Peer-review started: August 27, 2022
First decision: September 25, 2022
Revised: October 3, 2022
Accepted: November 21, 2022
Article in press: November 21, 2022
Published online: January 25, 2023
Processing time: 143 Days and 7.8 Hours
The liver has many significant functions, such as detoxification, the urea cycle, gluconeogenesis, and protein synthesis. Systemic diseases, hypoxia, infections, drugs, and toxins can easily affect the liver, which is extremely sensitive to injury. Systemic infection of severe acute respiratory syndrome coronavirus 2 can cause liver damage. The primary regulator of intracellular pH in the liver is the Na+/H+ exchanger (NHE). Physiologically, NHE protects hepatocytes from apoptosis by making the intracellular pH alkaline. Severe acute respiratory syndrome coronavirus 2 increases local angiotensin II levels by binding to angiotensin-converting enzyme 2. In severe cases of coronavirus disease 2019, high angi-otensin II levels may cause NHE overstimulation and lipid accumulation in the liver. NHE overstimulation can lead to hepatocyte death. NHE overstimulation may trigger a cytokine storm by increasing proinflammatory cytokines in the liver. Since the release of proinflammatory cytokines such as interleukin-6 increases with NHE activation, the virus may indirectly cause an increase in fibrinogen and D-dimer levels. NHE overstimulation may cause thrombotic events and systemic damage by increasing fibrinogen levels and cytokine release. Also, NHE overstimulation causes an increase in the urea cycle while inhibiting vitamin D synthesis and gluconeogenesis in the liver. Increasing NHE3 activity leads to Na+ loading, which impairs the containment and fluidity of bile acid. NHE overstimulation can change the gut microbiota composition by disrupting the structure and fluidity of bile acid, thus triggering systemic damage. Unlike other tissues, tumor necrosis factor-alpha and angiotensin II decrease NHE3 activity in the intestine. Thus, increased luminal Na+ leads to diarrhea and cytokine release. Severe acute respiratory syndrome coronavirus 2-induced local and systemic damage can be improved by preventing virus-induced NHE overstimulation in the liver.
Core Tip: Severe acute respiratory syndrome coronavirus 2 readily infects the liver by angiotensin-converting enzyme 2. Increased angiotensin II causes Na+/H+ exchanger (NHE) overstimulation allowing the accumulation of Na+ and Ca2+ in hepatocytes. Thus, hepatocytes are damaged and eventually die. Increased cytokine release increases fibrinogen levels, enhancing thrombotic events. Cytokine storms can be triggered by NHE overstimulation. Severe acute respiratory syndrome coronavirus 2-induced NHE overstimulation can change bile acid structure, which disrupts gut microbiota and can trigger cytokine storms. Liver damage from the virus can be considered the most important cause of disease progression and mortality.
- Citation: Cumhur Cure M, Cure E. Severe acute respiratory syndrome coronavirus 2 may cause liver injury via Na+/H+ exchanger. World J Virol 2023; 12(1): 12-21
- URL: https://www.wjgnet.com/2220-3249/full/v12/i1/12.htm
- DOI: https://dx.doi.org/10.5501/wjv.v12.i1.12
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused a worldwide pandemic and multisystem organ involvement, resulting in immense hospital costs and mortality. Although the virus settles in the lungs and causes infection, it spreads to other organs expressing angiotensin-converting enzyme 2 (ACE2), such as the heart, liver, and kidney, via the neighborhood route, systemic blood circulation, or vascular endothelium[1,2]. Liver involvement is also often seen in the course of severe novel coronavirus disease 2019 (COVID-19) and may advance the progression of the disease[3]. Understanding how SARS-CoV-2 spreads to these organs and solving the damage mechanism in the organs will provide an outstanding opportunity to reduce the severity and mortality of the disease.
Intracellular pH plays a vital role in SARS-CoV-2 infection[4]. Physiologically, the pH of endosomes is low. Therefore, acidic pH leads to the autophagy of viruses and harmful substances[4]. However, SARS-COV-2 can smoothly escape autophagy by manipulating cellular autophagy[5] and cause infection at low intracellular pH by fusing with ACE2[6]. When the pH of the endosomes becomes alkaline, SARS-CoV-2 cannot infect the cell since the configuration of ACE2 changes[7]. Hydroxychloroquine, which makes the intracellular pH alkaline, has been used until its harmful effects appear in patients with COVID-19[8]. The primary regulator of intracellular pH in the liver and many organs is the Na+/H+ exchanger (NHE)[9,10]. In a previous study, NHE activity was high in the blood of patients with COVID-19[11]. NHE activation in COVID-19 has been associated with cytokine storms and organ damage[12]. Unraveling the unique relationship between NHE and SARS-CoV-2 may illuminate liver involvement, mortality, and progression of COVID-19. Therefore, we should solve the mechanism of possible SARS-CoV-2 and NHE interaction in liver tissue.
The liver has many significant functions, such as detoxification, the urea cycle, gluconeogenesis, and protein synthesis. Many systemic diseases, hypoxia, infections, drugs, and toxins can easily affect the liver, which is extremely sensitive to injury. Systemic infection of SARS-CoV-2 or agents used in COVID-19 treatment can cause liver damage[13]. Liver enzyme elevation is seen in 16.1%-53.1% of patients with COVID-19[14]. Liver damage has been seen in approximately 20% of hospitalized patients with COVID-19[14]. Since hepatocytes express ACE2, SARS-CoV-2 can directly cause liver damage[15].
In COVID-19, gamma-glutamyl transferase (GGT) and alkaline phosphatase, which indicate cholestasis, increase. Aspartate aminotransferase (AST) and alanine transaminase (ALT), which are markers of hepatocyte damage, increase as well[16]. AST, ALT, GGT, and bilirubin indicate liver damage but also reflect the severity of COVID-19[13,17]. Liu et al[16] revealed that GGT and ACE2 share the same transcriptional machinery and speculated that GGT may indicate the in vivo expression level of ACE2. In addition, the finding of high bilirubin as a predictive marker for mortality in patients with COVID-19 reveals the importance of liver involvement in the disease[18,19]. On the other hand, the presence of microthrombus in most patients with COVID-19 and the fact that arterial thrombosis is responsible for mortality indicate that liver involvement is more significant in COVID-19.
Fibrinogen, the precursor of fibrin, is synthesized in the liver. D-dimer, a fibrin degradation product, was elevated in many critically ill COVID-19 patients[20]. D-dimer levels correlate with total bilirubin, AST, and ALT levels in patients with COVID-19[21]. Baroiu et al[21] reported that D-dimer might be a predictive marker of abnormal liver function parameters and liver injury in patients with COVID-19. Therefore, in most patients with COVID-19, liver involvement may be responsible for mortality and the disease progression.
There are many ion pumps in plasma membranes. They provide signaling and stabilization of ion concentrations between intracellular and extracellular areas. NHE is one of the most important and has nine isoforms. These isoforms are localized in different tissues and cell types and have various functions depending on their localization. NHE1 is the cleaning form found in almost all tissues and is expressed abundantly in the liver. NHE2 is located in the stomach and intestines. Liver, intestine, and kidney tissues have NHE3. NHE4 is in the stomach and kidney. The brain has NHE5. NHE6-9 are found in intracellular organelles[22-24]. NHEs are involved in the etiology of several gastrointestinal and liver diseases[22].
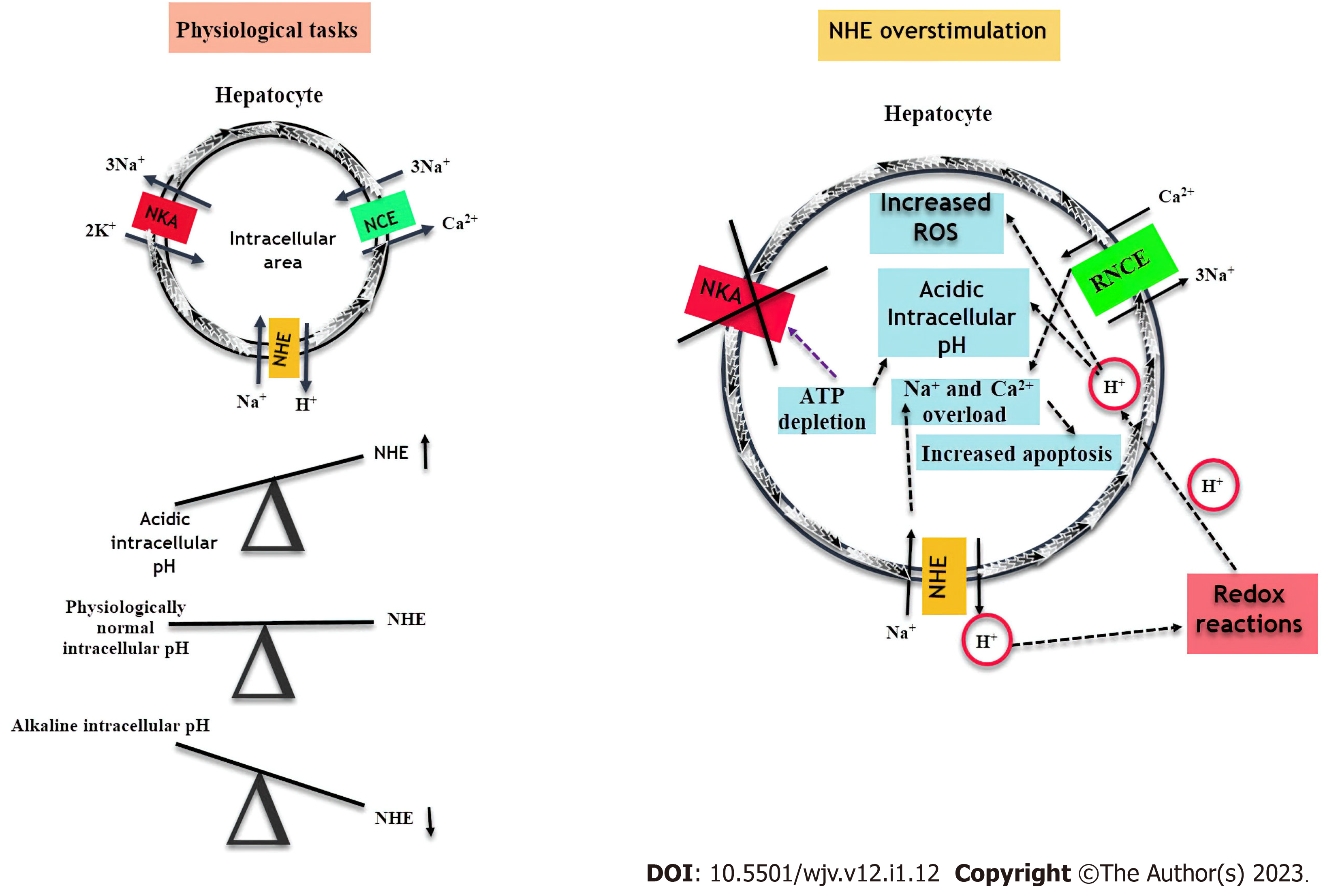
NHE is localized in the basolateral or sinusoidal membrane of hepatocytes[22]. The NHE, which provides the intracellular pH balance, is physiologically activated when the intracellular pH decreases and brings the intracellular pH to its physiological levels[25]. NHE causes the movement of Na+ into the cell and H+ out of the cell. As soon as the intracellular pH reaches physiological levels, the NHE activity reduces[12]. NHE provides the passage of Na+ into the cell. The Na+/K+/2Cl- cotransporters cause the influx of Na+, K+, and Cl- into the cell. Simultaneously, the Cl-/HCO3- antiporter ensures the influx of Cl- into the cell. Na+/K+-ATPase (NKA) pumps Na+ outward while K+ moves into the cell. Eventually, KCl increases in hepatocytes[25-27]. Na+/Ca2+ Exchanger (NCE) provides intracellular Ca2+ balance[28]. These pumps work in harmony in the liver. Physiological NHE, NCE, and NKA activities are summarized in Figure 1.
In acute ischemic events, NHE activation increases intracellular pH and prevents hepatocytes from undergoing apoptosis[10]. Arginine vasopressin activates NHE in hepatocytes by calcium/calmodulin-dependent processes[29]. Stimulation of NHE is involved in hepatocyte regeneration and growth[25]. However, if the stimulus is increased, hepatocyte apoptosis due to intracellular Ca2+ accumulation increases[22,25].
Chronic cytokine or platelet-derived growth factor-mediated NHE stimulation causes hepatic stellate cell proliferation and fibrosis[30]. NHE3 is located in the apical membranes of the hepatocytes and cholangiocytes. NHE3 is responsible for maintaining the fluid content of bile acid[31]. Increased NHE3 activity leads to Na+ loading and an increase in the concentration function of the gallbladder, resulting in gallstone formation[32-34].
While NHE does significant work in physiological conditions, its continuous activation causes serious problems. During NHE physiological function, NCE pumps Na+ into the cell and Ca2+ outward. As a result of NHE overstimulation, NCE activation stops, and reverse NCE becomes active due to increased Na+ in the cell. While Ca2+ is pumped into the cell, Na+ begins to be pumped outward. As a result, the concentration of Na+ and Ca2+ in the cell increases. The NKA pump, which pumps K+ inward and Na+ outward, loses its function due to increased intracellular Na+ and ATP depletion[4,12,35-37]. While edema occurs due to Na+ accumulation in hepatocytes, Ca2+ overload causes hepatocyte apoptosis[38]. In the prolonged activity of NHE, continuous pumping of H+ into the extracellular area causes some chain redox reactions, H+ begins to influx into the cell, and the intracellular pH drops[4,12,29]. NHE overstimulation and its outcomes are summarized in Figure 1. NHE activation also increases the influx of lipids and free fatty acids into the cell. Free fatty acid influx further increases intracellular acidity, and concomitant Ca2+ overload accelerates apoptosis[36]. As a result of all these events, acute hepatocyte damage may progress from an advanced level to liver failure. Tanaka et al[39] found that NHE suppression inhibited the nuclear factor kappa B pathway and proinflammatory cytokine release, thus preventing fatal acute liver failure. In addition, there is a strong interaction between NHE and low-density lipoprotein receptors (LDLR), which may cause liver damage. Non-physiological LDLR excess gives rise to cholesterol overload in hepatocytes. Lipid deposition causes hepatomegaly in the chronic process[40]. Although hepatocytes are resistant to cholesterol loading, hepatocytes begin to die with prolonged lipid deposition[40].
The alkalinity of the intracellular pH creates a barrier against SARS-CoV-2 infection. Therefore, this mechanism has been considered for COVID-19 treatment[6]. Hydroxychloroquine has been used for COVID-19 treatment since it makes the intracellular pH alkaline by inhibiting vacuolar H+-ATPase[41]. Also, metformin converts endosome pH to alkaline via vacuolar H+-ATPase[42-44]. At or slightly above the physiological NHE activation can shift the intracellular pH to alkaline, preventing ACE2-SARS-CoV-2 fusion. Using proton pump inhibitors in mild COVID-19 patients may worsen the course of the disease[45,46]. However, when the virus takes total control of the cells in patients with severe COVID-19, NHE overstimulation causes many detrimental effects as well as a decrease in intracellular pH.
Hitherto, it has not been determined whether SARS-CoV-2 directly affects NHE; however, NHE activity was elevated in the blood of patients with COVID-19[11]. SARS-CoV-2 can increase NHE activity by many mechanisms. SARS-CoV-2-infected mice, which have physiological angiotensin II levels, have been shown to have no damage in some of their tissues[47]. However, patients with severe COVID-19 have high angiotensin II levels[48]. ACE2 degrades angiotensin II and reduces its levels. When SARS-CoV-2 fuses with ACE2, the level of angiotensin II increases in the circulation and tissues since ACE2 cannot complete its task[4]. The liver has a local renin-angiotensin system that is not very important in physiological conditions, which is dissimilar to hepatic renin-angiotensin system that plays a significant role in pathological conditions[49,50]. Since the liver expresses ACE2, the virus increases the local angiotensin II level by binding to ACE2 in the liver. Angiotensin II is the substantial stimulus of NHE. Angiotensin II shows pro-oxidant, fibrogenic, and proinflammatory actions on the liver[51].
SARS-CoV-2 uses lipid rafts and cholesterol to fuse with ACE2 in membranes[52]. Lipid rafts play a remarkable role in the entry of SARS-CoV-2 into the cell[53]. In addition, there is a strong interaction among lipid rafts, cholesterol, and NHE. Therefore, the virus can activate NHE through cholesterol and lipid rafts[36]. The release of proinflammatory cytokines such as tumor necrosis factor-alpha and interleukin-6 and increased oxidative stress by SARS-CoV-2 can also stimulate NHE[12]. Fibrinogen produced in the liver provides activation of NHE1 by inducing NCE[54]. Fibrinogen increase in COVID-19 can also initiate NHE activation.
Since the liver has many vital functions, the virus infecting it will have many systemic consequences. Physiologically, NHE protects hepatocytes from apoptosis by making the intracellular pH alkaline[10]. However, NHE overstimulation can cause acute lethal liver damage[39]. Once the virus settles in the liver and infects hepatocytes, it can cause NHE overstimulation. The virus renders intracellular Ca2+ accumulation with reverse NCE activation after Na+ accumulation in hepatocytes. Apoptosis is induced in hepatocytes and cell death. Liver enzymes such as AST and ALT increase due to parenchymal damage[55]. NHE overstimulation may also trigger a cytokine storm by increasing proinflammatory cytokines in the liver[12].
On the other hand, NHE1 plays a role in urea synthesis[56]. Increased H+ extrusion increases urea synthesis[57]. The virus can increase the level of urea by NHE1 overstimulation. Increased urea in patients with COVID-19 indicates kidney damage[58]. However, urea may also be a marker of liver injury.
Gluconeogenesis in the liver is significantly reduced when the intracellular pH is lowered. Therefore, a hypoxic environment occurs[59,60]. In physiological conditions, NHE stimulates gluconeogenesis by increasing intracellular pH. NHE overstimulation decreases gluconeogenesis in the liver by reducing intracellular pH[61]. In patients with severe COVID-19, elevated blood glucose may be due to insulin resistance[61] or decreased insulin secretion related to NHE and proinflammatory cytokine-mediated pancreatic damage[62].
Other liver functions are vitamin B12 storage[63], iron storage[64], and vitamin D synthesis[65]. Vitamin D reduces acute liver injury[66]. Vitamin D is immunomodulatory, and low levels may worsen the progression of COVID-19[67,68]. Insulin-like growth factor-1 levels[69,70], which stimulate vitamin D synthesis in hepatocytes, was lower in COVID-19 patients[71]. While vitamin D increases circulating insulin-like growth factor-1 levels[72], insulin-like growth factor-1 inhibits NHE. Increased intracellular Ca2+ decreases vitamin D synthesis in the liver[73]. NHE-mediated hepatocyte injury in patients with COVID-19 may reduce vitamin D synthesis and worsen disease progression. Reduced intracellular pH leads to iron release from the liver[74], and excess iron causes local and systemic damage with the Fenton reaction[75]. Low intracellular pH caused by NHE overstimulation can lead to iron-mediated damage to cells in patients with COVID-19.
According to the LDLR mechanism we described earlier, the virus can cause lipid accumulation in the liver, leading to the fattening and enlargement of the liver and even loss of function[36]. Unfortunately, there is not enough information in the literature regarding the role of LDLR in patients with COVID-19. Although a study proved the opposite[76], Lange et al[77] reported that LDLR expression was higher in patients with COVID-19 than in healthy controls. Agirbasli et al[78] reported that LDLR-related protein 1, a member of the LDLR family, is increased in severe COVID-19. According to current findings, angiotensin II inhibits proprotein convertase subtilisin/kexin type 9[36], which disrupts LDLR[36]. Angiotensin II indirectly increases LDLR levels. In severe cases of COVID-19, increased angiotensin II may lead to NHE overstimulation and lipid accumulation in the liver. In addition, the virus needs cholesterol particles to form new virions after infecting cells[79]. Since the liver is the production and storage site of cholesterol, the virus can continue its life cycle in the liver for a lengthy time. Some studies have shown that high-density lipoprotein (HDL) binds to SARS-CoV-2[80]. Low HDL facilitates the virus binding to ACE2[80]; however, high HDL may play an active role in SARS-CoV-2 transfer from other infected tissues to the liver, as HDL is a cholesterol transporter of the liver.
The virus may settle in the liver and cause hepatocyte damage and multisystem disorder. Therefore, liver involvement may contribute significantly to mortality in COVID-19. There may be a close relationship between liver injury and fibrinogen and coagulation disorders in patients with COVID-19[3]. As we know, fibrinolysis increases liver necrosis[81]. Initially, fibrinogen and D-dimer levels are elevated in patients with severe COVID-19, but their levels decrease over the following days[82]. Fibrinogen is an acute phase reactant primarily released from the liver, and interleukin-6 increases fibrinogen synthesis[83]. Since the release of proinflammatory cytokines such as interleukin-6 increases with NHE activation, the virus may indirectly cause an increase in fibrinogen and D-dimer. In severe cases of COVID-19, fibrinogen and D-dimer levels were low after 10 d.
NHE increases the tendency for thrombosis mainly through platelets[84]; however, it may also indirectly lead to a tendency for thrombosis through the liver. Prothrombin synthesized in the liver is converted to thrombin when fibrinogen is transformed into fibrin. Thrombin levels are high in patients with COVID-19[85]. Thrombin activates NHE in vascular smooth muscle cells[86]. Thus, the virus can cause damage to distant organs with the help of NHE-mediated proinflammatory cytokine release and thrombin.
While the virus causes a Na+ load in the cell by NHE overstimulation, it overloads H+ in the extracellular area[4]. As a result of the subsequent redox reactions, H+ flows into the cell, and excessive ATP consumption by NKA results in hypoxia and ATP depletion in the cell[12]. Hypoxia and ATP depletion are prominent stimuli of heme oxidase[87,88]. Increased heme oxidase activation enhances carbon monoxide and bilirubin levels in the cell[89]. Carbon monoxide usually has a hepatoprotective effect, but sometimes it can also have a hepatotoxic effect[88]. Elevated bilirubin in patients with COVID-19 has been identified as a predictive marker for mortality rates[90]. Elevated bilirubin may indirectly reflect NHE-mediated cell hypoxia in patients with COVID-19.
The elevation of alkaline phosphatase and GGT in COVID-19 patients suggests that SARS-CoV-2 changes the structure of bile acid and may lead to cholestasis[91]. NHE1 on the basolateral surface of the bile ducts regulates intracellular pH, while NHE3 on the apical surface regulates bile acid structure and fluidity[92]. Increasing NHE3 activity leads to Na+ loading, which impairs the containment and fluidity of bile acid[32]. Bile acids play a primary role in maintaining gut microbiota composition[93]. We have recently described that disruption of NHE-mediated gut microbiota composition leads to cytokine release syndrome[12]. The virus disrupts the gut microbiota composition in the intestine and increases the release of proinflammatory cytokines like tumor necrosis factor-alpha. In addition, SARS-CoV-2 increases local angiotensin II levels by binding to ACE2. Unlike other tissues, tumor necrosis factor-alpha and angiotensin II decrease NHE3 activity in the intestine[12,94,95]. Thus, increased luminal Na+ leads to diarrhea and cytokine release[12,96]. This event may play a role in triggering cytokine storms[12]. The virus can cause intestinal-associated cytokine release by changing the structure and fluidity of bile acid before it has intestinal involvement. It is not yet known whether the changed content of bile acid will play a role in transferring the virus to the intestine. Detailed studies are needed on this subject.
Physiological NHE activation protects the liver against acute injury, whereas NHE overstimulation causes severe liver injury. NHE overstimulation can lead to hepatocyte death. Also, it increases the urea cycle and inhibits of gluconeogenesis and vitamin D synthesis in the liver. NHE overstimulation may cause thrombotic events and systemic damage by increasing fibrinogen levels and cytokine release. It can also change the gut microbiota composition by disrupting the structure and fluidity of bile acid, thus triggering systemic damage. SARS-CoV-2-induced local and systemic damage can be improved by preventing virus-induced NHE overstimulation in the liver.
We thank Fatma Cure for the English proofreading of the article.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Turkey
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Cidade JP, Portugal; Dey J, India; Teixeira KN, Brazil S-Editor: Chang KL L-Editor: Filipodia P-Editor: Chang KL
| 1. | Veleri S. Neurotropism of SARS-CoV-2 and neurological diseases of the central nervous system in COVID-19 patients. Exp Brain Res. 2022;240:9-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 2. | Lu RXZ, Lai BFL, Rafatian N, Gustafson D, Campbell SB, Banerjee A, Kozak R, Mossman K, Mubareka S, Howe KL, Fish JE, Radisic M. Vasculature-on-a-chip platform with innate immunity enables identification of angiopoietin-1 derived peptide as a therapeutic for SARS-CoV-2 induced inflammation. Lab Chip. 2022;22:1171-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 3. | D'Ardes D, Boccatonda A, Cocco G, Fabiani S, Rossi I, Bucci M, Guagnano MT, Schiavone C, Cipollone F. Impaired coagulation, liver dysfunction and COVID-19: Discovering an intriguing relationship. World J Gastroenterol. 2022;28:1102-1112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 43] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 4. | Cumhur Cure M, Cure E. Effects of the Na+/H+ Ion Exchanger on Susceptibility to COVID-19 and the Course of the Disease. J Renin Angiotensin Aldosterone Syst. 2021;2021:4754440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 5. | Koepke L, Hirschenberger M, Hayn M, Kirchhoff F, Sparrer KM. Manipulation of autophagy by SARS-CoV-2 proteins. Autophagy. 2021;17:2659-2661. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 66] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 6. | Jimenez L, Campos Codo A, Sampaio VS, Oliveira AER, Ferreira LKK, Davanzo GG, de Brito Monteiro L, Victor Virgilio-da-Silva J, Borba MGS, Fabiano de Souza G, Zini N, de Andrade Gandolfi F, Muraro SP, Luiz Proença-Modena J, Val FA, Cardoso Melo G, Monteiro WM, Nogueira ML, Lacerda MVG, Moraes-Vieira PM, Nakaya HI. Acid pH Increases SARS-CoV-2 Infection and the Risk of Death by COVID-19. Front Med (Lausanne). 2021;8:637885. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 7. | Pahan P, Pahan K. Smooth or Risky Revisit of an Old Malaria Drug for COVID-19? J Neuroimmune Pharmacol. 2020;15:174-180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 8. | Kapuy O, Korcsmáros T. Chloroquine and COVID-19-A systems biology model uncovers the drug's detrimental effect on autophagy and explains its failure. PLoS One. 2022;17:e0266337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 9. | Cheng PC, Lin HY, Chen YS, Cheng RC, Su HC, Huang RC. The Na+/H+-Exchanger NHE1 Regulates Extra- and Intracellular pH and Nimodipine-sensitive [Ca2+]i in the Suprachiasmatic Nucleus. Sci Rep. 2019;9:6430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Wang P, Wang X, Li L, Kan Q, Yu Z, Feng R, Chen Z, Shi Y, Gao J. Role of sodium-hydrogen exchanger isoform 1 in regulating hepatocyte apoptosis induced by hyperammonaemia. Gastroenterol Hepatol. 2018;41:490-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Mustroph J, Hupf J, Hanses F, Evert K, Baier MJ, Evert M, Meindl C, Wagner S, Hubauer U, Pietrzyk G, Leininger S, Staudner S, Vogel M, Wallner S, Zimmermann M, Sossalla S, Maier LS, Jungbauer C. Decreased GLUT1/NHE1 RNA expression in whole blood predicts disease severity in patients with COVID-19. ESC Heart Fail. 2021;8:309-316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Cure MC, Cure E. Prolonged NHE Activation may be both Cause and Outcome of Cytokine Release Syndrome in COVID-19. Curr Pharm Des. 2022;28:1815-1822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 13. | Ali N. Relationship Between COVID-19 Infection and Liver Injury: A Review of Recent Data. Front Med (Lausanne). 2020;7:458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (1)] |
| 14. | Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428-430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1348] [Cited by in RCA: 1292] [Article Influence: 258.4] [Reference Citation Analysis (4)] |
| 15. | Barnes E. Infection of liver hepatocytes with SARS-CoV-2. Nat Metab. 2022;4:301-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 16. | Liu J, Yu C, Yang Q, Yuan X, Yang F, Li P, Chen G, Liang W, Yang Y. The clinical implication of gamma-glutamyl transpeptidase in COVID-19. Liver Res. 2021;5:209-216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Shao T, Tong Y, Lu S, Jeyarajan AJ, Su F, Dai J, Shi J, Huang J, Hu C, Wu L, Dai X, Cheng Z, Yan J, Huang P, Tian Y, Li S, Chung RT, Chen D. Gamma-Glutamyltransferase Elevation Is Frequent in Patients With COVID-19: A Clinical Epidemiologic Study. Hepatol Commun. 2020;4:1744-1750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 18. | Russo A, Pisaturo M, Palladino R, Maggi P, Numis FG, Gentile I, Sangiovanni V, Esposito V, Punzi R, Calabria G, Rescigno C, Salomone Megna A, Masullo A, Manzillo E, Russo G, Parrella R, Dell'Aquila G, Gambardella M, Ponticiello A, Coppola N; On Behalf Of CoviCam Group. Prognostic Value of Transaminases and Bilirubin Levels at Admission to Hospital on Disease Progression and Mortality in Patients with COVID-19-An Observational Retrospective Study. Pathogens. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Seok H, Lim S, Kim JY, Park CH, Kim JH, Woo ML, Won H, Kang YM, Oh HS, Song KH, Jung YJ, Kim T, Jo S, Choi WS. Infectivity of Coronavirus Disease 2019: A Prospective Cohort Study in the Korean Metropolitan Area. J Korean Med Sci. 2022;37:e106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Rostami M, Khoshnegah Z, Mansouritorghabeh H. Hemostatic System (Fibrinogen Level, D-Dimer, and FDP) in Severe and Non-Severe Patients With COVID-19: A Systematic Review and Meta-Analysis. Clin Appl Thromb Hemost. 2021;27:10760296211010973. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Baroiu L, Lese AC, Stefanopol IA, Iancu A, Dumitru C, Ciubara AB, Bujoreanu FC, Baroiu N, Ciubara A, Nechifor A, Anghel L, Tatu AL. The Role of D-Dimers in the Initial Evaluation of COVID-19. Ther Clin Risk Manag. 2022;18:323-335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 22. | Cao L, Yuan Z, Liu M, Stock C. (Patho-)Physiology of Na+/H+ Exchangers (NHEs) in the Digestive System. Front Physiol. 2019;10:1566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Kinaneh S, Knany Y, Khoury EE, Ismael-Badarneh R, Hamoud S, Berger G, Abassi Z, Azzam ZS. Identification, localization and expression of NHE isoforms in the alveolar epithelial cells. PLoS One. 2021;16:e0239240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Kemp G, Young H, Fliegel L. Structure and function of the human Na+/H+ exchanger isoform 1. Channels (Austin). 2008;2:329-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 25. | Li T, Tuo B. Pathophysiology of hepatic Na+/H+ exchange (Review). Exp Ther Med. 2020;20:1220-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | Wehner F, Tinel H. Role of Na+ conductance, Na(+)-H+ exchange, and Na(+)-K(+)-2Cl- symport in the regulatory volume increase of rat hepatocytes. J Physiol. 1998;506 ( Pt 1):127-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Gleeson D, Smith ND, Boyer JL. Bicarbonate-dependent and -independent intracellular pH regulatory mechanisms in rat hepatocytes. Evidence for Na+-HCO3- cotransport. J Clin Invest. 1989;84:312-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 85] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | Liao QS, Du Q, Lou J, Xu JY, Xie R. Roles of Na+/Ca2+ exchanger 1 in digestive system physiology and pathophysiology. World J Gastroenterol. 2019;25:287-299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 29. | Anwer MS. Mechanism of activation of the Na+/H+ exchanger by arginine vasopressin in hepatocytes. Hepatology. 1994;20:1309-1317. [PubMed] |
| 30. | Benedetti A, Di Sario A, Casini A, Ridolfi F, Bendia E, Pigini P, Tonnini C, D'Ambrosio L, Feliciangeli G, Macarri G, Svegliati-Baroni G. Inhibition of the NA(+)/H(+) exchanger reduces rat hepatic stellate cell activity and liver fibrosis: an in vitro and in vivo study. Gastroenterology. 2001;120:545-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 55] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Mennone A, Biemesderfer D, Negoianu D, Yang CL, Abbiati T, Schultheis PJ, Shull GE, Aronson PS, Boyer JL. Role of sodium/hydrogen exchanger isoform NHE3 in fluid secretion and absorption in mouse and rat cholangiocytes. Am J Physiol Gastrointest Liver Physiol. 2001;280:G247-G254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 38] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 32. | Narins SC, Ramakrishnan R, Park EH, Smith PR, Meyers WC, Abedin MZ. Gallbladder Na+/H+ exchange activity is up-regulated prior to cholesterol crystal formation. Eur J Clin Invest. 2005;35:514-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 33. | Chen Y, Wu S, Qi L, Dai W, Tian Y, Kong J. Altered absorptive function in the gall bladder during cholesterol gallstone formation is associated with abnormal NHE3 complex formation. J Physiol Biochem. 2020;76:427-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 34. | Chen Y, Kong J, Wu S. Cholesterol gallstone disease: focusing on the role of gallbladder. Lab Invest. 2015;95:124-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 35. | Wu MY, Yiang GT, Liao WT, Tsai AP, Cheng YL, Cheng PW, Li CY, Li CJ. Current Mechanistic Concepts in Ischemia and Reperfusion Injury. Cell Physiol Biochem. 2018;46:1650-1667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 896] [Article Influence: 128.0] [Reference Citation Analysis (0)] |
| 36. | Cure E, Cumhur Cure M. Strong relationship between cholesterol, low-density lipoprotein receptor, Na+/H+ exchanger, and SARS-COV-2: this association may be the cause of death in the patient with COVID-19. Lipids Health Dis. 2021;20:179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 37. | Guan LY, Fu PY, Li PD, Li ZN, Liu HY, Xin MG, Li W. Mechanisms of hepatic ischemia-reperfusion injury and protective effects of nitric oxide. World J Gastrointest Surg. 2014;6:122-128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 121] [Cited by in RCA: 134] [Article Influence: 12.2] [Reference Citation Analysis (2)] |
| 38. | Carini R, de Cesaris MG, Bellomo G, Albano E. Role of Na+/Ca2+ exchanger in preventing Na+ overload and hepatocyte injury: opposite effects of extracellular and intracellular Ca2+ chelation. Biochem Biophys Res Commun. 1997;232:107-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 39. | Tanaka H, Uchida Y, Kaibori M, Hijikawa T, Ishizaki M, Yamada M, Matsui K, Ozaki T, Tokuhara K, Kamiyama Y, Nishizawa M, Ito S, Okumura T. Na+/H+ exchanger inhibitor, FR183998, has protective effect in lethal acute liver failure and prevents iNOS induction in rats. J Hepatol. 2008;48:289-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 40. | Cichon G, Willnow T, Herwig S, Uckert W, Löser P, Schmidt HH, Benhidjeb T, Schlag PM, Schnieders F, Niedzielska D, Heeren J. Non-physiological overexpression of the low density lipoprotein receptor (LDLr) gene in the liver induces pathological intracellular lipid and cholesterol storage. J Gene Med. 2004;6:166-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 41. | Rother N, Yanginlar C, Lindeboom RGH, Bekkering S, van Leent MMT, Buijsers B, Jonkman I, de Graaf M, Baltissen M, Lamers LA, Riksen NP, Fayad ZA, Mulder WJM, Hilbrands LB, Joosten LAB, Netea MG, Vermeulen M, van der Vlag J, Duivenvoorden R. Hydroxychloroquine Inhibits the Trained Innate Immune Response to Interferons. Cell Rep Med. 2020;1:100146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 42. | Glossmann HH, Lutz OMD. Metformin and Aging: A Review. Gerontology. 2019;65:581-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 98] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 43. | Zhang CS, Li M, Ma T, Zong Y, Cui J, Feng JW, Wu YQ, Lin SY, Lin SC. Metformin Activates AMPK through the Lysosomal Pathway. Cell Metab. 2016;24:521-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 207] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 44. | Kim J, Lee HY, Ahn J, Hyun M, Lee I, Min KJ, You YJ. NHX-5, an Endosomal Na+/H+ Exchanger, Is Associated with Metformin Action. J Biol Chem. 2016;291:18591-18599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 45. | Kim HB, Kim JH, Wolf BJ. Acid suppressant use in association with incidence and severe outcomes of COVID-19: a systematic review and meta-analysis. Eur J Clin Pharmacol. 2022;78:383-391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 46. | Fatima K, Almas T, Lakhani S, Jahangir A, Ahmed A, Siddiqui A, Rahim A, Qureshi SA, Arshad Z, Golani S, Musheer A. The Use of Proton Pump Inhibitors and COVID-19: A Systematic Review and Meta-Analysis. Trop Med Infect Dis. 2022;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 47. | Wang Y, Takeshita H, Yamamoto K, Huang Y, Wang C, Nakajima T, Nozato Y, Fujimoto T, Yokoyama S, Hongyo K, Nakagami F, Akasaka H, Takami Y, Takeya Y, Sugimoto K, Rakugi H. A pressor dose of angiotensin II has no influence on the angiotensin-converting enzyme 2 and other molecules associated with SARS-CoV-2 infection in mice. FASEB J. 2021;35:e21419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 48. | Xavier LL, Neves PFR, Paz LV, Neves LT, Bagatini PB, Timmers LFSM, Rasia-Filho AA, Mestriner RG, Wieck A. Does Angiotensin II Peak in Response to SARS-CoV-2? Front Immunol. 2020;11:577875. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 49. | Paul M, Poyan Mehr A, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev. 2006;86:747-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1160] [Cited by in RCA: 1237] [Article Influence: 65.1] [Reference Citation Analysis (0)] |
| 50. | Lubel JS, Herath CB, Burrell LM, Angus PW. Liver disease and the renin-angiotensin system: recent discoveries and clinical implications. J Gastroenterol Hepatol. 2008;23:1327-1338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 100] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 51. | Simões E Silva AC, Miranda AS, Rocha NP, Teixeira AL. Renin angiotensin system in liver diseases: Friend or foe? World J Gastroenterol. 2017;23:3396-3406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 81] [Cited by in RCA: 79] [Article Influence: 9.9] [Reference Citation Analysis (1)] |
| 52. | Dai J, Wang H, Liao Y, Tan L, Sun Y, Song C, Liu W, Qiu X, Ding C. Coronavirus Infection and Cholesterol Metabolism. Front Immunol. 2022;13:791267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 35] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 53. | Palacios-Rápalo SN, De Jesús-González LA, Cordero-Rivera CD, Farfan-Morales CN, Osuna-Ramos JF, Martínez-Mier G, Quistián-Galván J, Muñoz-Pérez A, Bernal-Dolores V, Del Ángel RM, Reyes-Ruiz JM. Cholesterol-Rich Lipid Rafts as Platforms for SARS-CoV-2 Entry. Front Immunol. 2021;12:796855. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 71] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 54. | Liu CL, Liu X, Wang Y, Deng Z, Liu T, Sukhova GK, Wojtkiewicz GR, Tang R, Zhang JY, Achilefu S, Nahrendorf M, Libby P, Wang X, Shi GP. Reduced Nhe1 (Na+-H+ Exchanger-1) Function Protects ApoE-Deficient Mice From Ang II (Angiotensin II)-Induced Abdominal Aortic Aneurysms. Hypertension. 2020;76:87-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 55. | Tokarczyk U, Kaliszewski K, Kopszak A, Nowak Ł, Sutkowska-Stępień K, Sroczyński M, Sępek M, Dudek A, Diakowska D, Trocha M, Gajecki D, Gawryś J, Matys T, Maciejiczek J, Kozub V, Szalast R, Madziarski M, Zubkiewicz-Zarębska A, Letachowicz K, Kiliś-Pstrusińska K, Matera-Witkiewicz A, Pomorski M, Protasiewicz M, Sokołowski J, Adamik B, Kujawa K, Doroszko A, Madziarska K, Jankowska EA. Liver Function Tests in COVID-19: Assessment of the Actual Prognostic Value. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Reference Citation Analysis (1)] |
| 56. | Pedersen SF, Counillon L. The SLC9A-C Mammalian Na+/H+ Exchanger Family: Molecules, Mechanisms, and Physiology. Physiol Rev. 2019;99:2015-2113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 123] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 57. | Zange J, Gronczewski J, Jans AW. NH4+ metabolism and the intracellular pH in isolated perfused rat liver. Biochem J. 1993;293 ( Pt 3):667-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 58. | Ye B, Deng H, Zhao H, Liang J, Ke L, Li W. Association between an increase in blood urea nitrogen at 24 h and worse outcomes in COVID-19 pneumonia. Ren Fail. 2021;43:347-350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 59. | Kashiwagura T, Deutsch CJ, Taylor J, Erecińska M, Wilson DF. Dependence of gluconeogenesis, urea synthesis, and energy metabolism of hepatocytes on intracellular pH. J Biol Chem. 1984;259:237-243. [PubMed] |
| 60. | Zhou FQ. Pyruvate in the correction of intracellular acidosis: a metabolic basis as a novel superior buffer. Am J Nephrol. 2005;25:55-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 61. | Kaloyianni M, Bourikas D, Koliakos G. The effect of insulin on Na+-H+ antiport activity of obese and normal subjects erythrocytes. Cell Physiol Biochem. 2001;11:253-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 62. | Cure E, Cumhur Cure M. COVID-19 may affect the endocrine pancreas by activating Na+/H+ exchanger 2 and increasing lactate levels. J Endocrinol Invest. 2020;43:1167-1168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 63. | Fedosov SN. Physiological and molecular aspects of cobalamin transport. Subcell Biochem. 2012;56:347-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 64. | Anderson ER, Shah YM. Iron homeostasis in the liver. Compr Physiol. 2013;3:315-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 160] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 65. | Rhyu J, Yu R. Newly discovered endocrine functions of the liver. World J Hepatol. 2021;13:1611-1628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (1)] |
| 66. | Kitson MT, Roberts SK. D-livering the message: the importance of vitamin D status in chronic liver disease. J Hepatol. 2012;57:897-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 159] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 67. | Martineau AR, Cantorna MT. Vitamin D for COVID-19: where are we now? Nat Rev Immunol. 2022;22:529-530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 68. | Dana N, Nasirian M, Vaseghi G, Heshmat-Ghahdarijani K, Ataei B, Mosayebi A, Manteghinejad A, Javanmard SH. Vitamin D Level in Laboratory Confirmed COVID-19 and Disease Progression. Eurasian J Med. 2022;54:206-212. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 69. | Theodoropoulos C, Demers C, Petit JL, Gascon-Barre M. High sensitivity of rat hepatic vitamin D3-25 hydroxylase CYP27A to 1,25-dihydroxyvitamin D3 administration. Am J Physiol Endocrinol Metab. 2003;284:E138-E147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 70. | Halupczok-Żyła J, Jawiarczyk-Przybyłowska A, Bolanowski M. Patients with Active Acromegaly are at High Risk of 25(OH)D Deficiency. Front Endocrinol (Lausanne). 2015;6:89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 71. | Ilias I, Diamantopoulos A, Botoula E, Athanasiou N, Zacharis A, Tsipilis S, Jahaj E, Vassiliou AG, Vassiliadi DA, Kotanidou A, Tsagarakis S, Dimopoulou I. Covid-19 and Growth Hormone/Insulin-Like Growth Factor 1: Study in Critically and Non-Critically Ill Patients. Front Endocrinol (Lausanne). 2021;12:644055. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 72. | Ameri P, Giusti A, Boschetti M, Bovio M, Teti C, Leoncini G, Ferone D, Murialdo G, Minuto F. Vitamin D increases circulating IGF1 in adults: potential implication for the treatment of GH deficiency. Eur J Endocrinol. 2013;169:767-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 73. | Corlett SC, Chaudhary MS, Tomlinson S, Care AD. The involvement of intracellular calcium ion concentration and calmodulin in the 25-hydroxylation of cholecalciferol in ovine and rat liver. Cell Calcium. 1987;8:247-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 74. | Tian Y, Tian Y, Yuan Z, Zeng Y, Wang S, Fan X, Yang D, Yang M. Iron Metabolism in Aging and Age-Related Diseases. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 60] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 75. | Habib HM, Ibrahim S, Zaim A, Ibrahim WH. The role of iron in the pathogenesis of COVID-19 and possible treatment with lactoferrin and other iron chelators. Biomed Pharmacother. 2021;136:111228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 163] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 76. | Lee W, Ahn JH, Park HH, Kim HN, Kim H, Yoo Y, Shin H, Hong KS, Jang JG, Park CG, Choi EY, Bae JS, Seo YK. COVID-19-activated SREBP2 disturbs cholesterol biosynthesis and leads to cytokine storm. Signal Transduct Target Ther. 2020;5:186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 95] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 77. | Lange C, Wolf J, Auw-Haedrich C, Schlecht A, Boneva S, Lapp T, Horres R, Agostini H, Martin G, Reinhard T, Schlunck G. Expression of the COVID-19 receptor ACE2 in the human conjunctiva. J Med Virol. 2020;92:2081-2086. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 97] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 78. | Agirbasli M, Korkmaz R, Isman FK. Abstract 9960: Soluble Low Density Lipoprotein Receptor-related Protein-1 Levels As A Potential Biomarker In Covid19. Circulation. 2021;144:A9960. [DOI] [Full Text] |
| 79. | Theken KN, Tang SY, Sengupta S, FitzGerald GA. The roles of lipids in SARS-CoV-2 viral replication and the host immune response. J Lipid Res. 2021;62:100129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 80. | Kluck GEG, Yoo JA, Sakarya EH, Trigatti BL. Good Cholesterol Gone Bad? Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 81. | Wardle EN. Fibrinogen in liver disease. Arch Surg. 1974;109:741-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 82. | Osawa I, Okamoto K, Ikeda M, Otani A, Wakimoto Y, Yamashita M, Shinohara T, Kanno Y, Jubishi D, Kurano M, Harada S, Okugawa S, Yatomi Y, Moriya K. Dynamic changes in fibrinogen and D-dimer levels in COVID-19 patients on nafamostat mesylate. J Thromb Thrombolysis. 2021;51:649-656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 83. | Surma S, Banach M. Fibrinogen and Atherosclerotic Cardiovascular Diseases-Review of the Literature and Clinical Studies. Int J Mol Sci. 2021;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 69] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 84. | Klinkhardt U, Kuczka K, Harder S. Effects of the NHE-1 inhibitor cariporide alone or together with the P2Y12 antagonist AR-C 69331 MX on CD62p expression and formation of platelet-leukocyte aggregates. Thromb Res. 2003;111:251-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 85. | Campello E, Bulato C, Spiezia L, Boscolo A, Poletto F, Cola M, Gavasso S, Simion C, Radu CM, Cattelan A, Tiberio I, Vettor R, Navalesi P, Simioni P. Thrombin generation in patients with COVID-19 with and without thromboprophylaxis. Clin Chem Lab Med. 2021;59:1323-1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 86. | Huang CL, Cogan MG, Cragoe EJ Jr, Ives HE. Thrombin activation of the Na+/H+ exchanger in vascular smooth muscle cells. Evidence for a kinase C-independent pathway which is Ca2+-dependent and pertussis toxin-sensitive. J Biol Chem. 1987;262:14134-14140. [PubMed] |
| 87. | Tift MS, Alves de Souza RW, Weber J, Heinrich EC, Villafuerte FC, Malhotra A, Otterbein LE, Simonson TS. Adaptive Potential of the Heme Oxygenase/Carbon Monoxide Pathway During Hypoxia. Front Physiol. 2020;11:886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 88. | Wagener FA, Volk HD, Willis D, Abraham NG, Soares MP, Adema GJ, Figdor CG. Different faces of the heme-heme oxygenase system in inflammation. Pharmacol Rev. 2003;55:551-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 430] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 89. | Di Noia MA, Van Driesche S, Palmieri F, Yang LM, Quan S, Goodman AI, Abraham NG. Heme oxygenase-1 enhances renal mitochondrial transport carriers and cytochrome C oxidase activity in experimental diabetes. J Biol Chem. 2006;281:15687-15693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 94] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 90. | Salık F, Uzundere O, Bıçak M, Akelma H, Akgündüz M, Korhan Z, Kandemir D, Kaçar CK. Liver function as a predictor of mortality in COVID-19: A retrospective study. Ann Hepatol. 2021;26:100553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 91. | Zhu X, Wang J, Du J, Chen S, Li J, Shen B. Changes in Serum Liver Function for Patients with COVID-19: A 1-Year Follow-Up Study. Infect Drug Resist. 2022;15:1857-1870. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 92. | Tabibian JH, Masyuk AI, Masyuk TV, O'Hara SP, LaRusso NF. Physiology of cholangiocytes. Compr Physiol. 2013;3:541-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 168] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 93. | Guzior DV, Quinn RA. Review: microbial transformations of human bile acids. Microbiome. 2021;9:140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 413] [Article Influence: 103.3] [Reference Citation Analysis (0)] |
| 94. | Amin MR, Malakooti J, Sandoval R, Dudeja PK, Ramaswamy K. IFN-gamma and TNF-alpha regulate human NHE3 gene expression by modulating the Sp family transcription factors in human intestinal epithelial cell line C2BBe1. Am J Physiol Cell Physiol. 2006;291:C887-C896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 95. | Nwia SM, Li XC, Leite APO, Hassan R, Zhuo JL. The Na+/H+ Exchanger 3 in the Intestines and the Proximal Tubule of the Kidney: Localization, Physiological Function, and Key Roles in Angiotensin II-Induced Hypertension. Front Physiol. 2022;13:861659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 96. | Gurney MA, Laubitz D, Ghishan FK, Kiela PR. Pathophysiology of Intestinal Na+/H+ exchange. Cell Mol Gastroenterol Hepatol. 2017;3:27-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |