Published online Nov 25, 2022. doi: 10.5501/wjv.v11.i6.435
Peer-review started: August 9, 2022
First decision: August 29, 2022
Revised: September 21, 2022
Accepted: October 19, 2022
Article in press: October 19, 2022
Published online: November 25, 2022
Processing time: 105 Days and 16.6 Hours
The 2019 coronavirus disease (COVID-19), resulting from the severe acute respiratory syndrome 2 virus, has transformed our globe and provided a new perspective on respiratory tract infections. However, COVID-19 would not be recognized as a condition restricted to only pneumonia. This narrative review was conducted by searching manuscripts in several databases, including PubMed/ MEDLINE, Web of Science, and Reference Citation Analysis, from December 2019 to July 2022. Many studies have revealed a broad spectrum of potential systemic symptoms, including biliary complications. Although biliary injury has been observed in a very low proportion of COVID-19 patients, it is associated with increased mortalities and long-term morbidities. We identify a cholangiopathy condition in individuals during infection and after recovering from severe COVID-19, defined by a significant increase in serum alkaline phosphatase and signs of bile duct injury. Understanding the pathogeneses behind this condition would help us develop new techniques to prevent these complications. This review thoroughly discusses and summarizes the current information regarding COVID-19-associated cholangiopathy. In addition, the possible explanations for COVID-19-associated cholangiopathy are presented. Since the exact pathogenesis may not be concluded, this review could provide relevant information to encourage additional investigations shortly.
Core Tip: The 2019 coronavirus disease (COVID-19) is not only regarded as a respiratory tract disease but also demonstrates a wide range of systemic consequences, including the biliary tract. A significant increase in serum alkaline phosphatase and signs of biliary injury on imaging and/or pathology are the hallmarks of COVID-19-associated cholangiopathy. Direct viral invasion, ischemic injury related to microvascular coagulopathy, drug-induced cholestatic liver injury, alteration of gut microbiota, and cytokine release syndrome are proposed as potential explanations for cholangiopathy associated with severe COVID-19 infection.
- Citation: Wongtanasarasin W. Cholestatic liver injury: A rare but fatal complication during and after COVID-19 infection. World J Virol 2022; 11(6): 435-442
- URL: https://www.wjgnet.com/2220-3249/full/v11/i6/435.htm
- DOI: https://dx.doi.org/10.5501/wjv.v11.i6.435
Since December 2019, the recent Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), later confirmed as the source of the 2019 coronavirus disease (COVID-19), has turned into a global threat to public health[1]. With a rapidly increasing number of overall cases, the World Health Organization announced the disease pandemic in March 2020. Currently, COVID-19 has caused about 577 million cases and over 6 million deaths worldwide[2].
SARS-CoV-2 is greatly transmitted by droplet transmission[3], with respiratory symptoms (i.e., sore throat, cough, dyspnea) being the most prevalent manifestation as a result of host seeding via angiotensin-converting enzyme 2 (ACE-2) receptors present primarily in type II alveolar cells of the lungs[4,5]. Although respiratory and non-specific symptoms such as fever, myalgia, and fatigue represented the most common presentations in patients with COVID-19 infection, gastrointestinal and hepatic symptoms have also been observed[6,7]. Infected individuals reported nausea, vomiting, and abdominal discomfort[6,8,9]. Current evidence has proposed pneumonia as a severe COVID-19 feature[10]. However, complications are notably distinguishable, and the virus has impacted different organ systems[11]. At initial presentation and in hospitalized patients, the incidence of abnormal serum liver function tests (LFTs) varies from 22% to 67%, with levels of elevation ranging from minor to severe[12-14]. Autopsy findings from the case series also demonstrated mild microvesicular steatosis and lobular with portal inflammation[15].
To date, the findings have concentrated on evidence of hepatocellular injury, serum aspartate aminotransferase (AST), and alanine aminotransferase (ALT) elevations[16-18]. Several studies also observed that abnormal LFTs during hospitalization had been linked with disease severity[19-22]. One article that included over 2000 patients in the United States investigated ALT increases and their associations with disease severity, also emphasizing the rarity of cholestasis[20]. Despite a myriad of research on the severe COVID-19 progression, we noticed a small number of reported reports on the consequences. Previously, Faruqui et al[11] described a condition characterized by increases in LFTs, particularly markedly elevated serum alkaline phosphatase (ALP), and radiographic findings indicating biliary tract inflammation, primarily bile duct stricture, similar to those seen in critically ill patients with secondary sclerosing cholangitis (SSC)[23]. Still, this condition named COVID-19-associated cholangiopathy is not antecedently reviewed and discussed. This review comprehensively summarizes up-to-date reports from studies highlighting this condition and its perspective. Moreover, possible explanations for COVID-19-associated cholangiopathy are provided and discussed. We anticipate that this review could underline the importance of this condition since it appears to have significantly negative effects on patients’ recovery and may potentially result in long-term morbidities.
This narrative review was performed considering articles published from December 2019 to July 2022. The manuscripts were searched electronically using several standard databases, including PubMed/MEDLINE, Web of Science, and Reference Citation Analysis. Various search terms and Medical Subject Headings (MeSH) were used to identify potential articles: “COVID-19”, “cholestasis”, “alkaline phosphatase”, and “obstructive jaundice” (Supplementary Table 1). This mini-review may only serve as a hypothesis-generation of all relevant articles existing in the literature. The extensive details of this condition may have been reviewed elsewhere. The included articles were only those that were published in English.
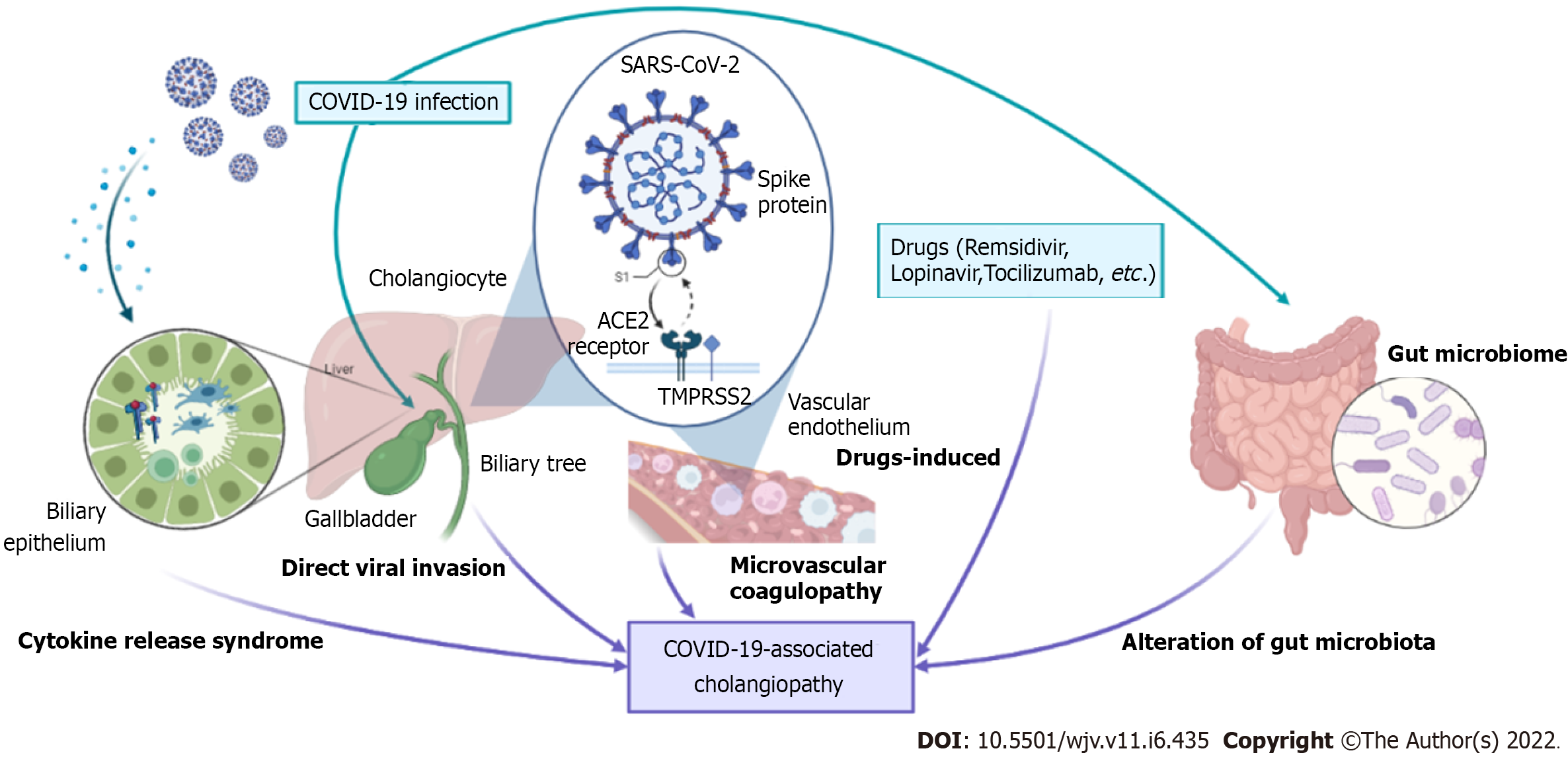
COVID-19-associated cholangiopathy (COVID-C) or COVID-19 cholestasis has been proposed to describe a condition that occurs in individuals during and after severe COVID-19 infection[11]. It is characterized by elevated liver enzymes, especially substantial increases in serum ALP, and imaging-based biliary tract inflammation[11]. This condition appears to have significant negative effects on patient recovery. After other indications of COVID-19 have recovered, it may cause delayed morbidity[17], the necessity for a liver transplant, and death[11].
COVID-19 is frequently linked with aberrant LFTs, despite the absence of disease-specific lesions on radiographic imaging or biopsy. Liver damage has been discovered to be a common feature of the highly deadly coronavirus-associated illness in humans[24]. Previous studies, mainly from China, have identified abnormal LFTs in infected individuals from the early stages of the recent SARS-CoV-2 pandemic[25-27]. Several systematic reviews with meta-analysis found that any abnormal LFTs were reported in 25%-47%[12-14,26]. Most abnormalities were elevated serum AST and ALT, representing hepatocellular injury[12,13]. Recent literature showed that acute hepatocellular injury during COVID-19 positively correlates with more severe COVID-19 disease[20]. Furthermore, SARS-CoV-2 can enter the liver via the ACE-2 receptor proteins found on the bile duct epithelium, which theoretically results in “direct viral cholangiocyte injury”. Supporting this concept, the findings from meta-analyses reported serum ALP elevations occurring in up to 4.0%-13.7% of patients[12-14]. In addition, recent studies identified serum ALP elevation as an independent predictor for unfavorable outcomes, including intensive care unit (ICU) admission and hospital mortality[13,28]. Furthermore, Da et al[17] documented that COVID-19 patients with increased ALP levels (> 3 times of normal upper limit) were correlated with a higher likelihood of prolonged mechanical ventilation and death. In the same way, a study conducted in Iraq reported that most SARS-CoV-2 patients had abnormal liver enzyme activities, which might be associated with viral replication in the liver[16].
Similarly, serum gamma-glutamyl transferase (GGT) activity represents a sign of hepatobiliary damage, particularly cholestasis and biliary impact[29]. Previous meta-analyses revealed that COVID-19 patients had higher GGT levels than those without, ranging from 15.0-22.5%[12,13]. Although the ACE-2 receptor is primarily expressed in the biliary tree, the evidence found that both abnormal serum ALP and GGT levels were lower than abnormal serum AST and ALT levels. We hypothesize that some abnormal hepatocellular enzymes may result from baseline chronic liver diseases. Furthermore, individuals with COVID-19 and concurrent advanced-stage liver disease may be more susceptible to severe liver damage than those without.
Faruqui et al[11] reported that only 0.6% of patients with severe COVID-19 infection developed aberrant radiographic findings consistent with cholestatic liver damage. All had severe pneumonia with sepsis and required mechanical ventilation during admission. Extracorporeal membrane oxygenation was used on three of them. All patients underwent magnetic resonance cholangiopancreatography, which indicated aberrant findings such as beaded intrahepatic channels, peribiliary diffusion high signal, bile duct wall thickening and hyperenhancement, and common bile duct dilatation[11]. These cholangiopathies described in that study are comparable to SSC observed in patients following prolonged ICU stays[23]. This disease has been encountered in critically ill patients with infection, polytrauma, burns, or after major surgery[30,31]. SSC also has been described in a case report or small case series[11,30,31]. It has been defined as a cholangiopathy with radiographic characteristics similar to those observed in primary SSC and comparable to ischemic cholangiopathy reported following liver transplantation[31]. Endoscopic retrograde cholangiopancreatography or liver histology was used to diagnose several individuals who had SSC following a severe illness. Gelbmann et al[30] recorded endoscopic observations of biliary casts with the reduced biliary flow and eventual cholangitis, as well as verified cholangitis and hemorrhagic exudates in bile ducts from liver biopsy. All 26 patients in that research had respiratory failure and required mechanical ventilation[30]. The relationship between severe SSC patients and COVID-19 cholangiopathy highlights a potential connection between hypoxic liver damage or ischemic liver failure and cholestatic liver injury[11]. The portal vein and the hepatic arteries supply the liver parenchyma or hepatocytes. On the other hand, the intrahepatic biliary tree is nourished only by hepatic artery branches via the peribiliary vascular plexus. Given its dependence on only arterial supply, the biliary epithelium appears more sensitive to ischemia than hepatocytes, which get dual supply[32,33]. This is illustrated by instances of hepatic artery thrombosis, which occurs in 9% of adult liver transplant patients following arterial blood supply termination, commonly leading in biliary ischemia lesions such as necrosis with biliary leakage and ischemic strictures[34].
Direct viral cholangiocyte injury is a hypothetically pathogenic mechanism of the virus leading to cholestatic liver injury since SARS-CoV-2 may enter the liver via the ACE-2 receptor protein found on the bile duct epithelium[35]. In liver tissues taken from 4 deceased donors of liver transplants, it is demonstrated that specific ACE-2 activity was expressed in 60% of cholangiocytes, compared with 3% of hepatocytes, suggesting that the virus might directly bind to specific ACE-2 receptors on cholangiocytes[36]. They discovered that ACE-2 expression in cholangiocytes is equivalent to ACE-2 expression in type II lung alveolar cells[36]. Also, subsequent reports have found that biliary epithelial cells exhibit a high level of ACE-2[35,37]. An in vitro investigation of human liver cells revealed that cholangiocytes might be more vulnerable to being infected with SARS-CoV-2 than other viruses[35]. Previous literature illustrated that viral particles in cholangiocytes had been found in ultrastructural and histological studies, highlighting the possibility that cholestatic damage may be caused by SARS-CoV-2 direct infection of biliary epithelial cells[11,38]. Furthermore, transmembrane protease serine 2 (TMPRSS2), the key host protease that allows several coronaviruses to enter the cells, including SARS-CoV-2, has been found to be associated with viral invasion mechanism since its activity was expressed in cholangiocytes[39]. Its actions lead to cell apoptosis, impaired transportation of bile acids, and epithelial barrier dysfunction[35]. On the other hand, another report documented that the proportion of cells expressing ACE-2 and TMPRSS2 was only 2.50% for cholangiocytes and 0.04% for hepatocytes, questioning the uncertain hypothesis of a direct viral effect on liver and bile duct cells[40].
The previously discussed cholestatic injury might result from ischemic damage caused by microvascular coagulopathy and/or hypotension during critical illness or sepsis[11,19,21]. Researchers have found that SARS-CoV-2 enters the host via the respiratory epithelial ACE-2 receptor[41]. ACE-2 is, nevertheless, widely expressed in endothelial cells of minor and major vessels across the body[37]. The expression of ACE-2 in vascular endothelium has been proposed as a key pathogenetic factor in the widespread coagulation that contributes considerably to COVID-19 morbidity and mortality[19,21]. A recent case series discovered many platelet-fibrin microthrombi in postmortem liver cells[36]. However, another case series of 40 COVID-19 cases found sinusoidal microthrombi in only 15%, whereas most reported macrovascular steatosis (75%) and mild lobular necroinflammation and portal inflammation (50%)[42]. These controversial issues, nonetheless, did not exclude the possibility of intravascular microthrombi and thrombosis theory. More research on this topic may be warranted.
Another possible explanation for COVID-C is drug-induced cholestatic liver injury. A wide range of medications has been investigated throughout this pandemic. Among these, remsidivir[43,44], lopinavir[45], ritonavir[45], and interleukin-6 antagonists (tocilizumab)[46] have been reported as a cause of increased ALT levels. However, the pattern of biliary injury from pathological examination strongly supports this hypothesis was insufficient[43,44,46]. Besides, no single medication was constantly delivered to all patients with COVID-19 infection, resulting in inconclusive confirmation of this issue.
Interestingly, changes in the gut microbiota may also lead to cholestatic damage[47]. When SARS-CoV-2 infected the enterocytes, it inhibited the absorption of intestinal tryptophan; therefore, resulting in the generation of antimicrobial peptides, mostly through the downregulation of ACE2 following viral entrance[48,49]. It has been proposed that disruption of the gut-liver axis may increase the likelihood of developing severe COVID-19 in patients with non-alcoholic fatty liver disease[50]. In addition, the gut microbiota has been used as a prospective target for adjuvant therapy during SARS-CoV-2 infection[51,52].
Moreover, cytokine release syndrome (CRS), which occurs in both SSC and COVID-19, is another sign that the pathophysiology of SSC-associated severe illnesses and COVID-C may be pathogenetically similar[32]. Documents indicating that CRS can produce severe cholestatic liver damage suggest that the biliary epithelium is partially sensitive to CRS-immune mediated damage[53]. Overall, we may assume that the inducers, such as SARS-CoV-2 epithelial infection, microthrombosis, or the magnitude of the COVID-19 CRS, aggravate the severity and frequency of COVID-19 infection[11].
This review provided some important and interesting points. Recently, many researchers raised the question of when the COVID-19 pandemic will end. One statistical report showed that the COVID-19 pandemic could terminate in 2022, but COVID-19 could be one or two times more fatal than seasonal influenza by 2023[54]. Understanding the complications and consequences after COVID-19 infection would help clinicians prevent such conditions and improve the quality of care during the post-infection period. Knowledge and evidence regarding COVID-19-associated cholangiopathy are comparably low despite the growing literature on COVID-19 and other complications. This review could pave the way for a better comprehension of this condition. Future research to completely explain the behind mechanism would advance the treatment and management paradigm. Furthermore, this mini-review will emphasize that all healthcare professionals recognize this disease and its circumstances better.
SARS-CoV-2 infection has taken our world into a disastrous situation. Severe COVID-19 patients may encounter COVID-19-associated cholangiopathy, similar to those with SSC after critical illness. COVID-19 infection initially signifies the virus's contact with ACE-2 receptors (expressed in cholangiocytes and vascular endothelium). Based on current evidence, several theories were described in this review, including direct viral invasion, microvascular coagulopathy, alteration of gut microbiota, drug-induced liver injury, and cytokine release syndrome (Figure 1). The exact underlining pathogenesis might not be concluded at this moment, raising the importance of further investigations into this issue. COVID-C may be rarely found in patients with severe COVID-19 infection but is associated with increased mortality and impaired quality of life. We anticipate that the findings described in this review will advance more translational research, resulting in a better understanding and improved treatment of COVID-C in the near future.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Thailand
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Laranjeira C, Portugal; Yu MC, Taiwan S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470-473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4848] [Cited by in RCA: 4388] [Article Influence: 877.6] [Reference Citation Analysis (1)] |
| 2. | World Health Organization. WHO Coronavirus (COVID-19) Dashboard With Vaccination Data. [cited September 30 2022]. Available from: https://covid19.who.int/. [RCA] [DOI] [Full Text] [Cited by in Crossref: 439] [Cited by in RCA: 294] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 3. | Ong SWX, Coleman KK, Chia PY, Thoon KC, Pada S, Venkatachalam I, Fisher D, Tan YK, Tan BH, Ng OT, Ang BSP, Leo YS, Wong MSY, Marimuthu K. Transmission modes of severe acute respiratory syndrome coronavirus 2 and implications for infection control: a review. Singapore Med J. 2022;63:61-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (1)] |
| 4. | Kuhn JH, Li W, Choe H, Farzan M. Angiotensin-converting enzyme 2: a functional receptor for SARS coronavirus. Cell Mol Life Sci. 2004;61:2738-2743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 184] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 5. | Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271-280.e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11946] [Cited by in RCA: 14273] [Article Influence: 2854.6] [Reference Citation Analysis (0)] |
| 6. | Patel AP, Sanders TK, Prakash P, Law J, Alvencar S, Choi A, Shah J, Patel K, Srivoleti P, Chauhan K, Weissman S, Holzwanger E, Dhingra R, Nguyen M, Kim D, Sidhu T, Stallwood C, Dickstein A, Parekh N, Altayar O, Ciorba MA, Yu J, Chen LA, Tabibian JH, Limketkai BN. Gastrointestinal Manifestations of Coronavirus Disease 2019 Across the United States: A Multicenter Cohort Study. Gastro Hep Adv. 2022;1:909-915. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Cheung KS, Hung IFN, Chan PPY, Lung KC, Tso E, Liu R, Ng YY, Chu MY, Chung TWH, Tam AR, Yip CCY, Leung KH, Fung AY, Zhang RR, Lin Y, Cheng HM, Zhang AJX, To KKW, Chan KH, Yuen KY, Leung WK. Gastrointestinal Manifestations of SARS-CoV-2 Infection and Virus Load in Fecal Samples From a Hong Kong Cohort: Systematic Review and Meta-analysis. Gastroenterology. 2020;159:81-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1113] [Cited by in RCA: 1133] [Article Influence: 226.6] [Reference Citation Analysis (1)] |
| 8. | Bangash MN, Patel J, Parekh D. COVID-19 and the liver: little cause for concern. Lancet Gastroenterol Hepatol. 2020;5:529-530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 324] [Cited by in RCA: 357] [Article Influence: 71.4] [Reference Citation Analysis (0)] |
| 9. | Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14113] [Cited by in RCA: 14767] [Article Influence: 2953.4] [Reference Citation Analysis (0)] |
| 10. | Feng Z, Yu Q, Yao S, Luo L, Zhou W, Mao X, Li J, Duan J, Yan Z, Yang M, Tan H, Ma M, Li T, Yi D, Mi Z, Zhao H, Jiang Y, He Z, Li H, Nie W, Liu Y, Zhao J, Luo M, Liu X, Rong P, Wang W. Early prediction of disease progression in COVID-19 pneumonia patients with chest CT and clinical characteristics. Nat Commun. 2020;11:4968. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 156] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 11. | Faruqui S, Okoli FC, Olsen SK, Feldman DM, Kalia HS, Park JS, Stanca CM, Figueroa Diaz V, Yuan S, Dagher NN, Sarkar SA, Theise ND, Kim S, Shanbhogue K, Jacobson IM. Cholangiopathy After Severe COVID-19: Clinical Features and Prognostic Implications. Am J Gastroenterol. 2021;116:1414-1425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 89] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 12. | Wijarnpreecha K, Ungprasert P, Panjawatanan P, Harnois DM, Zaver HB, Ahmed A, Kim D. COVID-19 and liver injury: a meta-analysis. Eur J Gastroenterol Hepatol. 2021;33:990-995. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 13. | Del Zompo F, De Siena M, Ianiro G, Gasbarrini A, Pompili M, Ponziani FR. Prevalence of liver injury and correlation with clinical outcomes in patients with COVID-19: systematic review with meta-analysis. Eur Rev Med Pharmacol Sci. 2020;24:13072-13088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 14. | Wu Y, Li H, Guo X, Yoshida EM, Mendez-Sanchez N, Levi Sandri GB, Teschke R, Romeiro FG, Shukla A, Qi X. Incidence, risk factors, and prognosis of abnormal liver biochemical tests in COVID-19 patients: a systematic review and meta-analysis. Hepatol Int. 2020;14:621-637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 92] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 15. | Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5228] [Cited by in RCA: 5785] [Article Influence: 1157.0] [Reference Citation Analysis (2)] |
| 16. | Hwaiz R, Merza M, Hamad B, HamaSalih S, Mohammed M, Hama H. Evaluation of hepatic enzymes activities in COVID-19 patients. Int Immunopharmacol. 2021;97:107701. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Da BL, Suchman K, Roth N, Rizvi A, Vincent M, Trindade AJ, Bernstein D, Satapathy SK; Northwell COVID-19 Research Consortium. Cholestatic liver injury in COVID-19 is a rare and distinct entity and is associated with increased mortality. J Intern Med. 2021;290:470-472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Goel H, Harmouch F, Garg K, Saraiya P, Daly T, Kumar A, Hippen JT. The liver in COVID-19: prevalence, patterns, predictors, and impact on outcomes of liver test abnormalities. Eur J Gastroenterol Hepatol. 2021;33:e274-e281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Ferm S, Fisher C, Pakala T, Tong M, Shah D, Schwarzbaum D, Cooley V, Hussain S, Kim SH. Analysis of Gastrointestinal and Hepatic Manifestations of SARS-CoV-2 Infection in 892 Patients in Queens, NY. Clin Gastroenterol Hepatol. 2020;18:2378-2379.e1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 20. | Phipps MM, Barraza LH, LaSota ED, Sobieszczyk ME, Pereira MR, Zheng EX, Fox AN, Zucker J, Verna EC. Acute Liver Injury in COVID-19: Prevalence and Association with Clinical Outcomes in a Large U.S. Cohort. Hepatology. 2020;72:807-817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 272] [Article Influence: 54.4] [Reference Citation Analysis (2)] |
| 21. | Fan Z, Chen L, Li J, Cheng X, Yang J, Tian C, Zhang Y, Huang S, Liu Z, Cheng J. Clinical Features of COVID-19-Related Liver Functional Abnormality. Clin Gastroenterol Hepatol. 2020;18:1561-1566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 559] [Cited by in RCA: 556] [Article Influence: 111.2] [Reference Citation Analysis (0)] |
| 22. | Hundt MA, Deng Y, Ciarleglio MM, Nathanson MH, Lim JK. Abnormal Liver Tests in COVID-19: A Retrospective Observational Cohort Study of 1,827 Patients in a Major U.S. Hospital Network. Hepatology. 2020;72:1169-1176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 197] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 23. | Laurent L, Lemaitre C, Minello A, Plessier A, Lamblin G, Poujol-Robert A, Gervais-Hasenknopf A, Pariente EA, Belenotti P, Mostefa-Kara N, Sogni P, Legrand M, Cournac JM, Tamion F, Savoye G, Bedossa P, Valla DC, Vilgrain V, Goria O. Cholangiopathy in critically ill patients surviving beyond the intensive care period: a multicentre survey in liver units. Aliment Pharmacol Ther. 2017;46:1070-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40:998-1004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 622] [Cited by in RCA: 575] [Article Influence: 115.0] [Reference Citation Analysis (0)] |
| 25. | Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708-1720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19202] [Cited by in RCA: 18877] [Article Influence: 3775.4] [Reference Citation Analysis (7)] |
| 26. | Mao R, Qiu Y, He JS, Tan JY, Li XH, Liang J, Shen J, Zhu LR, Chen Y, Iacucci M, Ng SC, Ghosh S, Chen MH. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:667-678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 741] [Cited by in RCA: 755] [Article Influence: 151.0] [Reference Citation Analysis (0)] |
| 27. | Wu J, Liu J, Zhao X, Liu C, Wang W, Wang D, Xu W, Zhang C, Yu J, Jiang B, Cao H, Li L. Clinical Characteristics of Imported Cases of Coronavirus Disease 2019 (COVID-19) in Jiangsu Province: A Multicenter Descriptive Study. Clin Infect Dis. 2020;71:706-712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 393] [Cited by in RCA: 413] [Article Influence: 82.6] [Reference Citation Analysis (1)] |
| 28. | Aghemo A, Piovani D, Parigi TL, Brunetta E, Pugliese N, Vespa E, Omodei PD, Preatoni P, Lleo A, Repici A, Voza A, Cecconi M, Malesci A, Bonovas S, Danese S; Humanitas COVID-19 Task Force. COVID-19 Digestive System Involvement and Clinical Outcomes in a Large Academic Hospital in Milan, Italy. Clin Gastroenterol Hepatol. 2020;18:2366-2368.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 29. | Chand K, Thakur S. "Significance of serum gamma glutamyl transpeptidase in cholestatic jaundice". Indian J Med Sci. 1997;51:270-274. [PubMed] |
| 30. | Gelbmann CM, Rümmele P, Wimmer M, Hofstädter F, Göhlmann B, Endlicher E, Kullmann F, Langgartner J, Schölmerich J. Ischemic-like cholangiopathy with secondary sclerosing cholangitis in critically ill patients. Am J Gastroenterol. 2007;102:1221-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 116] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 31. | Lin T, Qu K, Xu X, Tian M, Gao J, Zhang C, Di Y, Zhang Y, Liu C. Sclerosing cholangitis in critically ill patients: an important and easily ignored problem based on a German experience. Front Med. 2014;8:118-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 32. | Leonhardt S, Veltzke-Schlieker W, Adler A, Schott E, Hetzer R, Schaffartzik W, Tryba M, Neuhaus P, Seehofer D. Trigger mechanisms of secondary sclerosing cholangitis in critically ill patients. Crit Care. 2015;19:131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 33. | Gudnason HO, Björnsson ES. Secondary sclerosing cholangitis in critically ill patients: current perspectives. Clin Exp Gastroenterol. 2017;10:105-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 34. | Mourad MM, Liossis C, Gunson BK, Mergental H, Isaac J, Muiesan P, Mirza DF, Perera MT, Bramhall SR. Etiology and management of hepatic artery thrombosis after adult liver transplantation. Liver Transpl. 2014;20:713-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 157] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 35. | Zhao B, Ni C, Gao R, Wang Y, Yang L, Wei J, Lv T, Liang J, Zhang Q, Xu W, Xie Y, Wang X, Yuan Z, Zhang R, Lin X. Recapitulation of SARS-CoV-2 infection and cholangiocyte damage with human liver ductal organoids. Protein Cell. 2020;11:771-775. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 268] [Cited by in RCA: 312] [Article Influence: 62.4] [Reference Citation Analysis (0)] |
| 36. | Chai X, Hu L, Zhang Y, Han W, Lu Z, Ke A, Zhou J, Shi G, Fang N, Fan J, Cai J, Lan F. Specific ACE2 Expression in Cholangiocytes May Cause Liver Damage After 2019-nCoV Infection. bioRxiv (e-pub ahead of print 1 January 2020; DOI:10.1101/2020.02.03.931766).. [DOI] [Full Text] |
| 37. | Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631-637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3643] [Cited by in RCA: 4149] [Article Influence: 197.6] [Reference Citation Analysis (0)] |
| 38. | Wang Y, Liu S, Liu H, Li W, Lin F, Jiang L, Li X, Xu P, Zhang L, Zhao L, Cao Y, Kang J, Yang J, Li L, Liu X, Li Y, Nie R, Mu J, Lu F, Zhao S, Lu J, Zhao J. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J Hepatol. 2020;73:807-816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 353] [Cited by in RCA: 457] [Article Influence: 91.4] [Reference Citation Analysis (0)] |
| 39. | Schönfelder K, Breuckmann K, Elsner C, Dittmer U, Fistera D, Herbstreit F, Risse J, Schmidt K, Sutharsan S, Taube C, Jöckel KH, Siffert W, Kribben A, Möhlendick B. Transmembrane serine protease 2 Polymorphisms and Susceptibility to Severe Acute Respiratory Syndrome Coronavirus Type 2 Infection: A German Case-Control Study. Front Genet. 2021;12:667231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 40. | De Smet V, Verhulst S, van Grunsven LA. Single cell RNA sequencing analysis did not predict hepatocyte infection by SARS-CoV-2. J Hepatol. 2020;73:993-995. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 41. | Zamorano Cuervo N, Grandvaux N. ACE2: Evidence of role as entry receptor for SARS-CoV-2 and implications in comorbidities. Elife. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 225] [Cited by in RCA: 226] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 42. | Lagana SM, Kudose S, Iuga AC, Lee MJ, Fazlollahi L, Remotti HE, Del Portillo A, De Michele S, de Gonzalez AK, Saqi A, Khairallah P, Chong AM, Park H, Uhlemann AC, Lefkowitch JH, Verna EC. Hepatic pathology in patients dying of COVID-19: a series of 40 cases including clinical, histologic, and virologic data. Mod Pathol. 2020;33:2147-2155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 181] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 43. | Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, Hohmann E, Chu HY, Luetkemeyer A, Kline S, Lopez de Castilla D, Finberg RW, Dierberg K, Tapson V, Hsieh L, Patterson TF, Paredes R, Sweeney DA, Short WR, Touloumi G, Lye DC, Ohmagari N, Oh MD, Ruiz-Palacios GM, Benfield T, Fätkenheuer G, Kortepeter MG, Atmar RL, Creech CB, Lundgren J, Babiker AG, Pett S, Neaton JD, Burgess TH, Bonnett T, Green M, Makowski M, Osinusi A, Nayak S, Lane HC; ACTT-1 Study Group Members. Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med. 2020;383:1813-1826. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5711] [Cited by in RCA: 5119] [Article Influence: 1023.8] [Reference Citation Analysis (0)] |
| 44. | Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, Fu S, Gao L, Cheng Z, Lu Q, Hu Y, Luo G, Wang K, Lu Y, Li H, Wang S, Ruan S, Yang C, Mei C, Wang Y, Ding D, Wu F, Tang X, Ye X, Ye Y, Liu B, Yang J, Yin W, Wang A, Fan G, Zhou F, Liu Z, Gu X, Xu J, Shang L, Zhang Y, Cao L, Guo T, Wan Y, Qin H, Jiang Y, Jaki T, Hayden FG, Horby PW, Cao B, Wang C. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569-1578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2337] [Cited by in RCA: 2488] [Article Influence: 497.6] [Reference Citation Analysis (0)] |
| 45. | European Association for the Study of the Liver; Clinical Practice Guideline Panel: Chair:; Panel members; EASL Governing Board representative:. EASL Clinical Practice Guidelines: Drug-induced liver injury. J Hepatol. 2019;70:1222-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 715] [Cited by in RCA: 669] [Article Influence: 111.5] [Reference Citation Analysis (0)] |
| 46. | Guaraldi G, Meschiari M, Cozzi-Lepri A, Milic J, Tonelli R, Menozzi M, Franceschini E, Cuomo G, Orlando G, Borghi V, Santoro A, Di Gaetano M, Puzzolante C, Carli F, Bedini A, Corradi L, Fantini R, Castaniere I, Tabbì L, Girardis M, Tedeschi S, Giannella M, Bartoletti M, Pascale R, Dolci G, Brugioni L, Pietrangelo A, Cossarizza A, Pea F, Clini E, Salvarani C, Massari M, Viale PL, Mussini C. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol. 2020;2:e474-e484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 604] [Cited by in RCA: 688] [Article Influence: 137.6] [Reference Citation Analysis (0)] |
| 47. | Zuo T, Zhang F, Lui GCY, Yeoh YK, Li AYL, Zhan H, Wan Y, Chung ACK, Cheung CP, Chen N, Lai CKC, Chen Z, Tso EYK, Fung KSC, Chan V, Ling L, Joynt G, Hui DSC, Chan FKL, Chan PKS, Ng SC. Alterations in Gut Microbiota of Patients With COVID-19 During Time of Hospitalization. Gastroenterology. 2020;159:944-955.e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 739] [Cited by in RCA: 1067] [Article Influence: 213.4] [Reference Citation Analysis (0)] |
| 48. | Hashimoto T, Perlot T, Rehman A, Trichereau J, Ishiguro H, Paolino M, Sigl V, Hanada T, Hanada R, Lipinski S, Wild B, Camargo SM, Singer D, Richter A, Kuba K, Fukamizu A, Schreiber S, Clevers H, Verrey F, Rosenstiel P, Penninger JM. ACE2 Links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature. 2012;487:477-481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 787] [Cited by in RCA: 991] [Article Influence: 76.2] [Reference Citation Analysis (0)] |
| 49. | Liévin-Le Moal V, Servin AL. The front line of enteric host defense against unwelcome intrusion of harmful microorganisms: mucins, antimicrobial peptides, and microbiota. Clin Microbiol Rev. 2006;19:315-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 370] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 50. | Assante G, Williams R, Youngson NA. Is the increased risk for MAFLD patients to develop severe COVID-19 Linked to perturbation of the gut-liver axis? J Hepatol. 2021;74:487-488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 51. | Gasbarrini G, Dionisi T, Franceschi F, Gasbarrini A. Editorial - COVID-19 and the microbiota: new kids on the block. Eur Rev Med Pharmacol Sci. 2020;24:5189-5191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 52. | Di Renzo L, Merra G, Esposito E, De Lorenzo A. Are probiotics effective adjuvant therapeutic choice in patients with COVID-19? Eur Rev Med Pharmacol Sci. 2020;24:4062-4063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 53. | Abdalian R, Heathcote EJ. Sclerosing cholangitis: a focus on secondary causes. Hepatology. 2006;44:1063-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 134] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 54. | Chen JM. Novel statistics predict the COVID-19 pandemic could terminate in 2022. J Med Virol. 2022;94:2845-2848. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |