NON-MELANOMA SKIN CANCER IN GENERAL AND KIDNEY TRANSPLANT RECIPIENT POPULATIONS
Updated United States incidence estimates for non-melanoma cutaneous carcinoma, or skin cancer (NMSC) in the Medicare fee-for-service population indicate a pronounced rise of 100% for the entire two decade period from 1992 to 2012, and a sustained 6-year 2006 to 2012 elevation in NMSC rates of 35%[1]. Data recorded from kidney transplant recipient (KTR) populations spanning over four decades now, consistently yield 45- to 250-fold general population standardized incident rates for squamous cell carcinomas (SCCs), accompanied by 10-fold greater rates for basal cell carcinomas (BCCs)[2,3]. Analyzing data from 24088 United States KTRs who underwent an initial kidney transplant between 1995 and 2001, Kasiske et al[3] reported 3-year age-adjusted NMSC rate ratios of 90.0 for male KTRs, and 92.3 for female KTRs, relative to general population controls. Comparison of KTR and non-solid organ transplant recipient (SOTR) populations with a prior history of NMSC, undergoing surveillance for new NMSCs, also demonstrates a persistently greater risk for additional skin cancers in KTRs. While non-KTRs experience a 3-year cumulative 18% risk of a subsequent SCC after a first SCC[4], 3-year rates of 52%, and 59%, for a (at least one) new SCC in KTRs after an initial SCC, have been reported[5].
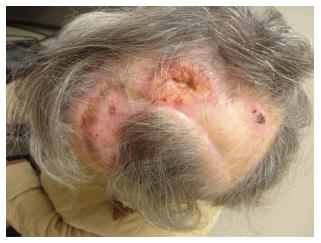
Pre-cancerous actinic keratoses (AKs), Bowen’s disease (SCC in situ), and keratoacanthomas are commonly associated with SCCs in KTRs[2]. Most SCCs among KTRs, as in non-SOTRs, develop, typically, on sun-exposed areas[2]. However, KTR SCCs have an increased tendency to be multiple, and aggressive, compared to SCCs which develop in non-SOTRs not exposed to chronic immunosuppression[2] (Figure 1). The characteristic aggressiveness of KTR SCCs exacerbates patient morbidity, because of the disproportionate rate of new lesions requiring, cryotherapy, electro-dessication and curettage, or surgical excision[2]. This increased morbidity, although non-fatal, also results in significant medical costs, reflecting the national United States economic burden of NMSC and AK care, tabulated, as of 2007-2011, at $4.8 billion, annually[6]. KTR SCCs, additionally, have a greater potential for metastasis, and death[2,5].
Figure 1 The unique aggressiveness of squamous cell carcinoma in kidney transplant recipients.
Deeply invasive, recurrent, poorly differentiated and ulcerated squamous cell carcinoma in a 73-year-old female kidney transplant recipient with background alopecia from prior radiation therapy for this squamous cell carcinoma.
Successful interventions to reduce the incidence and complications associated with all NMSCs, SCCs, in particular, as well as AKs, would represent a significant advance in the management of KTRs. Despite heroic “conversion” protocols from calcineurin inhibitor-based to mechanistic/mammalian target of rapamycin (mTOR) inhibitor-based (primarily, sirolimus) immunosuppressive regimens, KTRs with a predilection for NMSCs, especially SCCs, continue to develop new malignant skin lesions at grossly elevated rates[7,8]. The sirolimus converted group reported by Euvrard et al[7], for example, still experienced a 2-year incidence of 22% for SCC, and 47.6% for total NMSCs (71 new lesions in 20 patients). NMSC-prone KTRs converted to sirolimus also appear to increase their relative risk for death after mTOR conversion. A recent meta-analysis of such “conversion trials” underscored the lingering therapeutic dilemma: While sirolimus use significantly lowered SCC risk, it conferred an overall mortality penalty-a 1.59-fold excess risk of death[8].
Ultraviolet (UV) radiation, immunosuppressive therapy, and human papillomaviruses (the latter with an ostensible link to SCC, specifically), are all believed to contribute to the development of NMSC among KTRs, while their precise etiologic pathomechanisms, alone, or in concert, require elucidation[2]. Although topical sunscreens are an effective prophylactic against sunburn, their use may not afford protection from UV radiation-induced immunosuppression of the skin[9], and the incidence of skin carcinomas, especially SCCs, continues to climb, steeply[1]. The ideal chemopreventive treatment, as an adjunct to barrier sun protection, would be an oral agent that is safe, well-tolerated, inexpensive, and readily available. A potential candidate emerging to fill that therapeutic niche is oral nicotinamide (NAM).
