Published online Oct 28, 2019. doi: 10.5500/wjt.v9.i6.123
Peer-review started: June 9, 2019
First decision: August 2, 2019
Revised: September 17, 2019
Accepted: October 2, 2019
Article in press: October 2, 2019
Published online: October 28, 2019
Processing time: 145 Days and 5.3 Hours
The histopathological findings on the failing kidney allograft in the modern era is not well studied. In this study, we present our experience working with kidney transplant recipients with graft failure within one year of the biopsy.
To report the histopathological characteristics of failed kidney allografts in the current era of immunosuppression based on the time after transplant, cause of the end-stage renal disease and induction immunosuppressive medications.
In a single-center observational study, we characterized the histopathological findings of allograft biopsies in kidney transplant recipients with graft failure within one year after the biopsy.
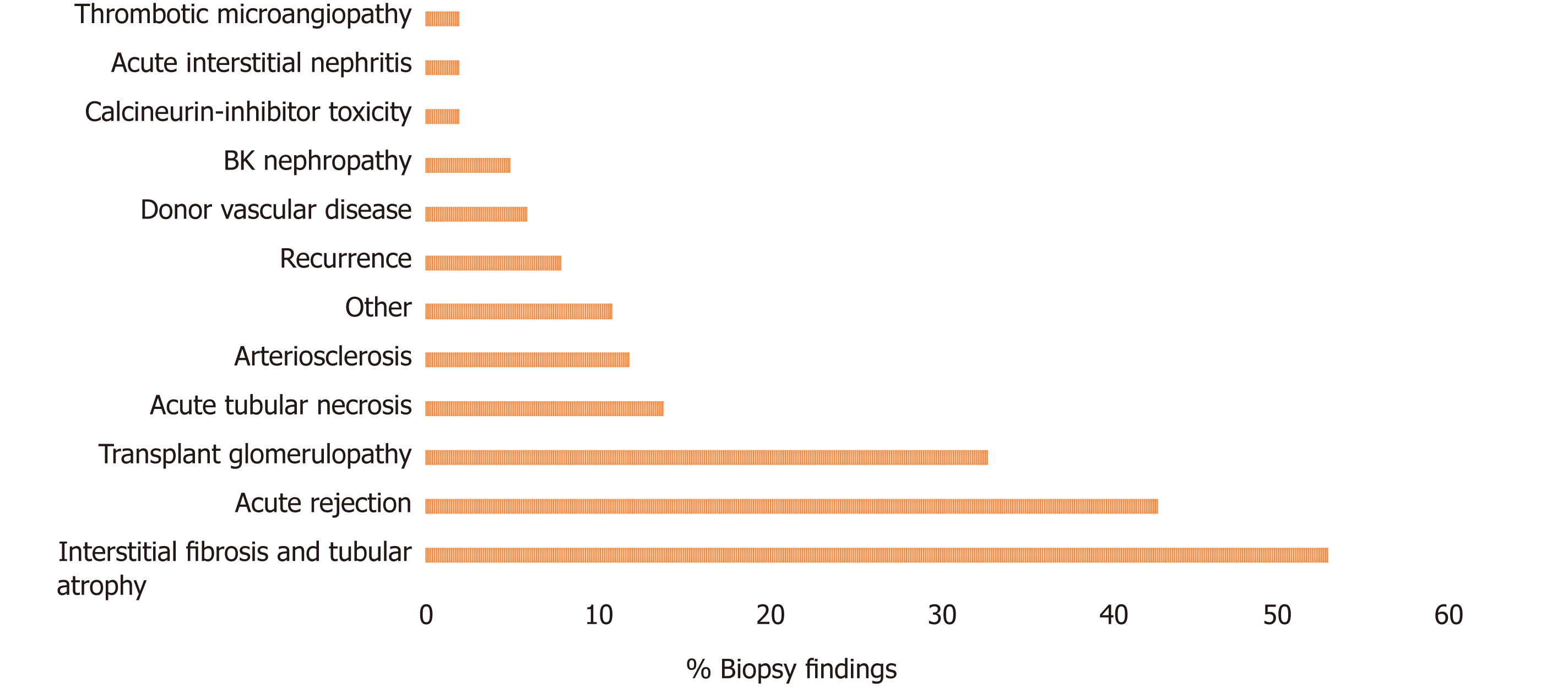
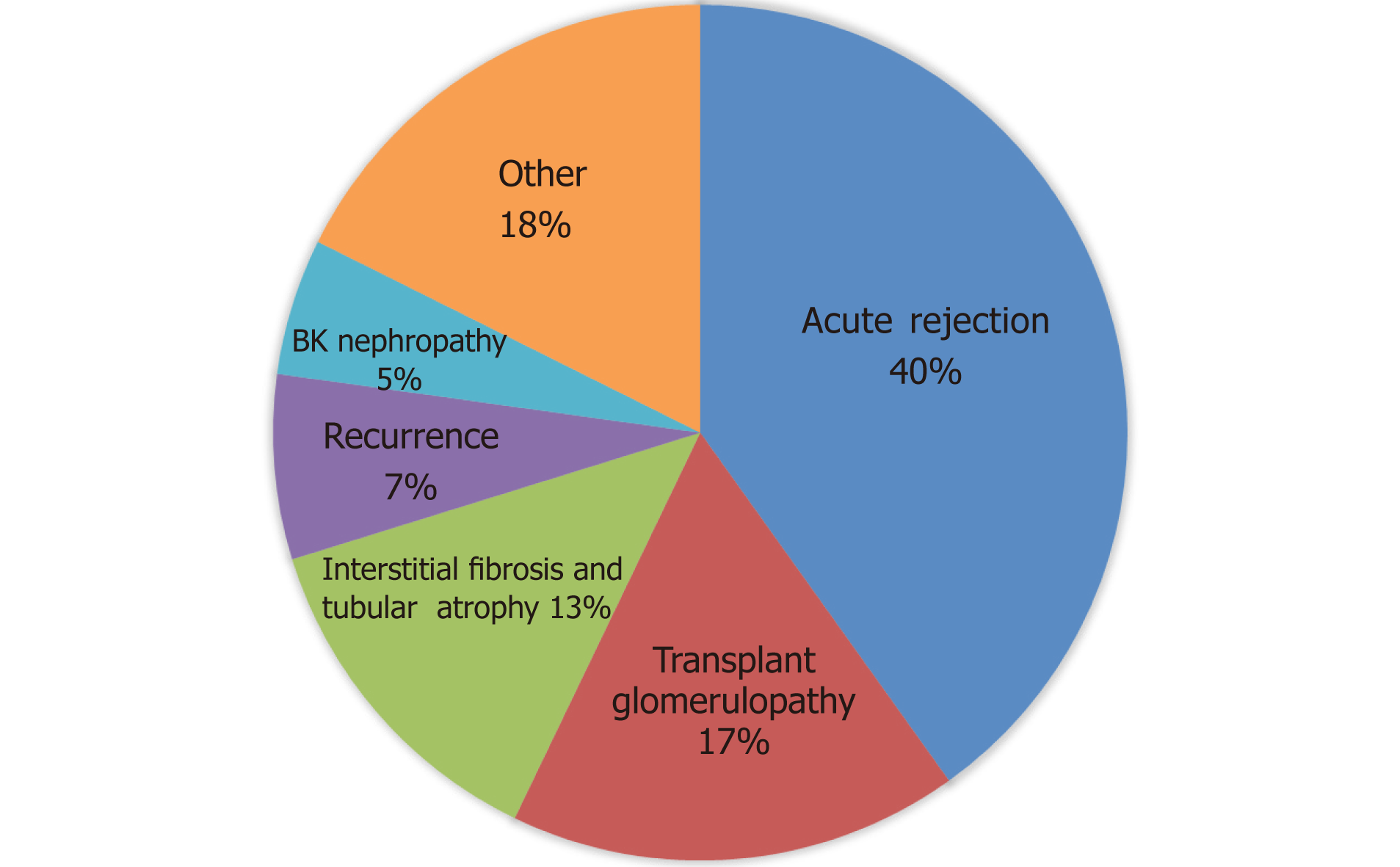
We identified 329 patients with graft failure that met the selection criteria between January 1, 2006 and December 31, 2016. The three most common biopsy findings were interstitial fibrosis and tubular atrophy (IFTA, 53%), acute rejection (AR, 43%) and transplant glomerulopathy (TG, 33%). Similarly, the three most common causes of graft failure based on the primary diagnosis were AR (40%), TG (17%), and IFTA (13%). Most grafts failed within two years of post-transplant (36%). Subsequently, approximately 10%-15% of grafts failed every two years: > 2-4 years (16%), > 4-6 years (13%), > 6-8 years (11%), > 8-10 years (9%) and > 10 years (16%). AR was the most common cause of graft failure in the first six years (48%), whereas TG was the most prevalent cause of graft failure after 6 years (32%) of transplant.
In the current era of immunosuppression, AR is still the most common cause of early graft failure, while TG is the most prevalent cause of late graft failure.
Core tip: There have been significant improvements in early graft survival. However, long-term graft survival has only had modest improvement. Causes of “true” late kidney allograft failure remain unclear. In this study, we explored the causes of graft failure based on the various factors, which may allow providers to determine interventions to prevent poor outcomes. We found, acute rejection, mainly antibody-mediated rejection, was the most common cause of early graft failure. And transplant glomerulopathy was a common cause of late graft failure, which occurred mainly after 6-7 years post-transplant even surpassed acute rejection.
- Citation: Parajuli S, Aziz F, Garg N, Panzer SE, Joachim E, Muth B, Mohamed M, Blazel J, Zhong W, Astor BC, Mandelbrot DA, Djamali A. Histopathological characteristics and causes of kidney graft failure in the current era of immunosuppression. World J Transplant 2019; 9(6): 123-133
- URL: https://www.wjgnet.com/2220-3230/full/v9/i6/123.htm
- DOI: https://dx.doi.org/10.5500/wjt.v9.i6.123
Kidney transplantation is the best form of treatment for patients with end-stage renal disease (ESRD) of any cause. Kidney transplant recipients (KTRs) experience survival benefits in all age groups have a better health-related quality of life and transplant is cost-effective compared to dialysis[1-3]. There have been significant improvements in early graft survival due to advances in immunosuppression and the overall medical care of transplant recipients. However, long-term graft survival has only had a modest improvement[4-6]. Allograft failure among transplanted kidney recipients is now the fourth leading cause of ESRD in the United States[7]. Studies from nearly a decade ago suggest that antibody-mediated rejection (ABMR) and disease recurrence are the most common causes of graft failure[7,8]. However, the causes of “true” late kidney allograft failure remain unclear[9]. In this study, we explored the causes of graft failure based on time after transplant, causes of ESRD and induction immuno-suppressive medication in the current era, which may allow providers to determine interventions to prevent poor outcomes.
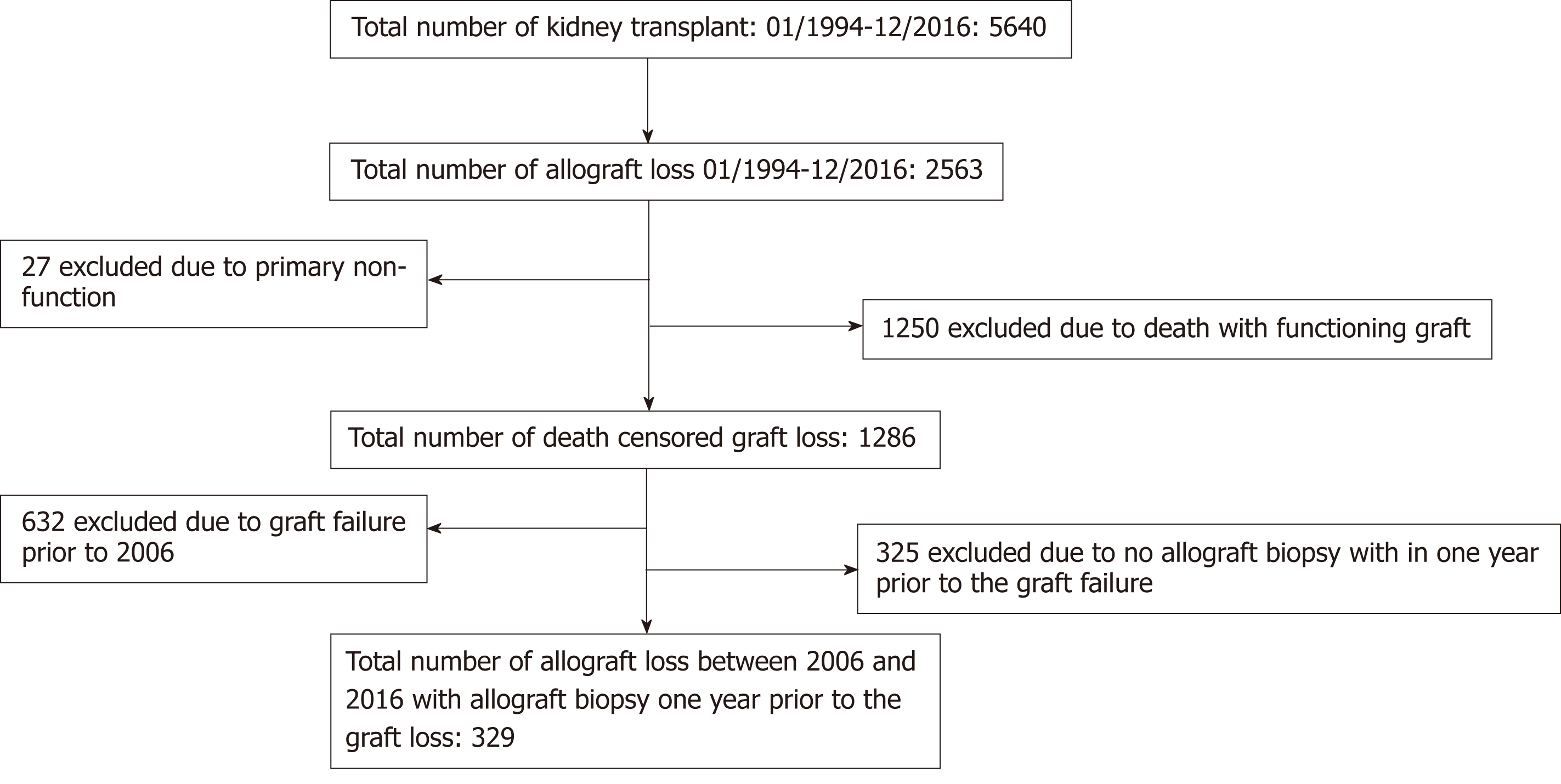
We study KTRs who were transplanted at the University of Wisconsin, and who had graft failure between January 1, 2006 and December 31, 2016 and transplanted between January 1, 1994 to December 31, 2016. We chose 2006 as a current era because at that time most of our clinical practice including histopathological reporting were protocolized. Patients were included if they underwent a kidney biopsy within one year prior to the graft failure. If they had multiple biopsies within one year prior to the graft failure, the biopsy closest to the graft failure was included in the analysis. Patients with primary graft dysfunction (defined as not having functional allograft and needing dialysis for at least 3 mo post-transplant or graft nephrectomy) or death with a functional graft were excluded from the study (Figure 1). This study was approved by the Health Sciences Institutional Review Board at the University of Wisconsin.
We analyzed data on age, gender, race, re-transplant status, the cause of ESRD, type of transplant, induction immunosuppression, organ failure method before graft failure (re-transplant vs initiation of dialysis). In cases where a patient had multiple biopsy diagnoses, all diagnoses were also reported separately, although the primary diagnosis (first diagnosis) was used for the cause of graft failure. We divided the causes of graft failure based on the post-transplant interval divided into 2 years interval, based on the causes of ESRD and also the types of induction immuno-suppressive medication.
Patients undergoing kidney transplant received induction immunosuppression with either a depleting (anti-thymocyte globulin, alemtuzumab or OKT3) or non-depleting (basiliximab or daclizumab) agent-based on immunological risk factors. Patients were typically maintained on a triple immunosuppressive regimen with a calcineurin inhibitor (CNI, usually tacrolimus), antiproliferative agent (usually mycophenolate mofetil or mycophenolic acid), and steroids. Some patients had early steroid withdrawal based on clinical judgment and the patient’s request. Doses and drug levels were individually adjusted at physician discretion based on the patient’s clinical condition, including infection, malignancy, and rejection. Patients were maintained on the same immunosuppressive medication until graft failure. However, if there was a feature of CNI toxicity on biopsy, then CNI trough goal was lowered or even discontinued based on physician discretion. Once the patient return on dialysis, immunosuppressive medication was tapered down and maintained only on low dose steroid. Switching to mTOR inhibitor among failing graft was not common practice.
The majority of the biopsies were performed for-cause, mainly for the unexplained rise in serum creatinine, concern for rejections, significant proteinuria, or the development of de novo donor-specific antibodies (DSA). Protocol biopsies were performed at months 3 and 12 for all patients with pre-transplant DSA, and 6-12 wk after treatment of rejection.
ABMR treatment protocols at our institution are based on both the severity of rejection and the time after transplant at which ABMR is diagnosed as described previously[10]. Briefly, for early rejection (within 3 mo post-transplant), treatment includes dexamethasone 100 mg bolus and taper, plasmapheresis (PP) 4-6 sessions, and intravenous immunoglobulin (IVIG) 100 mg/kg after each PP. Late rejection (> 3 mo post-transplant) is treated with dexamethasone 100 mg bolus and taper and IVIG 200 mg/kg every 2 wk × 3. Rituximab 375 mg/m2 as a single dose is added based on clinical and laboratory characteristics. The treatment regimen for both smoldering and clinical rejection is the same at our institution.
Treatment of acute cellular rejection (ACR) is also based on Banff criteria and severity. Borderline and Banff stage I rejection is treated with steroid pulse. Banff II and III ACR are treated with steroid pulse and Thymoglobulin 6-10.5 mg/kg in 4 to 7 divided doses. In mixed rejection, steroid pulse, IVIG, Thymoglobulin 10.5 mg/kg ± rituximab are used.
Continuous data were compared using Student’s t-test or the Wilcoxon rank-sum test, as appropriate, while categorical data were analyzed using Fisher’s exact test or chi-square test. P values < 0.05 were considered statistically significant. All analyses were performed using the MedCalc Statistical Software version 16.4.3 (MedCalc Software bvba, Ostend, Belgium; https://www.medcalc.org; 2016).
A total of 654 patients had death-censored graft failure during the study period. Of these, 329 (50%) fulfilled our selection criteria and were included in the study.
Out of the 329 KTRs included in the study, 127 (39%) were female and the majority were Caucasian (77%). Mean age at the time of transplant was 42.2 ± 13.7 years. Glomerulonephritis was the most common cause of ESRD and 33% were living KTRs. More than 50% had DSA around the time of graft failure. The mean interval from biopsy to graft failure was 106.5 ± 104.6 d (Table 1).
| Baseline characteristics | |
| Total number of graft failure | 329 (100) |
| Female gender | 127 (39) |
| Mean age at the time of transplant (yr) | 42.2 ± 13.7 |
| Caucasian | 253 (77) |
| Causes of end stage renal disease: | |
| Glomerulonephritis | 99 (30) |
| Diabetes | 71 (22) |
| Hypertension | 35 (11) |
| Polycystic kidney disease | 34 (10) |
| Congenital disorder | 9 (3) |
| Other | 81 (25) |
| Mean number of transplants (Range 1-3) | 1.29 ± 0.59 |
| Living donor transplant | 108 (33) |
| Induction Immunosuppression: | |
| Basiliximab | 179 (54) |
| Thymoglobulin | 52 (16) |
| Alemtuzumab | 66 (20) |
| Other | 32 (10) |
| Organ failure method: | |
| Resumption of dialysis | 319 (97) |
| Re-transplantation (preemptive re-transplant) | 10 (3) |
| DSA within a year prior to the graft failure: | |
| Present | 184 (56) |
| Absent | 89 (27) |
| Not tested | 56 (17) |
| Mean graft survival (yr) | 4.9 ± 4.4 |
| Mean interval between biopsy and graft failure (d) | 106.5 ± 104.6 |
Interstitial fibrosis and tubular atrophy (IFTA) was the most common biopsy finding in 53% of all failed grafts, followed by acute rejection (AR) in 43% and transplant glomerulopathy (TG) in 33%. Less common findings were acute tubular necrosis, arteriosclerosis, recurrence of disease, donor vascular disease and BK nephropathy (BKVN) (Figure 2).
AR was the most common cause of graft failure and accounted for 40% (32% ABMR or mixed rejection and 8% ACR) of all graft failure. TG (17%), IFTA (13%), disease recurrence (7%) including the recurrence of diabetic nephropathy and glomerular disease, and BKVN (5%) were the following common causes of graft failure. Other less common causes of graft failure were donor vascular disease, prolonged acute tubular necrosis, CNI toxicity, and renal infarction (18% total graft failures). Among patients with AR as a cause of graft failure, 74 % had human leukocyte antigen (HLA) DSA at time of a biopsy, while 17% did not have HLA DSA and in 9% HLA DSA was not tested (Figure 3).
We further analyzed the cause of graft failure based on the three most common causes of ESRD: Glomerulonephritis, diabetes, and hypertension. AR was significantly higher in the glomerulonephritis and hypertension group compared to diabetes, and acute tubular necrosis was higher in the hypertension group (Table 2).
| Glomerulonephritis (n = 99) | Diabetes (n = 71) | Hypertension (n = 35) | |
| Acute rejection | 49 (49) | 21 (30) | 19 (54) |
| Transplant glomerulopathy | 14 (14) | 14 (20) | 4 (11) |
| Interstitial fibrosis and tubular atrophy | 11 (11) | 12 (17) | 5 (14) |
| BK nephropathy | 3 (3) | 7 (10) | 2 (6) |
| Acute tubular necrosis | 1 (1) | 5 (7) | 3 (9) |
| Recurrence | 6 (6) | 6 (8) | 1 (3) |
| Other | 15 (15) | 6 (8) | 3 (9) |
Patients were divided into two groups based on the induction immunosuppressive medication they received at time of transplant: Depleting agents (Anti-thymocyte globulin or alemtuzumab or OKT3) and non-depleting agents (basiliximab or daclizumab), which also included patients who received no or unknown induction. In the non-depleting group, TG was a significantly higher cause of graft failure compared to depleting agent group 48% vs 24% (Table 3).
| Depleting (127) | Non-depleting (n = 202) | P value | |
| Acute rejection | 46 (36) | 86 (43) | 0.25 |
| Transplant glomerulopathy | 31 (24) | 96 (48) | 0.003 |
| Interstitial fibrosis and tubular atrophy | 13 (10) | 30 (15) | 0.23 |
| BK nephropathy | 7 (6) | 10 (5) | 0.82 |
| Acute tubular necrosis | 6 (5) | 10 (5) | 0.92 |
| Recurrence | 6 (5) | 8 (4) | 0.74 |
| Other | 18 (14) | 34 (17) | 0.52 |
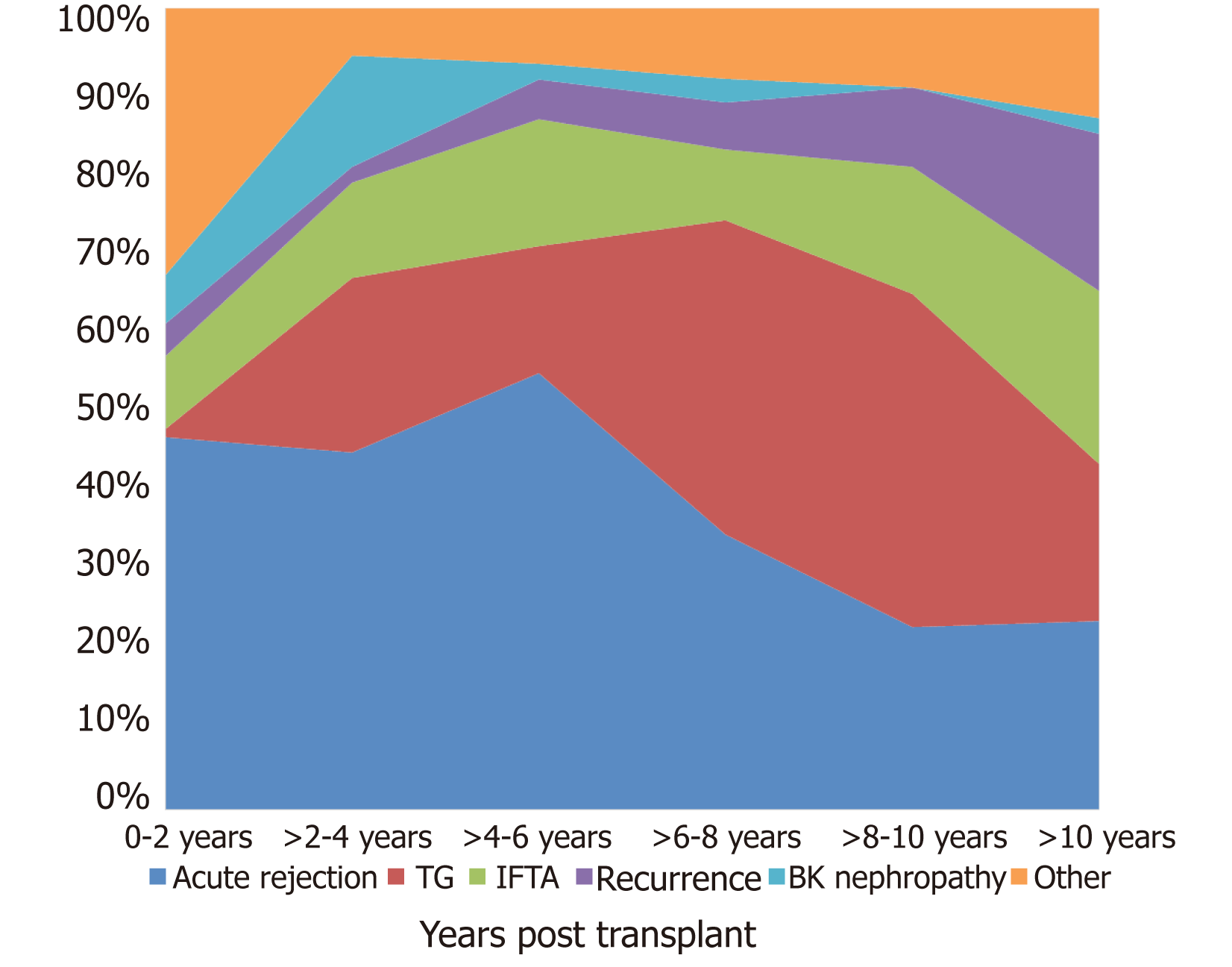
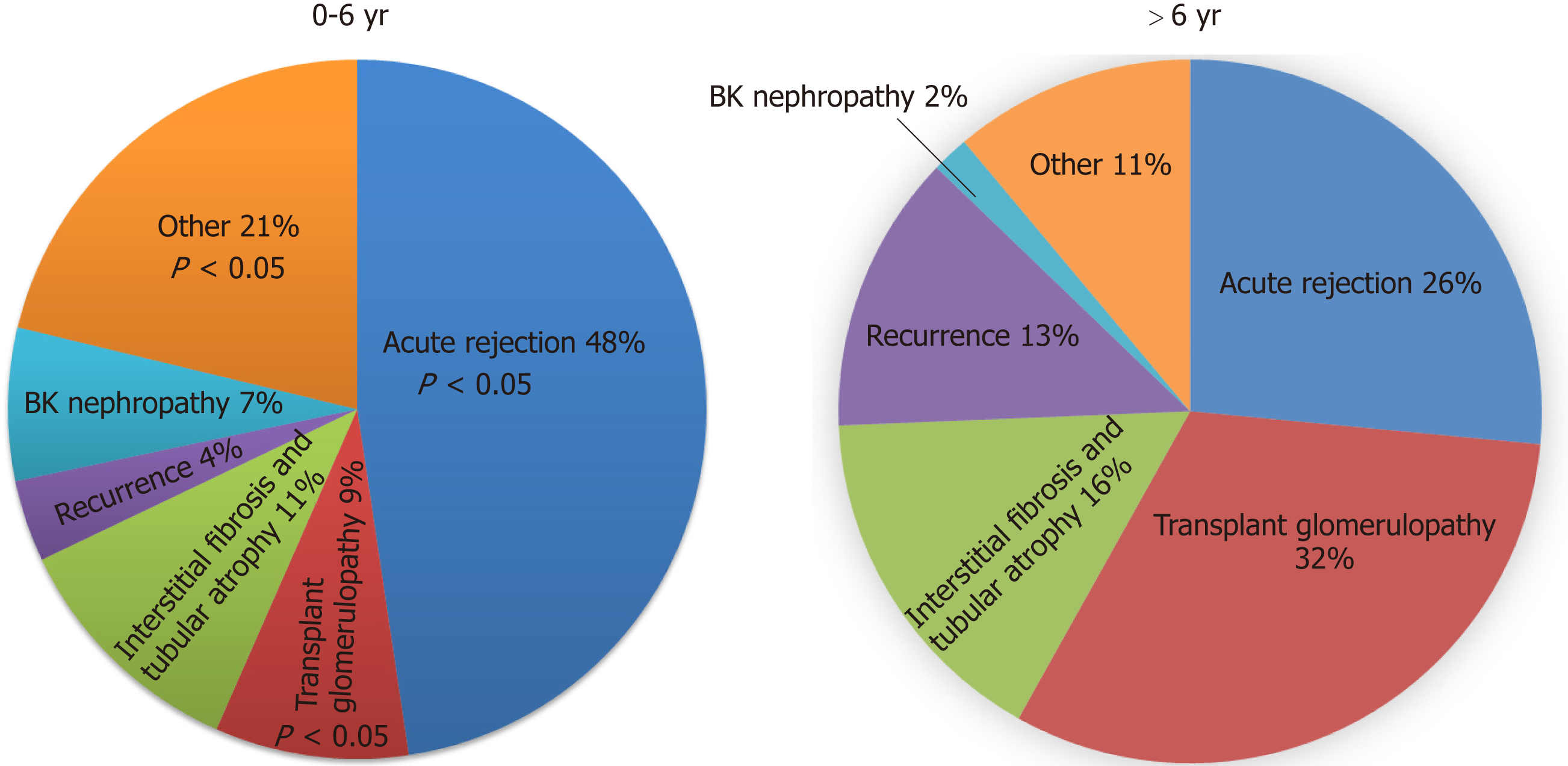
AR, was the most common cause of graft failure in the early post-transplant period (within six years post-transplant) and accounted for 31% of total graft failures. (23% ABMR or mixed rejection and 8% ACR). There was a significant trend for graft failure due to rejection in the early post-transplant period (P = 0.001), while in the late post-transplant period, TG was the most common cause of graft failure (P ≤ 0.001). The incidence of graft failure due to AR was higher up to 6 years post-transplant, with TG being the most common cause after 6 years (Figure 3). A total of 101 (48% of 212) graft failures within six years post-transplant were due to AR compared to 31 (26% of 117) after six years post-transplant (P = 0.01). TG was the primary cause of graft failure in 9% of patients within the first six years compared to 32% after six years (P < 0.001) (Figure 4). Rejection, TG, IFTA, and disease recurrence were evenly distributed as primary causes of graft failure after 10 years, each at approximately 20%-25%. Unsurprisingly, BKVN was more common in first 4 years post-transplant.
The most common time for graft failure was within two years post-transplant (n = 117, 36%). Subsequently, approximately 10%-15% of grafts failed every two years: > 2-4 years (n = 51, 16%), > 4-6 years (n = 44, 13%), > 6-8 years (n = 35, 11%), > 8-10 years (n = 31, 9%) and > 10 years (n = 51, 16%). Among 56 (17%) patients with the primary diagnosis of TG as a cause of graft failure, 25 (45%) had at least one episode of ABMR in the past. Similarly, around the time of last biopsy ( ± 3 mo), HLA-DSA was present in 30 (54%), DSA was not detected in 13 (23%) of the patients, and in 23% DSA was not checked around the time of biopsy (Figures 4 and 5).
In this study of the cause of graft failure among KTRs, we found that the primary cause of graft failure varies with time after transplantation. AR, mainly ABMR, was the most common cause of graft failure and accounted for 40% of graft failures, which peaked at 6 years post-transplant. After an AR, TG, one of the most specific histological findings of chronic ABMR[11], accounted for 17% of graft failure, which occurred mainly after 6-7 years post-transplant and was the most common cause of graft failure and even surpassed AR as a cause of graft failure. With careful adjustment of CNI dosing and with close monitoring of trough level, CNI toxicity was not a prevalent cause of graft failure in our cohort, which was considered one of the common cause of graft failure in the past.
There has been a dramatic improvement in the rate of AR. The half-life of a standard criteria deceased donor kidney in the United States has increased by almost 50%, from 10.6 years in 1989 to 15.5 years in 2005, and a similar pattern was seen with living donor transplantation[5]. This change was paralleled by a dramatic decline in graft failure within the first-year post-transplant period. Unfortunately, death-censored graft failure beyond the first year has remained unchanged since 1989[12]. During this time, our understanding of rejection and management have evolved, and graft failure due to hyperacute rejection is very rare. With newer protocols, ACR rates have decreased to less than 10% in the first year[5]. In the current era, our focus is on the prevention and treatment of ABMR. Certain newer therapeutics are considered for ABMR treatment based on their mechanism of action, such as anti-CD20 antibodies (e.g., ofatumumab and ocrelizumab), anti-CD22 antibody (epratuzumab), agents targeting B cell activation (e.g., atacicept and belimumab), and Anti-C5 antibody (eculizumab)[13-15], and others potentially in the investigational pipeline. Most of the work is being conducted in the fields of prevention and treatment of AR, and in time we may be able to effectively manage AR including acute ABMR. However, chronic changes and the lesser understood mechanisms of TG and IFTA may hinder our aim of prolonged graft survival.
TG has evolved as one of the histological features of chronic ABMR[16]. Overt TG is characterized histologically by glomerular basement membrane duplication in ≥ 1 of the capillary loops, mesangial expansion with or without mesangial hypercellularity, and mesangial cell interposition; glomerulitis can accompany these lesions[17]. The overall incidence of TG increases with time after transplant, occurring in approximately 20% by 5 years post-transplant[18,19]. TG is rarely diagnosed clinically within the first year of transplant, as TG lags behind the initial histologic stages of the disease[18]. In one study, subclinical TG (with stable renal function) was diagnosed in a protocol biopsy at a rate of 2.8% in the first year, which increased to 11.5% by 5 years post-transplant[18]. TG with significant proteinuria (> 2.5 g/day) is associated with worse graft survival outcomes compared with those with less proteinuria[20]. In the biopsy, TG is usually accompanied with the features of chronic damage to the allograft parenchyma mainly as fibrous intimal thickening of arteries, arteriolar hyalinosis and segmental and/or global glomerulosclerosis, IFTA and sometimes failure of peritubular capillaries[16]. Among patients with TG and active ABMR, outcomes are even worse; in one large observational study, 76% of the recipients lost their graft with a median survival of 1.9 years after the diagnosis of chronic active ABMR[21]. Overall, TG is associated with poor long-term graft survival, as grafts with TG fail sooner than those without[22]. Much effort is being made to investigate therapeutic options for the treatment of TG. Cooper et al., studied the effects of high-dose IVIG in chronic ABMR and did not find any favorable outcomes. Nine of 20 treated patients in their study had a follow-up biopsy and only 4 had no histological progression[23]. Similarly, in a recent randomized double-blinded clinical trial, the addition of IVIG and rituximab was not useful in patients with TG[24].
IFTA is a final common pathway involving a number of independent and overlapping cellular and molecular pathways[25]. In a recent study, prior ACR was associated with inflammation within IFTA and presence of inflammation within IFTA was associated with accelerated IFTA, arterial hyperplasia and chronic glomerulopathy along with reduced renal function compared to those without inflammation[26]. There is no reliable way to differentiate the cause of IFTA based on the morphology alone, or immunohistochemistry and molecular techniques[27]. Tubular atrophy and interstitial fibrosis progress in parallel[28]. In one surveillance biopsy among 321 KTRs, interstitial fibrosis was present in 71% of the graft at two years[28]. To date, there is no consensus about the mechanism or treatment for IFTA but chronic immune rejection and inflammation is considered one of the mechanisms[29]. Also, immune cell-derived and locally active complement has been associated with the progression of chronic fibrosis[30]. These suggest that although not as strong association as with TG, IFTA could be related to an immune-derived mechanism leading to graft loss.
Calcineurin inhibitor toxicity, thrombotic microangiopathy, and other causes of graft loss each contributed to 5% or less to graft failure. Our observations have the limitations inherent in this type of study. As a single-center study, it may not be possible to generalize our results to other centers. We looked for the specific causes of graft failure based on the primary biopsy diagnosis, but the specific management based on the biopsy findings was beyond the scope of this study. Similarly, around 50% of our patient population were excluded due to no biopsy within one year prior to the graft failure and it was not possible to determine the histopathological characteristics of those patients. We also excluded the small number of patients with primary graft dysfunction to avoid any surgical and technical issues for graft failure.
In summary, AR is still the most common cause of early graft failure in the current era of immunosuppression. Most early graft failures within the first six years of transplant are related to AR and are in theory preventable. Similarly, more effective diagnostic, monitoring, and therapeutic strategies for TG and IFTA are needed to improve long-term graft survival.
Although, there have been significant improvements in early graft survival due to advances in immunosuppression and the overall medical care of transplant recipients. However, long-term graft survival has only had modest improvement. The causes of “true” late kidney allograft failure remain unclear.
In this study, we explored the causes of graft failure based on various histopathological findings after transplant in the current era, which may allow providers to determine interventions to prevent poor outcomes.
The main objectives, of this study, was to identify the common causes of death censored graft failure among kidney transplant recipients. Knowing the causes may help provider to intervene on time and prevent for the graft loss.
This was a single-center, retrospective study among kidney transplant recipients who were transplanted at the University of Wisconsin, and who had graft failure between January 1, 2006 and December 31, 2016 and transplanted between January 1, 1994 to December 31, 2016. Patients were included if they underwent a kidney biopsy within one year prior to the graft failure. We divided histopathological causes of graft failure based on the post-transplant interval divided into 2 years interval, based on the causes of ESRD and also the types of induction immunosuppressive medication. In cases where a patient had multiple biopsy diagnoses, all diagnoses were also reported separately, although the primary diagnosis (first diagnosis) was used for the cause of graft failure.
A total of 329 kidney transplant recipients fulfilled our selection criteria and were included in the study. The three most common biopsy findings were interstitial fibrosis and tubular atrophy (IFTA, 53%), acute rejection (AR, 43%) and transplant glomerulopathy (TG, 33%). Similarly, the three most common causes of graft failure based on the primary diagnosis were AR (40%), TG (17%), and IFTA (13%). Most grafts failed within two years of post-transplant (36%). Subsequently, approximately 10%-15% of grafts failed every two years: > 2-4 years (16%), > 4-6 years (13%), > 6-8 years (11%), > 8-10 years (9%) and > 10 years (16%). AR was the most common cause of graft failure in the first six years (48%), whereas TG was the most prevalent cause of graft failure after 6 years (32%) of transplant. Most early graft failures within the first six years of transplant are related to AR and are in theory preventable. Similarly, more effective diagnostic, monitoring, and therapeutic strategies for TG and IFTA are needed to improve long-term graft survival.
In this study of the cause of graft failure among kidney transplant recipients, we found that the primary cause of graft failure varies with time after transplantation. AR, mainly antibody-mediated rejection (ABMR), was the most common cause of graft failure and accounted for 40% of graft failures, which peaked at 6 years post-transplant. After an AR, TG, one of the most specific histological findings of chronic ABMR, accounted for 17% of graft failure, which occurred mainly after 6-7 years post-transplant and was the most common cause of graft failure and even surpassed AR as a cause of graft failure. Interestingly, calcineurin inhibitor toxicity was not a common cause of graft failure.
Further studies in this field and specifically effective treatment of AR is needed to prolong the graft survival. Most of the work is being conducted in the fields of prevention and treatment of AR, and in time we may be able to effectively manage AR including acute ABMR. However, chronic changes and the lesser understood mechanisms of TG and IFTA may hinder our aim of prolonged graft survival and study should focus on the field of prevention or treatment of TG and IFTA.
Manuscript source: Invited manuscript
Specialty type: Transplantation
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gonzalez F S-Editor: Yan JP L-Editor: A E-Editor: Xing YX
| 1. | Pesavento TE. Kidney transplantation in the context of renal replacement therapy. Clin J Am Soc Nephrol. 2009;4:2035-2039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 2. | Fiebiger W, Mitterbauer C, Oberbauer R. Health-related quality of life outcomes after kidney transplantation. Health Qual Life Outcomes. 2004;2:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 103] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 3. | Parajuli S, Clark DF, Djamali A. Is Kidney Transplantation a Better State of CKD? Impact on Diagnosis and Management. Adv Chronic Kidney Dis. 2016;23:287-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Meier-Kriesche HU, Schold JD, Srinivas TR, Kaplan B. Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. Am J Transplant. 2004;4:378-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 945] [Cited by in RCA: 928] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 5. | Lamb KE, Lodhi S, Meier-Kriesche HU. Long-term renal allograft survival in the United States: a critical reappraisal. Am J Transplant. 2011;11:450-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 664] [Cited by in RCA: 696] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 6. | Hariharan S, Johnson CP, Bresnahan BA, Taranto SE, McIntosh MJ, Stablein D. Improved graft survival after renal transplantation in the United States, 1988 to 1996. N Engl J Med. 2000;342:605-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1431] [Cited by in RCA: 1432] [Article Influence: 57.3] [Reference Citation Analysis (0)] |
| 7. | Sellarés J, de Freitas DG, Mengel M, Reeve J, Einecke G, Sis B, Hidalgo LG, Famulski K, Matas A, Halloran PF. Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am J Transplant. 2012;12:388-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1088] [Cited by in RCA: 1246] [Article Influence: 95.8] [Reference Citation Analysis (0)] |
| 8. | El-Zoghby ZM, Stegall MD, Lager DJ, Kremers WK, Amer H, Gloor JM, Cosio FG. Identifying specific causes of kidney allograft loss. Am J Transplant. 2009;9:527-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 614] [Cited by in RCA: 631] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 9. | Chand S, Atkinson D, Collins C, Briggs D, Ball S, Sharif A, Skordilis K, Vydianath B, Neil D, Borrows R. The Spectrum of Renal Allograft Failure. PLoS One. 2016;11:e0162278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 10. | Parajuli S, Mandelbrot DA, Muth B, Mohamed M, Garg N, Aziz F, Redfield RR, Zhong W, Astor BC, Djamali A. Rituximab and Monitoring Strategies for Late Antibody-Mediated Rejection After Kidney Transplantation. Transplant Direct. 2017;3:e227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 11. | Husain S, Sis B. Advances in the understanding of transplant glomerulopathy. Am J Kidney Dis. 2013;62:352-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Stegall MD, Gaston RS, Cosio FG, Matas A. Through a glass darkly: seeking clarity in preventing late kidney transplant failure. J Am Soc Nephrol. 2015;26:20-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 104] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 13. | Djamali A, Kaufman DB, Ellis TM, Zhong W, Matas A, Samaniego M. Diagnosis and management of antibody-mediated rejection: current status and novel approaches. Am J Transplant. 2014;14:255-271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 286] [Cited by in RCA: 287] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 14. | Bentall A, Cornell LD, Gloor JM, Park WD, Gandhi MJ, Winters JL, Chedid MF, Dean PG, Stegall MD. Five-year outcomes in living donor kidney transplants with a positive crossmatch. Am J Transplant. 2013;13:76-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 156] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 15. | Stegall MD, Diwan T, Raghavaiah S, Cornell LD, Burns J, Dean PG, Cosio FG, Gandhi MJ, Kremers W, Gloor JM. Terminal complement inhibition decreases antibody-mediated rejection in sensitized renal transplant recipients. Am J Transplant. 2011;11:2405-2413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 442] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 16. | Remport A, Ivanyi B, Mathe Z, Tinckam K, Mucsi I, Molnar MZ. Better understanding of transplant glomerulopathy secondary to chronic antibody-mediated rejection. Nephrol Dial Transplant. 2015;30:1825-1833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Haas M, Sis B, Racusen LC, Solez K, Glotz D, Colvin RB, Castro MC, David DS, David-Neto E, Bagnasco SM, Cendales LC, Cornell LD, Demetris AJ, Drachenberg CB, Farver CF, Farris AB, Gibson IW, Kraus E, Liapis H, Loupy A, Nickeleit V, Randhawa P, Rodriguez ER, Rush D, Smith RN, Tan CD, Wallace WD, Mengel M; Banff meeting report writing committee. Banff 2013 meeting report: inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant. 2014;14:272-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1082] [Cited by in RCA: 1104] [Article Influence: 100.4] [Reference Citation Analysis (0)] |
| 18. | Gloor JM, Sethi S, Stegall MD, Park WD, Moore SB, DeGoey S, Griffin MD, Larson TS, Cosio FG. Transplant glomerulopathy: subclinical incidence and association with alloantibody. Am J Transplant. 2007;7:2124-2132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 270] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 19. | Cosio FG, Grande JP, Wadei H, Larson TS, Griffin MD, Stegall MD. Predicting subsequent decline in kidney allograft function from early surveillance biopsies. Am J Transplant. 2005;5:2464-2472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 245] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 20. | Banfi G, Villa M, Cresseri D, Ponticelli C. The clinical impact of chronic transplant glomerulopathy in cyclosporine era. Transplantation. 2005;80:1392-1397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Redfield RR, Ellis TM, Zhong W, Scalea JR, Zens TJ, Mandelbrot D, Muth BL, Panzer S, Samaniego M, Kaufman DB, Astor BC, Djamali A. Current outcomes of chronic active antibody mediated rejection - A large single center retrospective review using the updated BANFF 2013 criteria. Hum Immunol. 2016;77:346-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 22. | Cosio FG, Gloor JM, Sethi S, Stegall MD. Transplant glomerulopathy. Am J Transplant. 2008;8:492-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 183] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 23. | Cooper JE, Gralla J, Klem P, Chan L, Wiseman AC. High dose intravenous immunoglobulin therapy for donor-specific antibodies in kidney transplant recipients with acute and chronic graft dysfunction. Transplantation. 2014;97:1253-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Moreso F, Crespo M, Ruiz JC, Torres A, Gutierrez-Dalmau A, Osuna A, Perelló M, Pascual J, Torres IB, Redondo-Pachón D, Rodrigo E, Lopez-Hoyos M, Seron D. Treatment of chronic antibody mediated rejection with intravenous immunoglobulins and rituximab: A multicenter, prospective, randomized, double-blind clinical trial. Am J Transplant. 2018;18:927-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 134] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 25. | Farris AB, Colvin RB. Renal interstitial fibrosis: mechanisms and evaluation. Curr Opin Nephrol Hypertens. 2012;21:289-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 255] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 26. | Nankivell BJ, Shingde M, Keung KL, Fung CL, Borrows RJ, O'Connell PJ, Chapman JR. The causes, significance and consequences of inflammatory fibrosis in kidney transplantation: The Banff i-IFTA lesion. Am J Transplant. 2018;18:364-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 100] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 27. | Haas M. Chronic allograft nephropathy or interstitial fibrosis and tubular atrophy: what is in a name? Curr Opin Nephrol Hypertens. 2014;23:245-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 28. | Cosio FG, Grande JP, Larson TS, Gloor JM, Velosa JA, Textor SC, Griffin MD, Stegall MD. Kidney allograft fibrosis and atrophy early after living donor transplantation. Am J Transplant. 2005;5:1130-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 111] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 29. | Nakorchevsky A, Hewel JA, Kurian SM, Mondala TS, Campbell D, Head SR, Marsh CL, Yates JR, Salomon DR. Molecular mechanisms of chronic kidney transplant rejection via large-scale proteogenomic analysis of tissue biopsies. J Am Soc Nephrol. 2010;21:362-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 30. | Sheen JH, Heeger PS. Effects of complement activation on allograft injury. Curr Opin Organ Transplant. 2015;20:468-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |