Published online Mar 24, 2016. doi: 10.5500/wjt.v6.i1.249
Peer-review started: May 11, 2015
First decision: July 26, 2015
Revised: December 1, 2015
Accepted: December 13, 2015
Article in press: December 15, 2015
Published online: March 24, 2016
Processing time: 314 Days and 8.1 Hours
Lymphangioleiomyomatosis (LAM) is a rare, slowly progressive lethal lung disease primary afflicting young women. LAM is characterized by proliferation of abnormal smooth muscle cells that target the lungs, causing cystic destruction and eventual respiratory failure leading to death. Recent ten year mortality due to end stage LAM has been reported to be approximately 10%-20%, but may vary. The decline in lung function in LAM is gradual, occurring at a rate of about 3% to 15% per year but can vary from patient to patient. But recently therapy with mammalian target of rapamycin (mTOR) inhibitors such as sirolimus has shown promising results in the stabilization of lung function and reduction of chylous effusions in LAM. Lung transplantation is a viable option for patients who continue to have decline in lung function despite mTOR therapy. Unique issues that may occur post-transplant in a recipient with LAM include development of chylous effusion and a risk of recurrence. We describe a case of LAM recurrence in a bilateral lung transplant recipient who developed histological findings of LAM nine years after transplantation.
Core tip: Lymphangioleiomyomatosis (LAM) is a rare, slowly progressive lethal lung disease characterized by proliferation of abnormal smooth muscle cells that target the lungs, causing cystic destruction and eventual respiratory failure and death. Mammalian target of rapamycin (mTOR) inhibitors such as sirolimus have shown promise in stabilization of lung function. Lung transplantation is a viable option when lung function continues to decline despite use of mTOR inhibitors. However, recurrence of LAM in transplanted lung has been reported. We describe a case of LAM recurrence in a bilateral lung transplant recipient nine years after transplantation, our therapeutic approach once recurrence was documented with review of the literature.
- Citation: Zaki KS, Aryan Z, Mehta AC, Akindipe O, Budev M. Recurrence of lymphangioleiomyomatosis: Nine years after a bilateral lung transplantation. World J Transplant 2016; 6(1): 249-254
- URL: https://www.wjgnet.com/2220-3230/full/v6/i1/249.htm
- DOI: https://dx.doi.org/10.5500/wjt.v6.i1.249
Lymphangioleiomyomatosis (LAM) is a rare, progressive, cystic lung disease of young women characterized by abnormal proliferation of smooth muscle like LAM cells causing pulmonary tissue destruction and cystic changes[1]. LAM is commonly sporadic (S-LAM) however 30%-40% of cases are related with tuberous sclerosis complex (TSC-LAM) carrying mutations in TSC1 or TSC2 genes[1,2]. Interestingly, TSC2 mutation has also been reported in sporadic type which is indicative of genetic basis for LAM[1]. Patients with LAM can have several clinical findings including dyspnea on exertion, thoracic lymphadenopathy, recurrent pneumothorax, chylothorax and chylous ascites as well as angiomyolipomas and lymphangiomyomas[3]. Histologically, LAM is characterized by infiltration of abnormal spindle shaped smooth muscle cells called LAM cells. They express common melanoma related antigens (HMB-45, gp-100, MART-1) and smooth muscle antigens (S100) which are useful in histological identification[3]. Regardless of association with TSC, LAM cells have bi-allelic inactivation of TSC which is a tumor suppressor gene leading to activation of mammalian target of rapamycin (mTOR) pathway and uncontrolled proliferation and metastasis of LAM cells. Because of existence of genetic aberration in smooth muscle cell in organs other than the lungs and their ability to metastasize, recurrence of LAM after lung transplantation has been reported even in the absence of angiomyolipomas. Generally the lung function decline is extremely slow and may take up to 1-2 decades before LAM patients developed respiratory failure. Early hormonal treatment was thought to be beneficial but Oprescu et al[4] in 2013 showed that such therapy doesn’t improve the outcome. mTOR therapy with sirolimus has showed to stabilize lung function and improve quality of life. In patients that have exhausted all medical therapies, lung transplantation may be the only option. The recurrence of LAM following lung transplantation is rare and only nine cases have been reported in the literature[1,5-10]. The largest LAM database from Europe demonstrated only single digit recurrence rate of LAM after transplantation (6%-7%)[10,11]. Due to the rarity of LAM and low rate of recurrence following lung transplantation, there is a paucity in our current knowledge regarding the treatment and rate of its progression. Although looking at the LAM registry in general, out of the nine patients who underwent transplantation the most common cause of death was respiratory failure (44%) followed by infection but no documentation was noted regarding recurrence as a cause of death[4]. Here, we present the tenth case of recurrence of LAM following bilateral lung transplantation (BLT) and describe our therapeutic approach once the recurrence was demonstrated.
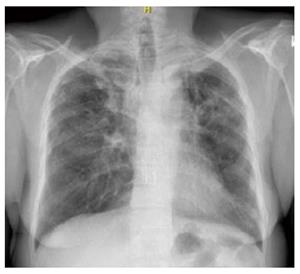
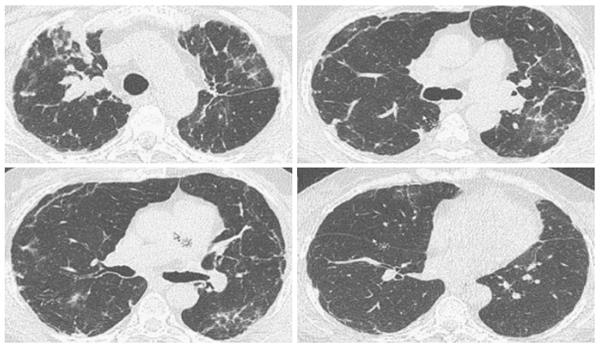
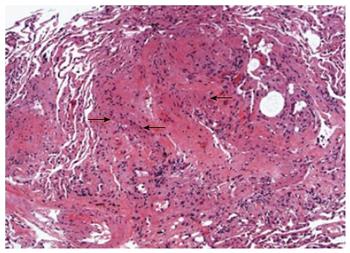
A 66-year-old African-American woman underwent sequential BLT for LAM in 1999. Her initial diagnosis of LAM was established at age 51 years when she was found to have cystic changes involving the lungs and histo-pathologic findings of abnormal proliferation of LAM cells on biopsy. The lung was the only organ involved with no evidence of angiomyolipomas before and after the transplant. Her early post-lung transplantation regimen included prednisone, tacrolimus, mycophenolate mofetil along with trimethoprim-sulfamethoxazole for pneumocystis jiroveci and acyclovir for viral prophylaxis. She underwent left upper lobe lobectomy for pseudomonas abscess in 2000 with no decline in her lung function or findings of chronic lung allograft dysfunction. Eight years later, she developed right upper lobe mass and nodules along with declining lung function and underwent BAL with transbronchial biopsy (TBBX). Her BAL demonstrated Aspergillus Ustis, Pseudomonas and Mycobacterium avium-intracellulare infection, which was treated with voriconazole, inhaled amphotericin-B, ciprofloxacin, azithromycin and ethambutol. There was no evidence of acute or chronic rejection at that time. Her symptoms improved with returning of FEV1 back to her baseline. Follow up bronchoscopy and TBBX in December 2008 revealed presence of bundles of smooth muscle cells with sparse atypical spindle/LAM cells without evidence of acute or chronic rejection or infection. Even though the immunohistochemical studies for HMB-45 were negative likely due to scant number of LAM cells, in the absence of other findings clinical diagnosis of LAM recurrence was made. She did well during the following years with stable lung function and her immunosuppression remained the same. In March 2011, she developed dyspnea on exertion despite stable lung functions which led to a bronchoscopy with TBBX which showed similar findings of LAM cells without rejection or infection. She was placed on sirolimus which was discontinued after six months of therapy due to the need for an urgent surgery. In December 2013, one year later she noticed worsening of dyspnea with gradual decline in FEV1 from 1.36 to 1.0 L (Table 1). On chest X-ray right upper lobe interstitial and nodular changes were noticed (Figure 1). A computed tomography (CT) of the chest showed right upper lobe nodules with bilateral interstitial thickening and scattered ground glass opacities which were unchanged from 2008 (Figure 2). A flexible bronchoscopy with BAL and TBBX again showed sparse LAM cells (Figure 3) negative for HMB-45 with no evidence of infection and acute or chronic rejection suggesting LAM recurrence as likely cause of her symptoms and findings on CT.
| PreTx-1999 | PostTx-2000 | 2009 | 2011 | 2013 | 2014 | |
| FVC | 0.81 (27%) | 1.70 (57%) | 2.06 (71%) | 1.90 (80%) | 1.83 (79%) | 1.76 (77%) |
| FEV1 | 0.26 (10%) | 1.39 (56%) | 1.36 (59%) | 1.33 (71%) | 1.12 (62%) | 1.0 (56%) |
| FEV1/FVC | 32.1 (39%) | 81.6 (100%) | 65.7 (83%) | 69.9 (89%) | 61.2 (78%) | 57.1 (73%) |
In an effort to stabilize lung function, tacrolimus was switched to sirolimus monotherapy resulting in brief stabilization of lung function. She subsequently developed respiratory failure due to HINI viral infection and mycoplasma pneumonia a few months later. However, despite therapy for the viral and mycoplasma infections her lung functions continued to deteriorate with a decline in her functional status, this was thought to be due to chronic lung allograft dysfunction of bronchiolitis obliterance type. She was not considered for re-transplantation due to her deconditioned state and age. She ultimately entered hospice care and died of complications likely due to chronic rejection along with LAM recurrence.
LAM is a rare disease with prevalence of 2 per 1 million of the population[3]. It almost exclusively affects young women. With respect to the rarity of LAM and limited knowledge on treatment and prognosis of these patients, here we presented a fifteen year follow up post-bilateral lung transplant of a patient with LAM recurrence. It is evident from the literature that LAM could recur as early as within two years after the lung transplantation. Although the recurrence of LAM is rare, the post-transplant survival of these patients when compared to all other indications of transplant is better[11]. But the number of patients that have undergone transplantation for LAM as the primary indication is very small and predications regarding this disease and survival post-transplant should be tempered.
To date lung transplantation represents one of the most effective and acceptable therapeutic option for LAM patients with respiratory failure. Both single and BLTs have been performed (Table 2). The estimated five year post lung transplant survival among LAM patients is between 60%-70%. The recurrence is rare, and the rate between 3.7%-7% has been reported in the largest European and United States studies[10,11]. It is likely that recurrence rate could be higher in long term survivors as early recurrence may be asymptomatic. These studies demonstrated that respiratory failure, BOS and infectious complications are the most common causes of death in the later period post-transplant similar to other cases of transplant. The LAM recurrence is rare and doesn’t compromise long term survival. As in our patient LAM recurrence diagnosis was made after nine years post-transplant and remained asymptomatic for at least four more years.
| Ref. | No. of patients | Type oftransplant | Age at transplantation(yr) | Donor | Post-transplant immunosuppressive drugs | Post-transplant complications | Outcomes |
| O'Brien et al[5] | 1 | Single right | NA | NA | NA | NA | NA |
| Bittmann et al[8,9] | 1 | Single right | 34 | Male Cadaveric | NA | Pneumothorax | Survival 2 yr COD: pneumothorax and hypoxemia |
| Karbowniczek et al[1] | 1 | Single right | 42 | Male cadaveric | Cyclosporine, Azathioprine, Prednisone | Chylous pleural effusion | Survival 2 yr COD: Aspergillus pneumonia, |
| Recurrence of LAM was confirmed on autopsy | |||||||
| Chen et al[7] | 1 | Bilateral Living-donor lobar | 23 | Mother and sister | NA | Massive chylous pleural effusion and ascites | Not known, but she was diagnosed with recurrence of LAM in left lung 2 yr after transplantation due to characteristics cystic changes and pathological confirmation |
| Sugimoto et al[6] | 1 | Bilateral Living-donor lobar | 23 | Brother | Tacrolimus, Prednisone | Un-eventful course | Dyspnea and pleural effusion following 5 yr post-transplant, sirolimus 1-2 mg/d helped resolve pleural effusion and improved lung function and symptoms |
| Benden et al[10] | 4 | NA | NA | NA | Cyclosporine, Tacrolimus, Prednisone, Azathioprine | Surgical complications, respiratory tract infections, pneumothorax, pulmonary embolism | Not specified for recurrence of LAM, 5 yr survival was estimated to be 34% |
Due to the limited knowledge regarding specific treatment of LAM, the goal remains relief of symptoms and management of complications. In 2011 MILES study showed promising results of sirolimus in LAM patients with stabilization of lung function with improvement in quality of life and functional performance[12]. In Europe, the dose of rapamycin varies individually from 0.5 mg every other day, to 2 mg daily while in MILES study the dose was adjusted by keeping serum levels between 5-15 μg/dL[10,12]. As LAM recurrence post lung transplant is mostly asymptomatic it is unclear when to start mTOR inhibitors. It is less likely that a large, randomized trial in this group of patients post-transplant can be carried out due to the rare nature of this disease; however our clinical acumen supports the notion that in lung transplant recipients with LAM, sirolimus should be considered as a primary anti-rejection medication either as mono or as dual therapy with a calcineurin inhibitors (CNI). Theoretically, therapy with mTOR inhibitors is likely to delay the progression or recurrence of LAM. However, there are no randomized trials to support the recommendation due to the rarity of the disease and its presentations. It is advisable to place the patients on lifelong mTOR inhibitors following the lung transplantation to delay the recurrence of LAM in the allograft. Intolerance or complications of mTOR inhibitors may limit their use in some patients, who may then require re-transplantation.
Our case highlights the possibility of LAM recurrence following BLT. Though rare, it remains asymptomatic and doesn’t seem to affect long term survival. The most common cause of death remains respiratory failure, development of BOS and infectious complications. Sirolimus should be considered as a primary anti-rejection medication either as monotherapy or as dual therapy with a CNI in this patient population but timing of initiation remains under debate.
A 66 year of women post bilateral lung transplantation for lymphangioleiomyomatosis (LAM) presented with dyspnea on exertions 9 years post transplantation.
Her clinical examination remained unremarkable and didn't change since prior visits.
Acute cellular rejection, chronic rejection, obliterative bronchiolitis syndrome, opportunistic infection, recurrence of LAM.
All laboratory work up was within normal limits.
Chest X-ray showed chronic right upper lobe interstitial and nodular changes. CT of the chest showed right upper lobe nodules with bilateral interstitial thickening and scattered ground glass opacities which were unchanged from prior studies.
Histopathological examination of the transbronchial biopsy revealing spindle shaped LAM cells without evidence of infection or rejection, suggestive of LAM recurrence.
Calcineurin inhibitor immunosuppressive therapy was switched to sirolimus monotherapy but had to be stopped due to surgery. Later again restarted resulted in brief stabilization of lung function. However the patient developed complications of infection and rejection which proved to be fatal.
Lung transplantation represents one of the most effective and acceptable therapeutic option for LAM patients with respiratory failure. The recurrence is rare and mostly remains asymptomatic. Sirolimus has shown to stabilized lung function in patients with LAM. However, post transplantation its role is not clear.
Broncholitis obliterans syndrome is a form of chronic lung allograft dysfunction that commonly presents with obstructive ventilatory defect and decline in forced expiratory volume in 1 s post lung transplantation.
LAM is a rare disease and its recurrence post lung transplantation is even rarer. Sirolimus therapy slows the progression of disease in patient with LAM. This clinical acumen supports the notion that in lung transplant recipients with LAM, sirolimus should be considered as a primary anti-rejection medication either as monotherapy or as dual therapy with a calcineurin inhibitors. Intolerance or complications of mammalian target of rapamycin inhibitors may limit their use in some patients, who may then require re-transplantation.
It is a very rare phenomenon.
P- Reviewer: Mehdi I S- Editor: Song XX L- Editor: A E- Editor: Wang CH
| 1. | Karbowniczek M, Astrinidis A, Balsara BR, Testa JR, Lium JH, Colby TV, McCormack FX, Henske EP. Recurrent lymphangiomyomatosis after transplantation: genetic analyses reveal a metastatic mechanism. Am J Respir Crit Care Med. 2003;167:976-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 174] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 2. | Maurer JR, Ryu J, Beck G, Moss J, Lee JC, Finlay G, Brown K, Chapman J, McMahan J, Olson E. Lung transplantation in the management of patients with lymphangioleiomyomatosis: baseline data from the NHLBI LAM Registry. J Heart Lung Transplant. 2007;26:1293-1299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Johnson SR, Cordier JF, Lazor R, Cottin V, Costabel U, Harari S, Reynaud-Gaubert M, Boehler A, Brauner M, Popper H. European Respiratory Society guidelines for the diagnosis and management of lymphangioleiomyomatosis. Eur Respir J. 2010;35:14-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 351] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 4. | Oprescu N, McCormack FX, Byrnes S, Kinder BW. Clinical predictors of mortality and cause of death in lymphangioleiomyomatosis: a population-based registry. Lung. 2013;191:35-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 5. | O’Brien JD, Lium JH, Parosa JF, Deyoung BR, Wick MR, Trulock EP. Lymphangiomyomatosis recurrence in the allograft after single-lung transplantation. Am J Respir Crit Care Med. 1995;151:2033-2036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 64] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Sugimoto R, Nakao A, Yamane M, Toyooka S, Okazaki M, Aoe M, Seyama K, Date H, Oto T, Sano Y. Sirolimus amelioration of clinical symptoms of recurrent lymphangioleiomyomatosis after living-donor lobar lung transplantation. J Heart Lung Transplant. 2008;27:921-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Chen F, Bando T, Fukuse T, Omasa M, Aoyama A, Hamakawa H, Fujinaga T, Shoji T, Sakai H, Hanaoka N. Recurrent lymphangioleiomyomatosis after living-donor lobar lung transplantation. Transplant Proc. 2006;38:3151-3153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Bittmann I, Rolf B, Amann G, Löhrs U. Recurrence of lymphangioleiomyomatosis after single lung transplantation: new insights into pathogenesis. Hum Pathol. 2003;34:95-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 81] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 9. | Bittmann I, Dose TB, Müller C, Dienemann H, Vogelmeier C, Löhrs U. Lymphangioleiomyomatosis: recurrence after single lung transplantation. Hum Pathol. 1997;28:1420-1423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 36] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Benden C, Rea F, Behr J, Corris PA, Reynaud-Gaubert M, Stern M, Speich R, Boehler A. Lung transplantation for lymphangioleiomyomatosis: the European experience. J Heart Lung Transplant. 2009;28:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 11. | Kpodonu J, Massad MG, Chaer RA, Caines A, Evans A, Snow NJ, Geha AS. The US experience with lung transplantation for pulmonary lymphangioleiomyomatosis. J Heart Lung Transplant. 2005;24:1247-1253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 12. | McCormack FX, Inoue Y, Moss J, Singer LG, Strange C, Nakata K, Barker AF, Chapman JT, Brantly ML, Stocks JM. Efficacy and safety of sirolimus in lymphangioleiomyomatosis. N Engl J Med. 2011;364:1595-1606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 882] [Cited by in RCA: 785] [Article Influence: 56.1] [Reference Citation Analysis (0)] |