Published online Dec 24, 2016. doi: 10.5500/wjt.v6.i4.703
Peer-review started: June 3, 2016
First decision: July 25, 2016
Revised: September 6, 2016
Accepted: September 21, 2016
Article in press: September 23, 2016
Published online: December 24, 2016
Processing time: 197 Days and 20.7 Hours
To evaluate the relationship between the state of transplanted liver graft and the recipient quality of life (QOL) of histologically proven lesions in a 10-year post liver transplantation (LT) cohort of patients.
Seventy-two recipients with a functional first graft at 10 years post-LT underwent liver biopsy and completed a QOL questionnaire. Logistic regression analysis was used to explore associations between histological, clinical and QOL criteria.
Ten years after LT, fibrosis was detected in 53% of patients, and affected the general health perception, while ductopenia, present in 36%, affected the well-being (P = 0.05). Hepatic steatosis (HS) was present in 33% of patients and was associated with the worst QOL score on multiple domains. When compared to patients without HS, patients with HS had significantly higher incidence of fibrosis (P = 0.03), hepatitis C virus (HCV) infection (P = 0.007), and more patients had retired from their job (P = 0.03). Recurrent or de novo HCV-associated fibrosis and patient retirement as objective variables, and abdominal pain or discomfort and joint aches or pains as subjective variables, emerged as independent determinants of HS.
Long-term liver graft lesions, mainly HS presumably as a surrogate marker of HCV infection, may have a substantial impact on QOL 10 years after LT.
Core tip: Objective and subjective parameters are helpful in the accurate assessment of long-term outcome in liver transplantation recipients. The main finding of this study was that histological lesions in the transplanted liver 10 years after liver transplantation can affect the recipient quality of life. Hepatic steatosis had the most significant impact on quality of life and this was independent of alcohol consumption, fibrosis, diabetes and body mass index. The strongest determinants of a worse quality of life in patients with hepatic steatosis were hepatitis C virus infection and retirement from job irrespective of patient-age.
- Citation: Karam V, Sebagh M, Rifai K, Yilmaz F, Bhangui P, Danet C, Saliba F, Samuel D, Castaing D, Adam R, Feray C. Quality of life 10 years after liver transplantation: The impact of graft histology. World J Transplant 2016; 6(4): 703-711
- URL: https://www.wjgnet.com/2220-3230/full/v6/i4/703.htm
- DOI: https://dx.doi.org/10.5500/wjt.v6.i4.703
The goal of liver transplantation (LT) is to ameliorate not only survival, but also quality of life (QOL) while minimizing the effects of disease and costs of care. Analysis of data from the European Liver Transplant Registry (ELTR) shows that 38% of the patients transplanted in 1991 were still alive with their first graft at least 10 years post LT[1]. The increasing proportion of recipients alive at long-term follow up has incited transplant professionals to focus on long term morbidity-free survival and an acceptable QOL.
The QOL is increasingly recognized as an important measure of outcome after solid organ transplantation[2-4]. We showed in a previous study that the challenge of maintaining long-term well-being is achieved to a greater extent in liver transplant recipients than in other solid-organ transplant recipients[4]. However, short term and long term QOL in liver transplant recipients is still inferior to that of the general population[3,4].
Studies of long-term survivors have been mainly based on clinical data and histological follow up at long term, or with respect to indication for LT, immunosuppressive regimen or recipient and donor criteria[5-10]. In previous studies, we justified the use of biopsies in the follow-up protocol to adjust treatments, not only in HCV-infected patients (in whom fibrosis progression was rapid and non-linear), but in all recipients[11,12].
No study has been published assessing the relationship between the state of transplanted liver graft and the recipient QOL of histologically proven lesions in a 10-year post LT cohort of patients.
Between September 1989 and December 1992, 485 LT were performed in 432 patients at Paul Brousse Hospital (Villejuif, France). During the 10th year post LT, among the 145 patients who were alive with a first functional graft, 126 accepted to complete the QOL questionnaire, and among these 72 accepted to have a liver biopsy done. For the purpose of this study, only the 72 subjects who underwent both a 10-year post LT liver biopsy and completed the QOL auto-questionnaire were included.
QOL data was obtained using the NIDDK questionnaire[2]. The questionnaire includes 21 disease-specific items assessing symptoms related to chronic liver disease. We used a validated French version of the questionnaire developed previously using the back-translation method
Five domains of QOL; physical distress (PHD), psychological distress (PD), personal function (PF), social/role function (SRF), and general health perception (GHP) are well represented in the questionnaire. Each symptom is numerically graded according to severity and then a composite overall score is calculated from all domains[3,4].