Nicotinamide as a potential non-melanoma skin cancer chemopreventive agent
Overt, pellagrous nicotinic acid (NA; niacin) deficiency has long been recognized as a cause of severe sunlight sensitivity in exposed skin[10]. Recent clinical trial reports from an Australian investigative group suggest that oral NAM can reduce the occurrence of both AKs and NMSCs in non-SOTRs who have a history of AKs, and/or NMSCs[11,12]. The initial 4-mo, placebo-controlled phase 2 studies (n = 35, and n = 41 participants, respectively) reported that oral NAM lowered the rate of appearance of new AKs (i.e., total AK counts) by 29% to 35%[11]. In a subsequent 12-mo phase 3, placebo-controlled trial of 386 patients with prior NMSCs, NAM treatment (n = 193 active; n = 193 placebo) reduced the average unadjusted new NMSC rate (total lesions per patient) by 27%. NAM treatment resulted in comparable rate reductions for SCCs (-30%), and BCCs (-20%), as separate outcomes[12]. Chen et al[13], from the same Australian investigative group which conducted these non-SOTR NAM chemoprevention studies[11,12], recently reported consistent results, in terms of effect sizes, from a phase 2 study of 22 KTRs. Patients at least 12 mo post-transplant, with stable renal function, and a history of ≥ 2 histologically-confirmed NMSCs in the previous 12 mo, were randomized 1:1 (n = 11 per group) to receive NAM 500 mg, or placebo, twice daily, for 6 mo. Skin exams and AK counts were performed at 2-mo intervals. The 6-mo NMSC rate (mean lesions per patient) was non-significantly lower for the NAM group (mean = 2.7; 95%CI: 1.4 to 5.3; total = 30 cancers), compared to placebo (mean = 4.2; 95%CI: 2.2 to 7.8; total = 45 cancers), although the numeric trend was dominated by one patient in the placebo group with 20 NMSCs (8 BCC and 12 SCC). The estimated relative rate difference was 0.35 (95%CI: -0.62 to 0.74, P = 0.36). Baseline AK counts (reported as means of 61.6 in the placebo, and 60.1 in the NAM groups, respectively), were also non-significantly lower in KTRs receiving NAM compared to placebo by 16% at 6 mo (95%CI: 7% to 34%; P = 0.15)[13]. Also of importance, there were no between groups differences for adverse clinical events, or changes in complete blood counts, liver function studies, eGFR, or urinary microalbumin/creatinine ratios[13]. Additional, if limited, independent confirmatory data have been provided in a research letter published by Drago et al[14], in The New England Journal of Medicine. These Genoa, Italy investigators studied NAM given at a dose of 250 mg thrice daily, relative to matched placebo, in 24 KTRs (n = 12 per group), also followed for 6-mo. The following is a verbatim description of their findings[14].
At baseline, no significant differences were observed between the sizes of light-damaged areas in patients (identified visually, by touch, and by means of polarized light dermoscopy), in the two groups. At 6 mo, 88% of the patients who received nicotinamide had partial regression of some or all actinic keratoses and surrounding light-damaged areas; in 44% of the patients who received nicotinamide, there was complete resolution in some of these areas (no lesions were detected on biopsy). In 91% of the patients who received placebo, the size of light-damaged areas increased, new light damaged areas developed, or both.
Two plausible anti-cancer biological effects of nicotinamide have been described by the Australian investigators which provide some independent validation of their clinical trial observations: Nicotinamide has been shown to promote DNA repair after UV exposure, and lessen local UV immunosuppression, in the skin[15].
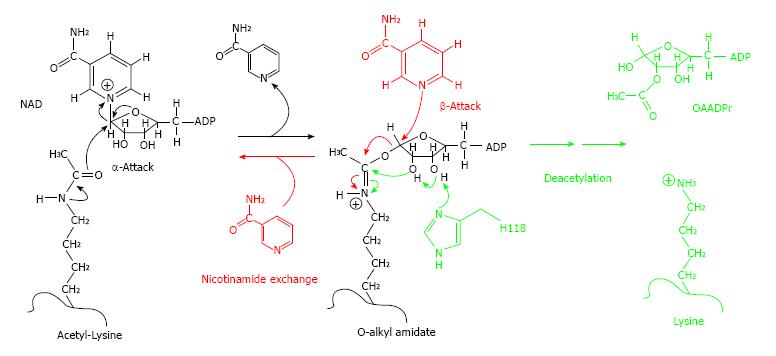
NAM’s reported effects on sirtuin enzymes and mediated pathways might also confer anti-cancer properties. Briefly, sirtuin (Sir2) enzymes, are an ancient class of nicotinamide adenine dinucleotide (NAD+)-dependent deacetylases which have been conserved from bacteria to humans (for example, SIRT1, etc.). They perform a myriad of biological functions, including transcriptional silencing, DNA recombination and repair, apoptosis, axonal protection, fat mobilization, and lifespan regulation[16-18]. Sirtuins deacetylate various proteins, notably p53, as well as histones, acetyl-coA synthetase, alpha-tubulin, Foxo3, Ku70, and NF kappa-beta. Lysine residues are the specific targets of sirtuin deacetylation reactions, allowing for tight cellular regulation. Sirtuin-catalyzed deacetylation is linked to the cleavage of NAD+, producing NAM, and O-acetyl ADP-ribose (OAADPr), in conjunction with deacetylated ribose. NAM produced from this reaction is a non-competitive inhibitor of sirtuins, which provides a mechanism for modulation of these enzymes by cellular nicotinamide concentrations[16-18]. Importantly, the inhibitory activity of NAM on sirtuin-mediated deacetylation is not conferred by nicotinic acid (NA)[18] (Figure 2). SIRT1 overexpression has been observed in human prostate cancer, adult T-cell leukemia, primary colon cancer, and, significantly, in skin tissue biopsies from patients with AK, Bowen’s disease, SCC, or BCC[16,17]. One mechanism by which elevated SIRT1 concentrations are believed to enhance malignant cell growth is via deacetylation of “tumor suppressor” p53 protein, inhibiting p53’s apoptotic activity[16,17]. SIRT1 interacts directly with p53 and deacetylates the protein’s C-terminal (Lysine)382 residue, which prevents p53 from trans-activating apoptotic genes, and promoting apoptosis[17]. NAM may also affect another of SIRT1’s downstream target proteins, the retinoblastoma tumor suppressor protein (Rb), reducing Rb phosphorylation (or “hyper”-phosphorylation)[19]. This has already been demonstrated, for example, in a mouse model of polycystic kidney disease, where NAM treatment reduced sirtuin-enhanced cyst formation[19]. Anti-cancer chemopreventive agents such as curcumin (a natural phenol responsible for the yellow color of turmeric), which suppresses Rb phosphorylation in prostate cancer cells, are under investigation[20]. Comparable NAM-induced p53 and Rb alterations could mediate the potential benefits of NAM treatment for the chemoprevention of AKs and NMSCs, in general, and KTR patient populations[11-14]. NAM’s putative in vivo role as an inhibitor of sirtuins, and sirtuin-catalyzed “pro-oncogenic” de-acetylation, and hyper-phosphorylation reactions[16-19] warrants investigation, as another pathophysiological correlate, in NAM-treated patients.
Figure 2 Nicotinamide-SIRT binding.
At high concentrations, NAM can bind to SIRT when it contains the O-alkyl-amidate intermediate, resulting in “NAM exchange”, reforming NAD+ and acetyl-lysine, and decreasing deacetylation activity - a process unique to NAM, not NA. Reproduced from[18]. NA: Nicotinic acid; NAM: Nicotinamide.
NICOTINAMIDE AND HYPOPHOSPHATEMIA: FROM TOXICITY MONITORING TO THERAPEUTIC INTERVENTION
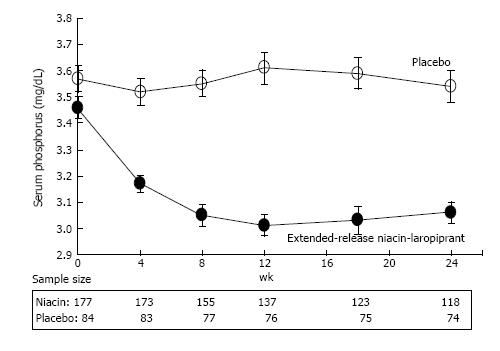
Extensive analyses by others[21], and our group[22-26] (Figure 3), demonstrate that both NAM and NA lower serum phosphorus in chronic kidney disease (CKD) patients, and indeed across the entire spectrum of renal function. Mediated via an elegant mechanism, i.e., direct inhibition of sodium-dependent transport of phosphorus in the small intestine[21], this consistent phosphorus-lowering effect mandates surveillance of serum phosphorus levels in patients on chronic oral NAM/NA to avoid the theoretical development of clinical hypophosphatemia[21]. But such potential toxicity might be counterbalanced by a distinctly positive clinical phenomenon: Since baseline serum phosphorus concentrations appear to predict total and/or cardiovascular disease (CVD) mortality in CKD and KTR populations[27-31], as well as native kidney, or kidney graft failure[27,30-32], conceivably, NAM-induced phosphorus-lowering could reduce such hard outcomes in these patients. Moreover, as NAM does not induce prostaglandin-mediated flushing, cause hyperuricemia, or adversely affect glucose tolerance, we believe it will have a better tolerability and safety profile relative to NA, confirming a substantive, decades old body of clinical evidence[33,34] from non-SOTR patient populations-now updated to include those studied on oral NAM therapy in the recent phase 2 and 3 Australian AK/NMSC prevention trials[11-13]. The sporadic occurrence of mild thrombocytopenia in end-stage renal disease (ESRD; stage 5 CKD) patients treated with NAM[35], has not been confirmed from either the recent placebo-controlled studies conducted among patients with normal renal function[11,12], or KTRs[13], for NMSC or AK prevention, nor was it reported in the large multicenter ENDIT trial[34], or a 30-year toxicity review of NAM trials which preceded ENDIT[33]. COMBINE, an ongoing, randomized, placebo-controlled trial (NCT0 2258074) in patients with an estimated glomerular filtration rate (eGFR) of 20-45 mL/min per 1.73 m2, which includes a NAM treatment arm (1.5 g/d, given as 0.75 g twice daily), will provide more definitive data on the incidence of this toxicity in stage 3b-4 CKD[35,36].
Figure 3 Phosphorus-lowering effect of nicotinic acid in chronic kidney disease.
Mean serum phosphorus concentrations in dyslipidemic patients with stage 3 chronic kidney disease receiving extended release niacin-laropiprant, or placebo. Error bars are standard errors. Reproduced from Ref. [24].
OVERVIEW OF DYSREGULATED CALCIUM-PHOSPHORUS HOMEOSTASIS AND ITS CLINICO-PATHOLOGIC IMPLICATIONS IN CKD AND KIDNEY TRANSPLANTATION
Deranged calcium-phosphorus metabolism often complicates CKD[36], worsens with the progressive development of ESRD[21,36], and is not fully reversed after kidney transplantation[28-30,37,38]. Moreover, notwithstanding abnormalities, particularly hyperparathyroidism (with resultant increased fractional excretion of urinary phosphorus/decreased tubular reabsorption of phosphorus)[37], which may persist long term despite successful transplantation, and excellent kidney graft function, chronic KTRs whose GFR subsequently declines to stage 4 (15-29 mL/min per 1.73 m2) to 5 (< 15 mL/min per 1.73 m2), or even 3b (30-44 mL/min per 1.73 m2) CKD, are prone to the hyperphosphatemia, inadequate vitamin D status (i.e., deficiencies of 25-hydoxy vitamin D3, and/or 1,25-dihydroxy vitamin D3), elevated concentrations of parathyroid hormone (PTH), and increased levels of the phosphatonin fibroblast growth factor 23 (FGF23), characteristic of stage 3b to 5 CKD in the native kidneys of non-KTRs[28-31,37,38].
Chronic upregulation of the hormonal mechanisms evolved to maintain calcium-phosphorus homeostasis, including normal serum phosphorus concentrations, may have adverse clinical sequelae. For example, higher serum concentrations of phosphorus (via increased dietary intake, and/or a reduced GFR) apparently stimulate production of the bone-derived hormone FGF23, which induces compensatory renal phosphorus excretion[30,36]. Elevated FGF23, in turn, may directly induce cardiac myocyte hypertrophy, clinically manifest as left ventricular hypertrophy (LVH)[36], and lower endogenous production of calcitriol (1,25-OH vitamin D3), from 25-OH vitamin D3[36]. Significant associations have been reported between FGF23 elevations LVH, CVD events, mortality, and progression to ESRD in patients with CKD stage 3-4[36]. Accordingly, sustained elevations in FGF23 concentrations, despite being a homeostatic adaptation to maintain normal serum phosphorus, may enhance the risk for developing LVH, CVD, and ESRD[36]. Higher phosphorus concentrations also stimulate chronic excess PTH secretion, eventually leading to clinical hyperparathyroidism[36,39]. Inexorable CKD progression, accompanied by further nephron loss, ultimately overwhelms these compensatory hormonal mechanisms, causing a preponderance of advanced CKD patients to manifest concurrent hyperphosphatemia, increased FGF23 and PTH levels, and suppressed 1,25-OH vitamin D3 concentrations[36,39].
The precise in vivo molecular mechanisms through which extracellular phosphate exerts its cytotoxic effects are not fully elucidated. However, investigations have demonstrated that extracellular phosphorus can form insoluble nanoparticles with calcium and fetuin-A, commonly dubbed calciprotein particles (CPPs)[39,40]. Highly bioactive ligands, CPPs can have cytotoxic effects such as causing cell death, or inducing osteogenic transformation of vascular endothelium, and renal tubular epithelium. CPPs, furthermore, are detectable in the circulation of both animal models, and humans, notably in patients with CKD, implicating their potential role in tissue injuries mediated by phosphatemia[39,40]. Recently, a novel assay of serum calcification propensity, the transformation time (“T50”) from primary calciprotein particles to secondary calciprotein particles, has been validated, and appears to reflect the pathophysiological milieu engendered by derangement of calcium-phosphorus metabolism which may predispose to ectopic, including vascular, calcification[29,30]. These interrelated perturbations-hyperphosphatemia, inadequate status of vitamin D, elevations in PTH and FGF23, and more recently, greater calcification propensity (reduced T50)-are of epidemiological, and potentially, clinical relevance, because they have been associated, with fatal CVD[26,35], graft failure[30-32,40] or rapid decline in eGFR[38], and total mortality[28-31,40], among KTRs.
Even after possible “over-adjustment” for these co-variable measures of disturbed calcium-phosphorus homeostasis-which may be in the causal pathway between “phosphorus toxicity”[39], and its clinical sequelae-the relationship between serum phosphorus concentrations, and outcomes, can persist in sizable observational KTR cohort studies. Pihlstrøm et al[31], for example, investigated the association between baseline phosphorus concentrations and major CVD events, kidney graft loss, and all-cause mortality by proportional hazard survival analyses in 1840 stable KTRs derived from the Assessment of LEscol in Renal Transplantation (ALERT) trial, a multicenter randomized, double-blind, placebo-controlled study examining the effect of fluvastatin (40-80 mg daily) on CVD (primarily coronary heart disease, CHD), and renal outcomes in 2102 KTRs. Patients were recruited a mean of 5.1 years after transplantation, and followed for 6 to 7 years. During a mean follow-up of 6.7 years, death censored graft loss was recorded in 333 patients, 277 patients experiencing a major CVD event (defined as time to cardiac death, nonfatal myocardial infarction, or undergoing a coronary revascularization procedure), and 342 died, 168 from CVD, including cerebrovascular, or other major (e.g., thoraco-abdominal aortic) vascular disease. Serum phosphorus (per 1 mg/dL increase) was associated with death from all causes, hazards ratio (HR) 1.23 (CI: 1.07-1.43, P = 0.005), and graft loss, HR 2.61 (CI: 2.25-3.04, P < 0.001), in unadjusted models. The relationship between serum phosphorus and mortality lost significance, HR 1.07 (CI: 0.89-1.28, P = 0.488), upon multivariable modeling (with PTH > 65 pg/mL), but persisted for graft loss, HR 1.52 (CI: 1.27-1.82, P < 0.001)[31]. Similarly, Wolf et al[30] studied a single center cohort of 984 chronic, stable Hungarian KTRs (median transplant vintage, 72 mo, interquartile range 40-114 mo; mean eGFR 51, SD = 21; 57% men). After a median follow-up of 37 mo (interquartile range, 35-39 mo), 87 patients died and 101 patients suffered kidney graft loss. Outcome data were analyzed in full models that adjusted for eGFR, age, gender, systolic blood pressure, body mass index, albumin, calcium, the modified Charlson Comorbidity index, and graft vintage, as well as serum phosphorus, PTH, and FGF23. These investigators reported that a 0.9 mg/dL (i.e., a 1 SD) increase in phosphorus predicted the composite endpoint of death or graft failure when analyzed as a continuous variable per SD increase (0.9 mg/dL), HR 1.23 (CI: 1.08-1.40, P = 0.002), in the fully adjusted modeling, which included FGF23[30].