Copyright
©The Author(s) 2023.
World J Virol. Mar 25, 2023; 12(2): 100-108
Published online Mar 25, 2023. doi: 10.5501/wjv.v12.i2.100
Published online Mar 25, 2023. doi: 10.5501/wjv.v12.i2.100
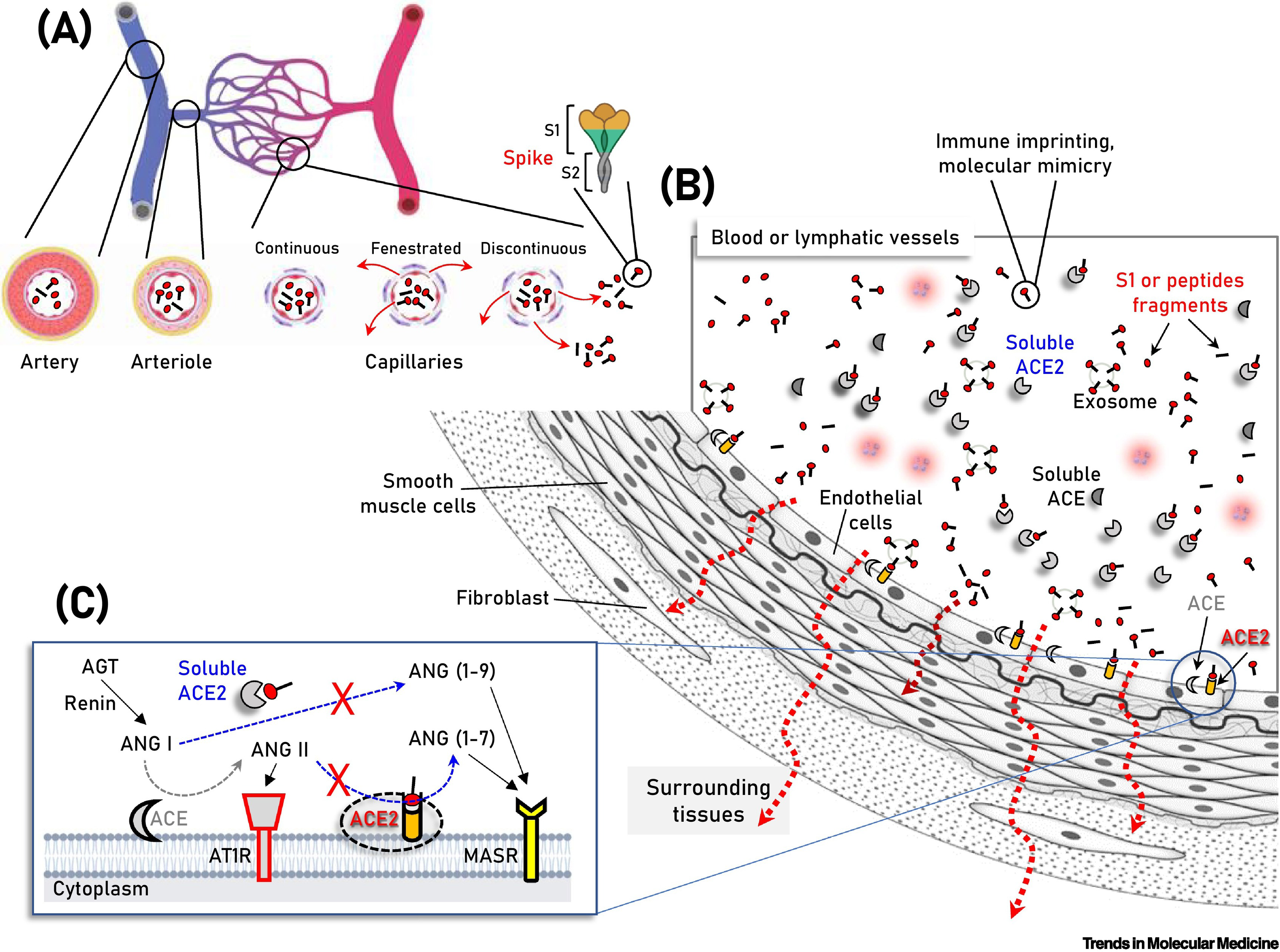
Figure 1 Schematic of the vasculature components showing vaccination-produced S protein/subunits/peptide fragments in the circulation, as well as soluble or endothelial cell membrane-attached angiotensin-converting enzyme 2.
A: Parallel to immune system activation, circulating S protein/subunits/peptide fragments; B: Binding to angiotensin-converting enzyme 2 (ACE2) may occur not only to ACE2-expressing endothelial cells, but also in multiple cell types of the vasculature and surrounding tissues due to antigen diffusion (e.g., in fenestrated or discontinuous capillary beds) (A, red arrows). These series of molecular events are unlikely for any severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-related antigen in the absence of severe coronavirus disease 2019, where SARS-CoV-2 is contained in the respiratory system; C: In the two counteracting pathways of the renin–angiotensin system (RAS), namely the ‘conventional’ arm, that involves ACE which generates angiotensin II (ANG II) from angiotensin I (ANG I), and the ACE2 arm which hydrolyzes ANG II to generate angiotensin (1–7) [ANG (1–7)] or ANG I to generate angiotensin (1–9) [ANG (1–9)] are depicted. ANG II binding and activation of the ANG II type 1 receptor (AT1R) promotes inflammation, fibrotic remodeling, and vasoconstriction, whereas the ANG (1–7) and ANG (1–9) peptides binding to MAS receptor (MASR) activate antifibrotic, anti-inflammatory pathways and vasodilation. Additional modules of the RAS (i.e., renin and angiotensinogen, AGT) are also shown. AT1R: Angiotensin II type 1 receptor. Citation: Trougakos IP, Terpos E, Alexopoulos H, Politou M, Paraskevis D, Scorilas A, Kastritis E, Andreakos E, Dimopoulos MA. Adverse effects of COVID-19 mRNA vaccines: the spike hypothesis. Trends Mol Med 2022; 28: 542-554. Copyright ©The Author(s) 2022. Published by Elsevier.
- Citation: Schinas G, Polyzou E, Dimakopoulou V, Tsoupra S, Gogos C, Akinosoglou K. Immune-mediated liver injury following COVID-19 vaccination. World J Virol 2023; 12(2): 100-108
- URL: https://www.wjgnet.com/2220-3249/full/v12/i2/100.htm
- DOI: https://dx.doi.org/10.5501/wjv.v12.i2.100