Published online Mar 25, 2024. doi: 10.5501/wjv.v13.i1.91149
Peer-review started: December 24, 2023
First decision: January 11, 2024
Revised: January 2, 2024
Accepted: February 6, 2024
Article in press: February 6, 2024
Published online: March 25, 2024
Processing time: 78 Days and 9.3 Hours
Metabolic dysfunction-associated steatotic liver disease (MASLD), formally known as nonalcoholic fatty liver disease, is the most common chronic liver disease in the United States. Patients with MASLD have been reported to be at a higher risk of developing severe coronavirus disease 2019 (COVID-19) and death. However, most studies are single-center studies, and nationwide data in the United States is lacking.
To study the influence of MASLD on COVID-19 hospitalizations during the initial phase of the pandemic.
We retrospectively analyzed the 2020 National Inpatient Sample (NIS) database to identify primary COVID-19 hospitalizations based on an underlying diagnosis of MASLD. A matched comparison cohort of COVID-19 hospitalizations without MASLD was identified from NIS after 1: N propensity score matching based on gender, race, and comorbidities, including hypertension, heart failure, diabetes, and cirrhosis. The primary outcomes included inpatient mortality, length of stay, and hospitalization costs. Secondary outcomes included the prevalence of systemic complications.
A total of 2210 hospitalizations with MASLD were matched to 2210 hospitalizations without MASLD, with a good comorbidity balance. Overall, there was a higher prevalence of severe disease with more intensive care unit admissions (9.5% vs 7.2%, P = 0.007), mechanical ventilation (7.2% vs 5.7%, P = 0.03), and septic shock (5.2% vs 2.7%, P <0.001) in the MASLD cohort than in the non-MASLD cohort. However, there was no difference in mortality (8.6% vs 10%, P = 0.49), length of stay (5 d vs 5 d, P = 0.25), and hospitalization costs (42081.5 $ vs 38614$, P = 0.15) between the MASLD and non-MASLD cohorts.
The presence of MAFLD with or without liver cirrhosis was not associated with increased mortality in COVID-19 hospitalizations; however, there was an increased incidence of severe COVID-19 infection. This data (2020) predates the availability of COVID-19 vaccines, and many MASLD patients have since been vaccinated. It will be interesting to see if these trends are present in the subsequent years of the pandemic.
Core Tip: This comprehensive study investigates the impact of metabolic dysfunction-associated steatotic liver disease (MASLD) on the severity of coronavirus disease 2019 (COVID-19) during the early stages of the pandemic, using the 2020 National Inpatient Sample database. It uniquely contrasts COVID-19 hospitalizations with and without MASLD, revealing that while MASLD is not linked to increased mortality, however, it is associated with a heightened risk of severe COVID-19 complications. This pivotal research offers valuable insights into the MASLD-COVID-19 relationship before the widespread availability of vaccines, setting the stage for further exploration into how these trends evolved in the later pandemic years.
- Citation: Sohail A, Ali H, Patel P, Subramanium S, Dahiya DS, Sohail AH, Gangwani MK, Satapathy SK. Impact of metabolic dysfunction-associated steatotic liver disease on COVID-19 hospitalizations: A propensity-matched analysis of the United States. World J Virol 2024; 13(1): 91149
- URL: https://www.wjgnet.com/2220-3249/full/v13/i1/91149.htm
- DOI: https://dx.doi.org/10.5501/wjv.v13.i1.91149
Coronavirus disease 2019 (COVID-19) is an ongoing public health emergency with long-term effects on mortality and morbidity. As of November 2023, the World Health Organization has reported over 770 million confirmed cases, resulting in more than 6.9 million deaths worldwide[1]. Although preventive health measures and vaccinations have mitigated the risk of contracting COVID-19 and experiencing adverse outcomes to some extent, individuals with chronic diseases continue to face an elevated likelihood of poor[2]. Among these, chronic liver disease, particularly metabolic dysfunction-associated steatotic liver disease (MASLD), formally known as nonalcoholic fatty liver disease, is presumed to be a high-risk comorbid condition for severe COVID-19 owing to its inherent immune dysregulation[3-5]. MASLD, the hepatic manifestation of metabolic syndrome, comprises a spectrum of diseases ranging from hepatocellular steatosis and steatohepatitis to fibrosis and eventual cirrhosis[6]. MASLD has quickly become the most prevalent etiology of chronic liver disease in the United States[7,8]. MASLD has been linked to severe infections such as community-acquired pneumonia and a decline in lung function[9,10].
A recent case-control study revealed that MASLD did not correlate with increased in-hospital mortality rates, ventilatory assistance requirements, intensive care unit (ICU) admissions, or the total duration of hospital stay[11]. In contrast, other studies have indicated an association between MASLD and severe COVID-19. A recent meta-analysis of 14 studies of 1851 patients with MASLD by Singh et al[12] has shed light on the relationship between MASLD and COVID-19, revealing a significantly increased risk of severe COVID-19 outcomes in patients with MASLD. These studies have shown a 1.80-fold increase in the incidence of severe COVID-19 among MASLD patients, although they did not find a significant correlation with COVID-19 mortality. Another meta-analysis by Tao et al[13] reported an increased risk of severe infection and higher ICU admissions for MASLD patients, with no significant difference in mortality compared with non-MASLD patients. However, these meta-analyses predominantly included studies outside the United States and were often conducted in single-center settings with small sample sizes. These studies also had a high degree of variability owing to differences in the pandemic stage, data availability, sample sizes, study designs, and healthcare settings. This limitation raises concerns about the generalizability of their findings, especially in diverse populations, such as those in the United States.
Considering these contradictions in the literature, utilizing a large national database, our study aimed to provide a more comprehensive and nationally representative analysis of the impact of MASLD on COVID-19 hospitalizations in the United States.
The present study utilized the National Inpatient Sample (NIS) 2020 database from the United States[14]. Detailed information on NIS's design and sampling methods is available at https://www.hcup-us.ahrq.gov. The NIS 2020 utilized the International Classification of Diseases (ICD) 10 coding system to store and report data. We used the "U07.1" ICD 10 code, introduced in March 2020 for COVID-19, to identify hospitalizations with a primary diagnosis (DX1) in the NIS 2020 database[15]. Hospitalizations were excluded if patients were < 18 years old, transferred, or had COVID-19 listed as a secondary diagnosis. Additionally, hospitalizations were excluded if there was any history of malignant neoplasms or liver and kidney transplant recipients, as these were deemed high-risk conditions that could confound the present analysis.
The primary outcomes included inpatient mortality in COVID-19 patients with MASLD (with and without cirrhosis) and resource utilization, including length of stay and hospitalization costs. Secondary outcomes included the prevalence of systemic complications, including acute hypoxic respiratory failure, the need for mechanical ventilation, septic shock, and cardiac arrhythmias (including supraventricular tachyarrhythmias, ventricular tachyarrhythmias (VT), atrial fibrillation/flutter (Afib/Aflutter), and deep venous thromboembolism.
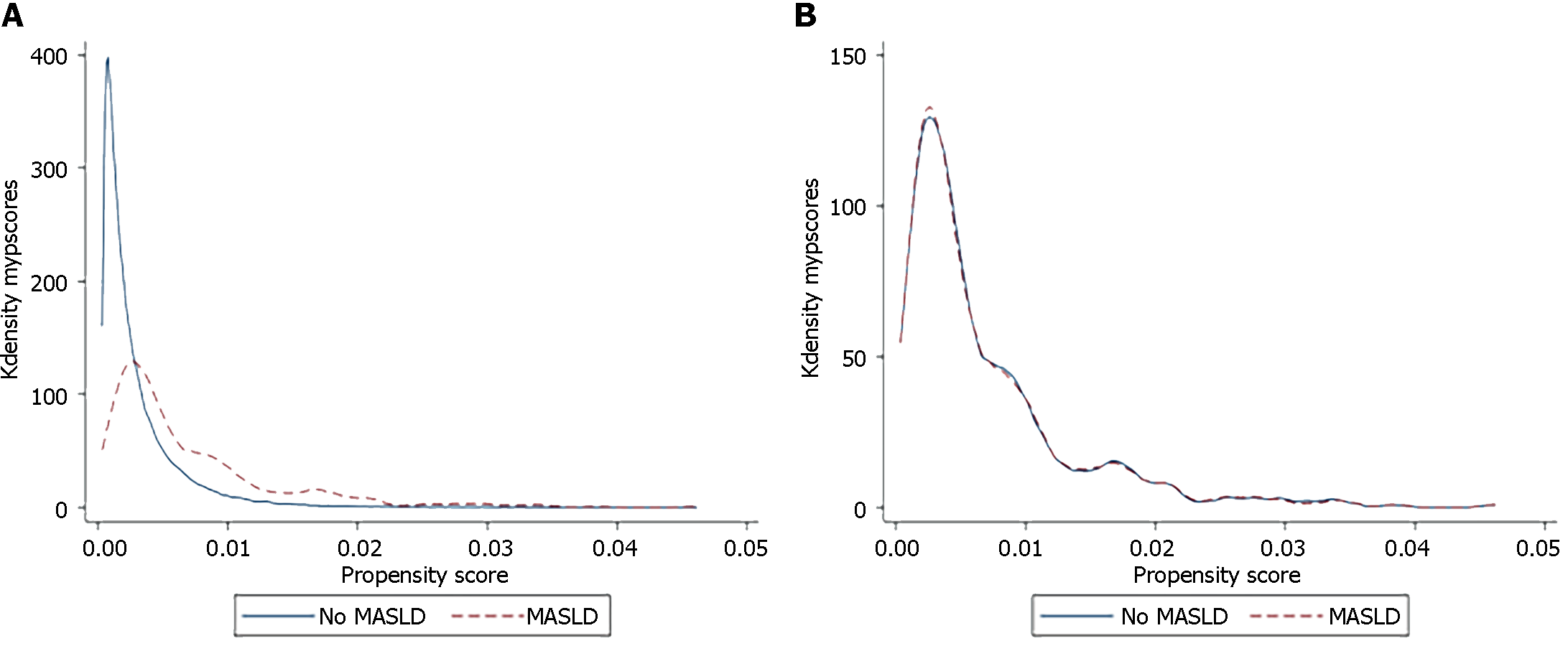
Statistical analysis was performed using statistical software for data science (STATA 16). We developed matched cohorts (MASLD and non-MASLD) using propensity score matching (PSM) to minimize the effects of hospital- and patient-level confounders. Propensity scores were generated with COVID-19 as the dependent variable and age, sex, cardiac comorbidities (heart failure and valvular dysfunction), hypertension, diabetes mellitus, and compensated and decompensated cirrhosis as independent variables. A 1:1 matching was performed using the command "clip match" (greedy matching) without replacement. Matched observations had a caliper width of 0.01 for the caliper matching variable (propensity scores). Cases and controls were matched 1:1 on age, sex, and Elixhauser comorbidities, including cardiac comorbidities (heart failure and valvular dysfunction), hypertension, diabetes, and cirrhosis (compensated and decompensated), as previously reported[16,17]. The covariate balance was visualized using the two-way plot shown in Figure 1. A two-sample Wilcoxon rank-sum (Mann-Whitney) test was used for continuous variables. The Chi-square test was used to compare categorical variables. The significance threshold was set at P < 0.05. For logistic regression, hierarchical models were designed using unbalanced variables in PSM (none), and outcomes were reported as odds ratios (OR) with 95% confidence intervals (95%CI) and P values. Patient consent and institutional review were not necessary, as the NIS is a de-identified, hospital-level, third-party database.
Using propensity matching, 2210 hospitalizations with MASLD were matched to 2210 hospitalizations without MASLD. After matching, there was no significant difference in gender, race, hospital region, or hospital location (Table 1). Additionally, there were no differences in comorbidities, including heart failure (P = 1.00), arrhythmias (P = 1.00), valvular disease (P = 1.00), chronic obstructive pulmonary disease (P = 1.00), hypertension (P = 1.00), diabetes mellitus type 2 (P = 1.00), compensated cirrhosis (P = 1.00), and decompensated cirrhosis (P = 1.00) (Table 2). The median age at admission was significantly lower in the MASLD group (58 years) than in the non-MASLD group (66 years). The gender distribution was identical in both groups, with the majority of patients being female in both groups. Most patients in both groups were white (58.8%). The regional distribution of hospitalizations showed slight variations that were not statistically significant, and most patients in both groups were admitted to urban teaching hospitals. Regarding primary payers, Medicare was the most common payer in the non-MASLD group (54.1%), followed by private payers (40.4%) in the MASLD group. In terms of patient disposition, a higher proportion of patients in both groups were discharged to their homes or self-care (66.3% in MASLD and 59.7% in non-MASLD) (Table 1). The list of the remaining comorbid conditions is presented in Supplementary Table 1.
| Characteristics | Non-MASLD | MASLD | P value |
| Total hospitalizations | 2210 | 2210 | |
| Age in years at admission, median (IQR) | 66.0 (56.0, 76.0) | 58.0 (47.0, 68.0) | < 0.001 |
| Gender | 1 | ||
| Male | 965 (43.7) | 965 (43.7) | |
| Female | 1245 (56.3) | 1245 (56.3) | |
| Race | 1 | ||
| White | 1300 (58.8) | 1300 (58.8) | |
| Black | 125 (5.7) | 125 (5.7) | |
| Hispanic | 615 (27.8) | 615 (27.8) | |
| Asian | 85 (3.8) | 85 (3.8) | |
| Native American | 20 (0.9) | 20 (0.9) | |
| Other | 65 (2.9) | 65 (2.9) | |
| Region of the hospital | 0.49 | ||
| Northeast | 375 (17.0) | 340 (15.4) | |
| Midwest | 535 (24.2) | 610 (27.6) | |
| South | 860 (38.9) | 885 (40.0) | |
| West | 440 (19.9) | 375 (17.0) | |
| Location/Teaching status of the hospital | 0.12 | ||
| Rural | 295 (13.3) | 250 (11.3) | |
| Urban nonteaching | 440 (19.9) | 345 (15.6) | |
| Urban teaching | 1475 (66.7) | 1615 (73.1) | |
| Primary payer | < 0.001 | ||
| Medicare | 1120 (54.1) | 820 (39.2) | |
| Medicaid | 285 (13.8) | 310 (14.8) | |
| Private | 595 (28.7) | 845 (40.4) | |
| Other | 70 (3.4) | 115 (5.5) | |
| Median household income national quartile for patient ZIP code | 0.55 | ||
| 1st (0-25th) | 710 (32.8) | 760 (35.1) | |
| 2nd (26th -50th) | 575 (26.6) | 615 (28.4) | |
| 3rd (51st -75th) | 540 (24.9) | 515 (23.8) | |
| 4th (76th -100th) | 340 (15.7) | 275 (12.7) | |
| Disposition of patient | 0.28 | ||
| Discharged to home or self-care (Routine discharge) | 1320 (59.7) | 1465 (66.3) | |
| Transfer to short-term hospital | 90 (4.1) | 50 (2.3) | |
| Transfer Other: Includes skilled nursing facility, intermediate care facility, Another Type of Facility | 265 (12.0) | 200 (9.0) | |
| Home health care | 295 (13.3) | 280 (12.7) | |
| Against medical advice | 20 (0.9) | 25 (1.1) | |
| Outcomes | |||
| Inpatient mortality | 220 (10.0) | 190 (8.6) | 0.49 |
| Inpatient mortality in cirrhosis | 418 (18.91) | 226 (10.22) | 0.05 |
| Mechanical ventilation | 150 (6.8) | 220 (10.0) | < 0.001 |
| ICU admission | 160 (7.2) | 210 (9.5) | 0.007 |
| Length of stay, median (IQR) | 5.0 (3.0, 8.0) | 5.0 (3.0, 8.0) | 0.25 |
| Total charges, median (IQR) | 38614.0 (22040.5, 70258.5) | 42081.5 (23021.0, 79820.5) | 0.15 |
| Secondary outcomes | No MASLD | MASLD | P value |
| Total hospitalizations | 2210 | 2210 | |
| ARDS | 125 (5.7) | 160 (7.2) | 0.032 |
| Hypoxic respiratory failure | 1105 (50.0) | 1220 (55.2) | < 0.001 |
| Bacterial pneumonia | 35 (1.6) | 45 (2.0) | 0.26 |
| Diarrhea | 185 (8.4) | 145 (6.6) | 0.022 |
| Septic shock | 60 (2.7) | 115 (5.2) | < 0.001 |
| Supraventricular tachycardia | 25 (1.1) | 30 (1.4) | 0.5 |
| Ventricular tachycardia | 10 (0.5) | 30 (1.4) | 0.001 |
| Atrial fibrillation | 165 (7.5) | 170 (7.7) | 0.78 |
| Aflutter | 100 (4.5) | 80 (3.6) | 0.13 |
| Pulmonary embolism | 35 (1.6) | 30 (1.4) | 0.53 |
| Deep venous thrombosis | 75 (3.4) | 65 (2.9) | 0.39 |
| Compensated cirrhosis | 635 (28.7) | 635 (28.7) | 1 |
| Decompensated cirrhosis | 45 (2.0) | 45 (2.0) | 1 |
Overall, there was no difference in mortality between the MASLD and non-MASLD cohorts (8.6% vs 10%, P = 0.49) (OR 0.85, 95%CI: 0.53-1.34, P = 0.48). Subgroup analysis among patients without liver cirrhosis revealed no difference in mortality between the MASLD and non-MASLD cohorts (7.94% vs 6.35%, P = 0.43) (OR 1.27, 95%CI: 0.96-1.67, P = 0.08). However, subgroup analysis among patients with liver cirrhosis revealed higher mortality in non-MASLD patients (cirrhosis due to other causes such as alcohol-related liver disease and chronic viral hepatitis) than in the MASLD cohort (18.91% vs 10.22, P = 0.05) (OR 2.03, 95%CI: 1.12-4.25, P = 0.05).
The prevalence of ICU admission (9.5% vs 7.2%, P = 0.007), mechanical ventilation (7.2% vs 5.7%, P = 0.03), septic shock (5.2% vs 2.7%, P < 0.001), and VT (1.4% vs 0.5%, P 0.001) was higher in the MASLD cohort than in the non-MASLD cohort. The two cohorts showed no differences in the median hospital stay (5 d vs 5 d, P = 0.25) or hospitalization costs ($42081 in the MASLD group and $38614 in the non-MASLD group) (Table 1).
Our study is among the first in the United States to analyze the outcomes of COVID-19 among patients with MASLD at the national level. The results revealed that overall mortality did not significantly differ between the MASLD and non-MASLD cohorts in the initial phase of the pandemic. This trend persisted in subgroup analyses; among patients without liver cirrhosis, mortality rates were similar across both cohorts. However, among patients with liver cirrhosis, we found higher mortality in the non-MASLD group, which included patients with cirrhosis due to other causes such as chronic viral hepatitis, alcohol, or autoimmune diseases. Notably, the MASLD cohort exhibited a higher prevalence of ICU admission, mechanical ventilation, septic shock, and arrhythmias, indicating more severe disease. However, despite these differences in clinical outcomes, the two cohorts showed no significant differences in median hospital stay or hospitalization costs.
Our results align with those of several previous studies describing the relationship between MASLD and increased severity of COVID-19. Mahamid et al[5] showed that MASLD was associated with increased severity of COVID-19 pneumonia, irrespective of metabolic syndrome. Another retrospective study by Zheng et al[18] showed that obesity is associated with a six-fold higher risk of severe COVID-19 in patients with MASLD. This finding was consistent even after adjusting for factors such as age, gender, smoking habits, diabetes, hypertension, and dyslipidemia. In a study of 202 COVID-19 patients, Ji et al[19] discovered a correlation between advanced age, male gender, increased body mass index, the presence of MASLD, and a higher rate of comorbidities with the severity of COVID-19. There are conflicting reports on whether MAFLD is independently associated with mortality in COVID-19 patients. Kim et al[20] performed a retrospective study involving 867 patients and showed that MAFLD was not an independent predictor of mortality in COVID-19 patients [hazard ratio (HR) = 1.08; 95%CI: 0.59–1.97; P = 0.80]. Lopez-Mendez et al[21] reported higher mortality in patients with MASLD and COVID-19 (81.8% vs 18.2%, P = 0.012). In the initial phase of the COVID-19 pandemic, the available data were predominantly sourced from limited case series and observational studies, offering restricted insights. However, with the escalation of the pandemic, there has been a subsequent emergence of large-scale studies. These studies have contributed to a more robust understanding of the implications of COVID-19 on patients with MASLD. Two recent meta-analyses, Singh et al[12] and Tao et al[13], reported increased severity of COVID-19 in patients with MASLD. However, these studies did not find a significant difference in mortality between MASLD and non-MASLD patients, which is consistent with our results. However, both meta-analyses we examined primarily included studies from outside the United States. They also showed considerable variation because of differences in the pandemic stages, data availability, sample sizes, research methods, and healthcare settings. In contrast, using the largest national-level database in the United States, which encompasses a substantial sample size and diverse demographic coverage, increases the generalizability of our findings. In subgroup analysis, patients without liver cirrhosis showed no significant difference in mortality rates between the MASLD and non-MASLD cohorts. However, among patients with liver cirrhosis in the non-MASLD group (e.g., alcohol-related liver disease and chronic viral hepatitis), there was a higher mortality rate than among those with MASLD-related cirrhosis. This could be explained by previously reported higher rates of complications and mortality among patients with alcohol-related liver diseases in the United States during the initial stages of the pandemic[22,23].
There is ongoing debate regarding the causes of poorer outcomes in certain patients, with various potential factors being studied. One theory suggests that MAFLD aggravates the cytokine storm associated with COVID-19 by promoting the release of inflammatory cytokines from the liver[24,25]. Conversely, it has been hypothesized that the shift in liver immune cells from pro-inflammatory M1 macrophages to pro-inflammatory M2 macrophages, which are regulatory, weakens innate immunity, thereby worsening the patient's condition[19,26]. Recent research supports both theories, showing that MAFLD patients exhibit elevated levels of specific inflammatory cytokines, such as interleukin-6, which plays a significant role in severe cases and treatment, and reduced levels of interferon-γ, which is essential for macrophage function[27]. Additionally, the upregulation of proteins facilitating severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) entry, such as ACE2 and TMPRSS2, particularly in obese patients with MASLD, is another factor[28]. The close association between fatty liver disease and metabolic syndrome suggests that similar harmful pathophysiological processes may contribute[29]. Our results also indicated that COVID-19 patients with MASLD are at a higher risk of cardiac arrhythmias, including VT. Several MASLD-related pathophysiological factors within the heart result in structural, electrical, and autonomic remodeling, causing arrhythmias[30]. Furthermore, cardiac arrhythmias place patients with COVID-19 at an increased risk of worse outcomes[31].
Regarding resource utilization in United States patients with MASLD and COVID-19 hospitalizations, despite a higher total hospitalization cost in the MASLD group than in the non-MASLD group (with costs of $42081 and $38614, respectively), this difference was not statistically significant (P = 0.15). Similarly, the mean length of hospital stay in both groups was similar at 5 d (P = 0.25). This apparent lack of significant cost disparity despite the presence of more complications in the MASLD group may be attributed to factors such as standardized treatment protocols and variations in cost calculation methodologies. Moreover, uniform insurance and billing practices and potential unaccounted-for confounding factors may have contributed to the unexpected similarity in hospitalization costs. Further investigations involving larger sample sizes and in-depth analyses are warranted to provide more precise insights. Our findings differ from those of previous studies on MASLD without COVID-19. For instance, Allen et al[32] reported annual care costs for MASLD patients with private insurance amounting to $7804 for new diagnoses and $3789 for long-term management, in contrast to the total annual cost of $2298 for matched controls without MASLD, underscoring the economic impact of this condition. Additionally, Adejumo et al[33] found differences in length of stay and total healthcare costs between genders, with females experiencing a shorter length of stay (4.55 vs 4.75 d) and lower total healthcare costs ($42848.00 vs $47026.00) compared to males. Unlike these studies, our study provides insights into the economic impact of COVID-19 on patients with MASLD. To the best of our knowledge, this is the first study to provide information on the costs associated with hospitalization for COVID-19 in individuals with MASLD across the United States.
While our findings contribute significantly to the current understanding of the relationship between MASLD and COVID-19, they also highlight the need to consider genetic variances as potential modulators of disease severity in patients with MASLD and COVID-19. Research indicates a significant genetic component in the severity of COVID-19, particularly in patients with MASLD. Genetic polymorphisms, such as PNPLA3 (rs738409), GCKR (rs780094), TM6SF2 (rs58542926), and LYPLAL1 (rs12137855), are associated with increased MAFLD risk and may influence COVID-19 outcomes. These genes are linked to lipid metabolism, glucose regulation, and liver function, which are vital for understanding COVID-19 severity in MAFLD patients with MAFLD. However, studies have reported mixed results regarding the impact of these genetic variants on COVID-19 severity, suggesting a complex relationship between these genetic factors and the disease[34]. A bioinformatics and systems biology approach also revealed differentially expressed genes shared between COVID-19 and MAFLD, highlighting the potential common pathogenesis and suggesting therapeutic targets for both conditions[35]. Future research should prioritize identifying genetic markers that predict severe COVID-19 in MASLD patients, aiming for more personalized treatment approaches to improve outcomes. Additionally, understanding the genetic interplay between MASLD and COVID-19 will aid in developing public health strategies and novel therapeutics for high-risk groups, ultimately easing healthcare burdens and enhancing patient care.
We acknowledge a few limitations to our study. We utilized the NIS database, which is subject to coding errors due to its dependence on the ICD-10 coding system. Second, the lack of information regarding medications and laboratory and radiological data has limited our ability to determine the specific baseline characteristics of patients with MASLD and individual patient management strategies. However, we used a validated tool for the Chronic Condition Indicator to assess the comorbidity burden of the patients as an essential prognostic factor. We also used PSM to minimize the effects of comorbid conditions in the comparison cohorts[36]. Third, the scope of this study was limited to inpatient data and did not include information on the outpatient outcomes of patients with MASLD who had non-severe COVID-19. In addition, the Agency of Healthcare Research and Quality recommends using an NIS to derive state-level data, owing to the relatively small contribution from several states. If state-level data were obtainable, it could have been helpful for policymakers to make certain public health efforts in certain states with more patients with MASLD and COVID-19 infection. Instead, we included geographical regions to study the outcome differences across the United States.
Despite some limitations, our study is the first to analyze and summarize national data on the impact of COVID-19-related hospitalizations in patients with MASLD during the early pandemic phase. The broad scope of our study, characterized by a large sample size and diverse range of participants, enhances the generalizability of our results. This diversity is reflected in the data from hospitals of varying sizes (small, medium, and large) and types (both teaching and non-teaching), as well as different settings, including rural and urban areas. Additionally, the variables included in the database allowed us to examine factors such as hospitalization costs, patient income, and other hospital characteristics, which are not often studied in single-center studies.
In conclusion, our study provides valuable insights into the existing and evolving body of evidence on the impact of COVID-19 in patients with MASLD. Our study showed that the presence of MASLD, with or without progression to liver cirrhosis, did not correlate with elevated mortality rates in patients hospitalized due to COVID-19. However, there was a notable increase in the severity of COVID-19 among this demographic group. It is important to note that this data, collected in 2020, predates the widespread distribution of COVID-19 vaccines. Since then, a significant proportion of patients with MASLD have received vaccination. Future analysis is required to determine whether these observed trends persist or are altered in the subsequent phases of the pandemic. This is important since MASLD is the most common cause of Chronic Liver disease in the US. Moreover, to mitigate the impact of COVID-19 on patients with MASLD, it is critical to promote preventive measures, such as vaccination and booster doses, and provide timely treatment with advanced COVID-19 therapies.
This study focused on the impact of metabolic dysfunction-associated steatotic liver disease (MASLD) on the severity and outcomes of coronavirus disease 2019 (COVID-19) hospitalizations. MASLD, formerly known as nonalcoholic fatty liver disease, is becoming increasingly prevalent and has been linked to more severe infectious diseases.
This study was motivated by a lack of comprehensive nationwide data on the relationship between the MASLD and COVID-19 outcomes in the United States. Previous studies have often been limited in scope, highlighting the need for more extensive research in this area.
The primary objective of this study was to analyze the impact of MASLD on the severity and outcomes of COVID-19 hospitalizations, particularly assessing the rates of intensive care unit (ICU) admission, mechanical ventilation, septic shock, and mortality in the United States.
We conducted a retrospective analysis of the National Inpatient Sample 2020 database. This study utilized propensity score matching to compare COVID-19 hospitalizations with and without MASLD, controlling for demographics and comorbidities.
The study found that patients have a higher rate of severe COVID-19 outcomes, such as increased ICU admissions, need for mechanical ventilation, and septic shock. However, no significant difference in mortality rate was observed between patients with and without MASLD.
MASLD is associated with an increased risk of severe COVID-19 complications but does not necessarily correlate with higher mortality. This finding is vital for healthcare providers in managing high-risk patient groups.
Future research should investigate the genetic factors influencing MASLD's impact on COVID-19 to identify specific genetic markers that predict severe outcomes. This could lead to more personalized healthcare strategies and inform public health policies, particularly for high-risk groups. Further studies are needed to explore the economic impact and develop effective treatment protocols for MASLD patients with COVID-19.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: American Association for the Study of Liver Diseases; American College of Gastroenterology; American Society for Gastrointestinal Endoscopy.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lim SYM, Malaysia S-Editor: Liu JH L-Editor: A P-Editor: Chen YX
| 1. | World Health Organization. Who coronavirus (COVID-19) dashboard. 2022. World Health Organization. 2023 [cited 2023 12]; Available from: https://covid19.who.int/. |
| 2. | Gao YD, Ding M, Dong X, Zhang JJ, Kursat Azkur A, Azkur D, Gan H, Sun YL, Fu W, Li W, Liang HL, Cao YY, Yan Q, Cao C, Gao HY, Brüggen MC, van de Veen W, Sokolowska M, Akdis M, Akdis CA. Risk factors for severe and critically ill COVID-19 patients: A review. Allergy. 2021;76:428-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 885] [Article Influence: 221.3] [Reference Citation Analysis (0)] |
| 3. | Nagarajan R, Krishnamoorthy Y, Rajaa S, Hariharan VS. COVID-19 Severity and Mortality Among Chronic Liver Disease Patients: A Systematic Review and Meta-Analysis. Prev Chronic Dis. 2022;19:E53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 51] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 4. | Anirvan P, Singh SP, Giammarino A, Satapathy SK. Association of non-alcoholic fatty liver disease and COVID-19: A literature review of current evidence. World J Hepatol. 2021;13:916-925. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (1)] |
| 5. | Mahamid M, Nseir W, Khoury T, Mahamid B, Nubania A, Sub-Laban K, Schifter J, Mari A, Sbeit W, Goldin E. Nonalcoholic fatty liver disease is associated with COVID-19 severity independently of metabolic syndrome: a retrospective case-control study. Eur J Gastroenterol Hepatol. 2021;33:1578-1581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 6. | Younossi ZM, Blissett D, Blissett R, Henry L, Stepanova M, Younossi Y, Racila A, Hunt S, Beckerman R. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology. 2016;64:1577-1586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 694] [Cited by in RCA: 916] [Article Influence: 101.8] [Reference Citation Analysis (0)] |
| 7. | Ahmed A, Wong RJ, Harrison SA. Nonalcoholic Fatty Liver Disease Review: Diagnosis, Treatment, and Outcomes. Clin Gastroenterol Hepatol. 2015;13:2062-2070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 238] [Article Influence: 23.8] [Reference Citation Analysis (2)] |
| 8. | Fassio E, Alvarez E, Domínguez N, Landeira G, Longo C. Natural history of nonalcoholic steatohepatitis: a longitudinal study of repeat liver biopsies. Hepatology. 2004;40:820-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 129] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 9. | Lee CH, Choi SH, Chung GE, Park B, Kwak MS. Nonalcoholic fatty liver disease is associated with decreased lung function. Liver Int. 2018;38:2091-2100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 10. | Nseir WB, Mograbi JM, Amara AE, Abu Elheja OH, Mahamid MN. Non-alcoholic fatty liver disease and 30-day all-cause mortality in adult patients with community-acquired pneumonia. QJM. 2019;112:95-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 11. | Madan K, Rastogi R, Bhargava R, Dagar V, Singla V, Sahu A, Singh P, Garg P, Aggarwal B, Singh RK. Is Fatty Liver Associated with Increased Mortality and Morbidity in Coronavirus Disease 2019 (COVID-19) Pneumonia? J Clin Exp Hepatol. 2022;12:1320-1327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Singh A, Hussain S, Antony B. Non-alcoholic fatty liver disease and clinical outcomes in patients with COVID-19: A comprehensive systematic review and meta-analysis. Diabetes Metab Syndr. 2021;15:813-822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 13. | Tao Z, Li Y, Cheng B, Zhou T, Gao Y. Risk of Severe COVID-19 Increased by Metabolic Dysfunction-associated Fatty Liver Disease: A Meta-analysis. J Clin Gastroenterol. 2021;55:830-835. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 14. | Khera R, Angraal S, Couch T, Welsh JW, Nallamothu BK, Girotra S, Chan PS, Krumholz HM. Adherence to Methodological Standards in Research Using the National Inpatient Sample. JAMA. 2017;318:2011-2018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 434] [Cited by in RCA: 577] [Article Influence: 72.1] [Reference Citation Analysis (0)] |
| 15. | Clausen S, Stahlman S, Cost A. Early use of ICD-10-CM code "U07.1, COVID-19" to identify 2019 novel coronavirus cases in Military Health System administrative data. MSMR. 2020;27:55-59. [PubMed] |
| 16. | Ali H, Shamoon S, Bolick NL, Manickam S, Sattar U, Poola S, Mudireddy P. Outcomes of endoscopic retrograde cholangiopancreatography-guided gallbladder drainage compared to percutaneous cholecystostomy in acute cholecystitis. Ann Hepatobiliary Pancreat Surg. 2023;27:56-62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 17. | Patel P, Ali H, Manickam S, Pamarthy R, Fatakhova K, Rajapakse R. Substance abuse and inpatient outcomes in inflammatory bowel disease hospitalizations in the United States: a propensity matched analysis. Ann Gastroenterol. 2023;36:32-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 18. | Zheng KI, Gao F, Wang XB, Sun QF, Pan KH, Wang TY, Ma HL, Chen YP, Liu WY, George J, Zheng MH. Letter to the Editor: Obesity as a risk factor for greater severity of COVID-19 in patients with metabolic associated fatty liver disease. Metabolism. 2020;108:154244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 250] [Cited by in RCA: 233] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 19. | Ji D, Qin E, Xu J, Zhang D, Cheng G, Wang Y, Lau G. Non-alcoholic fatty liver diseases in patients with COVID-19: A retrospective study. J Hepatol. 2020;73:451-453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 336] [Cited by in RCA: 408] [Article Influence: 81.6] [Reference Citation Analysis (2)] |
| 20. | Kim D, Adeniji N, Latt N, Kumar S, Bloom PP, Aby ES, Perumalswami P, Roytman M, Li M, Vogel AS, Catana AM, Wegermann K, Carr RM, Aloman C, Chen VL, Rabiee A, Sadowski B, Nguyen V, Dunn W, Chavin KD, Zhou K, Lizaola-Mayo B, Moghe A, Debes J, Lee TH, Branch AD, Viveiros K, Chan W, Chascsa DM, Kwo P, Dhanasekaran R. Predictors of Outcomes of COVID-19 in Patients With Chronic Liver Disease: US Multi-center Study. Clin Gastroenterol Hepatol. 2021;19:1469-1479.e19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 184] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 21. | Lopez-Mendez I, Aquino-Matus J, Gall SM, Prieto-Nava JD, Juarez-Hernandez E, Uribe M, Castro-Narro G. Association of liver steatosis and fibrosis with clinical outcomes in patients with SARS-CoV-2 infection (COVID-19). Ann Hepatol. 2021;20:100271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 22. | Yeo YH, He X, Lv F, Zhao Y, Liu Y, Yang JD, Zu J, Ji F, Nguyen MH. Trends of Cirrhosis-related Mortality in the USA during the COVID-19 Pandemic. J Clin Transl Hepatol. 2023;11:751-756. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (1)] |
| 23. | Gao X, Lv F, He X, Zhao Y, Liu Y, Zu J, Henry L, Wang J, Yeo YH, Ji F, Nguyen MH. Impact of the COVID-19 pandemic on liver disease-related mortality rates in the United States. J Hepatol. 2023;78:16-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 62] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 24. | Sharma P, Kumar A. Metabolic dysfunction associated fatty liver disease increases risk of severe Covid-19. Diabetes Metab Syndr. 2020;14:825-827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 25. | Targher G, Mantovani A, Byrne CD, Wang XB, Yan HD, Sun QF, Pan KH, Zheng KI, Chen YP, Eslam M, George J, Zheng MH. Risk of severe illness from COVID-19 in patients with metabolic dysfunction-associated fatty liver disease and increased fibrosis scores. Gut. 2020;69:1545-1547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 161] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 26. | Lefere S, Tacke F. Macrophages in obesity and non-alcoholic fatty liver disease: Crosstalk with metabolism. JHEP Rep. 2019;1:30-43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 190] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 27. | Tasoudis PT, Arvaniti CK, Adamou AT, Belios I, Stone JH, Horick N, Sagris D, Dalekos GN, Ntaios G. Interleukin-6 inhibitors reduce mortality in coronavirus disease-2019: An individual patient data meta-analysis from randomized controlled trials. Eur J Intern Med. 2022;101:41-48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 28. | Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, Zelber-Sagi S, Wai-Sun Wong V, Dufour JF, Schattenberg JM, Kawaguchi T, Arrese M, Valenti L, Shiha G, Tiribelli C, Yki-Järvinen H, Fan JG, Grønbæk H, Yilmaz Y, Cortez-Pinto H, Oliveira CP, Bedossa P, Adams LA, Zheng MH, Fouad Y, Chan WK, Mendez-Sanchez N, Ahn SH, Castera L, Bugianesi E, Ratziu V, George J. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol. 2020;73:202-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2883] [Cited by in RCA: 2836] [Article Influence: 567.2] [Reference Citation Analysis (1)] |
| 29. | Nowroozi A, Momtazmanesh S, Rezaei N. COVID-19 and MAFLD/NAFLD: An updated review. Front Med (Lausanne). 2023;10:1126491. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 30. | Targher G, Byrne CD, Tilg H. NAFLD and increased risk of cardiovascular disease: clinical associations, pathophysiological mechanisms and pharmacological implications. Gut. 2020;69:1691-1705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 514] [Article Influence: 102.8] [Reference Citation Analysis (0)] |
| 31. | Chen Z, Liu J, Zhou F, Li H, Zhang XJ, She ZG, Lu Z, Cai J. Nonalcoholic Fatty Liver Disease: An Emerging Driver of Cardiac Arrhythmia. Circ Res. 2021;128:1747-1765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 69] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 32. | Allen AM, Van Houten HK, Sangaralingham LR, Talwalkar JA, McCoy RG. Healthcare Cost and Utilization in Nonalcoholic Fatty Liver Disease: Real-World Data From a Large U.S. Claims Database. Hepatology. 2018;68:2230-2238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 112] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 33. | Adejumo AC, Samuel GO, Adegbala OM, Adejumo KL, Ojelabi O, Akanbi O, Ogundipe OA, Pani L. Prevalence, trends, outcomes, and disparities in hospitalizations for nonalcoholic fatty liver disease in the United States. Ann Gastroenterol. 2019;32:504-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 34. | Buchynskyi M, Oksenych V, Kamyshna I, Vari SG, Kamyshnyi A. Genetic Predictors of Comorbid Course of COVID-19 and MAFLD: A Comprehensive Analysis. Viruses. 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 35. | Huang T, Zheng D, Song Y, Pan H, Qiu G, Xiang Y, Wang Z, Wang F. Demonstration of the impact of COVID-19 on metabolic associated fatty liver disease by bioinformatics and system biology approach. Medicine (Baltimore). 2023;102:e34570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 36. | Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28:3083-3107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3915] [Cited by in RCA: 4437] [Article Influence: 277.3] [Reference Citation Analysis (1)] |