Prospectively obtained reperfusion biopsies and ten-year post LT liver biopsies were reviewed by the same experienced pathologist (MS) who was blinded to clinical information.
Portal tracts, hepatic veins and parenchyma were systematically analysed according to a preformed format. Fibrosis was staged on a five-point scale: 0, none; 1, portal fibrosis without septa; 2, few septa; 3, numerous septa without cirrhosis; 4, cirrhosis. Ductopenia evaluated on liver biopsy was analysed according to the Banff criteria[13], and ductopenia was considered as significant when the percentage of bile duct lost exceeded 20%. Steatosis was scored according to the percentage of biopsy tissue involved. Patients were considered in Hepatic Steatosis (HS) group when the percentage of steatosis exceeded 10%. Minimal changes were defined as the absence of all the above cited lesions or the existence of only one of the following criteria: steatosis < 10%, sinusoidal fibrosis, or minimal bile duct or lobular inflammation. The final diagnosis was established by joint review of records; biochemical, virological, and immuno-histochemical data.
Continuous variables are given as mean ± SD. Comparisons of continuous variables were performed with the Mann-Whitney test and those of nominal variables with χ2 contingency test or Fisher’s exact test when appropriate. Logistic regression was conducted to examine determinants of HS. We conducted two separate regressions: (1) with the objective (clinical) variables; and (2) with the subjective (QOL) variables. A P-value of < 0.05 was considered statistically significant.
Only 72 patients accepted to complete the QOL questionnaire and undergo liver biopsy amongst the 145 patients who survived for a minimum of 10 years with a first functional graft. Since this could create a selection bias in our study, we compared the selected and unselected patients with respect to characteristics at the time of LT and at 10-years post-transplant. Clinico-demographic characteristics like age at transplantation, sex, donor age, ABO group, CMV and reperfusion biopsy status, indication for LT, liver enzyme tests at the time of 10 year control of included patients were not statistically different from the non-included patients. Moreover, comparison of all domains of QOL has not shown any statistically significant difference between the selected and unselected patients (data not shown).
The mean age at time of transplantation was 35 ± 19 years and proportion of female patients was 52%. Mean donor age was 27 ± 11 years. The main indications for LT were PBC (25%), acute liver failure (24%) and viral cirrhosis (20%) [mostly hepatitis C virus (HCV) related (12%)]. The reperfusion biopsies showed steatosis (≥ 10%) in 18% of patients and reperfusion injury related lesions in 86% of reperfusion biopsies. Twenty-six percent of these lesions were classified as mild while 60% were of moderate to severe-degree (Table 1).
| All subjects n = 72 | No HS n = 48 | HS n = 24 | P1 | |
| Age (yr) | 35 ± 19 | 32 ± 21 | 42 ± 12 | 0.04 |
| Gender (female) | 52% | 60% | 71% | NS |
| Disease | ||||
| Acute hepatic failure | 24% | 27% | 17% | NS |
| Primary biliary cirrhosis | 25% | 25% | 25% | NS |
| HBV-related cirrhosis | 8% | 6% | 12% | NS |
| Autoimmune cirrhosis | 7% | 6% | 8% | NS |
| Biliary atresia | 5% | 8% | 0% | NS |
| HCV-related cirrhosis | 12% | 6% | 25% | NS |
| Metabolic disease (Wilson disease) | 1% | 2% | 0% | NS |
| Alcohol related cirrhosis | 1% | 2% | 0% | NS |
| Primary sclerosing cholangitis | 4% | 4% | 4% | NS |
| Cryptogenic cirrhosis | 2% | 4% | 0% | NS |
| Hepatocellular carcinoma | 8% | 8% | 8% | NS |
| ABO compatible | 97% | 96% | 100% | NS |
| Donor age (years) | 27 ± 11 | 27 ± 12 | 27 ± 10 | NS |
| Donor gender (female) | 41% | 42% | 39% | NS |
| Urgency | 25% | 27% | 21% | NS |
| Cold ischemic time (min) | 410 ± 212 | 406 ± 215 | 429 ± 214 | NS |
| Reperfusion biopsy2 | ||||
| Steatosis (≥ 10%) | 18% | 15% | 22% | NS |
| % of steatosis | 24 ± 15 | 31 ± 16 | 16 ± 8 | NS |
| Reperfusion lesions | ||||
| Mild | 26% | 31% | 17% | NS |
| Moderate to severe | 60% | 50% | 79% | NS |
As regards co-morbidities present in the recipients at follow-up, thirty eight (53%) patients had arterial hypertension and 7 (10%) suffered from diabetes mellitus (mostly type II). According to the body mass index (BMI), 9 (13%) patients were underweight, 50 (69%) patients were within normal limits, 10 (14%) were overweight and 3 (4%) were obese. Fifteen (21%) patients consumed alcohol with 1.1 ± 0.3 drinks/day (one drink = 1 bottle of beer or 1 glass of wine or 1 mixed drink, the equivalent of 1.25 grams of alcohol) and 12 (17%) were tobacco smokers with 1.9 ± 0.8 cigarettes/day (Table 2).
| All subjects n = 72 | No HS n = 48 | HS n = 24 | P1 | |
| Age at the time of survey | 49 ± 15 | 47 ± 15 | 53 ± 12 | NS |
| ≥ 60 yr aged patients | 28% | 27% | 29% | NS |
| Histological lesions | ||||
| Steatosis | 33% | - | 100% | |
| Macrovacuolar | 28% | - | 82% | |
| Microvacuolar | 1% | - | 4% | |
| Combined Mac-Mic | 4% | - | 14% | |
| Initial and 10-yr maintained steatosis | 8% (5 pat.) | 0% | 22% | 0.002 |
| Fibrosis (F1-F4) | 53% | 44% | 71% | 0.03 |
| F1-F2 | 40% | 35% | 50% | NS |
| F3-F4 | 13% | 8% | 21% | NS |
| Combined fibrosis-steatosis | 24% | 0% | 71% | < 0.0001 |
| HCV(+) Fibrosis | 44% | 31% | 71% | < 0.001 |
| Bile duct lesions | 36% | 42% | 25% | NS |
| Minimal change | 23% | 27% | 17% | NS |
| Other potential steatosis factors | ||||
| BMI (kg/m2) | 22.4 ± 3.8 | 22.3 ± 3.9 | 22.6 ± 3.4 | NS |
| Underweight (BMI ≤ 18.5) | 13% | 17% | 4% | |
| Normal weight (BMI = 18.5-24.9) | 69% | 65% | 79% | NS |
| Overweight (BMI = 25-29.9) | 14% | 14% | 13% | |
| Obesity (BMI ≥ 30) | 4% | 4% | 4% | |
| HCV infection (de novo or recurrence) | 57% | 46% | 79% | 0.007 |
| Arterial hypertension | 53% | 52% | 54% | NS |
| Glycemia (mmol/L) | 5.4 ± 2.0 | 5.1 ± 0.9 | 6.2 ± 3.2 | NS |
| Diabeties mellitus | 10% | 8% | 13% | NS |
| Maintenance immunosuppresssion | ||||
| Cyclosporine A | 96% | 96% | 96% | NS |
| Dosage (mg) | 129.8 ± 58.1 | 135.0 ± 61.5 | 119.5 ± 50.4 | NS |
| Prednisolone | 93% | 96% | 88% | NS |
| Dosage (mg) | 6.8 ± 3.1 | 6.9 ± 3.2 | 6.7 ± 2.9 | NS |
| Azathioprine | 43% | 40% | 50% | NS |
| Dosage (mg) | 48.4 ± 15.7 | 51.3 ± 15.5 | 43.8 ± 15.5 | NS |
Forty one patients (57%) had HCV infection, amongst them 35 (49%) had de novo infection whereas 6 (8%) had recurrent HCV infection. Most patients had been transplanted before the screening of blood and organ of donors for HCV serology began (pre HCV era). The predominant HCV genotype in our study cohort was genotype 1 (60%), mostly 1b subtype (51%). The proportion of other genotypes was; genotype 2 (12%), genotype 3 (9%) and genotype 4 (6%). In 13% of cases HCV-infection was established by RNA revelation. At the time of biopsy and QOL evaluation, none of the patients was being treated with interferon.
The immunosuppression was mainly using Cyclosporine-based (96%) in the study population.
The main histological findings were as follows: (1) fibrosis F1-F4 (n = 38, 53%), with F1 (n = 16, 22%), F2 (n = 13, 18%), F3 (n = 4, 6%). Cirrhosis (F4) was found in 7% (n = 5) of cases; (2) ductopenia (n = 26, 36%) with a mean percentage of bile duct loss of 40% ± 20%; and (3) steatosis (n = 24, 33%) with a mean percentage of 19 ± 17 %, which was mostly macrovacuolar (n = 23, 32%). Combined fibrosis and steatosis was found in 24% (n = 17) of patients. Only 23% (n = 16) of biopsies contained minimal-change lesions (as defined above).
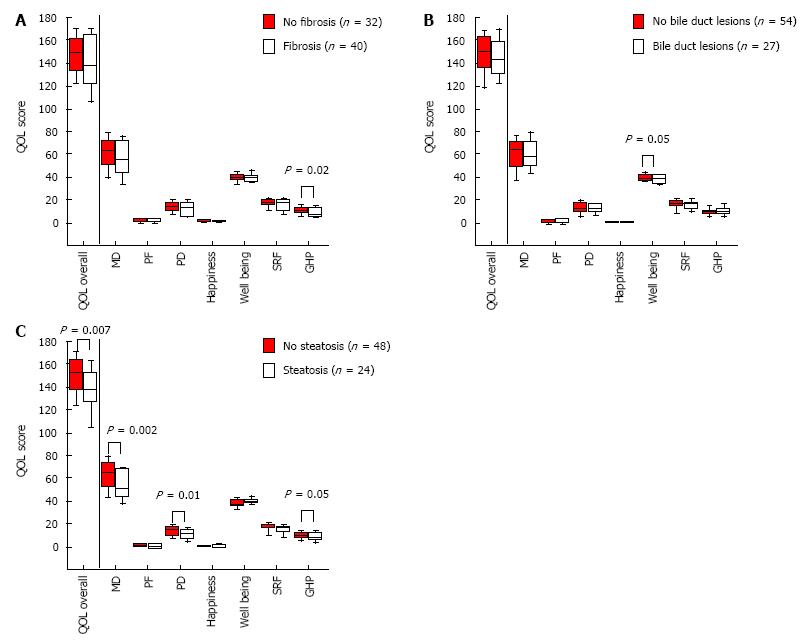
Overall-QOL was not affected by fibrosis or ductopenia (Figure 1A and B). Nevertheless, GHP score was lower in patients with fibrosis (P = 0.02) and well-being score was lower in patients with ductopenia (P = 0.05). The overall-QOL score was the lowest in HS patients (P = 0.007) (Figure 1C). HS impaired particularly the PHD (P = 0.002), PD (P = 0.01) and GHP (P = 0.05). According to these results, we focused our study on the group of patients with HS.
As the worst QOL score on multiple domains was associated strikingly with HS we made a detailed analysis to compare the group with steatosis on 10 year liver biopsy, with those without. There were no statistically significant differences between the groups with respect to data at the time of LT except for recipient age (32 ± 21 years vs 42 ± 12 years; P = 0.04) (Table 1). At 10 year post LT follow-up, the BMI (22.6 ± 3.4 vs 22.3 ± 3.9), rate of diabetes (13% vs 9%), rate of arterial hypertension (54% vs 54%) and immunosuppressive dosage were not statistically higher in HS group. No difference was found in liver function tests (Table 2).
For the 24 patients with HS, three of the studied objective variables were statistically significant when compared to patients without HS at 10 years post LT: Fibrosis (71% vs 44%, P = 0.03), HCV infection (79% vs 46%, P < 0.007) (Table 2), and patient retirement (50% vs 21%, P = 0.03) (Table 3). Fibrosis was present in 17 (71%) patients and was mainly related to HCV infection. The HCV genotype 1 was predominant and represented 63%, mostly 1b subtype (42%). Despite the equally distributed mean age and the percentage of more than 60 years old patients in the two groups (29% vs 27%, P = ns), retired recipients were more prevalent in the HS group (46% vs 21%, P = 0.03).
| All subjects n = 72 | No HS n = 48 | HS n = 24 | P1 | |
| Work | ||||
| Employed | 33% | 39% | 23% | NS |
| Homemaker | 13% | 17% | 4% | NS |
| Student full/part-time | 3% | 4% | 0% | NS |
| Unemployed | 20% | 19% | 23% | NS |
| Retired | 30% | 21% | 50% | 0.03 |
| No. of years worked | 17.9 ± 12.7 | 16.4 ± 12.6 | 20.9 ± 12.5 | NS |
| Alcohol and smoking | ||||
| Alcohol consumption | 21% | 17% | 30% | NS |
| No. of drinks2/d in drinkers | 1.1 ± 0.3 | 1.0 ± 0.0 | 1.2 ± 0.4 | NS |
| Tobacco smokers | 17% | 15% | 21% | NS |
| Cigarettes/d in smokers | 1.9 ± 0.7 | 2.1 ± 0.7 | 1.6 ± 0.9 | NS |
Regarding the subjective QOL variables, a detailed analysis showed that the HS has an impact on 17 symptoms belonging to each one of the 5 domains of QOL (Table 4). The most affected physical symptoms were: Abdominal pain or discomfort (P < 0.0001), joint aches or pains (P < 0.001) and change in facial appearance (P < 0.001). Nervousness/anxiety was the most affected psychological symptom followed by a feeling of being depressed, sad or blue (P < 0.01). As regards PF, the health of HS patients currently limits their ability to perform vigorous activities such as running, heavy lifting or sport (P < 0.001). The SRF was affected by the patients’ decreased interest in sex (P = 0.003). Finally, bodily pain during the last month represented the worst symptom of GHP (P < 0.01).
| QOL criteria | Univariate P |
| Physical distress | |
| Muscle weakness | 0.04 |
| Abdominal pains or discomfort | < 0.0001 |
| Abdominal swelling or bloating | 0.04 |
| Joint aches or pains | < 0.001 |
| Headaches | 0.03 |
| Poor or blurred vision | 0.03 |
| Change in facial appearance | < 0.001 |
| Fluid retention or swelling of ankles | 0.02 |
| Psychological distress | |
| Sleeplessness or insomnia | 0.03 |
| Nervousness, anxiety | 0.009 |
| Feeling depressed, sad or blue | < 0.01 |
| Low satisfaction with life as a whole | 0.02 |
| Personal function | |
| Health currently limits the kind of vigorous activities such as running, heavy lifting or sport | < 0.001 |
| Social and role function | |
| Decreased interest in sex | 0.003 |
| Problem with sex life | 0.04 |
| General health perception | |
| Bodily pain during the last month | < 0.01 |
In multivariate regression analysis, two objective variables emerged as independent determinants of HS: HCV infection (P < 0.01) and patient retirement (P = 0.04). So also, two subjective variables were significantly associated with HS: Abdominal pain or discomfort (P < 0.01) and joint aches or pains (P = 0.04) (Table 5).
| Factors | Multivariate P |
| Objective factors | |
| Retirement | 0.04 |
| Hepatitis C virus infection (de novo or recurrence) | < 0.01 |
| Subjective factors | |
| Abdominal pains or discomfort | < 0.01 |
| Joint aches or pains | 0.04 |
The developments in surgical techniques, immunosuppressive treatment modalities and better patient care have led to an increasing number of long-term survivors after LT, yet the QOL of transplant recipients does not always return to normal. The constant need for drug ingestion and monitoring the high incidence of recurrent or intervening diseases after LT, all seem to impair QOL[14,15]. Nevertheless, reported data shows that most of the QOL parameters are better after transplantation than before[2,3,16]. This study is an attempt to identify those factors which prevent long term liver transplant survivors from returning to a near normal lifestyle, with a specific focus on the relationship of QOL with graft histological status. One can recognize that a key challenge specific to this study could be its face validity, i.e., comparison of histologic changes to QOL, which in the absence of advanced histologic changes is not intuitively related. In order to attenuate the relative fluctuations liver biopsies were reviewed by the same experienced pathologist (MS) who was blinded to clinical information. Moreover, we used the NIDDK questionnaire considered as one of the most appropriate and validated instruments for QOL evaluation in transplant recipients[17].
The main finding of this study was that histological lesions (especially HS) in the transplanted liver 10 years after LT can affect the recipients’ QOL. Overall-QOL was not affected by fibrosis or ductopenia, but there was a significant decrease in GHP score in patients with fibrosis and in well-being score in patients with ductopenia. HS had the most significant impact on overall-QOL score and this was independent of alcohol consumption, fibrosis, diabetes and BMI.
Post-LT development of HS in recipients has only been analyzed in few studies so far[18]. Post-transplant metabolic syndrome and graft NAFLD are being increasingly recognized as long term problems in LT recipients[19,20]. Patients with post-LT NAFLD develop at the least an increased risk of cardiovascular events, rejection and infection[21]. Recently, a retrospective study reported that the reasons for long term steatosis in liver allografts may be related to seven factors either present alone or in combination, such as graft steatosis at the time of transplantation, HCV infection, recurrence of NAFLD or alcoholic liver disease, metabolic syndrome, diabetes mellitus and de novo NAFLD[22]. Most of these factors are known risk factors for NAFLD in the non-transplant setting also.
For the determination of such potential underlying factors, we compared the groups with and without HS in our series. We could not find any significant difference between the groups, neither with respect to known metabolic risk factors not related to LT (such as incidence of diabetes, hypertension or recipient BMI) nor with respect to transplant-related factors (such as donor liver steatosis, reperfusion injury, alcohol abuse and immunosuppressive dosage). Only three of the objective variables were significantly different; HCV infection, fibrosis and patient retirement irrespective of age.
The post-transplant setting is a good background for the development of one or several components of the metabolic syndrome[18], de novo NAFLD seems to be one of the most probable reasons for HS. For instance, high incidence of hypertension and hyperlipidemia in patients on Cyclosporine-based immunosuppressive regimen and diabetes mellitus in patients on Tacrolimus-based regimen are well-known side effects[23]. We acknowledge that at the time of this study almost all patients (96%) were on Cyclosporine and our results may not be applicable to patients who are on Tacrolimus.
Interestingly, in our series HS in the 10 year allograft biopsies were related to HCV infection, rather than NAFLD or other causes. HCV infection is well known to highly influence the rate of not only liver fibrosis but also HS. In the non-transplant setting steatosis is a very common lesion in chronic HCV infection[24], and the pathogenesis of steatosis may differ according to the genotype of HCV. Strong clinical and experimental evidence suggests that steatosis in patients infected with genotype 3 is partly related to a direct cytopathic effect, whereas in genotype 1, steatosis is mainly related to an associated metabolic syndrome and insulin resistance[25]. Because the predominant HCV genotype in our patients with HS was the genotype 1 (63%), mostly 1b subtype, and genotype 3 represented 16% (all in HS group), we can consider that both mechanisms were involved.
HCV seems to dominate other risk factors in our study. One explanation for this findings may be that a type II error occurred because of the relatively small sample size (n = 72 and only 24 in HS group). Otherwise, HS is presumably a surrogate for chronic hepatitis C, which is more directly affecting the QOL from chronic viral infection than the presence of histologic steatosis.
Unfortunately, HCV recurs in nearly all liver transplant recipients, and the reduction in long-term survival observed in these patients is the result of progressive fibrosis and evolution into cirrhosis[26-30]. Among the recipients with HCV infection, those who achieved 10 year post-transplant survival in our series can probably be categorized as “slow fibrosers”. Fibrosis was present in 71% of our patients with HS and was mainly related to HCV infection.
Other symptoms like changes in facial appearance, fluid retention or swelling of ankles, and headaches affected the QOL of long term survivors. These symptoms are probably associated to the long term medication that patients require after LT. Moreover, in addition to muscle weakness, these physical affections presumably had a repercussion on PD; predominantly nervousness, anxiety, sadness or depression associated with sleeplessness or insomnia. As a consequence, PD, SRF, and GHP also worsened in patients with HS.
The impact of HS on QOL has been already reported in non-transplanted patients. Recent studies demonstrated the negative impact of NAFLD on the physical and psychological function[31-33]. Newton et al[31] refuted the misconception that symptoms associated with NAFLD are entirely related to excessive weight, a concept that supported by our data. It is well recognized that the major risk factor for HS is excessive consumption of food, alcohol, or both. However, many people who over-consume do not have fatty livers, and steatosis can develop in those who do not engage in these behaviors. Thus, genetic or environmental factors or both could influence one’s susceptibility to hepatic triglyceride accumulation[34-36].
Future perspectives in the transplant setting must inevitably imply the host and the graft. At the present time, the gold standard for diagnosis remains liver biopsy but its costs and risks limit its practice in the non-transplant setting. Some demographic factors, blood tests, and imaging studies can be used to predict a higher risk of steato-hepatitis or advanced fibrosis, but are of limited sensitivity and specificity. More accurate predictors and scoring systems would allow identification of those who would benefit most from liver biopsy and monitor disease progression and response to therapy[19].
In conclusion, we could demonstrate that in patients with long-term follow-up after LT, HS is the most important histological finding that has an impact on the patients’ quality of life. Interventions are needed to restore and optimize QOL in patients with de novo or recurrent HS during long-term follow-up. Future research should focus on identifying factors that lead to the development of HS after LT.
The goal of liver transplantation is to ameliorate not only survival, but also quality of life (QOL) while minimizing the effects of disease and costs of care. The increasing proportion of recipients alive at long-term follow up has incited transplant professionals to focus on long term morbidity-free survival and an acceptable QOL. In this study the authors evaluated the relationship between the state of transplanted liver graft and the recipient QOL of histologically proven lesions in a 10-year post liver transplantation (LT) cohort of patients.
Studies of long-term survivors have been mainly based on clinical data and follow up at long term with respect to indication of LT, immunosuppressive regimen or recipient and donor criteria. Few studies assessed the graft histology by long-term graft biopsy protocol and, to our knowledge; no report assessing the relationship between the histological state of long-term transplanted liver graft and the recipient QOL has been published.
The results of this study showed a potential impact of graft’s steatosis on the QOL of transplant patients 10 years after surgery.
These results are encouraging and may represent the beginning of further studies in the area and, consequently the establishment of a specific care of these patients.
An interesting experience on the histological explore the outcome of 10-year liver transplantation. Manuscript is well written.
Manuscript source: Invited manuscript
Specialty type: Transplantation
Country of origin: France
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Chiu KW, Sipos F S- Editor: Kong JX L- Editor: A E- Editor: Lu YJ
| 1. | Adam R, Lucidi V, Karam V. Liver transplantation in Europe: is there a room for improvement? J Hepatol. 2005;42:33-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | Belle SH, Porayko MK, Hoofnagle JH, Lake JR, Zetterman RK. Changes in quality of life after liver transplantation among adults. National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Liver Transplantation Database (LTD). Liver Transpl Surg. 1997;3:93-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 105] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Karam V, Castaing D, Danet C, Delvart V, Gasquet I, Adam R, Azoulay D, Samuel D, Bismuth H. Longitudinal prospective evaluation of quality of life in adult patients before and one year after liver transplantation. Liver Transpl. 2003;9:703-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 62] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Karam VH, Gasquet I, Delvart V, Hiesse C, Dorent R, Danet C, Samuel D, Charpentier B, Gandjbakhch I, Bismuth H. Quality of life in adult survivors beyond 10 years after liver, kidney, and heart transplantation. Transplantation. 2003;76:1699-1704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 122] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 5. | Abbasoglu O, Levy MF, Brkic BB, Testa G, Jeyarajah DR, Goldstein RM, Husberg BS, Gonwa TA, Klintmalm GB. Ten years of liver transplantation: an evolving understanding of late graft loss. Transplantation. 1997;64:1801-1807. [PubMed] |
| 6. | Pappo O, Ramos H, Starzl TE, Fung JJ, Demetris AJ. Structural integrity and identification of causes of liver allograft dysfunction occurring more than 5 years after transplantation. Am J Surg Pathol. 1995;19:192-206. [PubMed] |
| 7. | Slapak GI, Saxena R, Portmann B, Gane E, Devlin J, Calne R, Williams R. Graft and systemic disease in long-term survivors of liver transplantation. Hepatology. 1997;25:195-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 81] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Duclos-Vallée JC, Sebagh M, Rifai K, Johanet C, Ballot E, Guettier C, Karam V, Hurtova M, Feray C, Reynes M. A 10 year follow up study of patients transplanted for autoimmune hepatitis: histological recurrence precedes clinical and biochemical recurrence. Gut. 2003;52:893-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 110] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | Lucey MR. Serial liver biopsies: a gateway into understanding the long-term health of the liver allograft. J Hepatol. 2001;34:762-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Berenguer M, Wright TL. Hepatitis C and liver transplantation. Gut. 1999;45:159-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Sebagh M, Rifai K, Féray C, Yilmaz F, Falissard B, Roche B, Bismuth H, Samuel D, Reynès M. All liver recipients benefit from the protocol 10-year liver biopsies. Hepatology. 2003;37:1293-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 90] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 12. | Rifai K, Sebagh M, Karam V, Saliba F, Azoulay D, Adam R, Castaing D, Bismuth H, Reynès M, Samuel D. Donor age influences 10-year liver graft histology independently of hepatitis C virus infection. J Hepatol. 2004;41:446-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 47] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Demetris A, Adams D, Bellamy C, Blakolmer K, Clouston A, Dhillon AP, Fung J, Gouw A, Gustafsson B, Haga H. Update of the International Banff Schema for Liver Allograft Rejection: working recommendations for the histopathologic staging and reporting of chronic rejection. An International Panel. Hepatology. 2000;31:792-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 360] [Article Influence: 14.4] [Reference Citation Analysis (1)] |
| 14. | Bravata DM, Olkin I, Barnato AE, Keeffe EB, Owens DK. Employment and alcohol use after liver transplantation for alcoholic and nonalcoholic liver disease: a systematic review. Liver Transpl. 2001;7:191-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 64] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | John PR, Thuluvath PJ. Outcome of liver transplantation in patients with diabetes mellitus: a case-control study. Hepatology. 2001;34:889-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 114] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 16. | Bryan S, Ratcliffe J, Neuberger JM, Burroughs AK, Gunson BK, Buxton MJ. Health-related quality of life following liver transplantation. Qual Life Res. 1998;7:115-120. [PubMed] |
| 17. | Jay CL, Butt Z, Ladner DP, Skaro AI, Abecassis MM. A review of quality of life instruments used in liver transplantation. J Hepatol. 2009;51:949-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 73] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 18. | Seo S, Maganti K, Khehra M, Ramsamooj R, Tsodikov A, Bowlus C, McVicar J, Zern M, Torok N. De novo nonalcoholic fatty liver disease after liver transplantation. Liver Transpl. 2007;13:844-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 124] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 19. | Pagadala M, Zein CO, McCullough AJ. Predictors of steatohepatitis and advanced fibrosis in non-alcoholic fatty liver disease. Clin Liver Dis. 2009;13:591-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Ong J, Younossi ZM. Non-alcoholic fatty liver disease after liver transplantation: a case of nurture and nature. Am J Gastroenterol. 2010;105:621-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Burke A, Lucey MR. Non-alcoholic fatty liver disease, non-alcoholic steatohepatitis and orthotopic liver transplantation. Am J Transplant. 2004;4:686-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 134] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 22. | Dumortier J, Giostra E, Belbouab S, Morard I, Guillaud O, Spahr L, Boillot O, Rubbia-Brandt L, Scoazec JY, Hadengue A. Non-alcoholic fatty liver disease in liver transplant recipients: another story of “seed and soil”. Am J Gastroenterol. 2010;105:613-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 163] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 23. | McAlister VC, Haddad E, Renouf E, Malthaner RA, Kjaer MS, Gluud LL. Cyclosporin versus tacrolimus as primary immunosuppressant after liver transplantation: a meta-analysis. Am J Transplant. 2006;6:1578-1585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 181] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 24. | Asselah T, Rubbia-Brandt L, Marcellin P, Negro F. Steatosis in chronic hepatitis C: why does it really matter? Gut. 2006;55:123-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 288] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 25. | Rubbia-Brandt L, Quadri R, Abid K, Giostra E, Malé PJ, Mentha G, Spahr L, Zarski JP, Borisch B, Hadengue A. Hepatocyte steatosis is a cytopathic effect of hepatitis C virus genotype 3. J Hepatol. 2000;33:106-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 401] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 26. | Charlton M, Wiesner R. Natural history and management of hepatitis C infection after liver transplantation. Semin Liver Dis. 2004;24 Suppl 2:79-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Charlton M. Obesity, hyperlipidemia, and metabolic syndrome. Liver Transpl. 2009;15 Suppl 2:S83-S89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Brown RS. Hepatitis C and liver transplantation. Nature. 2005;436:973-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 257] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 29. | Forman LM, Lewis JD, Berlin JA, Feldman HI, Lucey MR. The association between hepatitis C infection and survival after orthotopic liver transplantation. Gastroenterology. 2002;122:889-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 888] [Cited by in RCA: 829] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 30. | Singh N, Gayowski T, Wagener MM, Marino IR. Quality of life, functional status, and depression in male liver transplant recipients with recurrent viral hepatitis C. Transplantation. 1999;67:69-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 57] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Newton JL, Jones DE, Henderson E, Kane L, Wilton K, Burt AD, Day CP. Fatigue in non-alcoholic fatty liver disease (NAFLD) is significant and associates with inactivity and excessive daytime sleepiness but not with liver disease severity or insulin resistance. Gut. 2008;57:807-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 145] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 32. | Kistler KD, Molleston J, Unalp A, Abrams SH, Behling C, Schwimmer JB. Symptoms and quality of life in obese children and adolescents with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2010;31:396-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 33. | David K, Kowdley KV, Unalp A, Kanwal F, Brunt EM, Schwimmer JB. Quality of life in adults with nonalcoholic fatty liver disease: baseline data from the nonalcoholic steatohepatitis clinical research network. Hepatology. 2009;49:1904-1912. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 128] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 34. | Diehl AM. Genetic susceptibility to hepatic steatosis. N Engl J Med. 2010;362:1142-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 35. | Petersen KF, Dufour S, Hariri A, Nelson-Williams C, Foo JN, Zhang XM, Dziura J, Lifton RP, Shulman GI. Apolipoprotein C3 gene variants in nonalcoholic fatty liver disease. N Engl J Med. 2010;362:1082-1089. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 340] [Cited by in RCA: 320] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 36. | Feldstein AE, Wieckowska A, Lopez AR, Liu YC, Zein NN, McCullough AJ. Cytokeratin-18 fragment levels as noninvasive biomarkers for nonalcoholic steatohepatitis: a multicenter validation study. Hepatology. 2009;50:1072-1078. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 550] [Cited by in RCA: 516] [Article Influence: 32.3] [Reference Citation Analysis (0)] |