao D, Ou M, Han Y, Huang S, Liu D, Ye X, Zou X, Wu J, Wang H, Zeng EY, Jing C, Yang G. Global Epidemiology of Dengue Outbreaks in 1990-2015: A Systematic Review and Meta-Analysis. Front Cell Infect Microbiol. 2017;7:317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 235] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 15. | Stanaway JD, Shepard DS, Undurraga EA, Halasa YA, Coffeng LE, Brady OJ, Hay SI, Bedi N, Bensenor IM, Castañeda-Orjuela CA, Chuang TW, Gibney KB, Memish ZA, Rafay A, Ukwaja KN, Yonemoto N, Murray CJL. The global burden of dengue: an analysis from the Global Burden of Disease Study 2013. Lancet Infect Dis. 2016;16:712-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 567] [Cited by in RCA: 709] [Article Influence: 78.8] [Reference Citation Analysis (0)] |
| 16. | Delrieu M, Martinet JP, O’Connor O, Viennet E, Menkes C, Burtet-Sarramegna V, Frentiu FD, Dupont-Rouzeyrol M. Temperature and transmission of chikungunya, dengue, and Zika viruses: A systematic review of experimental studies on Aedes aegypti and Aedes albopictus. Curr Res Parasitol Vector Borne Dis. 2023;4:100139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 17. | Robinson M, Einav S. Towards Predicting Progression to Severe Dengue. Trends Microbiol. 2020;28:478-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Roy SK, Bhattacharjee S. Dengue virus: epidemiology, biology, and disease aetiology. Can J Microbiol. 2021;67:687-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 160] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 19. | Ngono AE, Shresta S. Immune Response to Dengue and Zika. Annu Rev Immunol. 2018;36:279-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 187] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 20. | Wong JM, Adams LE, Durbin AP, Muñoz-Jordán JL, Poehling KA, Sánchez-González LM, Volkman HR, Paz-Bailey G. Dengue: A Growing Problem With New Interventions. Pediatrics. 2022;149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 45] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 21. | O’Driscoll M, Buddhari D, Huang AT, Waickman A, Kaewhirun S, Iamsirithaworn S, Khampaen D, Farmer A, Fernandez S, Rodriguez-Barraquer I, Srikiatkhachorn A, Thomas S, Endy T, Rothman AL, Anderson K, Cummings DAT, Salje H. Maternally derived antibody titer dynamics and risk of hospitalized infant dengue disease. Proc Natl Acad Sci U S A. 2023;120:e2308221120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Vaughn DW, Green S, Kalayanarooj S, Innis BL, Nimmannitya S, Suntayakorn S, Endy TP, Raengsakulrach B, Rothman AL, Ennis FA, Nisalak A. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J Infect Dis. 2000;181:2-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1124] [Cited by in RCA: 1157] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 23. | Perdomo-Celis F, Salgado DM, Narváez CF. Magnitude of viremia, antigenemia and infection of circulating monocytes in children with mild and severe dengue. Acta Trop. 2017;167:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 24. | Soo KM, Khalid B, Ching SM, Tham CL, Basir R, Chee HY. Meta-analysis of biomarkers for severe dengue infections. PeerJ. 2017;5:e3589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 25. | Ten Bosch QA, Clapham HE, Lambrechts L, Duong V, Buchy P, Althouse BM, Lloyd AL, Waller LA, Morrison AC, Kitron U, Vazquez-Prokopec GM, Scott TW, Perkins TA. Contributions from the silent majority dominate dengue virus transmission. PLoS Pathog. 2018;14:e1006965. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 103] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 26. | Wu P, Yu X, Wang P, Cheng G. Arbovirus lifecycle in mosquito: acquisition, propagation and transmission. Expert Rev Mol Med. 2019;21:e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 27. | Rodenhuis-Zybert IA, Wilschut J, Smit JM. Dengue virus life cycle: viral and host factors modulating infectivity. Cell Mol Life Sci. 2010;67:2773-2786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 296] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 28. | Urcuqui-Inchima S, Patiño C, Torres S, Haenni AL, Díaz FJ. Recent developments in understanding dengue virus replication. Adv Virus Res. 2010;77:1-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 29. | Trivedi S, Chakravarty A. Neurological Complications of Dengue Fever. Curr Neurol Neurosci Rep. 2022;22:515-529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 70] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 30. | Chen Y, Maguire T, Hileman RE, Fromm JR, Esko JD, Linhardt RJ, Marks RM. Dengue virus infectivity depends on envelope protein binding to target cell heparan sulfate. Nat Med. 1997;3:866-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 760] [Cited by in RCA: 785] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 31. | Navarro-Sanchez E, Altmeyer R, Amara A, Schwartz O, Fieschi F, Virelizier JL, Arenzana-Seisdedos F, Desprès P. Dendritic-cell-specific ICAM3-grabbing non-integrin is essential for the productive infection of human dendritic cells by mosquito-cell-derived dengue viruses. EMBO Rep. 2003;4:723-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 388] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 32. | Reyes-Del Valle J, Chávez-Salinas S, Medina F, Del Angel RM. Heat shock protein 90 and heat shock protein 70 are components of dengue virus receptor complex in human cells. J Virol. 2005;79:4557-4567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 281] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 33. | Khandia R, Munjal A, Dhama K, Karthik K, Tiwari R, Malik YS, Singh RK, Chaicumpa W. Modulation of Dengue/Zika Virus Pathogenicity by Antibody-Dependent Enhancement and Strategies to Protect Against Enhancement in Zika Virus Infection. Front Immunol. 2018;9:597. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 91] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 34. | Nanaware N, Banerjee A, Mullick Bagchi S, Bagchi P, Mukherjee A. Dengue Virus Infection: A Tale of Viral Exploitations and Host Responses. Viruses. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 69] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 35. | Cruz-Oliveira C, Freire JM, Conceição TM, Higa LM, Castanho MA, Da Poian AT. Receptors and routes of dengue virus entry into the host cells. FEMS Microbiol Rev. 2015;39:155-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 233] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 36. | Marzinek JK, Holdbrook DA, Huber RG, Verma C, Bond PJ. Pushing the Envelope: Dengue Viral Membrane Coaxed into Shape by Molecular Simulations. Structure. 2016;24:1410-1420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 37. | Kuhn RJ, Zhang W, Rossmann MG, Pletnev SV, Corver J, Lenches E, Jones CT, Mukhopadhyay S, Chipman PR, Strauss EG, Baker TS, Strauss JH. Structure of dengue virus: implications for flavivirus organization, maturation, and fusion. Cell. 2002;108:717-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1145] [Cited by in RCA: 1176] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 38. | Tay MYF, Vasudevan SG. The Transactions of NS3 and NS5 in Flaviviral RNA Replication. Adv Exp Med Biol. 2018;1062:147-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 39. | Teramoto T, Balasubramanian A, Choi KH, Padmanabhan R. Serotype-specific interactions among functional domains of dengue virus 2 nonstructural proteins (NS) 5 and NS3 are crucial for viral RNA replication. J Biol Chem. 2017;292:9465-9479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 40. | Osawa T, Aoki M, Ehara H, Sekine SI. Structures of dengue virus RNA replicase complexes. Mol Cell. 2023;83:2781-2791.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 41. | Singh K, Martinez MG, Lin J, Gregory J, Nguyen TU, Abdelaal R, Kang K, Brennand K, Grünweller A, Ouyang Z, Phatnani H, Kielian M, Wendel HG. Transcriptional and Translational Dynamics of Zika and Dengue Virus Infection. Viruses. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 42. | Carter CC, Mast FD, Olivier JP, Bourgeois NM, Kaushansky A, Aitchison JD. Dengue activates mTORC2 signaling to counteract apoptosis and maximize viral replication. Front Cell Infect Microbiol. 2022;12:979996. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 43. | Chaudhary N, Srivastava S, Gupta S, Menon MB, Patel AK. Dengue virus induced autophagy is mediated by HMGB1 and promotes viral propagation. Int J Biol Macromol. 2023;229:624-635. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 44. | Frakolaki E, Kaimou P, Moraiti M, Kalliampakou KI, Karampetsou K, Dotsika E, Liakos P, Vassilacopoulou D, Mavromara P, Bartenschlager R, Vassilaki N. The Role of Tissue Oxygen Tension in Dengue Virus Replication. Cells. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 45. | Liang S, Wu YS, Li DY, Tang JX, Liu HF. Autophagy in Viral Infection and Pathogenesis. Front Cell Dev Biol. 2021;9:766142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 46. | Barnard TR, Abram QH, Lin QF, Wang AB, Sagan SM. Molecular Determinants of Flavivirus Virion Assembly. Trends Biochem Sci. 2021;46:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 66] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 47. | Alcon-LePoder S, Drouet MT, Roux P, Frenkiel MP, Arborio M, Durand-Schneider AM, Maurice M, Le Blanc I, Gruenberg J, Flamand M. The secreted form of dengue virus nonstructural protein NS1 is endocytosed by hepatocytes and accumulates in late endosomes: implications for viral infectivity. J Virol. 2005;79:11403-11411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 89] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 48. | Uno N, Ross TM. Dengue virus and the host innate immune response. Emerg Microbes Infect. 2018;7:167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 145] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 49. | Lee MF, Voon GZ, Lim HX, Chua ML, Poh CL. Innate and adaptive immune evasion by dengue virus. Front Cell Infect Microbiol. 2022;12:1004608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 35] [Reference Citation Analysis (0)] |
| 50. | Nasirudeen AM, Wong HH, Thien P, Xu S, Lam KP, Liu DX. RIG-I, MDA5 and TLR3 synergistically play an important role in restriction of dengue virus infection. PLoS Negl Trop Dis. 2011;5:e926. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 236] [Cited by in RCA: 245] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 51. | Mapalagamage M, Weiskopf D, Sette A, De Silva AD. Current Understanding of the Role of T Cells in Chikungunya, Dengue and Zika Infections. Viruses. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 52. | Castillo Ramirez JA, Urcuqui-Inchima S. Dengue Virus Control of Type I IFN Responses: A History of Manipulation and Control. J Interferon Cytokine Res. 2015;35:421-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 53. | Dalrymple NA, Cimica V, Mackow ER. Dengue Virus NS Proteins Inhibit RIG-I/MAVS Signaling by Blocking TBK1/IRF3 Phosphorylation: Dengue Virus Serotype 1 NS4A Is a Unique Interferon-Regulating Virulence Determinant. mBio. 2015;6:e00553-e00515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 162] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 54. | Aguirre S, Luthra P, Sanchez-Aparicio MT, Maestre AM, Patel J, Lamothe F, Fredericks AC, Tripathi S, Zhu T, Pintado-Silva J, Webb LG, Bernal-Rubio D, Solovyov A, Greenbaum B, Simon V, Basler CF, Mulder LC, García-Sastre A, Fernandez-Sesma A. Dengue virus NS2B protein targets cGAS for degradation and prevents mitochondrial DNA sensing during infection. Nat Microbiol. 2017;2:17037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 308] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 55. | Potisopon S, Priet S, Collet A, Decroly E, Canard B, Selisko B. The methyltransferase domain of dengue virus protein NS5 ensures efficient RNA synthesis initiation and elongation by the polymerase domain. Nucleic Acids Res. 2014;42:11642-11656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 56. | Thiemmeca S, Tamdet C, Punyadee N, Prommool T, Songjaeng A, Noisakran S, Puttikhunt C, Atkinson JP, Diamond MS, Ponlawat A, Avirutnan P. Secreted NS1 Protects Dengue Virus from Mannose-Binding Lectin-Mediated Neutralization. J Immunol. 2016;197:4053-4065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 57. | Okamoto T, Suzuki T, Kusakabe S, Tokunaga M, Hirano J, Miyata Y, Matsuura Y. Regulation of Apoptosis during Flavivirus Infection. Viruses. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 58. | Shukla R, Ramasamy V, Shanmugam RK, Ahuja R, Khanna N. Antibody-Dependent Enhancement: A Challenge for Developing a Safe Dengue Vaccine. Front Cell Infect Microbiol. 2020;10:572681. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 112] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 59. | Halstead S. Recent advances in understanding dengue. F1000Res. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 60. | Khanam A, Gutiérrez-Barbosa H, Lyke KE, Chua JV. Immune-Mediated Pathogenesis in Dengue Virus Infection. Viruses. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 46] [Reference Citation Analysis (0)] |
| 61. | Kuczera D, Assolini JP, Tomiotto-Pellissier F, Pavanelli WR, Silveira GF. Highlights for Dengue Immunopathogenesis: Antibody-Dependent Enhancement, Cytokine Storm, and Beyond. J Interferon Cytokine Res. 2018;38:69-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (1)] |
| 62. | Olagnier D, Peri S, Steel C, van Montfoort N, Chiang C, Beljanski V, Slifker M, He Z, Nichols CN, Lin R, Balachandran S, Hiscott J. Cellular oxidative stress response controls the antiviral and apoptotic programs in dengue virus-infected dendritic cells. PLoS Pathog. 2014;10:e1004566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 187] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 63. | Cockburn JJ, Navarro Sanchez ME, Goncalvez AP, Zaitseva E, Stura EA, Kikuti CM, Duquerroy S, Dussart P, Chernomordik LV, Lai CJ, Rey FA. Structural insights into the neutralization mechanism of a higher primate antibody against dengue virus. EMBO J. 2012;31:767-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 64. | Anandarao R, Swaminathan S, Khanna N. The identification of immunodominant linear epitopes of dengue type 2 virus capsid and NS4a proteins using pin-bound peptides. Virus Res. 2005;112:60-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 65. | Biering SB, Akey DL, Wong MP, Brown WC, Lo NTN, Puerta-Guardo H, Tramontini Gomes de Sousa F, Wang C, Konwerski JR, Espinosa DA, Bockhaus NJ, Glasner DR, Li J, Blanc SF, Juan EY, Elledge SJ, Mina MJ, Beatty PR, Smith JL, Harris E. Structural basis for antibody inhibition of flavivirus NS1-triggered endothelial dysfunction. Science. 2021;371:194-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 96] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 66. | Bournazos S, Gupta A, Ravetch JV. The role of IgG Fc receptors in antibody-dependent enhancement. Nat Rev Immunol. 2020;20:633-643. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 409] [Article Influence: 81.8] [Reference Citation Analysis (0)] |
| 67. | Xu Z, Zhang H, Yang D, Wei D, Demongeot J, Zeng Q. The Mathematical Modeling of the Host-Virus Interaction in Dengue Virus Infection: A Quantitative Study. Viruses. 2024;16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 68. | Waldran MJ, Wegman AD, Bahr LE, Roy NH, Currier JR, Waickman AT. Soluble NS1 Antagonizes IgG- and IgA- Mediated Monocytic Phagocytosis of DENV Infected Cells. J Infect Dis. 2023;228:70-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 69. | Chareonsirisuthigul T, Kalayanarooj S, Ubol S. Dengue virus (DENV) antibody-dependent enhancement of infection upregulates the production of anti-inflammatory cytokines, but suppresses anti-DENV free radical and pro-inflammatory cytokine production, in THP-1 cells. J Gen Virol. 2007;88:365-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 170] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 70. | Malavige GN, Jeewandara C, Alles KM, Salimi M, Gomes L, Kamaladasa A, Jayaratne SD, Ogg GS. Suppression of virus specific immune responses by IL-10 in acute dengue infection. PLoS Negl Trop Dis. 2013;7:e2409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 71. | Dowd KA, Sirohi D, Speer SD, VanBlargan LA, Chen RE, Mukherjee S, Whitener BM, Govero J, Aleshnick M, Larman B, Sukupolvi-Petty S, Sevvana M, Miller AS, Klose T, Zheng A, Koenig S, Kielian M, Kuhn RJ, Diamond MS, Pierson TC. prM-reactive antibodies reveal a role for partially mature virions in dengue virus pathogenesis. Proc Natl Acad Sci U S A. 2023;120:e2218899120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 72. | Tsai CL, Sun DS, Su MT, Lien TS, Chen YH, Lin CY, Huang CH, King CC, Li CR, Chen TH, Chiu YH, Lu CC, Chang HH. Suppressed humoral immunity is associated with dengue nonstructural protein NS1-elicited anti-death receptor antibody fractions in mice. Sci Rep. 2020;10:6294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 73. | Chuang YC, Lin YS, Liu HS, Yeh TM. Molecular mimicry between dengue virus and coagulation factors induces antibodies to inhibit thrombin activity and enhance fibrinolysis. J Virol. 2014;88:13759-13768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 74. | Lin YS, Lin CF, Lei HY, Liu HS, Yeh TM, Chen SH, Liu CC. Antibody-mediated endothelial cell damage via nitric oxide. Curr Pharm Des. 2004;10:213-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 75. | Liu C, Chu D, Kalantar-Zadeh K, George J, Young HA, Liu G. Cytokines: From Clinical Significance to Quantification. Adv Sci (Weinh). 2021;8:e2004433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 267] [Cited by in RCA: 426] [Article Influence: 106.5] [Reference Citation Analysis (0)] |
| 76. | Guzman MG, Alvarez M, Halstead SB. Secondary infection as a risk factor for dengue hemorrhagic fever/dengue shock syndrome: an historical perspective and role of antibody-dependent enhancement of infection. Arch Virol. 2013;158:1445-1459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 516] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 77. | Srikiatkhachorn A, Mathew A, Rothman AL. Immune-mediated cytokine storm and its role in severe dengue. Semin Immunopathol. 2017;39:563-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 180] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 78. | Modhiran N, Watterson D, Muller DA, Panetta AK, Sester DP, Liu L, Hume DA, Stacey KJ, Young PR. Dengue virus NS1 protein activates cells via Toll-like receptor 4 and disrupts endothelial cell monolayer integrity. Sci Transl Med. 2015;7:304ra142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 325] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 79. | Chao CH, Wu WC, Lai YC, Tsai PJ, Perng GC, Lin YS, Yeh TM. Dengue virus nonstructural protein 1 activates platelets via Toll-like receptor 4, leading to thrombocytopenia and hemorrhage. PLoS Pathog. 2019;15:e1007625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 125] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 80. | Ferreira RA, de Oliveira SA, Gandini M, Ferreira Lda C, Correa G, Abiraude FM, Reid MM, Cruz OG, Kubelka CF. Circulating cytokines and chemokines associated with plasma leakage and hepatic dysfunction in Brazilian children with dengue fever. Acta Trop. 2015;149:138-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 81. | Pan P, Li G, Shen M, Yu Z, Ge W, Lao Z, Fan Y, Chen K, Ding Z, Wang W, Wan P, Shereen MA, Luo Z, Chen X, Zhang Q, Lin L, Wu J. DENV NS1 and MMP-9 cooperate to induce vascular leakage by altering endothelial cell adhesion and tight junction. PLoS Pathog. 2021;17:e1008603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (1)] |
| 82. | Chen HR, Chuang YC, Lin YS, Liu HS, Liu CC, Perng GC, Yeh TM. Dengue Virus Nonstructural Protein 1 Induces Vascular Leakage through Macrophage Migration Inhibitory Factor and Autophagy. PLoS Negl Trop Dis. 2016;10:e0004828. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 83. | Chen HR, Chao CH, Liu CC, Ho TS, Tsai HP, Perng GC, Lin YS, Wang JR, Yeh TM. Macrophage migration inhibitory factor is critical for dengue NS1-induced endothelial glycocalyx degradation and hyperpermeability. PLoS Pathog. 2018;14:e1007033. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 84. | Puerta-Guardo H, Glasner DR, Harris E. Dengue Virus NS1 Disrupts the Endothelial Glycocalyx, Leading to Hyperpermeability. PLoS Pathog. 2016;12:e1005738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 252] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 85. | Seet RC, Chow AW, Quek AM, Chan YH, Lim EC. Relationship between circulating vascular endothelial growth factor and its soluble receptors in adults with dengue virus infection: a case-control study. Int J Infect Dis. 2009;13:e248-e253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 86. | Cardier JE, Mariño E, Romano E, Taylor P, Liprandi F, Bosch N, Rothman AL. Proinflammatory factors present in sera from patients with acute dengue infection induce activation and apoptosis of human microvascular endothelial cells: possible role of TNF-alpha in endothelial cell damage in dengue. Cytokine. 2005;30:359-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 82] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 87. | Bhatt P, Varma M, Sood V, Ambikan A, Jayaram A, Babu N, Gupta S, Mukhopadhyay C, Neogi U. Temporal cytokine storm dynamics in dengue infection predicts severity. Virus Res. 2024;341:199306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 88. | Malavige GN, Jeewandara C, Ogg GS. Dysfunctional Innate Immune Responses and Severe Dengue. Front Cell Infect Microbiol. 2020;10:590004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 89. | Schmid MA, Harris E. Monocyte recruitment to the dermis and differentiation to dendritic cells increases the targets for dengue virus replication. PLoS Pathog. 2014;10:e1004541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 90. | Simon AY, Sutherland MR, Pryzdial EL. Dengue virus binding and replication by platelets. Blood. 2015;126:378-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 130] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 91. | de Azeredo EL, Monteiro RQ, de-Oliveira Pinto LM. Thrombocytopenia in Dengue: Interrelationship between Virus and the Imbalance between Coagulation and Fibrinolysis and Inflammatory Mediators. Mediators Inflamm. 2015;2015:313842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 140] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 92. | Lin GL, Chang HH, Lien TS, Chen PK, Chan H, Su MT, Liao CY, Sun DS. Suppressive effect of dengue virus envelope protein domain III on megakaryopoiesis. Virulence. 2017;8:1719-1731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 93. | Lai JH, Wu DW, Wu CH, Hung LF, Huang CY, Ka SM, Chen A, Chang ZF, Ho LJ. Mitochondrial CMPK2 mediates immunomodulatory and antiviral activities through IFN-dependent and IFN-independent pathways. iScience. 2021;24:102498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 94. | Ojha A, Nandi D, Batra H, Singhal R, Annarapu GK, Bhattacharyya S, Seth T, Dar L, Medigeshi GR, Vrati S, Vikram NK, Guchhait P. Platelet activation determines the severity of thrombocytopenia in dengue infection. Sci Rep. 2017;7:41697. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 116] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 95. | Wan SW, Yang YW, Chu YT, Lin CF, Chang CP, Yeh TM, Anderson R, Lin YS. Anti-dengue virus nonstructural protein 1 antibodies contribute to platelet phagocytosis by macrophages. Thromb Haemost. 2016;115:646-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 96. | Tayal A, Kabra SK, Lodha R. Management of Dengue: An Updated Review. Indian J Pediatr. 2023;90:168-177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 51] [Reference Citation Analysis (0)] |
| 97. | Srichaikul T, Nimmannitya S. Haematology in dengue and dengue haemorrhagic fever. Baillieres Best Pract Res Clin Haematol. 2000;13:261-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 97] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 98. | World Health Organization. UNICEF/UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases. Handbook for clinical management of dengue. 2012. [cited 1 October 2024]. Available from: https://iris.who.int/handle/10665/76887. |
| 99. | Ministry of Health. Dengue: Diagnóstico e manejo clínico: adulto e criança. 2023. [cited 1 October 2024]. Available from: https://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/svsa/dengue/dengue-diagnostico-e-manejo-clinico-adulto-e-crianca/view. |
| 100. | World Health Organization. Dengue and severe dengue. April 23, 2024. [cited 1 October 2024]. Available from: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue. |
| 101. | Pan American Health Organization. Guidelines for the clinical diagnosis and treatment of dengue, chikungunya, and Zika. March 24, 2022. [cited 1 October 2024]. Available from: https://www.paho.org/en/documents/guidelines-clinical-diagnosis-and-treatment-dengue-chikungunya-and-zika. |
| 102. | Chuansumrit A, Chaiyaratana W. Hemostatic derangement in dengue hemorrhagic fever. Thromb Res. 2014;133:10-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 103. | Moore N, Pollack C, Butkerait P. Adverse drug reactions and drug-drug interactions with over-the-counter NSAIDs. Ther Clin Risk Manag. 2015;11:1061-1075. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 104. | Hussain H, Janaka KVC, Gunasekara H, Krishnan M, Perera I. Acute Psychosis Presenting With Dengue Fever Complicated by Dengue Encephalitis. Cureus. 2024;16:e55628. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 105. | Sandeep M, Padhi BK, Yella SST, Sruthi KG, Venkatesan RG, Krishna Sasanka KBS, Satapathy P, Mohanty A, Al-Tawfiq JA, Iqhrammullah M, Rabaan AA, Kabi A, Sah S, Rustagi S, Al-Qaim ZH, Barboza JJ, Waheed Y, Harapan H, Sah R. Myocarditis manifestations in dengue cases: A systematic review and meta-analysis. J Infect Public Health. 2023;16:1761-1768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 106. | Khan AA, Khan FU, Akhtar SA, Ghaffar R. Dengue beyond fever-fatal dengue myocarditis and complete heart block: A case report and brief overview of cardiac manifestations of dengue fever. SAGE Open Med Case Rep. 2023;11:2050313X231193983. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 107. | Ahmed M, Wasim MA, Kazi AN, Akber H, Sheikh M, Patel MJ. A curious case of expanded dengue syndrome. Trop Doct. 2024;54:179-181. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 108. | Kularatne SA, Dalugama C. Dengue infection: Global importance, immunopathology and management. Clin Med (Lond). 2022;22:9-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 79] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 109. | Kok BH, Lim HT, Lim CP, Lai NS, Leow CY, Leow CH. Dengue virus infection-a review of pathogenesis, vaccines, diagnosis and therapy. Virus Res. 2023;324:199018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 67] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 110. | Khetarpal N, Khanna I. Dengue Fever: Causes, Complications, and Vaccine Strategies. J Immunol Res. 2016;2016:6803098. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 154] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 111. | Grande AJ, Reid H, Thomas E, Foster C, Darton TC. Tourniquet Test for Dengue Diagnosis: Systematic Review and Meta-analysis of Diagnostic Test Accuracy. PLoS Negl Trop Dis. 2016;10:e0004888. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 112. | Gregory CJ, Lorenzi OD, Colón L, García AS, Santiago LM, Rivera RC, Bermúdez LJ, Báez FO, Aponte DV, Tomashek KM, Gutierrez J, Alvarado L. Utility of the tourniquet test and the white blood cell count to differentiate dengue among acute febrile illnesses in the emergency room. PLoS Negl Trop Dis. 2011;5:e1400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 113. | Ahmed NH, Broor S. Comparison of NS1 antigen detection ELISA, real time RT-PCR and virus isolation for rapid diagnosis of dengue infection in acute phase. J Vector Borne Dis. 2014;51:194-199. [PubMed] |
| 114. | Ohst C, Saschenbrecker S, Stiba K, Steinhagen K, Probst C, Radzimski C, Lattwein E, Komorowski L, Stöcker W, Schlumberger W. Reliable Serological Testing for the Diagnosis of Emerging Infectious Diseases. Adv Exp Med Biol. 2018;1062:19-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 115. | Xu H, Di B, Pan YX, Qiu LW, Wang YD, Hao W, He LJ, Yuen KY, Che XY. Serotype 1-specific monoclonal antibody-based antigen capture immunoassay for detection of circulating nonstructural protein NS1: Implications for early diagnosis and serotyping of dengue virus infections. J Clin Microbiol. 2006;44:2872-2878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 96] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 116. | Aquino VH, Fumagalli MJ, Silva A, de Moura Negrini BV, Rojas A, Guillen Y, Bernal C, Figueiredo LTM. Linear epitope mapping in the E and NS1 proteins of dengue and Zika viruses: Prospection of peptides for vaccines and diagnostics. PLoS One. 2023;18:e0292451. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 117. | Goncalves A, Peeling RW, Chu MC, Gubler DJ, de Silva AM, Harris E, Murtagh M, Chua A, Rodriguez W, Kelly C, Wilder-Smith A. Innovative and New Approaches to Laboratory Diagnosis of Zika and Dengue: A Meeting Report. J Infect Dis. 2018;217:1060-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 118. | Thomas SJ, Nisalak A, Anderson KB, Libraty DH, Kalayanarooj S, Vaughn DW, Putnak R, Gibbons RV, Jarman R, Endy TP. Dengue plaque reduction neutralization test (PRNT) in primary and secondary dengue virus infections: How alterations in assay conditions impact performance. Am J Trop Med Hyg. 2009;81:825-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 171] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 119. | Cascorbi I. The Uncertainties of Metamizole Use. Clin Pharmacol Ther. 2021;109:1373-1375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 120. | Yacoub S, Trung TH, Lam PK, Thien VHN, Hai DHT, Phan TQ, Nguyet OPK, Quyen NTH, Simmons CP, Broyd C, Screaton GR, Wills B. Cardio-haemodynamic assessment and venous lactate in severe dengue: Relationship with recurrent shock and respiratory distress. PLoS Negl Trop Dis. 2017;11:e0005740. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 121. | Chaurasia R, Zaman S, Chatterjee K, Das B. Retrospective Review of Platelet Transfusion Practices during 2013 Dengue Epidemic of Delhi, India. Transfus Med Hemother. 2015;42:227-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 122. | Lee TH, Wong JG, Leo YS, Thein TL, Ng EL, Lee LK, Lye DC. Potential Harm of Prophylactic Platelet Transfusion in Adult Dengue Patients. PLoS Negl Trop Dis. 2016;10:e0004576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 123. | Chia PY, Htun HL, Leo YS, Lye DC. Safety of temporary interruption of antiplatelet therapy in dengue fever with thrombocytopenia. J Infect. 2021;82:270-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 124. | Bandara SMR, Herath HMMTB. Effectiveness of corticosteroid in the treatment of dengue-A systemic review. Heliyon. 2018;4:e00816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 125. | Zhang F, Kramer CV. Corticosteroids for dengue infection. Cochrane Database Syst Rev. 2014;2014:CD003488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 126. | Lee MF, Wu YS, Poh CL. Molecular Mechanisms of Antiviral Agents against Dengue Virus. Viruses. 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 43] [Reference Citation Analysis (0)] |
| 127. | Kayesh MEH, Tsukiyama-Kohara K. Mammalian animal models for dengue virus infection: a recent overview. Arch Virol. 2022;167:31-44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 128. | Watanabe S, Low JG, Vasudevan SG. Preclinical Antiviral Testing for Dengue Virus Infection in Mouse Models and Its Association with Clinical Studies. ACS Infect Dis. 2018;4:1048-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 129. | Mahajan S, Choudhary S, Kumar P, Tomar S. Antiviral strategies targeting host factors and mechanisms obliging +ssRNA viral pathogens. Bioorg Med Chem. 2021;46:116356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 130. | Rather IA, Parray HA, Lone JB, Paek WK, Lim J, Bajpai VK, Park YH. Prevention and Control Strategies to Counter Dengue Virus Infection. Front Cell Infect Microbiol. 2017;7:336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 131. | Yakob L, Funk S, Camacho A, Brady O, Edmunds WJ. Aedes aegypti Control Through Modernized, Integrated Vector Management. PLoS Curr. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 132. | Buhler C, Winkler V, Runge-Ranzinger S, Boyce R, Horstick O. Environmental methods for dengue vector control-A systematic review and meta-analysis. PLoS Negl Trop Dis. 2019;13:e0007420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 133. | Dalpadado R, Gunathilaka N, Amarasinghe D, Udayanaga L. A Challenge for a Unique Dengue Vector Control Programme: Assessment of the Spatial Variation of Insecticide Resistance Status amongst Aedes aegypti and Aedes albopictus Populations in Gampaha District, Sri Lanka. Biomed Res Int. 2021;2021:6619175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 134. | Akter R, Tasneem F, Das S, Soma MA, Georgakopoulos-Soares I, Juthi RT, Sazed SA. Approaches of dengue control: vaccine strategies and future aspects. Front Immunol. 2024;15:1362780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 135. | Guy B, Barrere B, Malinowski C, Saville M, Teyssou R, Lang J. From research to phase III: preclinical, industrial and clinical development of the Sanofi Pasteur tetravalent dengue vaccine. Vaccine. 2011;29:7229-7241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 226] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 136. | Hou J, Ye W, Chen J. Current Development and Challenges of Tetravalent Live-Attenuated Dengue Vaccines. Front Immunol. 2022;13:840104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 137. | Dengue vaccine: WHO position paper, September 2018-Recommendations. Vaccine. 2019;37:4848-4849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 121] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 138. | Sridhar S, Luedtke A, Langevin E, Zhu M, Bonaparte M, Machabert T, Savarino S, Zambrano B, Moureau A, Khromava A, Moodie Z, Westling T, Mascareñas C, Frago C, Cortés M, Chansinghakul D, Noriega F, Bouckenooghe A, Chen J, Ng SP, Gilbert PB, Gurunathan S, DiazGranados CA. Effect of Dengue Serostatus on Dengue Vaccine Safety and Efficacy. N Engl J Med. 2018;379:327-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 556] [Article Influence: 79.4] [Reference Citation Analysis (0)] |
| 139. | Angelin M, Sjölin J, Kahn F, Ljunghill Hedberg A, Rosdahl A, Skorup P, Werner S, Woxenius S, Askling HH. Qdenga®-A promising dengue fever vaccine; can it be recommended to non-immune travelers? Travel Med Infect Dis. 2023;54:102598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 34] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 140. | Tricou V, Yu D, Reynales H, Biswal S, Saez-Llorens X, Sirivichayakul C, Lopez P, Borja-Tabora C, Bravo L, Kosalaraksa P, Vargas LM, Alera MT, Rivera L, Watanaveeradej V, Dietze R, Fernando L, Wickramasinghe VP, Moreira ED Jr, Fernando AD, Gunasekera D, Luz K, Oliveira AL, Tuboi S, Escudero I, Hutagalung Y, Lloyd E, Rauscher M, Zent O, Folschweiller N, LeFevre I, Espinoza F, Wallace D. Long-term efficacy and safety of a tetravalent dengue vaccine (TAK-003): 4·5-year results from a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Glob Health. 2024;12:e257-e270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 80] [Article Influence: 80.0] [Reference Citation Analysis (0)] |
| 141. | Musso D, Gubler DJ. Zika Virus. Clin Microbiol Rev. 2016;29:487-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1071] [Cited by in RCA: 1067] [Article Influence: 118.6] [Reference Citation Analysis (0)] |
| 142. | Kuno G, Chang GJ. Full-length sequencing and genomic characterization of Bagaza, Kedougou, and Zika viruses. Arch Virol. 2007;152:687-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 388] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 143. | Chambers TJ, Hahn CS, Galler R, Rice CM. Flavivirus genome organization, expression, and replication. Annu Rev Microbiol. 1990;44:649-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1441] [Cited by in RCA: 1473] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 144. | Heinz FX, Stiasny K. The Antigenic Structure of Zika Virus and Its Relation to Other Flaviviruses: Implications for Infection and Immunoprophylaxis. Microbiol Mol Biol Rev. 2017;81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 142] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 145. | Wang Y, Ling L, Zhang Z, Marin-Lopez A. Current Advances in Zika Vaccine Development. Vaccines (Basel). 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 49] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 146. | Pattnaik A, Sahoo BR, Pattnaik AK. Current Status of Zika Virus Vaccines: Successes and Challenges. Vaccines (Basel). 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 75] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 147. | Richner JM, Himansu S, Dowd KA, Butler SL, Salazar V, Fox JM, Julander JG, Tang WW, Shresta S, Pierson TC, Ciaramella G, Diamond MS. Modified mRNA Vaccines Protect against Zika Virus Infection. Cell. 2017;168:1114-1125.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 564] [Cited by in RCA: 592] [Article Influence: 74.0] [Reference Citation Analysis (0)] |
| 148. | Li L, Lok SM, Yu IM, Zhang Y, Kuhn RJ, Chen J, Rossmann MG. The flavivirus precursor membrane-envelope protein complex: structure and maturation. Science. 2008;319:1830-1834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 405] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 149. | Wu Y, Liu Q, Zhou J, Xie W, Chen C, Wang Z, Yang H, Cui J. Zika virus evades interferon-mediated antiviral response through the co-operation of multiple nonstructural proteins in vitro. Cell Discov. 2017;3:17006. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 168] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 150. | Dick GW, Kitchen SF, Haddow AJ. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg. 1952;46:509-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1908] [Cited by in RCA: 1927] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 151. | Cao-Lormeau VM, Roche C, Teissier A, Robin E, Berry AL, Mallet HP, Sall AA, Musso D. Zika virus, French polynesia, South pacific, 2013. Emerg Infect Dis. 2014;20:1085-1086. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 323] [Cited by in RCA: 560] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 152. | World Health Organization. Zika epidemiology update. June 3, 2024. [cited 1 October 2024]. Available from: https://www.who.int/publications/m/item/zika-epidemiology-update-may-2024. |
| 153. | Ioos S, Mallet HP, Leparc Goffart I, Gauthier V, Cardoso T, Herida M. Current Zika virus epidemiology and recent epidemics. Med Mal Infect. 2014;44:302-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 383] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 154. | Smith CE. Arthropod-borne viruses. Br Med Bull. 1959;15:235-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 155. | Lanciotti RS, Kosoy OL, Laven JJ, Velez JO, Lambert AJ, Johnson AJ, Stanfield SM, Duffy MR. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis. 2008;14:1232-1239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1606] [Cited by in RCA: 1673] [Article Influence: 98.4] [Reference Citation Analysis (0)] |
| 156. | Boccolini D, Toma L, Di Luca M, Severini F, Romi R, Remoli ME, Sabbatucci M, Venturi G, Rezza G, Fortuna C. Experimental investigation of the susceptibility of Italian Culex pipiens mosquitoes to Zika virus infection. Euro Surveill. 2016;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 157. | Mansuy JM, Dutertre M, Mengelle C, Fourcade C, Marchou B, Delobel P, Izopet J, Martin-Blondel G. Zika virus: high infectious viral load in semen, a new sexually transmitted pathogen? Lancet Infect Dis. 2016;16:405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 276] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 158. | Musso D, Roche C, Robin E, Nhan T, Teissier A, Cao-Lormeau VM. Potential sexual transmission of Zika virus. Emerg Infect Dis. 2015;21:359-361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 805] [Cited by in RCA: 792] [Article Influence: 79.2] [Reference Citation Analysis (0)] |
| 159. | D’Ortenzio E, Matheron S, Yazdanpanah Y, de Lamballerie X, Hubert B, Piorkowski G, Maquart M, Descamps D, Damond F, Leparc-Goffart I. Evidence of Sexual Transmission of Zika Virus. N Engl J Med. 2016;374:2195-2198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 554] [Cited by in RCA: 534] [Article Influence: 59.3] [Reference Citation Analysis (0)] |
| 160. | Major CG, Paz-Bailey G, Hills SL, Rodriguez DM, Biggerstaff BJ, Johansson M. Risk Estimation of Sexual Transmission of Zika Virus-United States, 2016-2017. J Infect Dis. 2021;224:1756-1764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 161. | Magnus MM, Espósito DLA, Costa VAD, Melo PS, Costa-Lima C, Fonseca BALD, Addas-Carvalho M. Risk of Zika virus transmission by blood donations in Brazil. Hematol Transfus Cell Ther. 2018;40:250-254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 162. | Paixao ES, Cardim LL, Costa MCN, Brickley EB, de Carvalho-Sauer RCO, Carmo EH, Andrade RFS, Rodrigues MS, Veiga RV, Costa LC, Moore CA, França GVA, Smeeth L, Rodrigues LC, Barreto ML, Teixeira MG. Mortality from Congenital Zika Syndrome-Nationwide Cohort Study in Brazil. N Engl J Med. 2022;386:757-767. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 55] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 163. | Wu KY, Zuo GL, Li XF, Ye Q, Deng YQ, Huang XY, Cao WC, Qin CF, Luo ZG. Vertical transmission of Zika virus targeting the radial glial cells affects cortex development of offspring mice. Cell Res. 2016;26:645-654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 225] [Cited by in RCA: 227] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 164. | Rabelo K, Gonçalves AJDS, Souza LJ, Sales AP, Lima SMB, Trindade GF, Ciambarella BT, Amorim Tasmo NR, Diaz BL, Carvalho JJ, Duarte MPO, Paes MV. Zika Virus Infects Human Placental Mast Cells and the HMC-1 Cell Line, and Triggers Degranulation, Cytokine Release and Ultrastructural Changes. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 165. | Miner JJ, Diamond MS. Zika Virus Pathogenesis and Tissue Tropism. Cell Host Microbe. 2017;21:134-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 314] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 166. | Atkinson B, Hearn P, Afrough B, Lumley S, Carter D, Aarons EJ, Simpson AJ, Brooks TJ, Hewson R. Detection of Zika Virus in Semen. Emerg Infect Dis. 2016;22:940. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 255] [Cited by in RCA: 244] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 167. | Foy BD, Kobylinski KC, Chilson Foy JL, Blitvich BJ, Travassos da Rosa A, Haddow AD, Lanciotti RS, Tesh RB. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg Infect Dis. 2011;17:880-882. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 906] [Cited by in RCA: 795] [Article Influence: 56.8] [Reference Citation Analysis (0)] |
| 168. | Deckard DT, Chung WM, Brooks JT, Smith JC, Woldai S, Hennessey M, Kwit N, Mead P. Male-to-Male Sexual Transmission of Zika Virus--Texas, January 2016. MMWR Morb Mortal Wkly Rep. 2016;65:372-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 237] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 169. | Fréour T, Mirallié S, Hubert B, Splingart C, Barrière P, Maquart M, Leparc-Goffart I. Sexual transmission of Zika virus in an entirely asymptomatic couple returning from a Zika epidemic area, France, April 2016. Euro Surveill. 2016;21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 110] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 170. | Bayer A, Lennemann NJ, Ouyang Y, Bramley JC, Morosky S, Marques ET Jr, Cherry S, Sadovsky Y, Coyne CB. Type III Interferons Produced by Human Placental Trophoblasts Confer Protection against Zika Virus Infection. Cell Host Microbe. 2016;19:705-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 441] [Cited by in RCA: 427] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 171. | Ouyang Y, Bayer A, Chu T, Tyurin VA, Kagan VE, Morelli AE, Coyne CB, Sadovsky Y. Isolation of human trophoblastic extracellular vesicles and characterization of their cargo and antiviral activity. Placenta. 2016;47:86-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 172. | Arora N, Sadovsky Y, Dermody TS, Coyne CB. Microbial Vertical Transmission during Human Pregnancy. Cell Host Microbe. 2017;21:561-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 260] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 173. | Mesci P, Macia A, Moore SM, Shiryaev SA, Pinto A, Huang CT, Tejwani L, Fernandes IR, Suarez NA, Kolar MJ, Montefusco S, Rosenberg SC, Herai RH, Cugola FR, Russo FB, Sheets N, Saghatelian A, Shresta S, Momper JD, Siqueira-Neto JL, Corbett KD, Beltrão-Braga PCB, Terskikh AV, Muotri AR. Blocking Zika virus vertical transmission. Sci Rep. 2018;8:1218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 174. | Hamel R, Dejarnac O, Wichit S, Ekchariyawat P, Neyret A, Luplertlop N, Perera-Lecoin M, Surasombatpattana P, Talignani L, Thomas F, Cao-Lormeau VM, Choumet V, Briant L, Desprès P, Amara A, Yssel H, Missé D. Biology of Zika Virus Infection in Human Skin Cells. J Virol. 2015;89:8880-8896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 902] [Cited by in RCA: 897] [Article Influence: 89.7] [Reference Citation Analysis (0)] |
| 175. | Meertens L, Carnec X, Lecoin MP, Ramdasi R, Guivel-Benhassine F, Lew E, Lemke G, Schwartz O, Amara A. The TIM and TAM families of phosphatidylserine receptors mediate dengue virus entry. Cell Host Microbe. 2012;12:544-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 397] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 176. | Pujhari S, Brustolin M, Macias VM, Nissly RH, Nomura M, Kuchipudi SV, Rasgon JL. Heat shock protein 70 (Hsp70) mediates Zika virus entry, replication, and egress from host cells. Emerg Microbes Infect. 2019;8:8-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 69] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 177. | Garcia-Vallejo JJ, van Kooyk Y. The physiological role of DC-SIGN: a tale of mice and men. Trends Immunol. 2013;34:482-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 146] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 178. | Tappe D, Pérez-Girón JV, Zammarchi L, Rissland J, Ferreira DF, Jaenisch T, Gómez-Medina S, Günther S, Bartoloni A, Muñoz-Fontela C, Schmidt-Chanasit J. Cytokine kinetics of Zika virus-infected patients from acute to reconvalescent phase. Med Microbiol Immunol. 2016;205:269-273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 125] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 179. | Mohd Ropidi MI, Khazali AS, Nor Rashid N, Yusof R. Endoplasmic reticulum: a focal point of Zika virus infection. J Biomed Sci. 2020;27:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 180. | Lennemann NJ, Coyne CB. Dengue and Zika viruses subvert reticulophagy by NS2B3-mediated cleavage of FAM134B. Autophagy. 2017;13:322-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 161] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 181. | Sirohi D, Kuhn RJ. Zika Virus Structure, Maturation, and Receptors. J Infect Dis. 2017;216:S935-S944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 182] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 182. | Beaver JT, Lelutiu N, Habib R, Skountzou I. Evolution of Two Major Zika Virus Lineages: Implications for Pathology, Immune Response, and Vaccine Development. Front Immunol. 2018;9:1640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 183. | Loo YM, Gale M Jr. Immune signaling by RIG-I-like receptors. Immunity. 2011;34:680-692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1581] [Cited by in RCA: 1432] [Article Influence: 102.3] [Reference Citation Analysis (0)] |
| 184. | Schneider WM, Chevillotte MD, Rice CM. Interferon-stimulated genes: a complex web of host defenses. Annu Rev Immunol. 2014;32:513-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2255] [Cited by in RCA: 2290] [Article Influence: 208.2] [Reference Citation Analysis (0)] |
| 185. | Riedl W, Acharya D, Lee JH, Liu G, Serman T, Chiang C, Chan YK, Diamond MS, Gack MU. Zika Virus NS3 Mimics a Cellular 14-3-3-Binding Motif to Antagonize RIG-I- and MDA5-Mediated Innate Immunity. Cell Host Microbe. 2019;26:493-503.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 92] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 186. | Ma J, Ketkar H, Geng T, Lo E, Wang L, Xi J, Sun Q, Zhu Z, Cui Y, Yang L, Wang P. Zika Virus Non-structural Protein 4A Blocks the RLR-MAVS Signaling. Front Microbiol. 2018;9:1350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 187. | Xia H, Luo H, Shan C, Muruato AE, Nunes BTD, Medeiros DBA, Zou J, Xie X, Giraldo MI, Vasconcelos PFC, Weaver SC, Wang T, Rajsbaum R, Shi PY. An evolutionary NS1 mutation enhances Zika virus evasion of host interferon induction. Nat Commun. 2018;9:414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 230] [Cited by in RCA: 231] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 188. | Winkler CW, Myers LM, Woods TA, Messer RJ, Carmody AB, McNally KL, Scott DP, Hasenkrug KJ, Best SM, Peterson KE. Adaptive Immune Responses to Zika Virus Are Important for Controlling Virus Infection and Preventing Infection in Brain and Testes. J Immunol. 2017;198:3526-3535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 87] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 189. | Lee LJ, Komarasamy TV, Adnan NAA, James W, Rmt Balasubramaniam V. Hide and Seek: The Interplay Between Zika Virus and the Host Immune Response. Front Immunol. 2021;12:750365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 190. | Stettler K, Beltramello M, Espinosa DA, Graham V, Cassotta A, Bianchi S, Vanzetta F, Minola A, Jaconi S, Mele F, Foglierini M, Pedotti M, Simonelli L, Dowall S, Atkinson B, Percivalle E, Simmons CP, Varani L, Blum J, Baldanti F, Cameroni E, Hewson R, Harris E, Lanzavecchia A, Sallusto F, Corti D. Specificity, cross-reactivity, and function of antibodies elicited by Zika virus infection. Science. 2016;353:823-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 575] [Cited by in RCA: 617] [Article Influence: 68.6] [Reference Citation Analysis (0)] |
| 191. | Wang Q, Yang H, Liu X, Dai L, Ma T, Qi J, Wong G, Peng R, Liu S, Li J, Li S, Song J, Liu J, He J, Yuan H, Xiong Y, Liao Y, Li J, Yang J, Tong Z, Griffin BD, Bi Y, Liang M, Xu X, Qin C, Cheng G, Zhang X, Wang P, Qiu X, Kobinger G, Shi Y, Yan J, Gao GF. Molecular determinants of human neutralizing antibodies isolated from a patient infected with Zika virus. Sci Transl Med. 2016;8:369ra179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 163] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 192. | Jurado KA, Yockey LJ, Wong PW, Lee S, Huttner AJ, Iwasaki A. Antiviral CD8 T cells induce Zika-virus-associated paralysis in mice. Nat Microbiol. 2018;3:141-147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 95] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 193. | Hassert M, Wolf KJ, Schwetye KE, DiPaolo RJ, Brien JD, Pinto AK. CD4+T cells mediate protection against Zika associated severe disease in a mouse model of infection. PLoS Pathog. 2018;14:e1007237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 194. | Li C, Xu D, Ye Q, Hong S, Jiang Y, Liu X, Zhang N, Shi L, Qin CF, Xu Z. Zika Virus Disrupts Neural Progenitor Development and Leads to Microcephaly in Mice. Cell Stem Cell. 2016;19:672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 148] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 195. | Gatherer D, Kohl A. Zika virus: a previously slow pandemic spreads rapidly through the Americas. J Gen Virol. 2016;97:269-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 201] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 196. | Paz-Bailey G, Rosenberg ES, Doyle K, Munoz-Jordan J, Santiago GA, Klein L, Perez-Padilla J, Medina FA, Waterman SH, Gubern CG, Alvarado LI, Sharp TM. Persistence of Zika Virus in Body Fluids-Final Report. N Engl J Med. 2018;379:1234-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 323] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 197. | Mladinich MC, Schwedes J, Mackow ER. Zika Virus Persistently Infects and Is Basolaterally Released from Primary Human Brain Microvascular Endothelial Cells. mBio. 2017;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 106] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 198. | Song J, Xian D, Yang L, Xiong X, Lai R, Zhong J. Pruritus: Progress toward Pathogenesis and Treatment. Biomed Res Int. 2018;2018:9625936. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 199. | Plourde AR, Bloch EM. A Literature Review of Zika Virus. Emerg Infect Dis. 2016;22:1185-1192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 313] [Cited by in RCA: 372] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 200. | Choi GB, Yim YS, Wong H, Kim S, Kim H, Kim SV, Hoeffer CA, Littman DR, Huh JR. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science. 2016;351:933-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 778] [Cited by in RCA: 866] [Article Influence: 96.2] [Reference Citation Analysis (0)] |
| 201. | Shahrizaila N, Lehmann HC, Kuwabara S. Guillain-Barré syndrome. Lancet. 2021;397:1214-1228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 343] [Article Influence: 85.8] [Reference Citation Analysis (0)] |
| 202. | Dos Santos T, Rodriguez A, Almiron M, Sanhueza A, Ramon P, de Oliveira WK, Coelho GE, Badaró R, Cortez J, Ospina M, Pimentel R, Masis R, Hernandez F, Lara B, Montoya R, Jubithana B, Melchor A, Alvarez A, Aldighieri S, Dye C, Espinal MA. Zika Virus and the Guillain-Barré Syndrome-Case Series from Seven Countries. N Engl J Med. 2016;375:1598-1601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 233] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 203. | Cao-Lormeau VM, Blake A, Mons S, Lastère S, Roche C, Vanhomwegen J, Dub T, Baudouin L, Teissier A, Larre P, Vial AL, Decam C, Choumet V, Halstead SK, Willison HJ, Musset L, Manuguerra JC, Despres P, Fournier E, Mallet HP, Musso D, Fontanet A, Neil J, Ghawché F. Guillain-Barré Syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet. 2016;387:1531-1539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1606] [Cited by in RCA: 1663] [Article Influence: 184.8] [Reference Citation Analysis (0)] |
| 204. | Rivera-Correa J, de Siqueira IC, Mota S, do Rosário MS, Pereira de Jesus PA, Alcantara LCJ, Ernst JD, Rodriguez A. Anti-ganglioside antibodies in patients with Zika virus infection-associated Guillain-Barré Syndrome in Brazil. PLoS Negl Trop Dis. 2019;13:e0007695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 205. | Nico D, Conde L, Rivera-Correa JL, Vasconcelos-Dos-Santos A, Mesentier-Louro L, Freire-de-Lima L, Arruda MB, Freire-de-Lima CG, Ferreira ODC Jr, Lopes Moreira ME, Zin AA, Vasconcelos ZFM, Otero RM, Palatnik-de-Sousa CB, Tanuri A, Todeschini AR, Savino W, Rodriguez A, Morrot A. Prevalence of IgG Autoantibodies against GD3 Ganglioside in Acute Zika Virus Infection. Front Med (Lausanne). 2018;5:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 206. | Lebeau G, Frumence E, Turpin J, Begue F, Hoarau JJ, Gadea G, Krejbich-Trotot P, Desprès P, Viranaicken W. Zika E Glycan Loop Region and Guillain-Barré Syndrome-Related Proteins: A Possible Molecular Mimicry to Be Taken in Account for Vaccine Development. Vaccines (Basel). 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 207. | Pielnaa P, Al-Saadawe M, Saro A, Dama MF, Zhou M, Huang Y, Huang J, Xia Z. Zika virus-spread, epidemiology, genome, transmission cycle, clinical manifestation, associated challenges, vaccine and antiviral drug development. Virology. 2020;543:34-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 137] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 208. | Baba SS, Fagbami AH, Ojeh CK. Preliminary studies on the use of solid-phase immunosorbent techniques for the rapid detection of Wesselsbron virus (WSLV) IgM by haemagglutination-inhibition. Comp Immunol Microbiol Infect Dis. 1999;22:71-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 209. | Oduyebo T, Igbinosa I, Petersen EE, Polen KN, Pillai SK, Ailes EC, Villanueva JM, Newsome K, Fischer M, Gupta PM, Powers AM, Lampe M, Hills S, Arnold KE, Rose LE, Shapiro-Mendoza CK, Beard CB, Muñoz JL, Rao CY, Meaney-Delman D, Jamieson DJ, Honein MA. Update: Interim Guidance for Health Care Providers Caring for Pregnant Women with Possible Zika Virus Exposure-United States, July 2016. MMWR Morb Mortal Wkly Rep. 2016;65:739-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 210. | De Moraes CG, Pettito M, Yepez JB, Sakuntabhai A, Simon-Loriere E, Zaidi MB, Prot M, Ruffie C, Kim SS, Allikmets R, Terwilliger JD, Lee JH, Maestre GE. Optic neuropathy and congenital glaucoma associated with probable Zika virus infection in Venezuelan patients. JMM Case Rep. 2018;5:e005145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 211. | Cauchemez S, Besnard M, Bompard P, Dub T, Guillemette-Artur P, Eyrolle-Guignot D, Salje H, Van Kerkhove MD, Abadie V, Garel C, Fontanet A, Mallet HP. Association between Zika virus and microcephaly in French Polynesia, 2013-15: a retrospective study. Lancet. 2016;387:2125-2132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 690] [Cited by in RCA: 669] [Article Influence: 74.3] [Reference Citation Analysis (0)] |
| 212. | Hennessey M, Fischer M, Staples JE. Zika Virus Spreads to New Areas-Region of the Americas, May 2015-January 2016. MMWR Morb Mortal Wkly Rep. 2016;65:55-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 301] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 213. | Schuler-Faccini L, Ribeiro EM, Feitosa IM, Horovitz DD, Cavalcanti DP, Pessoa A, Doriqui MJ, Neri JI, Neto JM, Wanderley HY, Cernach M, El-Husny AS, Pone MV, Serao CL, Sanseverino MT; Brazilian Medical Genetics Society-Zika Embryopathy Task Force. Possible Association Between Zika Virus Infection and Microcephaly-Brazil, 2015. MMWR Morb Mortal Wkly Rep. 2016;65:59-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 716] [Cited by in RCA: 733] [Article Influence: 81.4] [Reference Citation Analysis (0)] |
| 214. | Muñoz LS, Barreras P, Pardo CA. Zika Virus-Associated Neurological Disease in the Adult: Guillain-Barré Syndrome, Encephalitis, and Myelitis. Semin Reprod Med. 2016;34:273-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 215. | Halani S, Tombindo PE, O’Reilly R, Miranda RN, Erdman LK, Whitehead C, Bielecki JM, Ramsay L, Ximenes R, Boyle J, Krueger C, Willmott S, Morris SK, Murphy KE, Sander B. Clinical manifestations and health outcomes associated with Zika virus infections in adults: A systematic review. PLoS Negl Trop Dis. 2021;15:e0009516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 216. | Bido-Medina R, Wirsich J, Rodríguez M, Oviedo J, Miches I, Bido P, Tusen L, Stoeter P, Sadaghiani S. Impact of Zika Virus on adult human brain structure and functional organization. Ann Clin Transl Neurol. 2018;5:752-762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 217. | Pan American Health Organization. Epidemiological Alert, Neurological syndrome, congenital malformations, and Zika virus infection. Implications for public health in the American. December 1, 2015. [cited 1 October 2024]. Available from: https://iris.paho.org/handle/10665.2/50697. |
| 218. | Arzuza-Ortega L, Polo A, Pérez-Tatis G, López-García H, Parra E, Pardo-Herrera LC, Rico-Turca AM, Villamil-Gómez W, Rodríguez-Morales AJ. Fatal Sickle Cell Disease and Zika Virus Infection in Girl from Colombia. Emerg Infect Dis. 2016;22:925-927. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 83] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 219. | World Health Organization. Testes de laboratório para a infecção pelo vírus Zika. March 23, 2016. [cited 1 October 2024]. Available from: https://iris.who.int/bitstream/handle/10665/204671/WHO_ZIKV_LAB_16.1_por.pdf?sequence=6&isAllowed=y. |
| 220. | Johnson AJ, Martin DA, Karabatsos N, Roehrig JT. Detection of anti-arboviral immunoglobulin G by using a monoclonal antibody-based capture enzyme-linked immunosorbent assay. J Clin Microbiol. 2000;38:1827-1831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 158] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 221. | Sirikajornpan K, Suntarattiwong P, Suwanpakdee D, Tabprasit S, Buddhari D, Thaisomboonsuk B, Klungthong C, Poolpanichupatam Y, Buathong R, Srikiatkhachorn A, Jones A, Fernandez S, Hunsawong T. Standardization and Evaluation of an Anti-ZIKV IgM ELISA Assay for the Serological Diagnosis of Zika Virus Infection. Am J Trop Med Hyg. 2021;105:936-941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 222. | Martin DA, Muth DA, Brown T, Johnson AJ, Karabatsos N, Roehrig JT. Standardization of immunoglobulin M capture enzyme-linked immunosorbent assays for routine diagnosis of arboviral infections. J Clin Microbiol. 2000;38:1823-1826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 377] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 223. | Roehrig JT, Hombach J, Barrett AD. Guidelines for Plaque-Reduction Neutralization Testing of Human Antibodies to Dengue Viruses. Viral Immunol. 2008;21:123-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 277] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 224. | Faye O, Faye O, Diallo D, Diallo M, Weidmann M, Sall AA. Quantitative real-time PCR detection of Zika virus and evaluation with field-caught mosquitoes. Virol J. 2013;10:311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 280] [Cited by in RCA: 287] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 225. | Varghese J, De Silva I, Millar DS. Latest Advances in Arbovirus Diagnostics. Microorganisms. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 226. | Pedlar M, Emery MJ, Warburton PJ. Amplifying PCR productivity and environmental sustainability through shortened cycling protocols. Biochimie. 2024;221:60-64. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 227. | Gourinat AC, O’Connor O, Calvez E, Goarant C, Dupont-Rouzeyrol M. Detection of Zika virus in urine. Emerg Infect Dis. 2015;21:84-86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 570] [Cited by in RCA: 507] [Article Influence: 50.7] [Reference Citation Analysis (0)] |
| 228. | World Health Organization. Zika virus. December 8, 20222022. [cited 1 October 2024]. Available from: https://www.who.int/news-room/fact-sheets/detail/zika-virus. |
| 229. | Falcao MB, Cimerman S, Luz KG, Chebabo A, Brigido HA, Lobo IM, Timerman A, Angerami RN, da Cunha CA, Bacha HA, Alves JR, Barbosa AN, Teixeira RF, Weissmann L, Oliveira PR, Cyrillo MA, Bandeira AC. Management of infection by the Zika virus. Ann Clin Microbiol Antimicrob. 2016;15:57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 230. | Bernatchez JA, Tran LT, Li J, Luan Y, Siqueira-Neto JL, Li R. Drugs for the Treatment of Zika Virus Infection. J Med Chem. 2020;63:470-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 231. | Jemielity S, Wang JJ, Chan YK, Ahmed AA, Li W, Monahan S, Bu X, Farzan M, Freeman GJ, Umetsu DT, Dekruyff RH, Choe H. TIM-family proteins promote infection of multiple enveloped viruses through virion-associated phosphatidylserine. PLoS Pathog. 2013;9:e1003232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 244] [Cited by in RCA: 282] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 232. | Menéndez-Arias L, Andino R. Viral polymerases. Virus Res. 2017;234:1-3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 233. | Lin HH, Yip BS, Huang LM, Wu SC. Zika virus structural biology and progress in vaccine development. Biotechnol Adv. 2018;36:47-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 234. | Vannice KS, Cassetti MC, Eisinger RW, Hombach J, Knezevic I, Marston HD, Wilder-Smith A, Cavaleri M, Krause PR. Demonstrating vaccine effectiveness during a waning epidemic: A WHO/NIH meeting report on approaches to development and licensure of Zika vaccine candidates. Vaccine. 2019;37:863-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 235. | World Health Organization. WHO/UNICEF Zika Virus (ZIKV) Vaccine Target Product Profile (TPP). February 8, 2017. [cited 1 October 2024]. Available from: https://www.who.int/publications/m/item/who-unicef-zika-virus-(zikv)-vaccine-target-product-profile-(tpp). |
| 236. | Fortner A, Schumacher D. First COVID-19 Vaccines Receiving the US FDA and EMA Emergency Use Authorization. Discoveries (Craiova). 2021;9:e122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 237. | Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines-a new era in vaccinology. Nat Rev Drug Discov. 2018;17:261-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1620] [Cited by in RCA: 2799] [Article Influence: 399.9] [Reference Citation Analysis (0)] |
| 238. | Fiolet T, Kherabi Y, MacDonald CJ, Ghosn J, Peiffer-Smadja N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: a narrative review. Clin Microbiol Infect. 2022;28:202-221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 617] [Cited by in RCA: 606] [Article Influence: 202.0] [Reference Citation Analysis (0)] |
| 239. | Pollard AJ, Bijker EM. A guide to vaccinology: from basic principles to new developments. Nat Rev Immunol. 2021;21:83-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 296] [Cited by in RCA: 830] [Article Influence: 166.0] [Reference Citation Analysis (0)] |
| 240. | Rice CM, Lenches EM, Eddy SR, Shin SJ, Sheets RL, Strauss JH. Nucleotide sequence of yellow fever virus: implications for flavivirus gene expression and evolution. Science. 1985;229:726-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 681] [Cited by in RCA: 664] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 241. | Monath TP, Vasconcelos PF. Yellow fever. J Clin Virol. 2015;64:160-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 439] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 242. | Mukhopadhyay S, Kuhn RJ, Rossmann MG. A structural perspective of the flavivirus life cycle. Nat Rev Microbiol. 2005;3:13-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 828] [Cited by in RCA: 892] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 243. | Op De Beeck A, Rouillé Y, Caron M, Duvet S, Dubuisson J. The transmembrane domains of the prM and E proteins of yellow fever virus are endoplasmic reticulum localization signals. J Virol. 2004;78:12591-12602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 244. | Davis EH, Wang B, White M, Huang YS, Sarathy VV, Wang T, Bourne N, Higgs S, Barrett ADT. Impact of yellow fever virus envelope protein on wild-type and vaccine epitopes and tissue tropism. NPJ Vaccines. 2022;7:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 245. | Davis EH, Barrett ADT. Structure-Function of the Yellow Fever Virus Envelope Protein: Analysis of Antibody Epitopes. Viral Immunol. 2020;33:12-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 246. | Lindenbach BD, Rice CM. Molecular biology of flaviviruses. Adv Virus Res. 2003;59:23-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 553] [Cited by in RCA: 587] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 247. | Hanley KA, Monath TP, Weaver SC, Rossi SL, Richman RL, Vasilakis N. Fever versus fever: the role of host and vector susceptibility and interspecific competition in shaping the current and future distributions of the sylvatic cycles of dengue virus and yellow fever virus. Infect Genet Evol. 2013;19:292-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 142] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 248. | Ahmed QA, Memish ZA. Yellow fever from Angola and Congo: a storm gathers. Trop Doct. 2017;47:92-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 249. | World Health Organization. Eliminate Yellow Fever Epidemics (EYE) 2017-2026. January 21, 2018. [cited 1 October 2024]. Available from: https://www.who.int/publications/i/item/9789241513661. |
| 250. | Bryant JE, Holmes EC, Barrett AD. Out of Africa: a molecular perspective on the introduction of yellow fever virus into the Americas. PLoS Pathog. 2007;3:e75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 265] [Cited by in RCA: 213] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 251. | Staples JE, Monath TP. Yellow fever: 100 years of discovery. JAMA. 2008;300:960-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 88] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 252. | Pulendran B. Learning immunology from the yellow fever vaccine: innate immunity to systems vaccinology. Nat Rev Immunol. 2009;9:741-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 229] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 253. | Lloyd W, Theiler M, Ricci N. Modification of the virulence of yellow fever virus by cultivation in tissues in vitro. T Roy Soc Trop Med H. 1936;29:481-529. [RCA] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 34] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 254. | World Health Organization. Yellow fever [Internet]. 2023. [cited 1 October 2024]. Available from: https://www.who.int/news-room/fact-sheets/detail/yellow-fever. |
| 255. | Aitken TH, Tesh RB, Beaty BJ, Rosen L. Transovarial transmission of yellow fever virus by mosquitoes (Aedes aegypti). Am J Trop Med Hyg. 1979;28:119-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 63] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 256. | Dutary BE, Leduc JW. Transovarial transmission of yellow fever virus by a sylvatic vector, Haemagogus equinus. Trans R Soc Trop Med Hyg. 1981;75:128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 257. | Barrett AD, Higgs S. Yellow fever: a disease that has yet to be conquered. Annu Rev Entomol. 2007;52:209-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 236] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 258. | Gardner CL, Ryman KD. Yellow fever: a reemerging threat. Clin Lab Med. 2010;30:237-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 176] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 259. | World Health Organization. Laboratory manual for yellow fever. January 25, 2024. [cited 1 October 2024]. Available from: https://www.who.int/publications/i/item/9789240084476. |
| 260. | Pedrosa PB, Cardoso TA. Viral infections in workers in hospital and research laboratory settings: a comparative review of infection modes and respective biosafety aspects. Int J Infect Dis. 2011;15:e366-e376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 261. | Bentlin MR, de Barros Almeida RA, Coelho KI, Ribeiro AF, Siciliano MM, Suzuki A, Fortaleza CM. Perinatal transmission of yellow fever, Brazil, 2009. Emerg Infect Dis. 2011;17:1779-1780. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 262. | Diniz LMO, Romanelli RMC, de Carvalho AL, Teixeira DC, de Carvalho LFA, Ferreira Cury V, Filho MPL, Perígolo G, Heringer TP. Perinatal Yellow Fever: A Case Report. Pediatr Infect Dis J. 2019;38:300-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 263. | Centers for Disease Control and Prevention (CDC). Transfusion-related transmission of yellow fever vaccine virus--California, 2009. MMWR Morb Mortal Wkly Rep. 2010;59:34-37. [PubMed] |
| 264. | Gould CV, Free RJ, Bhatnagar J, Soto RA, Royer TL, Maley WR, Moss S, Berk MA, Craig-Shapiro R, Kodiyanplakkal RPL, Westblade LF, Muthukumar T, Puius YA, Raina A, Hadi A, Gyure KA, Trief D, Pereira M, Kuehnert MJ, Ballen V, Kessler DA, Dailey K, Omura C, Doan T, Miller S, Wilson MR, Lehman JA, Ritter JM, Lee E, Silva-Flannery L, Reagan-Steiner S, Velez JO, Laven JJ, Fitzpatrick KA, Panella A, Davis EH, Hughes HR, Brault AC, St George K, Dean AB, Ackelsberg J, Basavaraju SV, Chiu CY, Staples JE; Yellow Fever Vaccine Virus Transplant and Transfusion Investigation Team. Transmission of yellow fever vaccine virus through blood transfusion and organ transplantation in the USA in 2021: report of an investigation. Lancet Microbe. 2023;4:e711-e721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 265. | Hassan T, Bashir RA, Abdelrahman DN, Madni H, M El Hussein AR, Elkidir IM, Enan KA. Transmission of yellow fever vaccine virus from breast feeding mothers to their infants: reporting of yellow fever virus (YFV) RNA detection in milk specimens. F1000Res. 2022;11:76. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 266. | Centers for Disease Control and Prevention (CDC). Transmission of yellow fever vaccine virus through breast-feeding-Brazil, 2009. MMWR Morb Mortal Wkly Rep. 2010;59:130-132. [PubMed] |
| 267. | Simmonds P, Becher P, Bukh J, Gould EA, Meyers G, Monath T, Muerhoff S, Pletnev A, Rico-Hesse R, Smith DB, Stapleton JT; Ictv Report Consortium. ICTV Virus Taxonomy Profile: Flaviviridae. J Gen Virol. 2017;98:2-3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 391] [Cited by in RCA: 560] [Article Influence: 70.0] [Reference Citation Analysis (0)] |
| 268. | Kaufmann B, Rossmann MG. Molecular mechanisms involved in the early steps of flavivirus cell entry. Microbes Infect. 2011;13:1-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 101] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 269. | Douam F, Ploss A. Yellow Fever Virus: Knowledge Gaps Impeding the Fight Against an Old Foe. Trends Microbiol. 2018;26:913-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 124] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 270. | Chávez JH, Silva JR, Amarilla AA, Moraes Figueiredo LT. Domain III peptides from flavivirus envelope protein are useful antigens for serologic diagnosis and targets for immunization. Biologicals. 2010;38:613-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 271. | Lee E, Hall RA, Lobigs M. Common E protein determinants for attenuation of glycosaminoglycan-binding variants of Japanese encephalitis and West Nile viruses. J Virol. 2004;78:8271-8280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 129] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 272. | Hung JJ, Hsieh MT, Young MJ, Kao CL, King CC, Chang W. An external loop region of domain III of dengue virus type 2 envelope protein is involved in serotype-specific binding to mosquito but not mammalian cells. J Virol. 2004;78:378-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 181] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 273. | Huang YJ, Nuckols JT, Horne KM, Vanlandingham D, Lobigs M, Higgs S. Mutagenesis analysis of T380R mutation in the envelope protein of yellow fever virus. Virol J. 2014;11:60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 274. | Germi R, Crance JM, Garin D, Guimet J, Lortat-Jacob H, Ruigrok RW, Zarski JP, Drouet E. Heparan sulfate-mediated binding of infectious dengue virus type 2 and yellow fever virus. Virology. 2002;292:162-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 184] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 275. | Lee E, Lobigs M. E protein domain III determinants of yellow fever virus 17D vaccine strain enhance binding to glycosaminoglycans, impede virus spread, and attenuate virulence. J Virol. 2008;82:6024-6033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 83] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 276. | Harrison SC. The pH sensor for flavivirus membrane fusion. J Cell Biol. 2008;183:177-179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 277. | Fernandez-Garcia MD, Meertens L, Chazal M, Hafirassou ML, Dejarnac O, Zamborlini A, Despres P, Sauvonnet N, Arenzana-Seisdedos F, Jouvenet N, Amara A. Vaccine and Wild-Type Strains of Yellow Fever Virus Engage Distinct Entry Mechanisms and Differentially Stimulate Antiviral Immune Responses. mBio. 2016;7:e01956-e01915. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 278. | Bressanelli S, Stiasny K, Allison SL, Stura EA, Duquerroy S, Lescar J, Heinz FX, Rey FA. Structure of a flavivirus envelope glycoprotein in its low-pH-induced membrane fusion conformation. EMBO J. 2004;23:728-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 468] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 279. | Ramanathan HN, Zhang S, Douam F, Mar KB, Chang J, Yang PL, Schoggins JW, Ploss A, Lindenbach BD. A Sensitive Yellow Fever Virus Entry Reporter Identifies Valosin-Containing Protein (VCP/p97) as an Essential Host Factor for Flavivirus Uncoating. mBio. 2020;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 280. | Westaway EG, Brinton MA, Gaidamovich SYa, Horzinek MC, Igarashi A, Kääriäinen L, Lvov DK, Porterfield JS, Russell PK, Trent DW. Flaviviridae. Intervirology. 1985;24:183-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 291] [Article Influence: 7.3] [Reference Citation Analysis (1)] |
| 281. | Amberg SM, Rice CM. Mutagenesis of the NS2B-NS3-mediated cleavage site in the flavivirus capsid protein demonstrates a requirement for coordinated processing. J Virol. 1999;73:8083-8094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 96] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 282. | Chambers TJ, Droll DA, Tang Y, Liang Y, Ganesh VK, Murthy KHM, Nickells M. Yellow fever virus NS2B-NS3 protease: characterization of charged-to-alanine mutant and revertant viruses and analysis of polyprotein-cleavage activities. J Gen Virol. 2005;86:1403-1413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 283. | Chambers TJ, Nestorowicz A, Rice CM. Mutagenesis of the yellow fever virus NS2B/3 cleavage site: determinants of cleavage site specificity and effects on polyprotein processing and viral replication. J Virol. 1995;69:1600-1605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 284. | Dubankova A, Boura E. Structure of the yellow fever NS5 protein reveals conserved drug targets shared among flaviviruses. Antiviral Res. 2019;169:104536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 285. | Le Sommer C, Barrows NJ, Bradrick SS, Pearson JL, Garcia-Blanco MA. G protein-coupled receptor kinase 2 promotes flaviviridae entry and replication. PLoS Negl Trop Dis. 2012;6:e1820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 286. | Zhang R, Miner JJ, Gorman MJ, Rausch K, Ramage H, White JP, Zuiani A, Zhang P, Fernandez E, Zhang Q, Dowd KA, Pierson TC, Cherry S, Diamond MS. A CRISPR screen defines a signal peptide processing pathway required by flaviviruses. Nature. 2016;535:164-168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 270] [Cited by in RCA: 297] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 287. | Yi Z, Sperzel L, Nürnberger C, Bredenbeek PJ, Lubick KJ, Best SM, Stoyanov CT, Law LM, Yuan Z, Rice CM, MacDonald MR. Identification and characterization of the host protein DNAJC14 as a broadly active flavivirus replication modulator. PLoS Pathog. 2011;7:e1001255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 288. | Bozzacco L, Yi Z, Andreo U, Conklin CR, Li MM, Rice CM, MacDonald MR. Chaperone-Assisted Protein Folding Is Critical for Yellow Fever Virus NS3/4A Cleavage and Replication. J Virol. 2016;90:3212-3228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 289. | Campos RK, Wong B, Xie X, Lu YF, Shi PY, Pompon J, Garcia-Blanco MA, Bradrick SS. RPLP1 and RPLP2 Are Essential Flavivirus Host Factors That Promote Early Viral Protein Accumulation. J Virol. 2017;91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 290. | Klaitong P, Smith DR. Roles of Non-Structural Protein 4A in Flavivirus Infection. Viruses. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 291. | van den Elsen K, Quek JP, Luo D. Molecular Insights into the Flavivirus Replication Complex. Viruses. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 292. | Roby JA, Setoh YX, Hall RA, Khromykh AA. Post-translational regulation and modifications of flavivirus structural proteins. J Gen Virol. 2015;96:1551-1569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 84] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 293. | Ferreira MS, Sousa JR, Bezerra Júnior PS, Cerqueira VD, Oliveira Júnior CA, Rivero GRC, Castro PHG, Silva GA, Muniz JAPC, da Silva EVP, Casseb SMM, Pagliari C, Martins LC, Tesh RB, Quaresma JAS, Vasconcelos PFC. Experimental Yellow Fever in Squirrel Monkey: Characterization of Liver In Situ Immune Response. Viruses. 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 294. | Guo HY, Zhang XC, Jia RY. Toll-Like Receptors and RIG-I-Like Receptors Play Important Roles in Resisting Flavivirus. J Immunol Res. 2018;2018:6106582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 295. | Querec T, Bennouna S, Alkan S, Laouar Y, Gorden K, Flavell R, Akira S, Ahmed R, Pulendran B. Yellow fever vaccine YF-17D activates multiple dendritic cell subsets via TLR2, 7, 8, and 9 to stimulate polyvalent immunity. J Exp Med. 2006;203:413-424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 417] [Cited by in RCA: 424] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 296. | Sinigaglia L, Gracias S, Décembre E, Fritz M, Bruni D, Smith N, Herbeuval JP, Martin A, Dreux M, Tangy F, Jouvenet N. Immature particles and capsid-free viral RNA produced by Yellow fever virus-infected cells stimulate plasmacytoid dendritic cells to secrete interferons. Sci Rep. 2018;8:10889. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 297. | Beauclair G, Streicher F, Chazal M, Bruni D, Lesage S, Gracias S, Bourgeau S, Sinigaglia L, Fujita T, Meurs EF, Tangy F, Jouvenet N. Retinoic Acid Inducible Gene I and Protein Kinase R, but Not Stress Granules, Mediate the Proinflammatory Response to Yellow Fever Virus. J Virol. 2020;94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 298. | Miorin L, Laurent-Rolle M, Pisanelli G, Co PH, Albrecht RA, García-Sastre A, Morrison J. Host-Specific NS5 Ubiquitination Determines Yellow Fever Virus Tropism. J Virol. 2019;93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 299. | Laurent-Rolle M, Morrison J, Rajsbaum R, Macleod JML, Pisanelli G, Pham A, Ayllon J, Miorin L, Martinez C, tenOever BR, García-Sastre A. The interferon signaling antagonist function of yellow fever virus NS5 protein is activated by type I interferon. Cell Host Microbe. 2014;16:314-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 136] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 300. | Airo AM, Felix-Lopez A, Mancinelli V, Evseev D, Lopez-Orozco J, Shire K, Paszkowski P, Frappier L, Magor KE, Hobman TC. Flavivirus Capsid Proteins Inhibit the Interferon Response. Viruses. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 301. | Zoladek J, Nisole S. Mosquito-borne flaviviruses and type I interferon: catch me if you can! Front Microbiol. 2023;14:1257024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 302. | Quaresma JA, Pagliari C, Medeiros DB, Duarte MI, Vasconcelos PF. Immunity and immune response, pathology and pathologic changes: progress and challenges in the immunopathology of yellow fever. Rev Med Virol. 2013;23:305-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 303. | Quaresma JA, Barros VL, Pagliari C, Fernandes ER, Andrade HF Jr, Vasconcelos PF, Duarte MI. Hepatocyte lesions and cellular immune response in yellow fever infection. Trans R Soc Trop Med Hyg. 2007;101:161-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 304. | ter Meulen J, Sakho M, Koulemou K, Magassouba N, Bah A, Preiser W, Daffis S, Klewitz C, Bae HG, Niedrig M, Zeller H, Heinzel-Gutenbrunner M, Koivogui L, Kaufmann A. Activation of the cytokine network and unfavorable outcome in patients with yellow fever. J Infect Dis. 2004;190:1821-1827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 78] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 305. | Alberts B, Johnson A, Lewis J. Helper T Cells and Lymphocyte Activation. In: Molecular Biology of the Cell. 4th edition. New York: Garland Science, 2002. |
| 306. | Quaresma JA, Barros VL, Fernandes ER, Pagliari C, Takakura C, da Costa Vasconcelos PF, de Andrade HF Jr, Duarte MI. Reconsideration of histopathology and ultrastructural aspects of the human liver in yellow fever. Acta Trop. 2005;94:116-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 307. | Engelmann F, Josset L, Girke T, Park B, Barron A, Dewane J, Hammarlund E, Lewis A, Axthelm MK, Slifka MK, Messaoudi I. Pathophysiologic and transcriptomic analyses of viscerotropic yellow fever in a rhesus macaque model. PLoS Negl Trop Dis. 2014;8:e3295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 308. | Monath TP, Brinker KR, Chandler FW, Kemp GE, Cropp CB. Pathophysiologic correlations in a rhesus monkey model of yellow fever with special observations on the acute necrosis of B cell areas of lymphoid tissues. Am J Trop Med Hyg. 1981;30:431-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 74] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 309. | Xiao SY, Zhang H, Guzman H, Tesh RB. Experimental yellow fever virus infection in the Golden hamster (Mesocricetus auratus). II. Pathology. J Infect Dis. 2001;183:1437-1444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 88] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 310. | Hudson NP. The Pathology of Experimental Yellow Fever in the Macacus Rhesus: I. Gross Pathology. Am J Pathol. 1928;4:395-406.1. [PubMed] |
| 311. | Quaresma JA, Barros VL, Pagliari C, Fernandes ER, Guedes F, Takakura CF, Andrade HF Jr, Vasconcelos PF, Duarte MI. Revisiting the liver in human yellow fever: virus-induced apoptosis in hepatocytes associated with TGF-beta, TNF-alpha and NK cells activity. Virology. 2006;345:22-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 102] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 312. | Quaresma JA, Barros VL, Fernandes ER, Pagliari C, Guedes F, da Costa Vasconcelos PF, de Andrade Junior HF, Duarte MI. Immunohistochemical examination of the role of Fas ligand and lymphocytes in the pathogenesis of human liver yellow fever. Virus Res. 2006;116:91-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 313. | da Costa Lopes J, Falcão LFM, Martins Filho AJ, Carvalho MLG, Mendes CCH, Olímpio FA, do Socorro Cabral Miranda V, Dos Santos LC, Chiang JO, Cruz ACR, Galúcio VCA, do Socorro da Silva Azevedo R, Martins LC, Duarte MIS, de Sousa JR, da Costa Vasconcelos PF, Quaresma JAS. Factors Involved in the Apoptotic Cell Death Mechanism in Yellow Fever Hepatitis. Viruses. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 314. | Olímpio FA, Falcão LFM, Carvalho MLG, da Costa Lopes J, Mendes CCH, Filho AJM, da Silva CAM, Miranda VDSC, Santos LCD, da Silva Vilacoert FS, Cruz ACR, Galúcio VCA, da Silva Azevedo RDS, Martins LC, Duarte MIS, de Sousa JR, da Costa Vasconcelos PF, Quaresma JAS. Endothelium Activation during Severe Yellow Fever Triggers an Intense Cytokine-Mediated Inflammatory Response in the Liver Parenchyma. Pathogens. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 315. | Futosi K, Fodor S, Mócsai A. Neutrophil cell surface receptors and their intracellular signal transduction pathways. Int Immunopharmacol. 2013;17:638-650. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 355] [Cited by in RCA: 484] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 316. | Puerta-Guardo H, Glasner DR, Espinosa DA, Biering SB, Patana M, Ratnasiri K, Wang C, Beatty PR, Harris E. Flavivirus NS1 Triggers Tissue-Specific Vascular Endothelial Dysfunction Reflecting Disease Tropism. Cell Rep. 2019;26:1598-1613.e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 214] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 317. | Vieira WT, Gayotto LC, de Lima CP, de Brito T. Histopathology of the human liver in yellow fever with special emphasis on the diagnostic role of the Councilman body. Histopathology. 1983;7:195-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 34] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 318. | Carvalho MLG, Falcão LFM, Lopes JDC, Mendes CCH, Olímpio FA, Miranda VDSC, Santos LCD, de Moraes DDP, Bertonsin Filho MV, da Costa LD, da Silva Azevedo RDS, Cruz ACR, Galúcio VCA, Martins LC, Duarte MIS, Martins Filho AJ, Sousa JR, Vasconcelos PFDC, Quaresma JAS. Role of Th17 Cytokines in the Liver’s Immune Response during Fatal Yellow Fever: Triggering Cell Damage Mechanisms. Cells. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 319. | Lemos FO, França A, Lima Filho ACM, Florentino RM, Santos ML, Missiaggia DG, Rodrigues GOL, Dias FF, Souza Passos IB, Teixeira MM, Andrade AMF, Lima CX, Vidigal PVT, Costa VV, Fonseca MC, Nathanson MH, Leite MF. Molecular Mechanism for Protection Against Liver Failure in Human Yellow Fever Infection. Hepatol Commun. 2020;4:657-669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 320. | Domingo C, Charrel RN, Schmidt-Chanasit J, Zeller H, Reusken C. Yellow fever in the diagnostics laboratory. Emerg Microbes Infect. 2018;7:129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 321. | Monath TP. Yellow fever: an update. Lancet Infect Dis. 2001;1:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 335] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 322. | Johansson MA, Vasconcelos PF, Staples JE. The whole iceberg: estimating the incidence of yellow fever virus infection from the number of severe cases. Trans R Soc Trop Med Hyg. 2014;108:482-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 108] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 323. | Franco MB, Jardim LL, de Carvalho BN, Basques F, Ribeiro DD, Pereira LS, Rezende SM. Deficiency of coagulation factors is associated with the bleeding diathesis of severe yellow fever. Ann Hematol. 2023;102:1939-1949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 324. | Vasconcelos PF. [Yellow Fever]. Rev Soc Bras Med Trop. 2003;36:275-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 161] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 325. | Waggoner JJ, Rojas A, Pinsky BA. Yellow Fever Virus: Diagnostics for a Persistent Arboviral Threat. J Clin Microbiol. 2018;56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 326. | World Health Organization. Yellow Fever Vaccine-Preventable Diseases Surveillance Standards. April 30, 2020 [cited 1 October 2024]. Available from: https://www.who.int/publications/m/item/vaccine-preventable-diseases-surveillance-standards-yellow-fever. |
| 327. | Houghton-Triviño N, Montaña D, Castellanos J. Dengue-yellow fever sera cross-reactivity; challenges for diagnosis. Rev Salud Publica (Bogota). 2008;10:299-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 328. | Liu D, Chen D, Zhang T, Yu N, Ren R, Chen Y, Wang C. Preparation and application of yellow fever virus NS1 protein-specific monoclonal antibodies. J Med Virol. 2021;93:3374-3382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 329. | Dia M, Bob NS, Talla C, Dupressoir A, Escadafal C, Thiam MS, Diallo A, Ndiaye O, Heraud JM, Faye O, Sall AA, Faye O, Fall G. Performance assessment and validation of a plaque reduction neutralization test (PRNT) in support to yellow fever diagnostic and vaccine clinical trials. J Med Virol. 2023;95:e28700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 330. | Niedrig M, Kürsteiner O, Herzog C, Sonnenberg K. Evaluation of an indirect immunofluorescence assay for detection of immunoglobulin M (IgM) and IgG antibodies against yellow fever virus. Clin Vaccine Immunol. 2008;15:177-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 331. | Hughes HR, Russell BJ, Mossel EC, Kayiwa J, Lutwama J, Lambert AJ. Development of a Real-Time Reverse Transcription-PCR Assay for Global Differentiation of Yellow Fever Virus Vaccine-Related Adverse Events from Natural Infections. J Clin Microbiol. 2018;56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 332. | Fischer C, Torres MC, Patel P, Moreira-Soto A, Gould EA, Charrel RN, de Lamballerie X, Nogueira RMR, Sequeira PC, Rodrigues CDS, Kümmerer BM, Drosten C, Landt O, Bispo de Filippis AM, Drexler JF. Lineage-Specific Real-Time RT-PCR for Yellow Fever Virus Outbreak Surveillance, Brazil. Emerg Infect Dis. 2017;23:1867-1871. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 333. | Huang B, Montgomery BL, Adamczyk R, Ehlers G, van den Hurk AF, Warrilow D. A LAMP-based colorimetric assay to expedite field surveillance of the invasive mosquito species Aedes aegypti and Aedes albopictus. PLoS Negl Trop Dis. 2020;14:e0008130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 334. | Nunes MR, Vianez JL Jr, Nunes KN, da Silva SP, Lima CP, Guzman H, Martins LC, Carvalho VL, Tesh RB, Vasconcelos PF. Analysis of a Reverse Transcription Loop-mediated Isothermal Amplification (RT-LAMP) for yellow fever diagnostic. J Virol Methods. 2015;226:40-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 335. | Kwallah Ao, Inoue S, Muigai AW, Kubo T, Sang R, Morita K, Mwau M. A real-time reverse transcription loop-mediated isothermal amplification assay for the rapid detection of yellow fever virus. J Virol Methods. 2013;193:23-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 336. | Pan American Health Organization. Clinical Management of Yellow Fever in the Region of the Americas. Experiences and Recommendations for Health Services. 2023. [cited 1 October 2024]. Available from: https://iris.paho.org/handle/10665.2/57318. |
| 337. | Abbate M, Zoja C, Remuzzi G. How does proteinuria cause progressive renal damage? J Am Soc Nephrol. 2006;17:2974-2984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 514] [Cited by in RCA: 559] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 338. |
de Freitas CS, Higa LM, Sacramento CQ, Ferreira AC, Reis PA, Delvecchio R, Monteiro FL, Barbosa-Lima G, James Westgarth H, Vieira YR, Mattos M, Rocha N, Hoelz LVB, Leme RPP, Bastos MM, Rodrigues GOL, Lopes CEM, Queiroz-Junior CM, Lima CX, Costa VV, Teixeira MM, Bozza FA, Bozza PT, Boechat N, Tanuri A, Souza TML. Yellow fever virus is susceptible to sofosbuvir both in vitro and in |
| 339. | Mendes ÉA, Pilger DRB, Santos Nastri ACS, Malta FM, Pascoalino BDS, Carneiro D’Albuquerque LA, Balan A, Freitas LHG Jr, Durigon EL, Carrilho FJ, Rebello Pinho JR. Sofosbuvir inhibits yellow fever virus in vitro and in patients with acute liver failure. Ann Hepatol. 2019;18:816-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 340. | Rezende IM, Mendonça DC, Costa TA, de Oliveria GFG, Arruda MS, Gonçalves AP, Alves PA, Calzavara-Silva CE, Martins-Filho OA, Teixeira-Carvalho A, Bonjardim CA, Monath TP, LaBeaud AD, Drumond BP, Pascoal-Xavier MA, Pereira LS, Ramalho DB. Sofosbuvir Off-label Treatment of Yellow Fever Patients During an Outbreak in Brazil, 2018: A Cohort Study. Open Forum Infect Dis. 2024;11:ofae312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 341. | de Ávila RE, José Fernandes H, Barbosa GM, Araújo AL, Gomes TCC, Barros TG, Moreira RLF, Silva GLC, de Oliveira NR. Clinical profiles and factors associated with mortality in adults with yellow fever admitted to an intensive care unit in Minas Gerais, Brazil. Int J Infect Dis. 2020;93:90-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 342. | Kallas EG, D’Elia Zanella LGFAB, Moreira CHV, Buccheri R, Diniz GBF, Castiñeiras ACP, Costa PR, Dias JZC, Marmorato MP, Song ATW, Maestri A, Borges IC, Joelsons D, Cerqueira NB, Santiago E Souza NC, Morales Claro I, Sabino EC, Levi JE, Avelino-Silva VI, Ho YL. Predictors of mortality in patients with yellow fever: an observational cohort study. Lancet Infect Dis. 2019;19:750-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 343. | Ho YL, Joelsons D, Leite GFC, Malbouisson LMS, Song ATW, Perondi B, Andrade LC, Pinto LF, D’Albuquerque LAC, Segurado AAC; Hospital das Clínicas Yellow Fever Assistance Group. Severe yellow fever in Brazil: clinical characteristics and management. J Travel Med. 2019;26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 344. | Barrett AD, Teuwen DE. Yellow fever vaccine-how does it work and why do rare cases of serious adverse events take place? Curr Opin Immunol. 2009;21:308-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 130] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 345. | Querec TD, Akondy RS, Lee EK, Cao W, Nakaya HI, Teuwen D, Pirani A, Gernert K, Deng J, Marzolf B, Kennedy K, Wu H, Bennouna S, Oluoch H, Miller J, Vencio RZ, Mulligan M, Aderem A, Ahmed R, Pulendran B. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat Immunol. 2009;10:116-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 840] [Cited by in RCA: 882] [Article Influence: 51.9] [Reference Citation Analysis (0)] |
| 346. | Juan-Giner A, Kimathi D, Grantz KH, Hamaluba M, Kazooba P, Njuguna P, Fall G, Dia M, Bob NS, Monath TP, Barrett AD, Hombach J, Mulogo EM, Ampeire I, Karanja HK, Nyehangane D, Mwanga-Amumpaire J, Cummings DAT, Bejon P, Warimwe GM, Grais RF. Immunogenicity and safety of fractional doses of yellow fever vaccines: a randomised, double-blind, non-inferiority trial. Lancet. 2021;397:119-127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 347. | Cançado BLB, Aranda CS, Mallozi MC, Weckx LY, Solé D. Egg allergy and yellow fever vaccination. J Pediatr (Rio J). 2024;100:60-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 348. | Silva LA, Dermody TS. Chikungunya virus: epidemiology, replication, disease mechanisms, and prospective intervention strategies. J Clin Invest. 2017;127:737-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 265] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 349. | Caglioti C, Lalle E, Castilletti C, Carletti F, Capobianchi MR, Bordi L. Chikungunya virus infection: an overview. New Microbiol. 2013;36:211-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 350. | Althouse BM, Guerbois M, Cummings DAT, Diop OM, Faye O, Faye A, Diallo D, Sadio BD, Sow A, Faye O, Sall AA, Diallo M, Benefit B, Simons E, Watts DM, Weaver SC, Hanley KA. Role of monkeys in the sylvatic cycle of chikungunya virus in Senegal. Nat Commun. 2018;9:1046. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 351. | Horwood PF, Buchy P. Chikungunya. Rev Sci Tech. 2015;34:479-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 352. | Weaver SC. Evolutionary influences in arboviral disease. Curr Top Microbiol Immunol. 2006;299:285-314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 79] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 353. | Volk SM, Chen R, Tsetsarkin KA, Adams AP, Garcia TI, Sall AA, Nasar F, Schuh AJ, Holmes EC, Higgs S, Maharaj PD, Brault AC, Weaver SC. Genome-scale phylogenetic analyses of chikungunya virus reveal independent emergences of recent epidemics and various evolutionary rates. J Virol. 2010;84:6497-6504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 302] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 354. | Tsetsarkin KA, Chen R, Sherman MB, Weaver SC. Chikungunya virus: evolution and genetic determinants of emergence. Curr Opin Virol. 2011;1:310-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 133] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 355. | Tsetsarkin KA, Chen R, Leal G, Forrester N, Higgs S, Huang J, Weaver SC. Chikungunya virus emergence is constrained in Asia by lineage-specific adaptive landscapes. Proc Natl Acad Sci U S A. 2011;108:7872-7877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 183] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 356. | Tsetsarkin KA, McGee CE, Volk SM, Vanlandingham DL, Weaver SC, Higgs S. Epistatic roles of E2 glycoprotein mutations in adaption of chikungunya virus to Aedes albopictus and Ae. aegypti mosquitoes. PLoS One. 2009;4:e6835. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 181] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 357. | Tsetsarkin KA, Vanlandingham DL, McGee CE, Higgs S. A single mutation in chikungunya virus affects vector specificity and epidemic potential. PLoS Pathog. 2007;3:e201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1026] [Cited by in RCA: 1106] [Article Influence: 65.1] [Reference Citation Analysis (0)] |
| 358. | Ryan SJ, Carlson CJ, Mordecai EA, Johnson LR. Global expansion and redistribution of Aedes-borne virus transmission risk with climate change. PLoS Negl Trop Dis. 2019;13:e0007213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 499] [Cited by in RCA: 472] [Article Influence: 78.7] [Reference Citation Analysis (0)] |
| 359. | Messina JP, Brady OJ, Pigott DM, Golding N, Kraemer MU, Scott TW, Wint GR, Smith DL, Hay SI. The many projected futures of dengue. Nat Rev Microbiol. 2015;13:230-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 111] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 360. | Arif M, Tauran P, Kosasih H, Pelupessy NM, Sennang N, Mubin RH, Sudarmono P, Tjitra E, Murniati D, Alam A, Gasem MH, Aman AT, Lokida D, Hadi U, Parwati KTM, Lau CY, Neal A, Karyana M. Chikungunya in Indonesia: Epidemiology and diagnostic challenges. PLoS Negl Trop Dis. 2020;14:e0008355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 361. | Petersen LR, Powers AM. Chikungunya: epidemiology. F1000Res. 2016;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 362. | de Lima Cavalcanti TYV, Pereira MR, de Paula SO, Franca RFO. A Review on Chikungunya Virus Epidemiology, Pathogenesis and Current Vaccine Development. Viruses. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 113] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 363. | Sourisseau M, Schilte C, Casartelli N, Trouillet C, Guivel-Benhassine F, Rudnicka D, Sol-Foulon N, Le Roux K, Prevost MC, Fsihi H, Frenkiel MP, Blanchet F, Afonso PV, Ceccaldi PE, Ozden S, Gessain A, Schuffenecker I, Verhasselt B, Zamborlini A, Saïb A, Rey FA, Arenzana-Seisdedos F, Desprès P, Michault A, Albert ML, Schwartz O. Characterization of reemerging chikungunya virus. PLoS Pathog. 2007;3:e89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 336] [Cited by in RCA: 354] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 364. | Noret M, Herrero L, Rulli N, Rolph M, Smith PN, Li RW, Roques P, Gras G, Mahalingam S. Interleukin 6, RANKL, and osteoprotegerin expression by chikungunya virus-infected human osteoblasts. J Infect Dis. 2012;206 455-7:457-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 365. | Sun Q, Du X, Cheng W. Structures Unveil the Invasion Mechanism of Chikungunya Virus. Trends Microbiol. 2019;27:656-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 366. | Weber C, Berberich E, von Rhein C, Henß L, Hildt E, Schnierle BS. Identification of Functional Determinants in the Chikungunya Virus E2 Protein. PLoS Negl Trop Dis. 2017;11:e0005318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 367. | Basore K, Kim AS, Nelson CA, Zhang R, Smith BK, Uranga C, Vang L, Cheng M, Gross ML, Smith J, Diamond MS, Fremont DH. Cryo-EM Structure of Chikungunya Virus in Complex with the Mxra8 Receptor. Cell. 2019;177:1725-1737.e16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 121] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 368. | Kim AS, Zimmerman O, Fox JM, Nelson CA, Basore K, Zhang R, Durnell L, Desai C, Bullock C, Deem SL, Oppenheimer J, Shapiro B, Wang T, Cherry S, Coyne CB, Handley SA, Landis MJ, Fremont DH, Diamond MS. An Evolutionary Insertion in the Mxra8 Receptor-Binding Site Confers Resistance to Alphavirus Infection and Pathogenesis. Cell Host Microbe. 2020;27:428-440.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 369. | Song H, Zhao Z, Chai Y, Jin X, Li C, Yuan F, Liu S, Gao Z, Wang H, Song J, Vazquez L, Zhang Y, Tan S, Morel CM, Yan J, Shi Y, Qi J, Gao F, Gao GF. Molecular Basis of Arthritogenic Alphavirus Receptor MXRA8 Binding to Chikungunya Virus Envelope Protein. Cell. 2019;177:1714-1724.e12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 90] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 370. | De Caluwé L, Coppens S, Vereecken K, Daled S, Dhaenens M, Van Ostade X, Deforce D, Ariën KK, Bartholomeeusen K. The CD147 Protein Complex Is Involved in Entry of Chikungunya Virus and Related Alphaviruses in Human Cells. Front Microbiol. 2021;12:615165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 371. | Tanaka A, Tumkosit U, Nakamura S, Motooka D, Kishishita N, Priengprom T, Sa-Ngasang A, Kinoshita T, Takeda N, Maeda Y. Genome-Wide Screening Uncovers the Significance of N-Sulfation of Heparan Sulfate as a Host Cell Factor for Chikungunya Virus Infection. J Virol. 2017;91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 372. | McAllister N, Liu Y, Silva LM, Lentscher AJ, Chai W, Wu N, Griswold KA, Raghunathan K, Vang L, Alexander J, Warfield KL, Diamond MS, Feizi T, Silva LA, Dermody TS. Chikungunya Virus Strains from Each Genetic Clade Bind Sulfated Glycosaminoglycans as Attachment Factors. J Virol. 2020;94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 373. | Wintachai P, Wikan N, Kuadkitkan A, Jaimipuk T, Ubol S, Pulmanausahakul R, Auewarakul P, Kasinrerk W, Weng WY, Panyasrivanit M, Paemanee A, Kittisenachai S, Roytrakul S, Smith DR. Identification of prohibitin as a Chikungunya virus receptor protein. J Med Virol. 2012;84:1757-1770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 144] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 374. | Moller-Tank S, Kondratowicz AS, Davey RA, Rennert PD, Maury W. Role of the phosphatidylserine receptor TIM-1 in enveloped-virus entry. J Virol. 2013;87:8327-8341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 213] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 375. | Prado Acosta M, Geoghegan EM, Lepenies B, Ruzal S, Kielian M, Martinez MG. Surface (S) Layer Proteins of Lactobacillus acidophilus Block Virus Infection via DC-SIGN Interaction. Front Microbiol. 2019;10:810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 376. | Meertens L, Hafirassou ML, Couderc T, Bonnet-Madin L, Kril V, Kümmerer BM, Labeau A, Brugier A, Simon-Loriere E, Burlaud-Gaillard J, Doyen C, Pezzi L, Goupil T, Rafasse S, Vidalain PO, Bertrand-Legout A, Gueneau L, Juntas-Morales R, Ben Yaou R, Bonne G, de Lamballerie X, Benkirane M, Roingeard P, Delaugerre C, Lecuit M, Amara A. FHL1 is a major host factor for chikungunya virus infection. Nature. 2019;574:259-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 377. | Hoornweg TE, van Duijl-Richter MKS, Ayala Nuñez NV, Albulescu IC, van Hemert MJ, Smit JM. Dynamics of Chikungunya Virus Cell Entry Unraveled by Single-Virus Tracking in Living Cells. J Virol. 2016;90:4745-4756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 378. | Bernard E, Solignat M, Gay B, Chazal N, Higgs S, Devaux C, Briant L. Endocytosis of chikungunya virus into mammalian cells: role of clathrin and early endosomal compartments. PLoS One. 2010;5:e11479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 132] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 379. | Izumida M, Hayashi H, Tanaka A, Kubo Y. Cathepsin B Protease Facilitates Chikungunya Virus Envelope Protein-Mediated Infection via Endocytosis or Macropinocytosis. Viruses. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 380. | Lee CHR, Mohamed Hussain K, Chu JJH. Macropinocytosis dependent entry of Chikungunya virus into human muscle cells. PLoS Negl Trop Dis. 2019;13:e0007610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 381. | Krejbich-Trotot P, Denizot M, Hoarau JJ, Jaffar-Bandjee MC, Das T, Gasque P. Chikungunya virus mobilizes the apoptotic machinery to invade host cell defenses. FASEB J. 2011;25:314-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 108] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 382. | Constant LEC, Rajsfus BF, Carneiro PH, Sisnande T, Mohana-Borges R, Allonso D. Overview on Chikungunya Virus Infection: From Epidemiology to State-of-the-Art Experimental Models. Front Microbiol. 2021;12:744164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 383. | Sharma R, Kesari P, Kumar P, Tomar S. Structure-function insights into chikungunya virus capsid protein: Small molecules targeting capsid hydrophobic pocket. Virology. 2018;515:223-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 384. | Brown RS, Anastasakis DG, Hafner M, Kielian M. Multiple capsid protein binding sites mediate selective packaging of the alphavirus genomic RNA. Nat Commun. 2020;11:4693. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 385. | Chmielewski D, Schmid MF, Simmons G, Jin J, Chiu W. Chikungunya virus assembly and budding visualized in situ using cryogenic electron tomography. Nat Microbiol. 2022;7:1270-1279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 35] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 386. | Hakim MS, Aman AT. Understanding the Biology and Immune Pathogenesis of Chikungunya Virus Infection for Diagnostic and Vaccine Development. Viruses. 2022;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 387. | Weaver SC, Lecuit M. Chikungunya virus and the global spread of a mosquito-borne disease. N Engl J Med. 2015;372:1231-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 574] [Cited by in RCA: 584] [Article Influence: 58.4] [Reference Citation Analysis (0)] |
| 388. | Hoarau JJ, Jaffar Bandjee MC, Krejbich Trotot P, Das T, Li-Pat-Yuen G, Dassa B, Denizot M, Guichard E, Ribera A, Henni T, Tallet F, Moiton MP, Gauzère BA, Bruniquet S, Jaffar Bandjee Z, Morbidelli P, Martigny G, Jolivet M, Gay F, Grandadam M, Tolou H, Vieillard V, Debré P, Autran B, Gasque P. Persistent chronic inflammation and infection by Chikungunya arthritogenic alphavirus in spite of a robust host immune response. J Immunol. 2010;184:5914-5927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 443] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 389. | Couderc T, Chrétien F, Schilte C, Disson O, Brigitte M, Guivel-Benhassine F, Touret Y, Barau G, Cayet N, Schuffenecker I, Desprès P, Arenzana-Seisdedos F, Michault A, Albert ML, Lecuit M. A mouse model for Chikungunya: young age and inefficient type-I interferon signaling are risk factors for severe disease. PLoS Pathog. 2008;4:e29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 462] [Cited by in RCA: 465] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 390. | Labadie K, Larcher T, Joubert C, Mannioui A, Delache B, Brochard P, Guigand L, Dubreil L, Lebon P, Verrier B, de Lamballerie X, Suhrbier A, Cherel Y, Le Grand R, Roques P. Chikungunya disease in nonhuman primates involves long-term viral persistence in macrophages. J Clin Invest. 2010;120:894-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 426] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 391. | Gardner J, Anraku I, Le TT, Larcher T, Major L, Roques P, Schroder WA, Higgs S, Suhrbier A. Chikungunya virus arthritis in adult wild-type mice. J Virol. 2010;84:8021-8032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 326] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 392. | Wauquier N, Becquart P, Nkoghe D, Padilla C, Ndjoyi-Mbiguino A, Leroy EM. The acute phase of Chikungunya virus infection in humans is associated with strong innate immunity and T CD8 cell activation. J Infect Dis. 2011;204:115-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 181] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 393. | Her Z, Teng TS, Tan JJ, Teo TH, Kam YW, Lum FM, Lee WW, Gabriel C, Melchiotti R, Andiappan AK, Lulla V, Lulla A, Win MK, Chow A, Biswas SK, Leo YS, Lecuit M, Merits A, Rénia L, Ng LF. Loss of TLR3 aggravates CHIKV replication and pathology due to an altered virus-specific neutralizing antibody response. EMBO Mol Med. 2015;7:24-41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 394. | Rudd PA, Wilson J, Gardner J, Larcher T, Babarit C, Le TT, Anraku I, Kumagai Y, Loo YM, Gale M Jr, Akira S, Khromykh AA, Suhrbier A. Interferon response factors 3 and 7 protect against Chikungunya virus hemorrhagic fever and shock. J Virol. 2012;86:9888-9898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 149] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 395. | Cook LE, Locke MC, Young AR, Monte K, Hedberg ML, Shimak RM, Sheehan KCF, Veis DJ, Diamond MS, Lenschow DJ. Distinct Roles of Interferon Alpha and Beta in Controlling Chikungunya Virus Replication and Modulating Neutrophil-Mediated Inflammation. J Virol. 2019;94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 396. | Tanabe ISB, Santos EC, Tanabe ELL, Souza SJM, Santos FEF, Taniele-Silva J, Ferro JFG, Lima MC, Moura AA, Anderson L, Bassi ÊJ. Cytokines and chemokines triggered by Chikungunya virus infection in human patients during the very early acute phase. Trans R Soc Trop Med Hyg. 2019;113:730-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 397. | Wichit S, Hamel R, Yainoy S, Gumpangseth N, Panich S, Phuadraksa T, Saetear P, Monteil A, Morales Vargas R, Missé D. Interferon-inducible protein (IFI) 16 regulates Chikungunya and Zika virus infection in human skin fibroblasts. EXCLI J. 2019;18:467-476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 398. | Nair SR, Abraham R, Sundaram S, Sreekumar E. Interferon regulated gene (IRG) expression-signature in a mouse model of chikungunya virus neurovirulence. J Neurovirol. 2017;23:886-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 399. | Wichit S, Hamel R, Zanzoni A, Diop F, Cribier A, Talignani L, Diack A, Ferraris P, Liegeois F, Urbach S, Ekchariyawat P, Merits A, Yssel H, Benkirane M, Missé D. SAMHD1 Enhances Chikungunya and Zika Virus Replication in Human Skin Fibroblasts. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 400. | Valdés-López JF, Fernandez GJ, Urcuqui-Inchima S. Interleukin 27 as an inducer of antiviral response against chikungunya virus infection in human macrophages. Cell Immunol. 2021;367:104411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 401. | Bae S, Lee JY, Myoung J. Chikungunya Virus nsP2 Impairs MDA5/RIG-I-Mediated Induction of NF-κB Promoter Activation: A Potential Target for Virus-Specific Therapeutics. J Microbiol Biotechnol. 2020;30:1801-1809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 402. | Bae S, Lee JY, Myoung J. Chikungunya Virus-Encoded nsP2, E2 and E1 Strongly Antagonize the Interferon-β Signaling Pathway. J Microbiol Biotechnol. 2019;29:1852-1859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 403. | Teng TS, Kam YW, Lee B, Hapuarachchi HC, Wimal A, Ng LC, Ng LF. A Systematic Meta-analysis of Immune Signatures in Patients With Acute Chikungunya Virus Infection. J Infect Dis. 2015;211:1925-1935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 87] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 404. | Michlmayr D, Pak TR, Rahman AH, Amir ED, Kim EY, Kim-Schulze S, Suprun M, Stewart MG, Thomas GP, Balmaseda A, Wang L, Zhu J, Suaréz-Fariñas M, Wolinsky SM, Kasarskis A, Harris E. Comprehensive innate immune profiling of chikungunya virus infection in pediatric cases. Mol Syst Biol. 2018;14:e7862. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 405. | Chen W, Foo SS, Taylor A, Lulla A, Merits A, Hueston L, Forwood MR, Walsh NC, Sims NA, Herrero LJ, Mahalingam S. Bindarit, an inhibitor of monocyte chemotactic protein synthesis, protects against bone loss induced by chikungunya virus infection. J Virol. 2015;89:581-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 406. | Hornum L, Hansen AJ, Tornehave D, Fjording MS, Colmenero P, Wätjen IF, Søe Nielsen NH, Bliddal H, Bartels EM. C5a and C5aR are elevated in joints of rheumatoid and psoriatic arthritis patients, and C5aR blockade attenuates leukocyte migration to synovial fluid. PLoS One. 2017;12:e0189017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 407. | Andersson C, Wenander CS, Usher PA, Hebsgaard JB, Sondergaard BC, Rønø B, Mackay C, Friedrichsen B, Chang C, Tang R, Hornum L. Rapid-onset clinical and mechanistic effects of anti-C5aR treatment in the mouse collagen-induced arthritis model. Clin Exp Immunol. 2014;177:219-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 408. | Conroy AL, Gélvez M, Hawkes M, Rajwans N, Tran V, Liles WC, Villar-Centeno LA, Kain KC. Host biomarkers are associated with progression to dengue haemorrhagic fever: a nested case-control study. Int J Infect Dis. 2015;40:45-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 409. | Mahauad-Fernandez WD, Jones PH, Okeoma CM. Critical role for bone marrow stromal antigen 2 in acute Chikungunya virus infection. J Gen Virol. 2014;95:2450-2461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 410. | Lum FM, Teo TH, Lee WW, Kam YW, Rénia L, Ng LF. An essential role of antibodies in the control of Chikungunya virus infection. J Immunol. 2013;190:6295-6302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 127] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 411. | Hoarau JJ, Gay F, Pellé O, Samri A, Jaffar-Bandjee MC, Gasque P, Autran B. Identical strength of the T cell responses against E2, nsP1 and capsid CHIKV proteins in recovered and chronic patients after the epidemics of 2005-2006 in La Reunion Island. PLoS One. 2013;8:e84695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 412. | Teo TH, Lum FM, Claser C, Lulla V, Lulla A, Merits A, Rénia L, Ng LF. A pathogenic role for CD4+ T cells during Chikungunya virus infection in mice. J Immunol. 2013;190:259-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 157] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 413. | Dias CNS, Gois BM, Lima VS, Guerra-Gomes IC, Araújo JMG, Gomes JAS, Araújo DAM, Medeiros IA, Azevedo FLAA, Veras RC, Janebro DI, Amaral IPGD, Keesen TSL. Human CD8 T-cell activation in acute and chronic chikungunya infection. Immunology. 2018;155:499-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 414. | Davenport BJ, Bullock C, McCarthy MK, Hawman DW, Murphy KM, Kedl RM, Diamond MS, Morrison TE. Chikungunya Virus Evades Antiviral CD8(+) T Cell Responses To Establish Persistent Infection in Joint-Associated Tissues. J Virol. 2020;94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 415. | Jain J, Okabayashi T, Kaur N, Nakayama E, Shioda T, Gaind R, Kurosu T, Sunil S. Evaluation of an immunochromatography rapid diagnosis kit for detection of chikungunya virus antigen in India, a dengue-endemic country. Virol J. 2018;15:84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 416. | Chua CL, Sam IC, Chiam CW, Chan YF. The neutralizing role of IgM during early Chikungunya virus infection. PLoS One. 2017;12:e0171989. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 417. | Prince HE, Seaton BL, Matud JL, Batterman HJ. Chikungunya virus RNA and antibody testing at a National Reference Laboratory since the emergence of Chikungunya virus in the Americas. Clin Vaccine Immunol. 2015;22:291-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 418. | Kam YW, Simarmata D, Chow A, Her Z, Teng TS, Ong EK, Rénia L, Leo YS, Ng LF. Early appearance of neutralizing immunoglobulin G3 antibodies is associated with chikungunya virus clearance and long-term clinical protection. J Infect Dis. 2012;205:1147-1154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 153] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 419. | Reddy V, Mani RS, Desai A, Ravi V. Correlation of plasma viral loads and presence of Chikungunya IgM antibodies with cytokine/chemokine levels during acute Chikungunya virus infection. J Med Virol. 2014;86:1393-1401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 420. | Tumkosit U, Siripanyaphinyo U, Takeda N, Tsuji M, Maeda Y, Ruchusatsawat K, Shioda T, Mizushima H, Chetanachan P, Wongjaroen P, Matsuura Y, Tatsumi M, Tanaka A. Anti-Chikungunya Virus Monoclonal Antibody That Inhibits Viral Fusion and Release. J Virol. 2020;94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 421. | Kam YW, Lum FM, Teo TH, Lee WW, Simarmata D, Harjanto S, Chua CL, Chan YF, Wee JK, Chow A, Lin RT, Leo YS, Le Grand R, Sam IC, Tong JC, Roques P, Wiesmüller KH, Rénia L, Rötzschke O, Ng LF. Early neutralizing IgG response to Chikungunya virus in infected patients targets a dominant linear epitope on the E2 glycoprotein. EMBO Mol Med. 2012;4:330-343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 155] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 422. | Henss L, Yue C, Von Rhein C, Tschismarov R, Lewis-Ximenez LL, Dölle A, Baylis SA, Schnierle BS. Analysis of Humoral Immune Responses in Chikungunya Virus (CHIKV)-Infected Patients and Individuals Vaccinated With a Candidate CHIKV Vaccine. J Infect Dis. 2020;221:1713-1723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 423. | Quiroz JA, Malonis RJ, Thackray LB, Cohen CA, Pallesen J, Jangra RK, Brown RS, Hofmann D, Holtsberg FW, Shulenin S, Nyakatura EK, Durnell LA, Rayannavar V, Daily JP, Ward AB, Aman MJ, Dye JM, Chandran K, Diamond MS, Kielian M, Lai JR. Human monoclonal antibodies against chikungunya virus target multiple distinct epitopes in the E1 and E2 glycoproteins. PLoS Pathog. 2019;15:e1008061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 424. | Anfasa F, Lim SM, Fekken S, Wever R, Osterhaus ADME, Martina BEE. Characterization of antibody response in patients with acute and chronic chikungunya virus disease. J Clin Virol. 2019;117:68-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 425. | Murillo-Zamora E, Mendoza-Cano O, Trujillo-Hernández B, Guzmán-Esquivel J, Higareda-Almaraz E, Higareda-Almaraz MA, Sánchez-Piña RA, Lugo-Radillo A. Persistent Arthralgia and Related Risks Factors: A Cohort Study at 12 Months from Laboratory-Confirmed Chikungunya Infection. Arch Med Res. 2018;49:65-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 426. | Jacob-Nascimento LC, Carvalho CX, Silva MMO, Kikuti M, Anjos RO, Fradico JRB, Campi-Azevedo AC, Tauro LB, Campos GS, Moreira PSDS, Portilho MM, Martins-Filho OA, Ribeiro GS, Reis MG. Acute-Phase Levels of CXCL8 as Risk Factor for Chronic Arthralgia Following Chikungunya Virus Infection. Front Immunol. 2021;12:744183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 427. | Sissoko D, Malvy D, Ezzedine K, Renault P, Moscetti F, Ledrans M, Pierre V. Post-epidemic Chikungunya disease on Reunion Island: course of rheumatic manifestations and associated factors over a 15-month period. PLoS Negl Trop Dis. 2009;3:e389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 255] [Cited by in RCA: 271] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 428. | Suhrbier A. Rheumatic manifestations of chikungunya: emerging concepts and interventions. Nat Rev Rheumatol. 2019;15:597-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 127] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 429. | Chang AY, Martins KAO, Encinales L, Reid SP, Acuña M, Encinales C, Matranga CB, Pacheco N, Cure C, Shukla B, Ruiz Arteta T, Amdur R, Cazares LH, Gregory M, Ward MD, Porras A, Rico Mendoza A, Dong L, Kenny T, Brueggemann E, Downey LG, Kamalapathy P, Lichtenberger P, Falls O, Simon GL, Bethony JM, Firestein GS. Chikungunya Arthritis Mechanisms in the Americas: A Cross-Sectional Analysis of Chikungunya Arthritis Patients Twenty-Two Months After Infection Demonstrating No Detectable Viral Persistence in Synovial Fluid. Arthritis Rheumatol. 2018;70:585-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 430. | Borgherini G, Poubeau P, Staikowsky F, Lory M, Le Moullec N, Becquart JP, Wengling C, Michault A, Paganin F. Outbreak of chikungunya on Reunion Island: early clinical and laboratory features in 157 adult patients. Clin Infect Dis. 2007;44:1401-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 359] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 431. | Chaaitanya IK, Muruganandam N, Sundaram SG, Kawalekar O, Sugunan AP, Manimunda SP, Ghosal SR, Muthumani K, Vijayachari P. Role of proinflammatory cytokines and chemokines in chronic arthropathy in CHIKV infection. Viral Immunol. 2011;24:265-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 432. | Kelvin AA, Banner D, Silvi G, Moro ML, Spataro N, Gaibani P, Cavrini F, Pierro A, Rossini G, Cameron MJ, Bermejo-Martin JF, Paquette SG, Xu L, Danesh A, Farooqui A, Borghetto I, Kelvin DJ, Sambri V, Rubino S. Inflammatory cytokine expression is associated with chikungunya virus resolution and symptom severity. PLoS Negl Trop Dis. 2011;5:e1279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 133] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 433. | Thanapati S, Ganu M, Giri P, Kulkarni S, Sharma M, Babar P, Ganu A, Tripathy AS. Impaired NK cell functionality and increased TNF-α production as biomarkers of chronic chikungunya arthritis and rheumatoid arthritis. Hum Immunol. 2017;78:370-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 434. | Simon F, Javelle E, Cabie A, Bouquillard E, Troisgros O, Gentile G, Leparc-Goffart I, Hoen B, Gandjbakhch F, Rene-Corail P, Franco JM, Caumes E, Combe B, Poiraudeau S, Gane-Troplent F, Djossou F, Schaerverbeke T, Criquet-Hayot A, Carrere P, Malvy D, Gaillard P, Wendling D; Société de pathologie infectieuse de langue francaise. French guidelines for the management of chikungunya (acute and persistent presentations). November 2014. Med Mal Infect. 2015;45:243-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 152] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 435. | Simon F, Parola P, Grandadam M, Fourcade S, Oliver M, Brouqui P, Hance P, Kraemer P, Mohamed AA, de Lamballerie X, Charrel R, Tolou H. Chikungunya infection: an emerging rheumatism among travelers returned from Indian Ocean islands. Report of 47 cases. Medicine (Baltimore). 2007;86:123-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 192] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 436. | Zaid A, Gérardin P, Taylor A, Mostafavi H, Malvy D, Mahalingam S. Chikungunya Arthritis: Implications of Acute and Chronic Inflammation Mechanisms on Disease Management. Arthritis Rheumatol. 2018;70:484-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 87] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 437. | Javelle E, Tiong TH, Leparc-Goffart I, Savini H, Simon F. Inflammation of the external ear in acute chikungunya infection: Experience from the outbreak in Johor Bahru, Malaysia, 2008. J Clin Virol. 2014;59:270-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 438. | Blettery M, Brunier L, Polomat K, Moinet F, Deligny C, Arfi S, Jean-Baptiste G, De Bandt M. Brief Report: Management of Chronic Post-Chikungunya Rheumatic Disease: The Martinican Experience. Arthritis Rheumatol. 2016;68:2817-2824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 439. | Godaert L, Najioullah F, Bartholet S, Colas S, Yactayo S, Cabié A, Fanon JL, Césaire R, Dramé M. Atypical Clinical Presentations of Acute Phase Chikungunya Virus Infection in Older Adults. J Am Geriatr Soc. 2017;65:2510-2515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 440. | Mehta R, Gerardin P, de Brito CAA, Soares CN, Ferreira MLB, Solomon T. The neurological complications of chikungunya virus: A systematic review. Rev Med Virol. 2018;28:e1978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 149] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 441. | Torres JR, Leopoldo Códova G, Castro JS, Rodríguez L, Saravia V, Arvelaez J, Ríos-Fabra A, Longhi MA, Marcano M. Chikungunya fever: Atypical and lethal cases in the Western hemisphere: A Venezuelan experience. IDCases. 2015;2:6-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 442. | Economopoulou A, Dominguez M, Helynck B, Sissoko D, Wichmann O, Quenel P, Germonneau P, Quatresous I. Atypical Chikungunya virus infections: clinical manifestations, mortality and risk factors for severe disease during the 2005-2006 outbreak on Réunion. Epidemiol Infect. 2009;137:534-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 379] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 443. | Imad HA, Phadungsombat J, Nakayama EE, Suzuki K, Ibrahim AM, Afaa A, Azeema A, Nazfa A, Yazfa A, Ahmed A, Saeed A, Waheed A, Shareef F, Islam MM, Anees SM, Saleem S, Aroosha A, Afzal I, Leaungwutiwong P, Piyaphanee W, Phumratanaprapin W, Shioda T. Clinical Features of Acute Chikungunya Virus Infection in Children and Adults during an Outbreak in the Maldives. Am J Trop Med Hyg. 2021;105:946-954. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 444. | Tandale BV, Sathe PS, Arankalle VA, Wadia RS, Kulkarni R, Shah SV, Shah SK, Sheth JK, Sudeep AB, Tripathy AS, Mishra AC. Systemic involvements and fatalities during Chikungunya epidemic in India, 2006. J Clin Virol. 2009;46:145-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 135] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 445. | Ramos R, Viana R, Brainer-Lima A, FloreÂncio T, Carvalho MD, van der Linden V, Amorim A, Rocha MA, Medeiros F. Perinatal Chikungunya Virus-associated Encephalitis Leading to Postnatal-Onset Microcephaly and Optic Atrophy. Pediatr Infect Dis J. 2018;37:94-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 446. | Simon F, Javelle E, Oliver M, Leparc-Goffart I, Marimoutou C. Chikungunya virus infection. Curr Infect Dis Rep. 2011;13:218-228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 141] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 447. | Mohan A, Kiran DH, Manohar IC, Kumar DP. Epidemiology, clinical manifestations, and diagnosis of Chikungunya fever: lessons learned from the re-emerging epidemic. Indian J Dermatol. 2010;55:54-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 94] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 448. | Bertolotti A, Thioune M, Abel S, Belrose G, Calmont I, Césaire R, Cervantes M, Fagour L, Javelle É, Lebris C, Najioullah F, Pierre-François S, Rozé B, Vigan M, Laouénan C, Cabié A; Chronic Chikungunya working group of University Medical Center of Martinique. Prevalence of chronic chikungunya and associated risks factors in the French West Indies (La Martinique): A prospective cohort study. PLoS Negl Trop Dis. 2020;14:e0007327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 449. | Johnson BW, Russell BJ, Goodman CH. Laboratory Diagnosis of Chikungunya Virus Infections and Commercial Sources for Diagnostic Assays. J Infect Dis. 2016;214:S471-S474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 450. | Wikan N, Sakoonwatanyoo P, Ubol S, Yoksan S, Smith DR. Chikungunya virus infection of cell lines: analysis of the East, Central and South African lineage. PLoS One. 2012;7:e31102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 451. | Thirion L, Pezzi L, Corcostegui I, Dubot-Pérès A, Falchi A, de Lamballerie X, Charrel RN. Development and Evaluation of a Duo Chikungunya Virus Real-Time RT-PCR Assay Targeting Two Regions within the Genome. Viruses. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 452. | Edwards T, Del Carmen Castillo Signor L, Williams C, Larcher C, Espinel M, Theaker J, Donis E, Cuevas LE, Adams ER. Analytical and clinical performance of a Chikungunya qRT-PCR for Central and South America. Diagn Microbiol Infect Dis. 2017;89:35-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 453. | Chiam CW, Chan YF, Loong SK, Yong SS, Hooi PS, Sam IC. Real-time polymerase chain reaction for diagnosis and quantitation of negative strand of chikungunya virus. Diagn Microbiol Infect Dis. 2013;77:133-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 454. | Broeders S, Garlant L, Fraiture MA, Vandermassen E, Suin V, Vanhomwegen J, Dupont-Rouzeyrol M, Rousset D, Van Gucht S, Roosens N. A new multiplex RT-qPCR method for the simultaneous detection and discrimination of Zika and chikungunya viruses. Int J Infect Dis. 2020;92:160-170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 455. | Giry C, Roquebert B, Li-Pat-Yuen G, Gasque P, Jaffar-Bandjee MC. Improved detection of genus-specific Alphavirus using a generic TaqMan® assay. BMC Microbiol. 2017;17:164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 456. | Appassakij H, Khuntikij P, Kemapunmanus M, Wutthanarungsan R, Silpapojakul K. Viremic profiles in asymptomatic and symptomatic chikungunya fever: a blood transfusion threat? Transfusion. 2013;53:2567-2574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 100] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 457. | Manzoor KN, Javed F, Ejaz M, Ali M, Mujaddadi N, Khan AA, Khattak AA, Zaib A, Ahmad I, Saeed WK, Manzoor S. The global emergence of Chikungunya infection: An integrated view. Rev Med Virol. 2022;32:e2287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 458. | Andrew A, Navien TN, Yeoh TS, Citartan M, Mangantig E, Sum MSH, Ch’ng ES, Tang TH. Diagnostic accuracy of serological tests for the diagnosis of Chikungunya virus infection: A systematic review and meta-analysis. PLoS Negl Trop Dis. 2022;16:e0010152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 459. | Clements TL, Rossi CA, Irish AK, Kibuuka H, Eller LA, Robb ML, Kataaha P, Michael NL, Hensley LE, Schoepp RJ. Chikungunya and O’nyong-nyong Viruses in Uganda: Implications for Diagnostics. Open Forum Infect Dis. 2019;6:ofz001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 460. | Fischer C, Bozza F, Merino Merino XJ, Pedroso C, de Oliveira Filho EF, Moreira-Soto A, Schwalb A, de Lamballerie X, Netto EM, Bozza PT, Sarno M, Brites C, Gotuzzo E, Talledo M, Drexler JF. Robustness of Serologic Investigations for Chikungunya and Mayaro Viruses following Coemergence. mSphere. 2020;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 461. | Huits R, Okabayashi T, Cnops L, Barbé B, Van Den Berg R, Bartholomeeusen K, Ariën KK, Jacobs J, Bottieau E, Nakayama EE, Shioda T, Van Esbroeck M. Diagnostic accuracy of a rapid E1-antigen test for chikungunya virus infection in a reference setting. Clin Microbiol Infect. 2018;24:78-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 462. | Reddy A, Bosch I, Salcedo N, Herrera BB, de Puig H, Narváez CF, Caicedo-Borrero DM, Lorenzana I, Parham L, García K, Mercado M, Turca AMR, Villar-Centeno LA, Gélvez-Ramírez M, Ríos NAG, Hiley M, García D, Diamond MS, Gehrke L. Development and Validation of a Rapid Lateral Flow E1/E2-Antigen Test and ELISA in Patients Infected with Emerging Asian Strain of Chikungunya Virus in the Americas. Viruses. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 463. | Tuekprakhon A, Nakayama EE, Bartholomeeusen K, Puiprom O, Sasaki T, Huits R, Luplertlop N, Kosoltanapiwat N, Maneekan P, Ariën KK, Shioda T, Leaungwutiwong P. Variation at position 350 in the Chikungunya virus 6K-E1 protein determines the sensitivity of detection in a rapid E1-antigen test. Sci Rep. 2018;8:1094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 464. | Vairo F, Haider N, Kock R, Ntoumi F, Ippolito G, Zumla A. Chikungunya: Epidemiology, Pathogenesis, Clinical Features, Management, and Prevention. Infect Dis Clin North Am. 2019;33:1003-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 121] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 465. | Millsapps EM, Underwood EC, Barr KL. Development and Application of Treatment for Chikungunya Fever. Res Rep Trop Med. 2022;13:55-66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 466. | Couderc T, Khandoudi N, Grandadam M, Visse C, Gangneux N, Bagot S, Prost JF, Lecuit M. Prophylaxis and therapy for Chikungunya virus infection. J Infect Dis. 2009;200:516-523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 188] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 467. | Khan M, Santhosh SR, Tiwari M, Lakshmana Rao PV, Parida M. Assessment of in vitro prophylactic and therapeutic efficacy of chloroquine against Chikungunya virus in vero cells. J Med Virol. 2010;82:817-824. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 146] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 468. | Pohjala L, Utt A, Varjak M, Lulla A, Merits A, Ahola T, Tammela P. Inhibitors of alphavirus entry and replication identified with a stable Chikungunya replicon cell line and virus-based assays. PLoS One. 2011;6:e28923. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 185] [Cited by in RCA: 209] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 469. | Kaur P, Thiruchelvan M, Lee RC, Chen H, Chen KC, Ng ML, Chu JJ. Inhibition of chikungunya virus replication by harringtonine, a novel antiviral that suppresses viral protein expression. Antimicrob Agents Chemother. 2013;57:155-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 131] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 470. | Bourjot M, Leyssen P, Neyts J, Dumontet V, Litaudon M. Trigocherrierin A, a potent inhibitor of chikungunya virus replication. Molecules. 2014;19:3617-3627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 471. | Albulescu IC, Tas A, Scholte FEM, Snijder EJ, van Hemert MJ. An in vitro assay to study chikungunya virus RNA synthesis and the mode of action of inhibitors. J Gen Virol. 2014;95:2683-2692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 472. | Schilte C, Couderc T, Chretien F, Sourisseau M, Gangneux N, Guivel-Benhassine F, Kraxner A, Tschopp J, Higgs S, Michault A, Arenzana-Seisdedos F, Colonna M, Peduto L, Schwartz O, Lecuit M, Albert ML. Type I IFN controls chikungunya virus via its action on nonhematopoietic cells. J Exp Med. 2010;207:429-442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 225] [Cited by in RCA: 243] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 473. | Kumar M, Sudeep AB, Arankalle VA. Evaluation of recombinant E2 protein-based and whole-virus inactivated candidate vaccines against chikungunya virus. Vaccine. 2012;30:6142-6149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 474. | Wang E, Kim DY, Weaver SC, Frolov I. Chimeric Chikungunya viruses are nonpathogenic in highly sensitive mouse models but efficiently induce a protective immune response. J Virol. 2011;85:9249-9252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 475. | López-Camacho C, Kim YC, Blight J, Lazaro Moreli M, Montoya-Diaz E, Huiskonen JT, Kümmerer BM, Reyes-Sandoval A. Assessment of Immunogenicity and Neutralisation Efficacy of Viral-Vectored Vaccines Against Chikungunya Virus. Viruses. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 476. | Tretyakova I, Hearn J, Wang E, Weaver S, Pushko P. DNA vaccine initiates replication of live attenuated chikungunya virus in vitro and elicits protective immune response in mice. J Infect Dis. 2014;209:1882-1890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 477. | Zhao Z, Deng Y, Niu P, Song J, Wang W, Du Y, Huang B, Wang W, Zhang L, Zhao P, Tan W. Co-Immunization With CHIKV VLP and DNA Vaccines Induces a Promising Humoral Response in Mice. Front Immunol. 2021;12:655743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 478. | Plante KS, Rossi SL, Bergren NA, Seymour RL, Weaver SC. Extended Preclinical Safety, Efficacy and Stability Testing of a Live-attenuated Chikungunya Vaccine Candidate. PLoS Negl Trop Dis. 2015;9:e0004007. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 479. | Schneider M, Narciso-Abraham M, Hadl S, McMahon R, Toepfer S, Fuchs U, Hochreiter R, Bitzer A, Kosulin K, Larcher-Senn J, Mader R, Dubischar K, Zoihsl O, Jaramillo JC, Eder-Lingelbach S, Buerger V, Wressnigg N. Safety and immunogenicity of a single-shot live-attenuated chikungunya vaccine: a double-blind, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2023;401:2138-2147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 89] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 480. | Roques P, Fritzer A, Dereuddre-Bosquet N, Wressnigg N, Hochreiter R, Bossevot L, Pascal Q, Guehenneux F, Bitzer A, Corbic Ramljak I, Le Grand R, Lundberg U, Meinke A. Effectiveness of CHIKV vaccine VLA1553 demonstrated by passive transfer of human sera. JCI Insight. 2022;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 43] [Reference Citation Analysis (0)] |
| 481. | Wressnigg N, Hochreiter R, Zoihsl O, Fritzer A, Bézay N, Klingler A, Lingnau K, Schneider M, Lundberg U, Meinke A, Larcher-Senn J, Čorbic-Ramljak I, Eder-Lingelbach S, Dubischar K, Bender W. Single-shot live-attenuated chikungunya vaccine in healthy adults: a phase 1, randomised controlled trial. Lancet Infect Dis. 2020;20:1193-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 482. | Bennett SR, McCarty JM, Ramanathan R, Mendy J, Richardson JS, Smith J, Alexander J, Ledgerwood JE, de Lame PA, Royalty Tredo S, Warfield KL, Bedell L. Safety and immunogenicity of PXVX0317, an aluminium hydroxide-adjuvanted chikungunya virus-like particle vaccine: a randomised, double-blind, parallel-group, phase 2 trial. Lancet Infect Dis. 2022;22:1343-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 37] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 483. | Chen GL, Coates EE, Plummer SH, Carter CA, Berkowitz N, Conan-Cibotti M, Cox JH, Beck A, O’Callahan M, Andrews C, Gordon IJ, Larkin B, Lampley R, Kaltovich F, Gall J, Carlton K, Mendy J, Haney D, May J, Bray A, Bailer RT, Dowd KA, Brockett B, Gordon D, Koup RA, Schwartz R, Mascola JR, Graham BS, Pierson TC, Donastorg Y, Rosario N, Pape JW, Hoen B, Cabié A, Diaz C, Ledgerwood JE; VRC 704 Study Team. Effect of a Chikungunya Virus-Like Particle Vaccine on Safety and Tolerability Outcomes: A Randomized Clinical Trial. JAMA. 2020;323:1369-1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 74] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 484. | Chang LJ, Dowd KA, Mendoza FH, Saunders JG, Sitar S, Plummer SH, Yamshchikov G, Sarwar UN, Hu Z, Enama ME, Bailer RT, Koup RA, Schwartz RM, Akahata W, Nabel GJ, Mascola JR, Pierson TC, Graham BS, Ledgerwood JE; VRC 311 Study Team. Safety and tolerability of chikungunya virus-like particle vaccine in healthy adults: a phase 1 dose-escalation trial. Lancet. 2014;384:2046-2052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 185] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 485. | Akahata W, Yang ZY, Andersen H, Sun S, Holdaway HA, Kong WP, Lewis MG, Higgs S, Rossmann MG, Rao S, Nabel GJ. A virus-like particle vaccine for epidemic Chikungunya virus protects nonhuman primates against infection. Nat Med. 2010;16:334-338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 324] [Cited by in RCA: 367] [Article Influence: 24.5] [Reference Citation Analysis (0)] |