Published online Mar 24, 2016. doi: 10.5500/wjt.v6.i1.10
Peer-review started: August 5, 2015
First decision: September 21, 2015
Revised: November 4, 2015
Accepted: December 3, 2015
Article in press: December 4, 2015
Published online: March 24, 2016
Processing time: 227 Days and 17.3 Hours
Corneal transplantation is the most common surgical procedure amongst solid organ transplants with a high survival rate of 86% at 1-year post-grafting. This high success rate has been attributed to the immune privilege of the eye. However, mechanisms originally thought to promote immune privilege, such as the lack of antigen presenting cells and vessels in the cornea, are challenged by recent studies. Nevertheless, the immunological and physiological features of the cornea promoting a relatively weak alloimmune response is likely responsible for the high survival rate in “low-risk” settings. Furthermore, although corneal graft survival in “low-risk” recipients is favourable, the prognosis in “high-risk” recipients for corneal graft is poor. In “high-risk” grafts, the process of indirect allorecognition is accelerated by the enhanced innate and adaptive immune responses due to pre-existing inflammation and neovascularization of the host bed. This leads to the irreversible rejection of the allograft and ultimately graft failure. Many therapeutic measures are being tested in pre-clinical and clinical studies to counter the immunological challenge of “high-risk” recipients. Despite the prevailing dogma, recent data suggest that tissue matching together with use of systemic immunosuppression may increase the likelihood of graft acceptance in “high-risk” recipients. However, immunosuppressive drugs are accompanied with intolerance/side effects and toxicity, and therefore, novel cell-based therapies are in development which target host immune cells and restore immune homeostasis without significant side effect of treatment. In addition, developments in regenerative medicine may be able to solve both important short comings of allotransplantation: (1) graft rejection and ultimate graft failure; and (2) the lack of suitable donor corneas. The advances in technology and research indicate that wider therapeutic choices for patients may be available to address the worldwide problem of corneal blindness in both “low-risk” and “high-risk” hosts.
Core tip: Corneal grafts enjoy a high acceptance rate when performed in “low-risk” host graft beds. This is associated with a relatively weak alloimmune response. However, in “high-risk” hosts where the immunologically quiescent homeostatic environment of the cornea is compromised prior to graft procedure, heightened immune responses significantly increase the risk of graft rejection. Clinical approaches such as tissue matching and long-term immunosuppression could be beneficial in preventing graft rejection especially in “high-risk” settings. In addition, promotion of transplant tolerance by cell-based therapies and use of corneal “substitutes” such as collagen-based hydrogels are promising alternatives for “high-risk” recipients.
- Citation: Yu T, Rajendran V, Griffith M, Forrester JV, Kuffová L. High-risk corneal allografts: A therapeutic challenge. World J Transplant 2016; 6(1): 10-27
- URL: https://www.wjgnet.com/2220-3230/full/v6/i1/10.htm
- DOI: https://dx.doi.org/10.5500/wjt.v6.i1.10
Corneal transplantation is the most common and successful form of solid organ transplantation[1]. It is considered the primary treatment to restore vision to patients with corneal blindness - a leading cause of blindness worldwide[1]. In the year 2014-2015, 3520 cases of corneal transplantation were performed in the United Kingdom compared to 2069 cases of kidney and 842 liver transplantations[2]. The corneal graft survival rate is 86% at 1-year for penetrating keratoplasty (PK), despite the fact that corneal grafts are rarely tissue matched for histocompatibility leukocyte antigens (HLA) and systemic immunosuppressant medications are not routinely used[3]. However, the 15-year graft acceptance declines to 55%, which is similar to survival rates in other forms of solid organ transplantation[3,4]. More importantly, corneal grafts performed in “high-risk” recipients have a much reduced acceptance rate with a 5-year survival of 54.2% compared to 91.3% in recipient eyes that have not been overtly inflamed. The “high-risk” recipients were defined by the Collaborative Corneal Transplantation Studies Research Group as two or more quadrants of the cornea vascularized or a previous graft had been rejected[5,6]. Unfortunately, any previous inflammatory response in the ocular surface such as corneal infectious diseases (e.g., herpetic simplex keratitis or trachoma), severe trauma, alkali burn and previously failed graft place the host cornea at risk of corneal neovascularization[7,8]. Furthermore, “high-risk” recipients not only experience higher graft failure rate but also present with more frequent acute rejection episodes compared to “low-risk” grafts[7].
It is worth emphasizing here the difference between corneal graft failure and corneal graft rejection. In brief, clinical corneal graft failure is the irreversible loss of graft clarity, and rejection is one of the causes of corneal graft failure. However, the loss of graft clarity can be due to a number of reasons including infection, surgical trauma, glaucoma, aging as well as rejection, which is an exclusively immunological event. Graft rejection is moreover the most common cause of graft failure accounting for over 30% of cases[3,4]. The characteristic features of corneal graft rejection in which there is an immunological response against donor antigens are graft oedema, keratic precipitates on the endothelium of the transplanted graft and the presence of rejection lines [formed due to accumulation of inflammatory cells on corneal epithelium or endothelium (Khodadoust line)] together with the presence of inflammatory cells in the anterior chamber (AC) of the eye[9,10]. This review article focuses on the mechanism of corneal graft rejection revealed through experimental studies as well as current and potential treatments for corneal graft rejection.
The immunological responses mediating corneal graft rejection have been studied extensively using animal models, and especially in the well-established murine model of full-thickness orthotopic corneal transplantation. Similar to human corneal grafting, murine corneal allografts performed in an uninflamed graft bed, despite being mismatched for both major and minor histocompatibility complex antigens, half of the grafts failed, whereas in the inflamed “high-risk” graft bed, almost all of the grafts failed and with an increased tempo depending on the level of major histocompatibility complex (MHC)/non-MHC antigen mismatch[11,12].
Corneal allograft rejection represents a form of delayed-type hypersensitivity (DTH) response, predominantly mediated by allospecific CD4+ T cells. The response can affect one or more of the three cellular layers in the cornea (epithelium, stroma and endothelium)[13-15]. However, the endothelial layer is the main target in PK with graft failure occurring when > 50% of the corneal endothelium is lost[16,17]. As the corneal endothelium possesses limited regenerative property and is the essential layer responsible for maintaining corneal deturgescence, alloimmune responses directed at the corneal endothelium eventually result in stromal and epithelial oedema and with irreversible corneal opacification[16].
During the surgical procedure, trauma to corneal tissues induces local production of cytokines and chemokines such as interferon (IFN)-γ, interleukin (IL)-1β, IL-6, IL-10 and CXCL2 which initially peaks at day 3-5 post graft procedure[18]. Meanwhile, infiltration of innate immune cells occurs into the cornea including dendritic cells (DC), macrophages, natural killer (NK) cells and neutrophils[19]. A unique feature of corneal allograft compared to other forms of solid organ transplantation is that the rejection response is mediated almost exclusively through the indirect pathway as the healthy central donor cornea possesses low numbers of antigen presenting cell (APC). Therefore, the activation of naïve T cells occurs predominantly through host APC newly recruited from the bone marrow and presenting donor antigenic peptides, including HLA antigens to host naïve T cells. In contrast, the direct pathway involves the direct recognition of alloantigen on donor origin APC which have migrated from the graft tissue to the local draining lymph nodes (DLN), by host naïve T cells[20,21]. Newly recruited bone marrow APC after processing antigens from the corneal allograft then migrate via lymphatic vessels to the DLN where they activate naïve T cells and mediate immune rejection against corneal graft.
Corneal allograft rejection is predominantly mediated through CD4+ Th1 cells that secrete cytokines IFN-γ, tumour necrosis factor (TNF)-α and IL-2[14,22]. In the rejected graft, abundant neutrophils, macrophages and CD4+ T cells are present[23]. Furthermore, studies have suggested that CD4+ T cells may function directly as effector cells mediating graft rejection as adoptive transfer of allogeneic CD4+ T cells to beige nude mice (impaired T cell production, but do produce macrophages) resulted in graft rejection even when macrophages were depleted[24]. Although in vitro experiments showed the ability of allo-specific CD4+ T cells to induce apoptosis of corneal endothelial and epithelial cells, investigations of the involvement of perforin or Fas-induced apoptosis by CD4+ T cells have eliminated both mechanisms[24]. In addition, allografts deficient in Fas-ligand (FasL or CD95L) demonstrated 100% rejection, further indicating that mechanisms other than Fas-FasL were used by CD4+ T cells in mediating graft rejection while FasL expressed in the cornea was more likely to promote immune privilege[25]. Nevertheless, prolonged exposure to proinflammatory Th1 type cytokines IFN-γ, TNF-α and IL-1 was shown to induce apoptosis of corneal endothelium and upregulation of inducible nitric oxide synthase, the latter generating nitric oxide which causes direct cytotoxicity to endothelial cells[26]. In addition, inhibition of inducible nitric oxide synthase showed protection against cytokine-mediated corneal tissue damage as well as prolonged allograft survival when administered systemically[26,27]. However, studies investigating the role of Th17 cells in mediating corneal allograft rejection have shown controversial results. While some studies showed that IL-17 demonstrated pathological effect during early corneal allograft rejection[28], recent findings have suggested that Th17 cells are involved in promoting allograft acceptance in the early post graft stages followed by a Th1 dominant response mediating graft rejection[29,30]. Interestingly, further investigation also indicated that enhanced expression of IL-17 at a late stage (> 45 d) post corneal allograft impaired graft survival. Late stage anti-IL-17 treatment not only reversed corneal opacity but also reduced the level of neovascularization[30]. Strikingly, IL-17 knockout mice that received anti-IFN-γ treatment failed to reveal any significant difference in graft survival compared to wild type mice. This indicates that mechanisms other than Th1 and Th17 cells were involved, which may be due to the redundancy of the immune system promoting an alternative and exaggerated Th2 response capable of mediating graft damage[29,31].
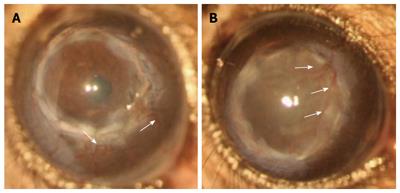
The relatively high acceptance of corneal allografts compared to other forms of solid organ transplantation has been largely ascribed to the immune privilege of the eye[32,33]. Immune privilege was a term coined by Sir Peter Medawar in the 1940s where skin allografts placed in the AC of the eye evaded immunological rejection but only if the graft was not invaded by blood vessels[34]. Extensive study of this phenomenon ascribed immune privilege especially in the context of corneal allograft to: (1) the reduced expression of MHC class I molecules in corneal tissue and the lack of constitutive MHC class II expression; (2) the absence of both blood and lymphatic vessels in the cornea; (3) the lack of “passenger leukocytes” in the cornea; (4) presence of immunoregulatory molecules in the AC and on corneal cells; and (5) anterior chamber-associated immune deviation (ACAID) induced post corneal allograft[32,33]. However, recent studies have shown that the corneal tissue possesses a population of MHCII+ leukocytes with increased numbers towards the peripheral cornea[20,35-40]. Furthermore, corneal neovascularization rapidly develops post corneal grafting; within 1 wk, both blood and lymphatic vessels are already invading the donor cornea thus providing access of immune cells to the cornea as well as increasing homing of APC to the DLN. Furthermore, vessels persist regardless of the fate of the graft (Figure 1)[11]. This means that unmatched corneal allografts are accepted in 50% cases indefinitely despite the presence of blood and lymphatic vessels and infiltration of host immune cells.
In contrast to immune privilege, which describes the local acceptance of grafts within the eye, ACAID is a systemic immune response. ACAID is an unusual suppression of the systemic immune system whereby alloantigen placed in the AC of the eye elicits a regulatory response in the spleen, which upon further exposure suppresses the immune response to the alloantigen (e.g., skin graft), and prevents graft rejection[41]. This phenomenon has been shown to be mediated through CD8+ T regulatory cells (Treg) generated in the spleen[33]. It was believed that ACAID is induced not only when alloantigen is inoculated into the AC but also post corneal allograft due to shedding of alloantigenic materials from graft endothelial cells[42]. However, growing evidence suggested that Treg induced after corneal allograft show a phenotype of CD4+CD25+Foxp3+ whereas effector Treg in ACAID is CD8+ Treg[13,43,44]. Furthermore, blockade of CD8+ T cells only abrogated ACAID but with no effect on corneal allograft survival while blockade of IL-17A which reportedly impaired allograft induced Treg suppressive function also reduced corneal graft survival, but did not alter the induction of ACAID[43,45].
It is clear therefore that most of the proposed mechanisms to explain the phenomenon of immune privilege have proven not to be true. Instead, the prolonged acceptance in “low-risk” corneal allograft compared to other solid organ transplants may simply be due to the effect of an overall weak indirect alloimmune response as a result of the low levels of alloantigen acting together with local and systemic regulatory mechanisms. First, the insufficient strength of the alloimmune response in the initial stages of allosensitization is likely due to the limited number of donor derived passenger leukocytes particularly in the central cornea, and low expression of histocompatibility antigens. In addition, while other forms of solid organ transplants are rich in vascular networks and donor passenger leukocytes undergo both acute (direct pathway) and chronic (indirect pathway) rejection[46], corneal allograft rejection is predominantly mediated through the indirect pathway[47-50]. In the healthy cornea, the majority of MHCII+ cells are CD11b+ and CD11c+ cells distributed at the peripheral cornea whereas the central cornea which is used as donor cornea during corneal allograft procedure was believed to be devoid of MHCII+ cells but contains a population of MHC class II negative immature DC and Langerhans cells[20,36-39]. Recently, studies using CD11c-eGFP mice have shown that a reduced number of MHCII+CD11c+ cells are present in the central cornea and exclusively located in the corneal epithelial basal layer beneath which a layer of MHCII+CD11b+ cells were also observed[40]. However, the expression level of MHC class II molecules on these cells was found to be at a relatively low level indicating that these cells together with MHC class II negative DC and Langerhans cells are more likely to promote immune tolerance rather than immunity[40]. We reported that in a “low-risk” setting, there was no evidence of donor leukocyte migration to the DLN[20]. Therefore, corneal allograft rejection in “low-risk” setting is exclusively mediated by indirect allorecognition. The lack of both blood and lymphatic vessels in initial stages post graft may delay the infiltration of host innate immune cells including APC, thus becoming a limiting factor for initiating a sufficient rejection response before the development of an established vessel network. Second, while new vessels invade the graft, other regulatory mechanisms including the induction of Treg come into play. It was found that rather than changes in frequency, the expression level of Foxp3 was significantly higher in the DLN of accepted allografts compare to either rejected or syngeneic grafts[44]. Moreover, adoptive transfer of Treg has been shown to promote corneal graft survival[51], associated with production of IFN-γ and IL-17A[45,52]. It was shown that IL-17A is required for the effective suppressive function of Treg in promoting allograft survival and unusually supports a protective role for Th17 cells during corneal allograft rejection[45]. Interestingly, IFN-γ was required for generation of Treg under fully MHC and minor histocompatibility antigen mismatched condition, whereas IFN-γ inhibited the generation of allospecific Treg when only MHC or minor histocompatibility antigen was mismatched[52]. These somewhat puzzling findings suggest that possibly the balance between Th1, Th17 and Treg responses largely dictates the outcome of the graft. Consequently, when an effective peripheral tolerance response fails to be induced, the default balance favours a Th1 response and as such, promotes allograft rejection.
Lastly, the physiological milieu of the cornea and the anterior segment of the eye possess many immunoregulatory molecules that protect the cornea from immune mediated attack. For instance, FasL is expressed extensively in ocular compartments including all three cellular layers of the cornea[53,54]. Several studies have reported that FasL expressed in the eye is responsible for inducing apoptosis of infiltrating Fas-bearing leukocytes, especially lymphocytes. Furthermore, its expression in particular on corneal endothelial cells plays an important role in corneal allograft survival, since donor corneas lacking FasL in the endothelium and stroma but not epithelium were rejected vigorously compared to normal FasL expressing donor corneas[25,53,55,56]. Moreover, the interaction of Fas-FasL induced apoptotic cell death was shown to be an important mechanism in the induction of immunological tolerance to antigens injected into the AC, as in the absence of apoptotic cell death, immune tolerance failed to be elicited[55]. Tumour necrosis factor-related apoptosis-inducing ligand (TRAIL) is also capable of inducing apoptosis of various tumour cells and its functional expression was demonstrated in corneal tissue[57]. Overexpression of TRAIL in donor corneal tissue has been shown to significantly delay graft rejection, accompanied by an increased number of apoptotic cells in the graft[58]. However, other groups in attempts to establish a correlation between TRAIL expression and allograft survival have not found an effect[13].
Programmed death ligand-1 (PD-L1 or B7-H1) is another molecule with similar functions to FasL and TRAIL by promoting apoptosis of infiltrating PD-1 positive CD4 and CD8 T lymphocytes[59]. PD-L1 belongs to the B7 superfamily providing costimulatory signals to T cells and is constitutively expressed in both murine and human corneal tissues[59-61]. Its blockade or deficiency is associated with increased corneal graft rejection whereas strong ligation between PD-L1 and PD-1 revealed prolonged allograft survival[59-62].
Complement regulatory proteins were found to be expressed by corneal tissues and in the AC, which protects the cornea from being the target of complement-fixing antibodies[63,64]. One such molecule strongly expressed in the corneal epithelium is decay-accelerating factor (DAF) which function is to inhibit complement deposition on the cell surface, thus preventing autologous complement activation[63,65]. Further studies have suggested that DAF shows regulatory properties towards the T cell response[66]. DAF deficiency on donor or recipient cornea accelerated graft rejection together with increased numbers of IFN-γ producing T cells, reduced levels of transforming growth factor (TGF)-β and IL-10[66]. Furthermore, NK cells attack cells that lack the expression of MHC class I molecules and the poor expression of MHC class I by corneal endothelial cells makes them prone to NK cells mediated tissue damage[13,67]. However, studies have shown that the AC contains NK cell inhibitory factors such as macrophage migration inhibitory factor and TGF-β, which prevent corneal endothelial cells becoming targets for NK cells[13,68,69]. Galectin-9 was demonstrated as another immunosuppressive molecule constitutively expressed on corneal tissues, which significantly promoted corneal allograft survival by inducing apoptosis of alloreactive T cells[70].
Many other immunoregulatory molecules present in the anterior segment of the eye have also been demonstrated to have potential in prolonging corneal allograft survival including alpha-melanocyte stimulating hormone, calcitonin gene-related peptide, vasointestinal peptide, somatostatin or indoleamine dioxygenase[71-73].
Although clinically and experimentally, there are many causes of a “high-risk” graft bed, a common denominator is an already activated immune system both systemically and locally (cornea and eye-DLN) providing a proinflammatory milieu unlike the situation in “low-risk” dormant recipients. In general, murine corneal allografts performed in “high-risk” recipients not only experience over 95% graft rejection rates compared to 50% in “low-risk” recipients, but in addition grafts are usually rejected rapidly, 2 wk post-surgery compared to 3-4 wk in uninflamed corneas[12]. As early as 24 h post corneal allograft, increased levels of chemokine mRNA expression including CCL2 and CXCL2 were observed in “high-risk” recipients compared to “low-risk” recipients[74]. No difference in the number of infiltrating leukocytes was observed between “high-risk” and “low-risk” recipients at day 1 suggesting the source of the early increased chemokine levels was from resident corneal cells[74]. Increased numbers of infiltrating macrophages and neutrophils in “high-risk” recipients were found at day 3 recruited by CCL2 and CXCL2 which leads to a dramatic increase in chemokine levels in the “high-risk” group at day 6 post graft with a broader spectrum of chemokines including CCL2-CCL5, CCL11, CXCL2 and to a lesser extent CXCL10[74]. Furthermore, the local proinflammatory environment in “high-risk” recipients post-surgery contains high levels of vascular adhesion molecules further increasing the recruitment of both innate immune cells and memory T cells to the cornea[75]. Accordingly, the increased levels of innate leukocytes especially macrophages and DC which serve as APC together with pre-existing vascularization significantly increases the number of APC reaching the DLN within a shorter period compared to “low-risk” recipients. In addition, although the presence of donor APC in the DLN as well as their ability to upregulate expression of MHC class II post “high-risk” allograft were reported in several studies, it remains controversial whether direct pathway-activated allospecific T cells play a role in mediating corneal allograft rejection[76] or rather promotes tolerance to the allograft[77]. Depletion of leukocytes from donor corneas prior to “high-risk” corneal allograft as well as using CCR7-/- donor corneas failed to demonstrate a significant difference in allograft survival[77,78]. Thus, these studies indicate that the frequency of donor APC is unlikely to be sufficient to mediate significant acute graft rejection through direct antigen presentation during corneal allograft rejection. Therefore, it remains likely that the heightened innate immune responses leading to increased infiltration of host APC presenting alloantigen to host T cells is (indirect pathway) responsible for the increased rejection of “high-risk” grafts, as well as “low-risk” grafts as described in previous sections.
Neovascularization is the common feature that distinguishes “high-risk” and “low-risk” host graft beds. In “high-risk” corneal allografts, despite vascularization of the cornea prior to the graft procedure, further vascularization is also induced after grafting[79]. Lymphatic vessels in the cornea act as conduits for efferent migration of APC to DLN while blood vessels provide afferent access of inflammatory leukocytes to the cornea; infiltrating leukocytes then act as a further source of pro-angiogenic factors. Studies have shown that inhibition of either blood or lymphatic vessels was able to significantly prolong graft survival comparable to “low-risk” recipients suggesting that either disruption of efferent or afferent access of leukocytes can suppress alloimmune responses[80-82]. Furthermore, although the definition of “high-risk” recipients included corneas with two or more quadrants with evidence of vascularization, clinically the incidence of graft rejection has been shown to increase with increased levels of vascularization present prior to the corneal graft procedure[83], further suggesting that increased corneal vascularization shifted the balance towards immune rejection.
The adaptive immune response was also shown to be elevated in various ways among “high-risk” recipients. One of the consequences of an increased innate immune response is the increased number of APC with the ability to activate naïve T cells. Indeed, the DTH response in “high-risk” recipients was found significantly accelerated compared to “low-risk” recipients[12,47]. Furthermore, the allograft was rejected promptly if the recipient had been previously sensitized with a previous corneal graft or skin graft[84]. It was clearly shown that in “high-risk” recipients which previously experienced graft rejection, the effector/memory T cell response promoted accelerated rejection of regraft of the same donor origin[85]. It is also possible that memory T cells due to a previous infectious disease of the cornea such as herpes keratitis becomes activated by bystander mechanisms, when a subsequent corneal graft procedure is performed (Kuffova et al, in press). Thus, two types of increased adaptive immune responses are present in “high-risk” recipients to promote graft rejection, namely, enhanced activation of allospecific T cells as well as reactivation of memory T cells due to previous immune mediated conditions of the cornea such as infection or previous graft.
Tissue matching is not routinely performed clinically for patients undergoing corneal transplantation due to its remarkable success rate in “low-risk” recipients[3,86,87]. However, the markedly poorer prognosis of “high-risk” grafts suggests this should be reconsidered, although, the controversy has not been resolved[6,7,88]. Some of the studies addressing this issue are reviewed below: In clinical practice, matching for HLA class I antigens under ”low-risk” and HLA class II antigens under “high-risk” conditions have both been shown to significantly reduce the risk of rejection[89,90]. In a pre-clinical model, minor H antigen incompatibility has been shown to have higher rates of rejection even in “low-risk” grafts than MHC mismatches, and similarly, improvement in prognosis of “high-risk” grafts were demonstrated in a clinical study as well, when matched for minor H antigens[91,92]. Differences in donor-recipient blood groups may also contribute to graft rejection in “high-risk” recipients as ABO antigens are expressed in the corneal epithelium and endothelium[93]. ABO and Rh ± incompatibility were shown to have a significant influence on corneal allograft rejection in earlier clinical studies[6,94], but recently, no influence in allograft failure due to immune rejection was shown in a 5-year follow up clinical study in “low-risk” corneal transplants. However, conflicting results were reported in “high-risk” cases[93,95]. The major reasons for differences in success rates of allografts in humans are thought to be due to surgical techniques, competency of surgeons and properly distinguished risk factors associated with graft bed[96]. Furthermore, a recent review identified the lack of specificity and low sensitivity in tissue typing methods compromise the quality of HLA matching in different centres performing clinical studies[97].
A possible reason behind the high success rates of acceptance of corneal allograft in “low-risk” recipients without tissue matching is, regardless of the technical factors discussed above, the relative weakness of the alloimmune response (as discussed above), which is relatively easily controlled with daily application of topical steroidal drops. This concept is supported by the observation that more frequent graft rejection “episodes” and eventual graft failure develop after topical steroids are discontinued in “low-risk” graft recipients (e.g., after first year post corneal transplantation)[98-100].
The shortage of donor corneas worldwide, the high demand and the long wait time for the “right” donor match restricts the wider application of corneal grafts, while on some occasions, it has to be performed as an emergency procedure with high risk of failure[101,102]. As the immunological events behind the “high-risk” grafts lead inevitably to irreversible graft failure, a treatment protocol is currently being developed which will assess and compare the HLA matching along with longer wait time for the surgery, but may be associated with more favourable graft survival outcome especially in “high-risk” graft recipients[101].
Support for tissue matching comes from experimental studies using a “high-risk” regraft model, with single antigen disparity, in which antigen-specific memory T cell activation was directly correlated with accelerated graft rejection. Thus matching is advised to prevent risk of rejection by ensuring that a donor regraft has no or minimal concordance with the original graft[85].
Generally, for “low-risk” patients, treatment with topical steroids will prevent rejection as indicated above. Daily application of steroid drops plays a major role in local control of the host immune system by preventing the invasion of IL-1 and IL-6 producing macrophages and subsequent initiation of adaptive T cell responses[103]. However, topical steroids alone are not sufficient in preventing rejection in “high-risk” recipients due to much stronger immune response generated by unfavourable microenvironment of the graft bed[103]. Though clinical studies have shown improvement of graft outcome by administering systemic (oral) steroids, steroid treatment alone is not advised in the long-term due to side effects[104-106]. Further studies have shown that use of systemic immunosuppressive therapy with either cyclosporine A (CsA) or mycophenolate mofetil (MMF) is successful in preventing corneal allograft rejection, but MMF has shown greater success than CsA[104,107-109]. Intraocular delivery of immunosuppressants has been shown to prevent “high-risk” graft rejection in rabbits while topical treatment did not show any significant effect[110,111].
Biologics, the novel immunosuppressive agents, comprised mainly of recombinant antibodies and fusion proteins, bind to receptors and block immune cells; similarly inhibitors of mediators of corneal inflammation and vascularization like IL-2 receptor (IL-2R), TNF-α, vascular endothelial growth factor (VEGF) and CCL2, all of which are involved in allograft rejection may be effective[112]. Local anti-VEGF treatment is a proficient strategy to reduce corneal angiogenesis and lymphangiogenesis and this may reduce the incidence of rejection especially in “high-risk” recipients[113-116]. Some biologics like anti-VEGF, anti-TNF-α or anti-IL-2R are already in use to inhibit “high-risk” graft rejection while potent blockers of TNF receptors are currently being evaluated in clinical trials[112].
Corneal allograft survival would be greatly improved if, in addition to tissue matching and topical steroids, an appropriate low dose immunosuppressant was also used[98]. However, alternative therapies should also be considered as discussed below.
Currently, cell-based therapies such as stem cells, tolerogenic DC or Treg are proposed as alternative treatments especially for “high-risk” corneal grafts and they function by promoting immune tolerance.
Stem cells are undifferentiated cells which give rise to two daughter cells comprising one self-renewing and one differentiating progenitor generated by asymmetric cell division[117]. Stem cells include embryonic stem cells (ESC), induced pluripotent stem cells (iPSC) and mesenchymal stem cells (MSC) and they have been investigated as a therapeutic strategy in promoting transplant tolerance[118] and in ocular surface reconstruction[119].
ESC and iPSC: The most fascinating breakthrough of the last decade is the generation of iPSC from adult somatic cells. This is a novel method of generating stem cell which ensures a continuous supply of self-renewing PSC. The process of reprogramming somatic cells ex vivo by transmitting the signalling cues through four well-defined transcription factors such as Oct3/4, Sox2, c-Myc, and Klf4 has opened the way for a wide range of clinical applications[120,121]. Like ESC, iPSC are also capable of trans-differentiating into cells of different lineages. Several in vitro, in vivo studies and even phase I clinical trials were initiated using ESC and iPSC to treat sequelae of sight threatening intraocular inflammation or retinal degenerative diseases[122-126].
In the context of corneal reconstruction and repair, in vitro studies have shown the feasibility of differentiating ESC and iPSC into corneal epithelial, keratocytes and endothelial cells individually as an option to treat corneal scarring, stromal opacity and malfunctioned endothelial cells[127-130]. Furthermore, ex vivo transplantation of ESC derived cells onto partially de-epithelialized cornea led to regeneration of normal stratified layers of the corneal epithelium[131]. iPSC are able to differentiate into limbal stem cells (LSC) in vitro, confirmed by expression of LSC markers ABCG-2 and p63α at both cellular and molecular levels[119]. The successful engraftment of a differentiated LSC-seeded scaffold demonstrated significant reconstruction of the ocular surface with functional re-epithelization, minimal corneal scars and corneal vascularization in an experimental model of alkali burn in rabbits[132]. Hence, PSC could potentially be used to replace damaged LSC which is a characteristic feature found in many “high-risk” ocular pathologies[119,132].
Though there is much to be explored, the therapeutic impact of PSC is remarkable. The advantages of PSC are they do not induce allogenecity and related immune rejection[126]. However, problems with insufficient supply of cells as well as the possibility of differentiating into the malignant cells still remain[133,134].
LSC: LSC play a vital role in maintaining corneal integrity and renewal of epithelial cells. The limbus, reservoir of LSC, is responsible for homeostasis of the corneal epithelium[135,136]. Damage to LSC occurs during severe burns, injury or infection to the ocular surface and results in a “high-risk” cornea with limbal stem cell deficiency (LSCD) features such as chronic inflammation, severe corneal vascularization, persistent epithelial defects, conjunctivalization of the cornea and increased risk of corneal perforation[137].
Autologous transplantation of limbal epithelial sheets is considered a long-term effective clinical solution for unilateral corneal stem cell deficiency; and for bilateral deficiency, LSC from deceased donors is a possible option but raises the problems of matching and increased chance of rejection[138-140]. In addition, autologous limbal transplantation was shown to be performed in a 2 step approach, with PK performed at a later date. However, the outcome of these procedures were not satisfactory in bilaterally deficient patients with severe ocular damage[139,140]. Nevertheless, a large clinical study reported that autologous LSC transplantation was effective even in “high-risk” patients post alkali burn or with previously failed corneal graft where the outcome was restoration of a stable ocular surface and vision[141].
Currently, LSC therapy is a promising strategy clinically to improve the chance of normalization of ocular surface and later acceptance of “high-risk” corneal grafts[142,143]. However, there are still considerable obstacles to overcome such as methods to isolate/prepare cells, expand the cells in culture and avoiding damaging cells due to the surgical procedure and immune reaction. As such, the procedure is limited to clinics that have a specialized laboratory for cell expansion, operating at a level conforming to guidelines for good manufacturing practice. A new simpler method that has been recently developed, termed simple limbal epithelial transplantation combines existing know-how but allows for the entire grafting procedure to be performed in the operating room[144].
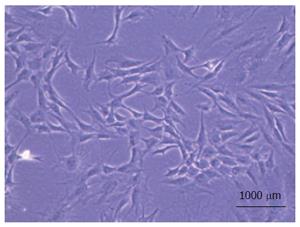
MSC: MSC are multipotent stem cells mainly isolated from bone marrow amongst other sources[145-151] (Figure 2). These cells are being tested currently in repairing tissue defects by attenuating scar formation and in immunomodulation[152]. MSC have the capability of differentiating into cells of mesenchymal and non-mesenchymal origin induced by paracrine and autocrine signals according to the local microenvironment[153]. Several in vitro studies have shown MSC capable of reducing T cell immune responses by promoting the activation of Treg and production of IL-10, TGF-β, prostaglandin E2 and thrombospondin-1[154,155]. Likewise, in vivo studies of different solid organ transplantation models also suggested significant reduction of adaptive immune response and promotion of immune tolerance in the presence of MSC[156-159].
Initial studies demonstrated that MSC are promising candidates to treat corneal blindness by restoring corneal transparency in a congenital keratocyte dysfunction model[160] and differentiating into keratocytes in corneal stroma, thereby facilitating tissue repair[161]. Based on these studies, MSC therapy has been promoted in many acquired corneal disease and injury models. Recent studies have shown that systemic injection of MSC prolonged corneal allograft survival by homing into the inflamed graft site and DLN and suppressing APC function thus inhibiting allosensitization[162-165]. Local administration of MSC was also able to induce anti-inflammatory and anti-angiogenic effects and prevent LSCD in models of acute alkali burn[166,167].
Despite relative scarcity and difficulties with isolation and expansion, MSC are safer than PSC for treatment in pre-clinical studies as no adverse effects such as a tumour formation (teratoma), have so far been observed[168].
DC possess both immunogenic and tolerogenic functions[169]. Activated mature immunogenic DC have been used in cancer immunotherapy for more than a decade and found to be efficacious. In this setting, DC are used as natural adjuvants carrying tumour specific peptides and induce antigen specific T cells in the DLN with subsequent tumour lysis[170,171]. DC based immunotherapy can also be used as vaccination to protect against tumours by promoting tumour antigen specific immunity and prevent cancer recurrence[172,173].
However, in contrast to their immunogenicity when activated, DC mainly maintain immune homeostasis by immune regulatory action against self-antigen specific T effector cells and so prevent autoimmunity[174]. This tolerogenic feature of DC presents them as a possible candidate for treatment in autoimmune disease and allograft rejection[175]. Phenotypically immature DC remain tolerogenic as they fail to deliver an adequate costimulatory signal required for specific T cell activation. These non-activated or partially activated T cells undergo optimally low proliferation, cell death, anergy or develop the phenotype of Treg[176,177]. In vitro manipulation of DC by exposing them to an antigen at a sub-optimal level or treating them with anti-inflammatory cytokines such as IL-10 and TGF-β leads to alternatively activated DC which are poor stimulators of the alloimmune response but promote immune tolerance[174,176]. The in vitro manipulated immature DC have been shown to impair CD4+ effector T cell induction and enrich CD4+CD25+Foxp3+ Treg by inducing hyporesponsiveness of the DC to the antigenic stimuli through toll-like receptors[178].
This phenomenon of inducing or restoring tolerance by DC therapy has been applied in transplantation models in an attempt to enhance allograft survival[179]. A number of pre-clinical studies on rodents and non-human primate transplantation models have shown long-term survival and function of allograft by administering ex vivo manipulated DC[175,177,180]. The efficacy of donor derived DC based therapy was tested in a pre-clinical “high-risk” corneal transplantation model and was reportedly effective by significant reduction in IFN-γ and increased production of Foxp3+ Treg[181,182].
Treg are crucial in maintaining self-tolerance and their absence leads to autoimmune diseases[183,184]. The in vitro generation, phenotype and immunosuppressive function of Treg have been reviewed in detail previously[185]. In vitro manipulated donor-derived CD8+Foxp3+ Treg were infused and found to induce CD4+CD25+Foxp3+ to provide donor specific tolerance to allografts and protect from aggressive host immune rejection in a fully mismatched skin graft murine model[186]. Similarly, production of Treg is critical for the survival of corneal allografts[44] (as discussed above) and interestingly, even the local administration of naïve Treg prolongs corneal allograft survival in infant rats[187].
DC and Treg are recognised as promising candidates for the clinical application of immunosuppressive therapy to promote corneal graft survival. It has been demonstrated that autologous DC are safe with no toxic or immunogenic effects[188,189] while graft versus host disease (GVHD) was not observed when allogeneic cells were used[173,190]. Instead, they were shown to inhibit GVHD after bone marrow transplantation in pre-clinical and clinical studies of leukemia[173,190]. Though already in clinical trials, efficient isolation without manipulation of their phenotype and function is still under development for potential application, especially in “high-risk” grafts.
The use of artificial corneas is an exciting option, which would overcome the problems with shortage of donors and frequent graft rejection in “high-risk” hosts[191,192]. Two approaches have been used to replace the damaged corneal tissue so far: (1) keratoprosthesis; and (2) bioengineered scaffolds that serve as templates for promoting corneal regeneration[193].
Keratoprostheses are synthetically generated corneas made of artificial materials which are not fully biocompatible and “only” provide central vision, yet are a viable option for patients who are at the end stage of severe corneal disease where grafting a donor cornea is almost certain to fail[194-196]. The Boston Keratoprosthesis (BKPro) is the most commonly used artificial cornea in clinical practice. Though the device is made of synthetic material, a donor cornea still has to be used as the carrier of the central optical device[197,198]. Patients with “high-risk” herpetic keratitis transplanted with BKPro were shown to have better outcomes than transplanted allografts only[199]. Nevertheless, several postoperative complications including keratolysis (corneal melt), tissue necrosis which may result in corneal perforation in both host and donor cornea, and retro-prosthetic membrane formation have been reported[197,200,201]. In addition, lack of bio-integration of the prosthesis seems to be the major reason for BKPro extrusion, instability and ultimate failure[195,197]. The other type of prosthesis known as the osteo-odonto-keratoprosthesis (OOKP) was designed with an autologous tooth that forms the frame for central transparent optical cylinder[196]. This is a complicated procedure, and an end stage choice for patients with severe dry eye disease. Retro-prosthetic membrane is not a significant complication in OOKP unlike BKPro[202] but, the osteo-dental lamina resorption is a specific problem of OOKP as it compromises integrity of the eye[202] while glaucoma and retinal detachment are the secondary complications of both types[203].
The persisting problem of stable integration of corneal implants with host and implant extrusion may be better addressed by developing tissue engineered biomimetic collagen-based corneal equivalents as discussed below.
Bioengineered equivalents of the corneal stromal extracellular matrix have also been tested clinically. These biosynthetic implants are based on chemically crosslinked collagen designed as regeneration templates[204-206].
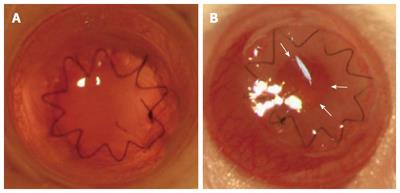
Pre-clinical studies were performed in a murine full-thickness orthotopic corneal transplantation model using porcine collagen and recombinant human collagen (RHC) (Figure 3), the latter of which, by using fully biologically synthetic material, reduces the risk of transmission of disease across species as well as reducing the chance of inducing adaptive immune responses[207,208]. Studies show a strong local innate immune response associated with excessive fibrin production and deposition in the AC. This may represent an exaggerated tissue repair/wound healing response[207]. Interestingly, only minimal or no activation of APC or CD4+ and CD8+ T lymphocytes in eye-DLN as well as a minimal systemic humoral response was detected[204,207]. Thus, the main problem seems to be the generation of a retro-hydrogel membrane (Figure 3, arrows), which ultimately reduces the clarity of the graft. Surprisingly, neither an immune response to the hydrogel nor retro-hydrogel membrane formation was detected in a guinea pig model of PK[209]. Additionally, regeneration of endogenous corneal layers and functional corneal nerves were also determined in the collagen matrix[209]. Similar findings were demonstrated when the structurally reinforced collagen-based hydrogels were transplanted in a “high-risk” graft model of ocular alkali burn in rabbits[210]. Furthermore, additional advancements were made in the fabrication of biomimetic, acellular, corneal implants by incorporating biocompatible silica (Sio2) nanoparticle (NP) carriers for sustained release of anti-viral drugs such as acyclovir and LL-37 for use in “high-risk” grafts due to herpetic keratitis to prevent re-activation/re-infection of virus and this was supported by low viral copy numbers in in vitro experiments[211,212].
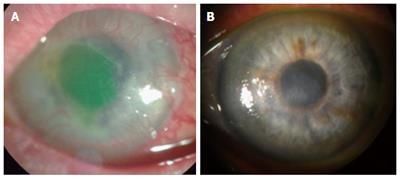
Hydrogel implants have also had their premiere in clinical medicine. A phase I human clinical study using the biosynthetically designed corneal hydrogel substitutes made of RHC which were shown to mirror the natural cornea structurally, mechanistically and functionally by promoting active regeneration of endogenous corneal epithelial and stromal cells has been reported[213]. In addition, recent outcomes of the 4-year follow-up clinical study show high acceptance/adaptation of the hydrogel to the ocular surface with improved visual acuity and sensory nerve ingrowth[214]. A most recent clinical observation (case report) in three patients with severe corneal ulcers and recurrent erosions suggests that RHCIII hydrogels reinforced with phosphorylcholine polymer networks potentially withstand the “high-risk” environment (Figure 4) and is a safe and efficient alternative to donor corneal allografts in emergency situations where a corneal allograft is not available, as the corneal integrity can be well maintained in recipients[215].
Instead of fully in vitro generated hydrogel matrixes, decellularized corneas have also been tested in a clinical study[216]. This study showed promising clinical results in “high-risk” fungal keratitic patients where the implanted decellularized porcine corneas caused regression of corneal vascularization and improved corneal clarity. Although no safety problems were demonstrated, immunogenicity still could be a problem and so further studies addressing this issue may be required[216].
Thus, bioengineered collagen-based corneal equivalents have shown to be a promising alternative to keratoprosthesis. Though collagen hydrogels show promise in the clinic, this applies mainly to lamellar keratoplasty, which is a partial thickness replacement of damaged cornea, where host endothelium is intact. Thus, the complications observed in experimental models - fibrin deposition and retro-hydrogel membranes formation are eliminated as the integrity of the anterior segment microenvironment is preserved. For PK, the “holy grail” of full-thickness artificial cornea remains the ultimate aim of current research.
In full-thickness corneal transplantation in “low-risk” settings - the balance between the strength of alloimmune response and regulatory mechanisms dictates the outcome of the graft, whereas in “high-risk” settings heightened innate and adaptive immune responses significantly tilt the balance to favour graft rejection. Though highly debated, tissue matching with long-term immunosuppression is recommended to reduce the rejection of “high-risk” grafts. Meanwhile, alternative approaches are being explored to avoid the side effects of prolonged use of systemic immunosuppressants. Such approaches including cell-based therapies and development of collagen-based corneal equivalents appear to be promising. Research continues to refine the available therapies for the betterment of the clinical outcomes. The recent surgical advances made in endothelial and stromal lamellar keratoplasty would be a potential realistic option to increase the success rates of some “high-risk” grafts. Manipulation of immunomodulatory molecules like TGF-β and IL-10 in the donor corneal layers by gene therapy might facilitate weakening the aggravated host immune response in “high-risk” grafts. The combined approach of cell or gene therapy along with allograft transplantation might render a better preventive measure for “high-risk” corneal graft rejection.
All experiments on animals were performed according to the guidelines described in the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Vision and Ophthalmic Research and Animal License Act (United Kingdom).
P- Reviewer: Holan V S- Editor: Qiu S L- Editor: A E- Editor: Wang CH
| 1. | Tan DT, Dart JK, Holland EJ, Kinoshita S. Corneal transplantation. Lancet. 2012;379:1749-1761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 496] [Cited by in RCA: 548] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 2. | National Health Services Blood and Transplant. Organ Donation and Transplantation Activity Report. [accessed 2015 Jul 27]. Available from: http//www.odt.nhs.uk/uk-transplant-registry/annual-activity-report/. |
| 3. | Williams KA, Esterman AJ, Bartlett C, Holland H, Hornsby NB, Coster DJ. How effective is penetrating corneal transplantation? Factors influencing long-term outcome in multivariate analysis. Transplantation. 2006;81:896-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 132] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 4. | Williams KA, Lowe M, Bartlett C, Kelly TL, Coster DJ. Risk factors for human corneal graft failure within the Australian corneal graft registry. Transplantation. 2008;86:1720-1724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 181] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 5. | Garg P, Krishna PV, Stratis AK, Gopinathan U. The value of corneal transplantation in reducing blindness. Eye (Lond). 2005;19:1106-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 93] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 6. | The collaborative corneal transplantation studies (CCTS). Effectiveness of histocompatibility matching in high-risk corneal transplantation. The Collaborative Corneal Transplantation Studies Research Group. Arch Ophthalmol. 1992;110:1392-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 253] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 7. | Coster DJ, Williams KA. Management of high-risk corneal grafts. Eye (Lond). 2003;17:996-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Chang JH, Garg NK, Lunde E, Han KY, Jain S, Azar DT. Corneal neovascularization: an anti-VEGF therapy review. Surv Ophthalmol. 2012;57:415-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 287] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 9. | Inoue K, Amano S, Oshika T, Tsuru T. Risk factors for corneal graft failure and rejection in penetrating keratoplasty. Acta Ophthalmol Scand. 2001;79:251-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 90] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 10. | Perera C, Jhanji V, Vajpayee RB. Factors influencing outcomes of the treatment of allograft corneal rejection. Am J Ophthalmol. 2011;152:358-363.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Sonoda Y, Streilein JW. Orthotopic corneal transplantation in mice--evidence that the immunogenetic rules of rejection do not apply. Transplantation. 1992;54:694-704. [PubMed] |
| 12. | Sano Y, Ksander BR, Streilein JW. Fate of orthotopic corneal allografts in eyes that cannot support anterior chamber-associated immune deviation induction. Invest Ophthalmol Vis Sci. 1995;36:2176-2185. [PubMed] |
| 13. | Niederkorn JY. Corneal transplantation and immune privilege. Int Rev Immunol. 2013;32:57-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 14. | Qazi Y, Hamrah P. Corneal Allograft Rejection: Immunopathogenesis to Therapeutics. J Clin Cell Immunol. 2013;2013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Yamada J, Kurimoto I, Streilein JW. Role of CD4+ T cells in immunobiology of orthotopic corneal transplants in mice. Invest Ophthalmol Vis Sci. 1999;40:2614-2621. [PubMed] |
| 16. | Plskova J, Kuffova L, Filipec M, Holan V, Forrester JV. Quantitative evaluation of the corneal endothelium in the mouse after grafting. Br J Ophthalmol. 2004;88:1209-1216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Hori J, Streilein JW. Dynamics of donor cell persistence and recipient cell replacement in orthotopic corneal allografts in mice. Invest Ophthalmol Vis Sci. 2001;42:1820-1828. [PubMed] |
| 18. | King WJ, Comer RM, Hudde T, Larkin DF, George AJ. Cytokine and chemokine expression kinetics after corneal transplantation. Transplantation. 2000;70:1225-1233. [PubMed] |
| 19. | Niederkorn JY. Immune mechanisms of corneal allograft rejection. Curr Eye Res. 2007;32:1005-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 94] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 20. | Kuffová L, Netuková M, Duncan L, Porter A, Stockinger B, Forrester JV. Cross presentation of antigen on MHC class II via the draining lymph node after corneal transplantation in mice. J Immunol. 2008;180:1353-1361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Auchincloss H, Sultan H. Antigen processing and presentation in transplantation. Curr Opin Immunol. 1996;8:681-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 96] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 22. | Chong EM, Dana MR. Graft failure IV. Immunologic mechanisms of corneal transplant rejection. Int Ophthalmol. 2008;28:209-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 77] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 23. | Larkin DF, Calder VL, Lightman SL. Identification and characterization of cells infiltrating the graft and aqueous humour in rat corneal allograft rejection. Clin Exp Immunol. 1997;107:381-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 90] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 24. | Hegde S, Beauregard C, Mayhew E, Niederkorn JY. CD4(+) T-cell-mediated mechanisms of corneal allograft rejection: role of Fas-induced apoptosis. Transplantation. 2005;79:23-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 74] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 25. | Stuart PM, Griffith TS, Usui N, Pepose J, Yu X, Ferguson TA. CD95 ligand (FasL)-induced apoptosis is necessary for corneal allograft survival. J Clin Invest. 1997;99:396-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 265] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 26. | Sagoo P, Chan G, Larkin DF, George AJ. Inflammatory cytokines induce apoptosis of corneal endothelium through nitric oxide. Invest Ophthalmol Vis Sci. 2004;45:3964-3973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 102] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 27. | Strestíková P, Plsková J, Filipec M, Farghali H. FK 506 and aminoguanidine suppress iNOS induction in orthotopic corneal allografts and prolong graft survival in mice. Nitric Oxide. 2003;9:111-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Chen H, Wang W, Xie H, Xu X, Wu J, Jiang Z, Zhang M, Zhou L, Zheng S. A pathogenic role of IL- 17 at the early stage of corneal allograft rejection. Transpl Immunol. 2009;21:155-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 29. | Cunnusamy K, Chen PW, Niederkorn JY. IL-17 promotes immune privilege of corneal allografts. J Immunol. 2010;185:4651-4658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 30. | Yin XT, Zobell S, Jarosz JG, Stuart PM. Anti-IL-17 therapy restricts and reverses late-term corneal allorejection. J Immunol. 2015;194:4029-4038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 31. | Yamada J, Hamuro J, Fukushima A, Ohteki T, Terai K, Iwakura Y, Yagita H, Kinoshita S. MHC-matched corneal allograft rejection in an IFN-gamma/IL-17-independent manner in C57BL/6 mice. Invest Ophthalmol Vis Sci. 2009;50:2139-2146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Streilein JW, Yamada J, Dana MR, Ksander BR. Anterior chamber-associated immune deviation, ocular immune privilege, and orthotopic corneal allografts. Transplant Proc. 1999;31:1472-1475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 33. | Streilein JW. Ocular immune privilege: therapeutic opportunities from an experiment of nature. Nat Rev Immunol. 2003;3:879-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 545] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 34. | Medawar PB. Immunity to homologous grafted skin; the fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. Br J Exp Pathol. 1948;29:58-69. [PubMed] |
| 35. | Sosnová M, Bradl M, Forrester JV. CD34+ corneal stromal cells are bone marrow-derived and express hemopoietic stem cell markers. Stem Cells. 2005;23:507-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 36. | Novak N, Siepmann K, Zierhut M, Bieber T. The good, the bad and the ugly--APCs of the eye. Trends Immunol. 2003;24:570-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 37. | Hamrah P, Liu Y, Zhang Q, Dana MR. The corneal stroma is endowed with a significant number of resident dendritic cells. Invest Ophthalmol Vis Sci. 2003;44:581-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 234] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 38. | Hamrah P, Huq SO, Liu Y, Zhang Q, Dana MR. Corneal immunity is mediated by heterogeneous population of antigen-presenting cells. J Leukoc Biol. 2003;74:172-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 234] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 39. | Brissette-Storkus CS, Reynolds SM, Lepisto AJ, Hendricks RL. Identification of a novel macrophage population in the normal mouse corneal stroma. Invest Ophthalmol Vis Sci. 2002;43:2264-2271. [PubMed] |
| 40. | Knickelbein JE, Watkins SC, McMenamin PG, Hendricks RL. Stratification of Antigen-presenting Cells within the Normal Cornea. Ophthalmol Eye Dis. 2009;1:45-54. [PubMed] |
| 41. | Kaplan HJ, Streilein JW. Immune response to immunization via the anterior chamber of the eye. I. F. lymphocyte-induced immune deviation. J Immunol. 1977;118:809-814. [PubMed] |
| 42. | Niederkorn JY. Role of NKT cells in anterior chamber-associated immune deviation. Expert Rev Clin Immunol. 2009;5:137-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 43. | Cunnusamy K, Paunicka K, Reyes N, Yang W, Chen PW, Niederkorn JY. Two different regulatory T cell populations that promote corneal allograft survival. Invest Ophthalmol Vis Sci. 2010;51:6566-6574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 44. | Chauhan SK, Saban DR, Lee HK, Dana R. Levels of Foxp3 in regulatory T cells reflect their functional status in transplantation. J Immunol. 2009;182:148-153. [PubMed] |
| 45. | Cunnusamy K, Chen PW, Niederkorn JY. IL-17A-dependent CD4+CD25+ regulatory T cells promote immune privilege of corneal allografts. J Immunol. 2011;186:6737-6745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 46. | Ali JM, Bolton EM, Bradley JA, Pettigrew GJ. Allorecognition pathways in transplant rejection and tolerance. Transplantation. 2013;96:681-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 47. | Sano Y, Ksander BR, Streilein JW. Langerhans cells, orthotopic corneal allografts, and direct and indirect pathways of T-cell allorecognition. Invest Ophthalmol Vis Sci. 2000;41:1422-1431. [PubMed] |
| 48. | Sano Y, Streilein JW, Ksander BR. Detection of minor alloantigen-specific cytotoxic T cells after rejection of murine orthotopic corneal allografts: evidence that graft antigens are recognized exclusively via the “indirect pathway”. Transplantation. 1999;68:963-970. [PubMed] |
| 49. | Boisgérault F, Liu Y, Anosova N, Dana R, Benichou G. Differential roles of direct and indirect allorecognition pathways in the rejection of skin and corneal transplants. Transplantation. 2009;87:16-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 50. | Forrester JV. Privilege revisited: an evaluation of the eye’s defence mechanisms. Eye (Lond). 2009;23:756-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 51. | He Y, Jie Y, Wang B, Zeng H, Zhang Y, Pan Z. Adoptive transfer of donor corneal antigen-specific regulatory T cells can prolong mice corneal grafts survival. Cornea. 2010;29 Suppl 1:S25-S31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 52. | Cunnusamy K, Niederkorn JY. IFN-γ blocks CD4+CD25+ Tregs and abolishes immune privilege of minor histocompatibility mismatched corneal allografts. Am J Transplant. 2013;13:3076-3084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 53. | Griffith TS, Brunner T, Fletcher SM, Green DR, Ferguson TA. Fas ligand-induced apoptosis as a mechanism of immune privilege. Science. 1995;270:1189-1192. [PubMed] |
| 54. | Ferguson TA, Griffith TS. The role of Fas ligand and TNF-related apoptosis-inducing ligand (TRAIL) in the ocular immune response. Chem Immunol Allergy. 2007;92:140-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 55. | Griffith TS, Yu X, Herndon JM, Green DR, Ferguson TA. CD95-induced apoptosis of lymphocytes in an immune privileged site induces immunological tolerance. Immunity. 1996;5:7-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 273] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 56. | Osawa H, Maruyama K, Streilein JW. CD95 ligand expression on corneal epithelium and endothelium influences the fates of orthotopic and heterotopic corneal allografts in mice. Invest Ophthalmol Vis Sci. 2004;45:1908-1915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 57. | Lee HO, Herndon JM, Barreiro R, Griffith TS, Ferguson TA. TRAIL: a mechanism of tumor surveillance in an immune privileged site. J Immunol. 2002;169:4739-4744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 58. | Xie L, Shi W, Guo P. Roles of tumor necrosis factor-related apoptosis-inducing ligand in corneal transplantation. Transplantation. 2003;76:1556-1559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 59. | Hori J, Wang M, Miyashita M, Tanemoto K, Takahashi H, Takemori T, Okumura K, Yagita H, Azuma M. B7-H1-induced apoptosis as a mechanism of immune privilege of corneal allografts. J Immunol. 2006;177:5928-5935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 159] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 60. | Yang W, Li H, Chen PW, Alizadeh H, He Y, Hogan RN, Niederkorn JY. PD-L1 expression on human ocular cells and its possible role in regulating immune-mediated ocular inflammation. Invest Ophthalmol Vis Sci. 2009;50:273-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 61. | Shen L, Jin Y, Freeman GJ, Sharpe AH, Dana MR. The function of donor versus recipient programmed death-ligand 1 in corneal allograft survival. J Immunol. 2007;179:3672-3679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 82] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 62. | Watson MP, George AJ, Larkin DF. Differential effects of costimulatory pathway modulation on corneal allograft survival. Invest Ophthalmol Vis Sci. 2006;47:3417-3422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 63. | Bora NS, Gobleman CL, Atkinson JP, Pepose JS, Kaplan HJ. Differential expression of the complement regulatory proteins in the human eye. Invest Ophthalmol Vis Sci. 1993;34:3579-3584. [PubMed] |
| 64. | Goslings WR, Prodeus AP, Streilein JW, Carroll MC, Jager MJ, Taylor AW. A small molecular weight factor in aqueous humor acts on C1q to prevent antibody-dependent complement activation. Invest Ophthalmol Vis Sci. 1998;39:989-995. [PubMed] |
| 65. | Lass JH, Walter EI, Burris TE, Grossniklaus HE, Roat MI, Skelnik DL, Needham L, Singer M, Medof ME. Expression of two molecular forms of the complement decay-accelerating factor in the eye and lacrimal gland. Invest Ophthalmol Vis Sci. 1990;31:1136-1148. [PubMed] |
| 66. | Esposito A, Suedekum B, Liu J, An F, Lass J, Strainic MG, Lin F, Heeger P, Medof ME. Decay accelerating factor is essential for successful corneal engraftment. Am J Transplant. 2010;10:527-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 67. | Ljunggren HG, Kärre K. In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol Today. 1990;11:237-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1849] [Cited by in RCA: 1956] [Article Influence: 55.9] [Reference Citation Analysis (0)] |
| 68. | Apte RS, Niederkorn JY. Isolation and characterization of a unique natural killer cell inhibitory factor present in the anterior chamber of the eye. J Immunol. 1996;156:2667-2673. [PubMed] |
| 69. | Apte RS, Sinha D, Mayhew E, Wistow GJ, Niederkorn JY. Cutting edge: role of macrophage migration inhibitory factor in inhibiting NK cell activity and preserving immune privilege. J Immunol. 1998;160:5693-5696. [PubMed] |
| 70. | Shimmura-Tomita M, Wang M, Taniguchi H, Akiba H, Yagita H, Hori J. Galectin-9-mediated protection from allo-specific T cells as a mechanism of immune privilege of corneal allografts. PLoS One. 2013;8:e63620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 71. | Hamrah P, Haskova Z, Taylor AW, Zhang Q, Ksander BR, Dana MR. Local treatment with alpha-melanocyte stimulating hormone reduces corneal allorejection. Transplantation. 2009;88:180-187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 72. | Beutelspacher SC, Pillai R, Watson MP, Tan PH, Tsang J, McClure MO, George AJ, Larkin DF. Function of indoleamine 2,3-dioxygenase in corneal allograft rejection and prolongation of allograft survival by over-expression. Eur J Immunol. 2006;36:690-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 132] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 73. | Taylor AW. Ocular immunosuppressive microenvironment. Chem Immunol Allergy. 2007;92:71-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 68] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 74. | Yamagami S, Hamrah P, Zhang Q, Liu Y, Huq S, Dana MR. Early ocular chemokine gene expression and leukocyte infiltration after high-risk corneal transplantation. Mol Vis. 2005;11:632-640. [PubMed] |
| 75. | Clark R, Kupper T. Old meets new: the interaction between innate and adaptive immunity. J Invest Dermatol. 2005;125:629-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 115] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 76. | Liu Y, Hamrah P, Zhang Q, Taylor AW, Dana MR. Draining lymph nodes of corneal transplant hosts exhibit evidence for donor major histocompatibility complex (MHC) class II-positive dendritic cells derived from MHC class II-negative grafts. J Exp Med. 2002;195:259-268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 164] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 77. | Jin Y, Chauhan SK, Saban DR, Dana R. Role of CCR7 in facilitating direct allosensitization and regulatory T-cell function in high-risk corneal transplantation. Invest Ophthalmol Vis Sci. 2010;51:816-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 78. | Zhang X, Shen L, Jin Y, Saban DR, Chauhan SK, Dana R. Depletion of passenger leukocytes from corneal grafts: an effective means of promoting transplant survival? Invest Ophthalmol Vis Sci. 2009;50:3137-3144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 79. | Bachmann BO, Bock F, Wiegand SJ, Maruyama K, Dana MR, Kruse FE, Luetjen-Drecoll E, Cursiefen C. Promotion of graft survival by vascular endothelial growth factor a neutralization after high-risk corneal transplantation. Arch Ophthalmol. 2008;126:71-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 97] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 80. | Zhang H, Grimaldo S, Yuen D, Chen L. Combined blockade of VEGFR-3 and VLA-1 markedly promotes high-risk corneal transplant survival. Invest Ophthalmol Vis Sci. 2011;52:6529-6535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 81. | Dietrich T, Bock F, Yuen D, Hos D, Bachmann BO, Zahn G, Wiegand S, Chen L, Cursiefen C. Cutting edge: lymphatic vessels, not blood vessels, primarily mediate immune rejections after transplantation. J Immunol. 2010;184:535-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 231] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 82. | Dohlman TH, Omoto M, Hua J, Stevenson W, Lee SM, Chauhan SK, Dana R. VEGF-trap aflibercept significantly improves long-term graft survival in high-risk corneal transplantation. Transplantation. 2015;99:678-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 83. | Hill JC. High risk corneal grafting. Br J Ophthalmol. 2002;86:945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 84. | Plsková J, Holán V, Filipec M, Forrester JV. Lymph node removal enhances corneal graft survival in mice at high risk of rejection. BMC Ophthalmol. 2004;4:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 85. | Vitova A, Kuffová L, Klaska IP, Holan V, Cornall RJ, Forrester JV. The high-risk corneal regraft model: a justification for tissue matching in humans. Transpl Int. 2013;26:453-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 86. | Sheldon S, Poulton K. HLA typing and its influence on organ transplantation. Methods Mol Biol. 2006;333:157-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 87. | Coster DJ, Williams KA. The impact of corneal allograft rejection on the long-term outcome of corneal transplantation. Am J Ophthalmol. 2005;140:1112-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 156] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 88. | Armitage WJ. HLA matching and corneal transplantation. Eye (Lond). 2004;18:231-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 89. | Bartels MC, Otten HG, van Gelderen BE, Van der Lelij A. Influence of HLA-A, HLA-B, and HLA-DR matching on rejection of random corneal grafts using corneal tissue for retrospective DNA HLA typing. Br J Ophthalmol. 2001;85:1341-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 90. | Böhringer D, Reinhard T, Duquesnoy RJ, Böhringer S, Enczmann J, Lange P, Claas F, Sundmacher R. Beneficial effect of matching at the HLA-A and -B amino-acid triplet level on rejection-free clear graft survival in penetrating keratoplasty. Transplantation. 2004;77:417-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 91. | Böhringer D, Spierings E, Enczmann J, Böhringer S, Sundmacher R, Goulmy E, Reinhard T. Matching of the minor histocompatibility antigen HLA-A1/H-Y may improve prognosis in corneal transplantation. Transplantation. 2006;82:1037-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 92. | Sano Y, Ksander BR, Streilein JW. Minor H, rather than MHC, alloantigens offer the greater barrier to successful orthotopic corneal transplantation in mice. Transpl Immunol. 1996;4:53-56. [PubMed] |
| 93. | Dunn SP, Stark WJ, Stulting RD, Lass JH, Sugar A, Pavilack MA, Smith PW, Tanner JP, Dontchev M, Gal RL. The effect of ABO blood incompatibility on corneal transplant failure in conditions with low-risk of graft rejection. Am J Ophthalmol. 2009;147:432-438.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 94. | Inoue K, Tsuru T. ABO antigen blood-group compatibility and allograft rejection in corneal transplantation. Acta Ophthalmol Scand. 1999;77:495-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 95. | Stulting RD, Sugar A, Beck R, Belin M, Dontchev M, Feder RS, Gal RL, Holland EJ, Kollman C, Mannis MJ. Effect of donor and recipient factors on corneal graft rejection. Cornea. 2012;31:1141-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 96. | Reinhard T, Böhringer D, Enczmann J, Kögler G, Mayweg S, Wernet P, Sundmacher R. Improvement of graft prognosis in penetrating normal-risk keratoplasty by HLA class I and II matching. Eye (Lond). 2004;18:269-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 97. | van Essen TH, Roelen DL, Williams KA, Jager MJ. Matching for Human Leukocyte Antigens (HLA) in corneal transplantation - to do or not to do. Prog Retin Eye Res. 2015;46:84-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 98. | Nguyen NX, Seitz B, Martus P, Langenbucher A, Cursiefen C. Long-term topical steroid treatment improves graft survival following normal-risk penetrating keratoplasty. Am J Ophthalmol. 2007;144:318-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 87] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 99. | Nguyen NX, Pham HN, Langenbucher A, Cursiefen C, Seitz B. Impact of short-term versus longterm topical steroid treatment on ‘idiopathic’ endothelial cell loss after normal-risk penetrating keratoplasty. Acta Ophthalmol Scand. 2007;85:209-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 100. | Shimazaki J, Iseda A, Satake Y, Shimazaki-Den S. Efficacy and safety of long-term corticosteroid eye drops after penetrating keratoplasty: a prospective, randomized, clinical trial. Ophthalmology. 2012;119:668-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 101. | Böhringer D, Ihorst G, Grotejohann B, Maurer J, Spierings E, Reinhard T. Functional antigen matching in corneal transplantation: matching for the HLA-A, -B and -DRB1 antigens (FANCY) - study protocol. BMC Ophthalmol. 2014;14:156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 102. | Böhringer D, Reinhard T, Böhringer S, Enczmann J, Godehard E, Sundmacher R. Predicting time on the waiting list for HLA matched corneal grafts. Tissue Antigens. 2002;59:407-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 103. | Reis A, Reinhard T. Current Systemic Immunosuppressive Strategies in Penetrating Keratoplasty. Cornea and External Eye Disease. Berlin Heidelberg: Springer 2006; 109-122. |
| 104. | Hill JC. Systemic cyclosporine in high-risk keratoplasty: long-term results. Eye (Lond). 1995;9:422-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 31] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 105. | Kharod-Dholakia B, Randleman JB, Bromley JG, Stulting RD. Prevention and treatment of corneal graft rejection: current practice patterns of the Cornea Society (2011). Cornea. 2015;34:609-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 106. | Stanbury RM, Graham EM. Systemic corticosteroid therapy--side effects and their management. Br J Ophthalmol. 1998;82:704-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 257] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 107. | Rumelt S, Bersudsky V, Blum-Hareuveni T, Rehany U. Systemic cyclosporin A in high failure risk, repeated corneal transplantation. Br J Ophthalmol. 2002;86:988-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 108. | Birnbaum F, Mayweg S, Reis A, Böhringer D, Seitz B, Engelmann K, Messmer EM, Reinhard T. Mycophenolate mofetil (MMF) following penetrating high-risk keratoplasty: long-term results of a prospective, randomised, multicentre study. Eye (Lond). 2009;23:2063-2070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 109. | Reis A, Reinhard T, Voiculescu A, Kutkuhn B, Godehardt E, Spelsberg H, Althaus C, Sundmacher R. Mycophenolate mofetil versus cyclosporin A in high risk keratoplasty patients: a prospectively randomised clinical trial. Br J Ophthalmol. 1999;83:1268-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 110. | Wei X, Chen XM, Wang L, Song JP, Deng YP. Effects of immunosuppressants after penetrating keratoplasty: meta-analysis of randomized controlled trials. Int J Ophthalmol. 2011;4:529-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 111. | Shi W, Gao H, Xie L, Wang S. Sustained intraocular rapamycin delivery effectively prevents high-risk corneal allograft rejection and neovascularization in rabbits. Invest Ophthalmol Vis Sci. 2006;47:3339-3344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 112. | Thiel MA, Kaufmann C, Coster DJ, Williams KA. Antibody-based immunosuppressive agents for corneal transplantation. Eye (Lond). 2009;23:1962-1965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 113. | Bock F, Onderka J, Dietrich T, Bachmann B, Kruse FE, Paschke M, Zahn G, Cursiefen C. Bevacizumab as a potent inhibitor of inflammatory corneal angiogenesis and lymphangiogenesis. Invest Ophthalmol Vis Sci. 2007;48:2545-2552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 233] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 114. | Dastjerdi MH, Saban DR, Okanobo A, Nallasamy N, Sadrai Z, Chauhan SK, Hajrasouliha AR, Dana R. Effects of topical and subconjunctival bevacizumab in high-risk corneal transplant survival. Invest Ophthalmol Vis Sci. 2010;51:2411-2417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 115. | Rayner SA, King WJ, Comer RM, Isaacs JD, Hale G, George AJ, Larkin DF. Local bioactive tumour necrosis factor (TNF) in corneal allotransplantation. Clin Exp Immunol. 2000;122:109-116. [PubMed] |
| 116. | Dana R. Comparison of topical interleukin-1 vs tumor necrosis factor-alpha blockade with corticosteroid therapy on murine corneal inflammation, neovascularization, and transplant survival (an American Ophthalmological Society thesis). Trans Am Ophthalmol Soc. 2007;105:330-343. [PubMed] |
| 117. | Watt FM, Hogan BL. Out of Eden: stem cells and their niches. Science. 2000;287:1427-1430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1325] [Cited by in RCA: 1167] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 118. | Guo K, Ikehara S, Meng X. Mesenchymal stem cells for inducing tolerance in organ transplantation. Front Cell Dev Biol. 2014;2:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 119. | Casaroli-Marano RP, Nieto-Nicolau N, Martínez-Conesa EM, Edel M, B Álvarez-Palomo A. Potential Role of Induced Pluripotent Stem Cells (IPSCs) for Cell-Based Therapy of the Ocular Surface. J Clin Med. 2015;4:318-342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 120. | Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14327] [Cited by in RCA: 14319] [Article Influence: 842.3] [Reference Citation Analysis (0)] |
| 121. | Takahashi K, Okita K, Nakagawa M, Yamanaka S. Induction of pluripotent stem cells from fibroblast cultures. Nat Protoc. 2007;2:3081-3089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 752] [Cited by in RCA: 745] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 122. | Forrester JV, Steptoe RJ, Klaska IP, Martin-Granados C, Dua HS, Degli-Esposti MA, Wikstrom ME. Cell-based therapies for ocular inflammation. Prog Retin Eye Res. 2013;35:82-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 123. | Wright LS, Phillips MJ, Pinilla I, Hei D, Gamm DM. Induced pluripotent stem cells as custom therapeutics for retinal repair: progress and rationale. Exp Eye Res. 2014;123:161-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 124. | Kamao H, Mandai M, Okamoto S, Sakai N, Suga A, Sugita S, Kiryu J, Takahashi M. Characterization of human induced pluripotent stem cell-derived retinal pigment epithelium cell sheets aiming for clinical application. Stem Cell Reports. 2014;2:205-218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 414] [Cited by in RCA: 439] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 125. | Okamoto S, Takahashi M. Induction of retinal pigment epithelial cells from monkey iPS cells. Invest Ophthalmol Vis Sci. 2011;52:8785-8790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 126. | Schwartz SD, Regillo CD, Lam BL, Eliott D, Rosenfeld PJ, Gregori NZ, Hubschman JP, Davis JL, Heilwell G, Spirn M. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet. 2015;385:509-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 846] [Cited by in RCA: 884] [Article Influence: 88.4] [Reference Citation Analysis (0)] |
| 127. | Hayashi R, Ishikawa Y, Ito M, Kageyama T, Takashiba K, Fujioka T, Tsujikawa M, Miyoshi H, Yamato M, Nakamura Y. Generation of corneal epithelial cells from induced pluripotent stem cells derived from human dermal fibroblast and corneal limbal epithelium. PLoS One. 2012;7:e45435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 107] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 128. | Chan AA, Hertsenberg AJ, Funderburgh ML, Mann MM, Du Y, Davoli KA, Mich-Basso JD, Yang L, Funderburgh JL. Differentiation of human embryonic stem cells into cells with corneal keratocyte phenotype. PLoS One. 2013;8:e56831. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 129. | Zhang K, Pang K, Wu X. Isolation and transplantation of corneal endothelial cell-like cells derived from in-vitro-differentiated human embryonic stem cells. Stem Cells Dev. 2014;23:1340-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 130. | Ahmad S, Stewart R, Yung S, Kolli S, Armstrong L, Stojkovic M, Figueiredo F, Lako M. Differentiation of human embryonic stem cells into corneal epithelial-like cells by in vitro replication of the corneal epithelial stem cell niche. Stem Cells. 2007;25:1145-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 146] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 131. | Hanson C, Hardarson T, Ellerström C, Nordberg M, Caisander G, Rao M, Hyllner J, Stenevi U. Transplantation of human embryonic stem cells onto a partially wounded human cornea in vitro. Acta Ophthalmol. 2013;91:127-130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 132. | Zhu J, Zhang K, Sun Y, Gao X, Li Y, Chen Z, Wu X. Reconstruction of functional ocular surface by acellular porcine cornea matrix scaffold and limbal stem cells derived from human embryonic stem cells. Tissue Eng Part A. 2013;19:2412-2425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 133. | Herberts CA, Kwa MS, Hermsen HP. Risk factors in the development of stem cell therapy. J Transl Med. 2011;9:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 404] [Cited by in RCA: 516] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 134. | Medina RJ, Archer DB, Stitt AW. Eyes open to stem cells: safety trial may pave the way for cell therapy to treat retinal disease in patients. Stem Cell Res Ther. 2011;2:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 135. | Townsend WM. The limbal palisades of Vogt. Trans Am Ophthalmol Soc. 1991;89:721-756. [PubMed] |
| 136. | Dua HS, Azuara-Blanco A. Limbal stem cells of the corneal epithelium. Surv Ophthalmol. 2000;44:415-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 396] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 137. | Lavker RM, Tseng SC, Sun TT. Corneal epithelial stem cells at the limbus: looking at some old problems from a new angle. Exp Eye Res. 2004;78:433-446. [PubMed] |
| 138. | Pellegrini G, Traverso CE, Franzi AT, Zingirian M, Cancedda R, De Luca M. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet. 1997;349:990-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 939] [Cited by in RCA: 946] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 139. | Ozdemir O, Tekeli O, Ornek K, Arslanpençe A, Yalçindağ NF. Limbal autograft and allograft transplantations in patients with corneal burns. Eye (Lond). 2004;18:241-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 140. | Dua HS, Azuara-Blanco A. Autologous limbal transplantation in patients with unilateral corneal stem cell deficiency. Br J Ophthalmol. 2000;84:273-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 133] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 141. | Rama P, Matuska S, Paganoni G, Spinelli A, De Luca M, Pellegrini G. Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med. 2010;363:147-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 929] [Cited by in RCA: 834] [Article Influence: 55.6] [Reference Citation Analysis (0)] |
| 142. | Qi X, Xie L, Cheng J, Zhai H, Zhou Q. Characteristics of immune rejection after allogeneic cultivated limbal epithelial transplantation. Ophthalmology. 2013;120:931-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 143. | Shortt AJ, Tuft SJ, Daniels JT. Ex vivo cultured limbal epithelial transplantation. A clinical perspective. Ocul Surf. 2010;8:80-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 144. | Sangwan VS, Basu S, MacNeil S, Balasubramanian D. Simple limbal epithelial transplantation (SLET): a novel surgical technique for the treatment of unilateral limbal stem cell deficiency. Br J Ophthalmol. 2012;96:931-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 310] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 145. | Branch MJ, Hashmani K, Dhillon P, Jones DR, Dua HS, Hopkinson A. Mesenchymal stem cells in the human corneal limbal stroma. Invest Ophthalmol Vis Sci. 2012;53:5109-5116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 112] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 146. | Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2248] [Cited by in RCA: 2347] [Article Influence: 123.5] [Reference Citation Analysis (0)] |
| 147. | Qiao C, Xu W, Zhu W, Hu J, Qian H, Yin Q, Jiang R, Yan Y, Mao F, Yang H. Human mesenchymal stem cells isolated from the umbilical cord. Cell Biol Int. 2008;32:8-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 177] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 148. | Seeberger KL, Dufour JM, Shapiro AM, Lakey JR, Rajotte RV, Korbutt GS. Expansion of mesenchymal stem cells from human pancreatic ductal epithelium. Lab Invest. 2006;86:141-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 127] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 149. | Zhang W, Walboomers XF, Shi S, Fan M, Jansen JA. Multilineage differentiation potential of stem cells derived from human dental pulp after cryopreservation. Tissue Eng. 2006;12:2813-2823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 270] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 150. | Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279-4295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4817] [Cited by in RCA: 5014] [Article Influence: 218.0] [Reference Citation Analysis (0)] |
| 151. | Ponnaiyan D, Jegadeesan V. Comparison of phenotype and differentiation marker gene expression profiles in human dental pulp and bone marrow mesenchymal stem cells. Eur J Dent. 2014;8:307-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 152. | Hoogduijn MJ, Popp F, Verbeek R, Masoodi M, Nicolaou A, Baan C, Dahlke MH. The immunomodulatory properties of mesenchymal stem cells and their use for immunotherapy. Int Immunopharmacol. 2010;10:1496-1500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 180] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 153. | Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15372] [Cited by in RCA: 15201] [Article Influence: 584.7] [Reference Citation Analysis (0)] |
| 154. | Ghannam S, Bouffi C, Djouad F, Jorgensen C, Noël D. Immunosuppression by mesenchymal stem cells: mechanisms and clinical applications. Stem Cell Res Ther. 2010;1:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 351] [Cited by in RCA: 379] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 155. | Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815-1822. [PubMed] |
| 156. | Casiraghi F, Azzollini N, Cassis P, Imberti B, Morigi M, Cugini D, Cavinato RA, Todeschini M, Solini S, Sonzogni A. Pretransplant infusion of mesenchymal stem cells prolongs the survival of a semiallogeneic heart transplant through the generation of regulatory T cells. J Immunol. 2008;181:3933-3946. [PubMed] |
| 157. | Zhang W, Qin C, Zhou ZM. Mesenchymal stem cells modulate immune responses combined with cyclosporine in a rat renal transplantation model. Transplant Proc. 2007;39:3404-3408. [PubMed] |
| 158. | Hong ZF, Huang XJ, Yin ZY, Zhao WX, Wang XM. Immunosuppressive function of bone marrow mesenchymal stem cells on acute rejection of liver allografts in rats. Transplant Proc. 2009;41:403-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 159. | Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, Hardy W, Devine S, Ucker D, Deans R. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42-48. [PubMed] |
| 160. | Liu H, Zhang J, Liu CY, Wang IJ, Sieber M, Chang J, Jester JV, Kao WW. Cell therapy of congenital corneal diseases with umbilical mesenchymal stem cells: lumican null mice. PLoS One. 2010;5:e10707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 112] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 161. | Liu H, Zhang J, Liu CY, Hayashi Y, Kao WW. Bone marrow mesenchymal stem cells can differentiate and assume corneal keratocyte phenotype. J Cell Mol Med. 2012;16:1114-1124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 162. | Treacy O, O’Flynn L, Ryan AE, Morcos M, Lohan P, Schu S, Wilk M, Fahy G, Griffin MD, Nosov M. Mesenchymal stem cell therapy promotes corneal allograft survival in rats by local and systemic immunomodulation. Am J Transplant. 2014;14:2023-2036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 163. | Oh JY, Lee RH, Yu JM, Ko JH, Lee HJ, Ko AY, Roddy GW, Prockop DJ. Intravenous mesenchymal stem cells prevented rejection of allogeneic corneal transplants by aborting the early inflammatory response. Mol Ther. 2012;20:2143-2152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 134] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 164. | Jia Z, Jiao C, Zhao S, Li X, Ren X, Zhang L, Han ZC, Zhang X. Immunomodulatory effects of mesenchymal stem cells in a rat corneal allograft rejection model. Exp Eye Res. 2012;102:44-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 165. | Omoto M, Katikireddy KR, Rezazadeh A, Dohlman TH, Chauhan SK. Mesenchymal stem cells home to inflamed ocular surface and suppress allosensitization in corneal transplantation. Invest Ophthalmol Vis Sci. 2014;55:6631-6638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 166. | Oh JY, Kim MK, Shin MS, Lee HJ, Ko JH, Wee WR, Lee JH. The anti-inflammatory and anti-angiogenic role of mesenchymal stem cells in corneal wound healing following chemical injury. Stem Cells. 2008;26:1047-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 298] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 167. | Yao L, Li ZR, Su WR, Li YP, Lin ML, Zhang WX, Liu Y, Wan Q, Liang D. Role of mesenchymal stem cells on cornea wound healing induced by acute alkali burn. PLoS One. 2012;7:e30842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 125] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 168. | Wei X, Yang X, Han ZP, Qu FF, Shao L, Shi YF. Mesenchymal stem cells: a new trend for cell therapy. Acta Pharmacol Sin. 2013;34:747-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 586] [Cited by in RCA: 691] [Article Influence: 57.6] [Reference Citation Analysis (0)] |
| 169. | Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10867] [Cited by in RCA: 10721] [Article Influence: 397.1] [Reference Citation Analysis (0)] |
| 170. | Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12:265-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1427] [Cited by in RCA: 1555] [Article Influence: 119.6] [Reference Citation Analysis (0)] |
| 171. | Schuler G, Schuler-Thurner B, Steinman RM. The use of dendritic cells in cancer immunotherapy. Curr Opin Immunol. 2003;15:138-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 468] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 172. | Fong L, Engleman EG. Dendritic cells in cancer immunotherapy. Annu Rev Immunol. 2000;18:245-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 514] [Cited by in RCA: 490] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 173. | Sato K, Yamashita N, Yamashita N, Baba M, Matsuyama T. Regulatory dendritic cells protect mice from murine acute graft-versus-host disease and leukemia relapse. Immunity. 2003;18:367-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 255] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 174. | Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2370] [Cited by in RCA: 2338] [Article Influence: 106.3] [Reference Citation Analysis (0)] |
| 175. | Moreau A, Varey E, Bouchet-Delbos L, Cuturi MC. Cell therapy using tolerogenic dendritic cells in transplantation. Transplant Res. 2012;1:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 176. | Morelli AE, Thomson AW. Tolerogenic dendritic cells and the quest for transplant tolerance. Nat Rev Immunol. 2007;7:610-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 672] [Cited by in RCA: 699] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 177. | Fu F, Li Y, Qian S, Lu L, Chambers F, Starzl TE, Fung JJ, Thomson AW. Costimulatory molecule-deficient dendritic cell progenitors (MHC class II+, CD80dim, CD86-) prolong cardiac allograft survival in nonimmunosuppressed recipients. Transplantation. 1996;62:659-665. [PubMed] |
| 178. | Turnquist HR, Raimondi G, Zahorchak AF, Fischer RT, Wang Z, Thomson AW. Rapamycin-conditioned dendritic cells are poor stimulators of allogeneic CD4+ T cells, but enrich for antigen-specific Foxp3+ T regulatory cells and promote organ transplant tolerance. J Immunol. 2007;178:7018-7031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 348] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 179. | Moreau A, Varey E, Bériou G, Hill M, Bouchet-Delbos L, Segovia M, Cuturi MC. Tolerogenic dendritic cells and negative vaccination in transplantation: from rodents to clinical trials. Front Immunol. 2012;3:218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 180. | Lan YY, Wang Z, Raimondi G, Wu W, Colvin BL, de Creus A, Thomson AW. “Alternatively activated” dendritic cells preferentially secrete IL-10, expand Foxp3+CD4+ T cells, and induce long-term organ allograft survival in combination with CTLA4-Ig. J Immunol. 2006;177:5868-5877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 121] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 181. | Hattori T, Saban DR, Emami-Naeini P, Chauhan SK, Funaki T, Ueno H, Dana R. Donor-derived, tolerogenic dendritic cells suppress immune rejection in the indirect allosensitization-dominant setting of corneal transplantation. J Leukoc Biol. 2012;91:621-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 182. | Gao XW, Fu Y, Li WJ, Du AJ, Li X, Zhao XD. Mechanism of immune tolerance induced by donor derived immature dendritic cells in rat high-risk corneal transplantation. Int J Ophthalmol. 2013;6:269-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 183. | Jonuleit H, Schmitt E. The regulatory T cell family: distinct subsets and their interrelations. J Immunol. 2003;171:6323-6327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 295] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 184. | Rudensky AY. Regulatory T cells and Foxp3. Immunol Rev. 2011;241:260-268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 671] [Cited by in RCA: 652] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 185. | Schmitt EG, Williams CB. Generation and function of induced regulatory T cells. Front Immunol. 2013;4:152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 156] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 186. | Lerret NM, Houlihan JL, Kheradmand T, Pothoven KL, Zhang ZJ, Luo X. Donor-specific CD8+ Foxp3+ T cells protect skin allografts and facilitate induction of conventional CD4+ Foxp3+ regulatory T cells. Am J Transplant. 2012;12:2335-2347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 187. | Hildebrand A, Jarsch C, Kern Y, Böhringer D, Reinhard T, Schwartzkopff J. Subconjunctivally applied naïve Tregs support corneal graft survival in baby rats. Mol Vis. 2014;20:1749-1757. [PubMed] |
| 188. | Giannoukakis N, Phillips B, Finegold D, Harnaha J, Trucco M. Phase I (safety) study of autologous tolerogenic dendritic cells in type 1 diabetic patients. Diabetes Care. 2011;34:2026-2032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 284] [Cited by in RCA: 325] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 189. | Benham H, Nel HJ, Law SC, Mehdi AM, Street S, Ramnoruth N, Pahau H, Lee BT, Ng J, G Brunck ME. Citrullinated peptide dendritic cell immunotherapy in HLA risk genotype-positive rheumatoid arthritis patients. Sci Transl Med. 2015;7:290ra87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 279] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 190. | Brunstein CG, Fuchs EJ, Carter SL, Karanes C, Costa LJ, Wu J, Devine SM, Wingard JR, Aljitawi OS, Cutler CS. Alternative donor transplantation after reduced intensity conditioning: results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood. 2011;118:282-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 466] [Cited by in RCA: 456] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 191. | Lam FC, Liu C. The future of keratoprostheses (artificial corneae). Br J Ophthalmol. 2011;95:304-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 192. | Lagali N, Fagerholm P, Griffith M. Biosynthetic corneas: prospects for supplementing the human donor cornea supply. Expert Rev Med Devices. 2011;8:127-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 193. | Polisetti N, Islam MM, Griffith M. The artificial cornea. Methods Mol Biol. 2013;1014:45-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 194. | Magalhães FP, Sousa LB, Oliveira LA. Boston type I keratoprosthesis: Review. Arq Bras Oftalmol. 2012;75:218-222. [PubMed] |
| 195. | Ciolino JB, Belin MW, Todani A, Al-Arfaj K, Rudnisky CJ. Retention of the Boston keratoprosthesis type 1: multicenter study results. Ophthalmology. 2013;120:1195-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 121] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 196. | Gomaa A, Comyn O, Liu C. Keratoprostheses in clinical practice - a review. Clin Experiment Ophthalmol. 2010;38:211-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 197. | Sivaraman KR, Hou JH, Allemann N, de la Cruz J, Cortina MS. Retroprosthetic membrane and risk of sterile keratolysis in patients with type I Boston Keratoprosthesis. Am J Ophthalmol. 2013;155:814-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 198. | Bond B, Williams CA, Jackman SR, Woodward A, Armstrong N, Barker AR. Accumulating exercise and postprandial health in adolescents. Metabolism. 2015;64:1068-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 199. | Khan BF, Harissi-Dagher M, Pavan-Langston D, Aquavella JV, Dohlman CH. The Boston keratoprosthesis in herpetic keratitis. Arch Ophthalmol. 2007;125:745-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 200. | Akpek EK, Harissi-Dagher M, Petrarca R, Butrus SI, Pineda R, Aquavella JV, Dohlman CH. Outcomes of Boston keratoprosthesis in aniridia: a retrospective multicenter study. Am J Ophthalmol. 2007;144:227-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 92] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 201. | Stacy RC, Jakobiec FA, Michaud NA, Dohlman CH, Colby KA. Characterization of retrokeratoprosthetic membranes in the Boston type 1 keratoprosthesis. Arch Ophthalmol. 2011;129:310-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 202. | Avadhanam VS, Liu CS. A brief review of Boston type-1 and osteo-odonto keratoprostheses. Br J Ophthalmol. 2015;99:878-887. [PubMed] |
| 203. | Wong HS, Then KY, Ramli R. Osteo-odonto-keratoprosthesis for end-stage cornea blindness. Med J Malaysia. 2011;66:369-370. [PubMed] |
| 204. | Ahn JI, Kuffova L, Merrett K, Mitra D, Forrester JV, Li F, Griffith M. Crosslinked collagen hydrogels as corneal implants: effects of sterically bulky vs. non-bulky carbodiimides as crosslinkers. Acta Biomater. 2013;9:7796-7805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 205. | Mirazul Islam M, Cėpla V, He C, Edin J, Rakickas T, Kobuch K, Ruželė Ž, Jackson WB, Rafat M, Lohmann CP. Functional fabrication of recombinant human collagen-phosphorylcholine hydrogels for regenerative medicine applications. Acta Biomater. 2015;12:70-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 206. | Liu W, Deng C, McLaughlin CR, Fagerholm P, Lagali NS, Heyne B, Scaiano JC, Watsky MA, Kato Y, Munger R. Collagen-phosphorylcholine interpenetrating network hydrogels as corneal substitutes. Biomaterials. 2009;30:1551-1559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 137] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 207. | Liu L, Kuffová L, Griffith M, Dang Z, Muckersie E, Liu Y, McLaughlin CR, Forrester JV. Immunological responses in mice to full-thickness corneal grafts engineered from porcine collagen. Biomaterials. 2007;28:3807-3814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 208. | Liu Y, Griffith M, Watsky MA, Forrester JV, Kuffova L, Grant D, Merrett K, Carlsson DJ. Properties of porcine and recombinant human collagen matrices for optically clear tissue engineering applications. Biomacromolecules. 2006;7:1819-1828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 69] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 209. | McLaughlin CR, Acosta MC, Luna C, Liu W, Belmonte C, Griffith M, Gallar J. Regeneration of functional nerves within full thickness collagen-phosphorylcholine corneal substitute implants in guinea pigs. Biomaterials. 2010;31:2770-2778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 210. | Hackett JM, Lagali N, Merrett K, Edelhauser H, Sun Y, Gan L, Griffith M, Fagerholm P. Biosynthetic corneal implants for replacement of pathologic corneal tissue: performance in a controlled rabbit alkali burn model. Invest Ophthalmol Vis Sci. 2011;52:651-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 211. | Bareiss B, Ghorbani M, Li F, Blake JA, Scaiano JC, Zhang J, Deng C, Merrett K, Harden JL, Diaz-Mitoma F. Controlled Release of Acyclovir Through Bioengineered Corneal Implants with Silica Nanoparticle Carriers. Open Open Tissue Eng Regen Med J. 2010;3:10-17. [RCA] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 212. | Lee CJ, Buznyk O, Kuffova L, Rajendran V, Forrester JV, Phopase J, Islam MM, Skog M, Ahlqvist J, Griffith M. Cathelicidin LL-37 and HSV-1 Corneal Infection: Peptide Versus Gene Therapy. Transl Vis Sci Technol. 2014;3:4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 213. | Fagerholm P, Lagali NS, Carlsson DJ, Merrett K, Griffith M. Corneal regeneration following implantation of a biomimetic tissue-engineered substitute. Clin Transl Sci. 2009;2:162-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 214. | Fagerholm P, Lagali NS, Ong JA, Merrett K, Jackson WB, Polarek JW, Suuronen EJ, Liu Y, Brunette I, Griffith M. Stable corneal regeneration four years after implantation of a cell-free recombinant human collagen scaffold. Biomaterials. 2014;35:2420-2427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 195] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 215. | Buznyk O, Pasyechnikova N, Islam MM, Iakymenko S, Fagerholm P, Griffith M. Bioengineered Corneas Grafted as Alternatives to Human Donor Corneas in Three High-Risk Patients. Clin Transl Sci. 2015;8:558-562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 216. | Zhang MC, Liu X, Jin Y, Jiang DL, Wei XS, Xie HT. Lamellar keratoplasty treatment of fungal corneal ulcers with acellular porcine corneal stroma. Am J Transplant. 2015;15:1068-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 106] [Article Influence: 10.6] [Reference Citation Analysis (0)] |