Published online Dec 24, 2015. doi: 10.5500/wjt.v5.i4.329
Peer-review started: August 11, 2015
First decision: September 21, 2015
Revised: October 10, 2015
Accepted: November 23, 2015
Article in press: November 25, 2015
Published online: December 24, 2015
Processing time: 136 Days and 4 Hours
AIM: To investigate the long-term results of ABO-incompatible (ABOi) kidney transplantation in a single center in Greece.
METHODS: Thirty consecutive ABOi kidney transplantations were performed from June 2005 to December 2013. All patients received rituximab one month prior to transplantation. Immunoadsorption therapy was performed for the removal of anti-A/B IgG antibodies until the titer was ≤ 1:16. Additional apheresis sessions were performed post-operatively. Intravenous immunoglobulin and oral immunosuppression consisting of tacrolimus (TAC) in combination with either everolimus or mycophenolate acid was administered. We compared the long term results of our ABOi group to those of a matched group of 30 ABO compatible (ABOc) living kidney recipients with similar baseline characteristics. The ABOc recipients received an immunosuppressive regimen consisting of TAC and mycophenolate acid. All patients in both groups received induction therapy with Basiliximab or Daclizumab, whereas corticosteroids were instituted on the day of surgery. During the follow-up period, indication biopsies were performed and interpreted by an experienced nephropathologist. The parameters we analyzed included the following: Donor/recipient age, gender, blood type, human leukocyte antigen mismatches, panel reactive antibodies, primary cause of renal failure, mean time on dialysis, immunosuppressive regimen, patient survival, graft outcome, incidence of rejections, surgical and infectious complications.
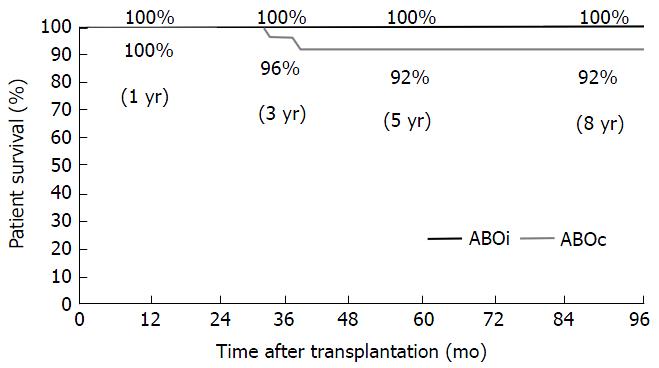
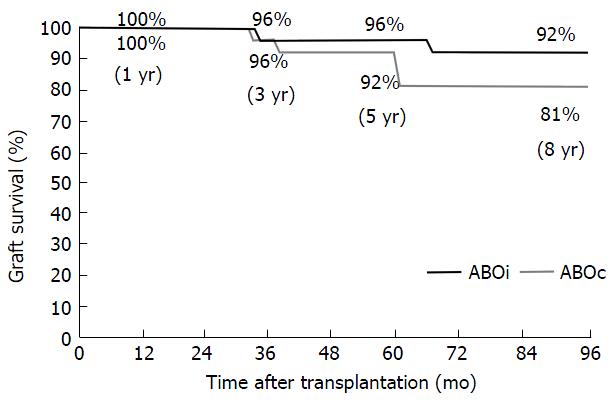
RESULTS: The mean follow-up period was 6 years (range 1 to 9 years). A mean of 5.0 ± 3.0 (range 0-14) pre-transplant immunoadsorptions were required in order to reach the target titer. Patient survival in ABOi group in comparison to ABOc group at 1, 3, 5 and 8 years did not differ significantly (100% vs 100%, 96% vs 100%, 92% vs 100% and 92% vs 100%, P = ns). Additionally, graft survival was similar in the two groups at the same time points (100% vs 100%, 96% vs 96%, 92% vs 96% and 81% vs 92%, P = ns). The mean serum creatinine and the estimated glomerular filtration rate by the modification of diet in renal disease formula at 1, 3, 5 and 8 years did not differ significantly between ABOi and ABOc group. None of the patients in the ABOi group developed acute or chronic antibody-mediated rejection evidenced by histological signs. Four patients (13.3%) in the ABOi group and 3 (10%) in the ABOc group experienced acute cellular rejection, which was treated successfully in all cases. Bacterial and viral infections were also similar between the two groups.
CONCLUSION: ABOi kidney transplantation is a safe and effective alternative that enables kidney transplantation in countries with unacceptably long deceased-donor waiting lists.
Core tip: These excellent long term results further establish ABO-incompatible (ABOi) kidney transplantation as a safe and effective therapeutic strategy for the management of end-stage renal disease patients. Various immunosuppressants including Everolimus could be potentially selected based on patient’s profile. ABOi kidney transplantation could contribute to the enlargement of the living donor pool, particularly in countries with organ shortage.
- Citation: Melexopoulou C, Marinaki S, Liapis G, Skalioti C, Gavalaki M, Zavos G, Boletis JN. Excellent long term patient and renal allograft survival after ABO-incompatible kidney transplantation: Experience of one center. World J Transplant 2015; 5(4): 329-337
- URL: https://www.wjgnet.com/2220-3230/full/v5/i4/329.htm
- DOI: https://dx.doi.org/10.5500/wjt.v5.i4.329
Considering the shortage of available organs for transplantation, efforts have been made worldwide to expand the donor pool. Attempts to expand the deceased donor pool include “expanded criteria donors”, “non-heart beating donors” as well as programs such as the “old for old” Eurotransplant program[1]. For living donor kidney transplantation, the expansion of new and potent immunosuppressive drugs, allowed us to overcome traditional “immunologic barriers” as blood group incompatibility and transplantation to recipients with preformed donor specific antibodies, which had previously been considered as “impossible”. Especially in countries with long waiting lists for patients on maintenance dialysis, ABO-incompatible (ABOi) kidney transplantation constitutes an attractive alternative therapeutic option[2,3]. In 2014, approximately 1100 patients with end-stage renal disease were awaiting for a kidney transplant in Greece. Unfortunately, only 88 (8%) of them were transplanted from a deceased donor, whereas 40 (3.6%) died during the same period while awaiting for a transplant. The mean time on the waiting list for an available organ in our country comprises about 5 years and it is growing every year. Given the shortage of deceased donors, efforts have been made to expand the living donor pool.
In 2005 our center started the ABOi kidney transplant program, using for recipient preconditioning protocols that had been used successfully in other European centers[4]. We herein analyze the long term results from 30 consecutive ABOi kidney transplantations performed at our transplant center. We compared them with a control group comprising of matched recipients transplanted during the same period from ABO compatible (ABOc) living kidney donors.
From June 2005 to December 2013, a total of 30 ABOi kidney transplantations have been performed at the Renal Transplantation Unit of “Laiko” General hospital, in Athens, Greece. Our center is the only one in Greece that performs ABOi transplantations. The mean follow-up period after renal transplantation was 6 years (range 1 to 9 years). In this study we retrospectively analyzed data of those patients. The parameters we analyzed included the following: Donor/recipient age, gender, blood type, human leukocyte antigen (HLA) mismatches, Panel Reactive Antibodies (PRAs), primary cause of renal failure, mean time on dialysis, immunosuppressive regimen, patient survival, graft outcome, incidence of rejections, surgical and infectious complications. We additionally reviewed the histological findings of the performed renal biopsies. We compared the clinical and laboratory findings of the ABOi kidney transplant recipients with those of a control group consisting of ABOc living kidney recipients. The control group comprised 30 patients who were transplanted at the same time period and were randomly selected on the basis of similar baseline demographic and clinical characteristics of donors and recipients.
The preconditioning regimen used for ABOi kidney transplantation in our center was based on the Swedish protocol[4].
A single dose of anti-CD20 monoclonal antibody Rituximab (375 mg/m2 body surface area) was given one month (day 30) before transplantation. This was followed by a double drug immunosuppressive regimen initiated on day-15. Twenty two recipients (73.3%) received an immunosuppressive regimen consisting of tacrolimus (TAC) with a targeted through level of 4 to 6 ng/mL and everolimus (EVR) with trough levels of 6 to 8 ng/mL, while 8 recipients (26.7%) received TAC aiming at trough levels of 6 to 8 ng/mL and MPA (mycophenolate mofetil, MMF 1000 mg bid or mycophenolate sodium 720 mg bid).
All patients received induction therapy with either Basiliximab (20 mg on days 0 and 4 of transplantation) or Daclizumab (1 mg/kg on days 0, 15, 30, 45 post-transplantation). Twenty one patients (70%) received Basiliximab and 9 patients (30%) Daclizumab. Methylprednisolone was administered at a dose of 500 mg intraoperatively followed by 20 or 30 mg/d postoperatively.
Three months post-transplantation EVR was switched to MPA. At that time point TAC target trough levels were 5 to 7 ng/mL. Steroids were tapered to a dose of 4mg/d during the first three months of transplantation.
Preoperatively, the anti-A or anti-B antibodies (abs) were removed using repeated immunoadsorption (IA) or double filtration plasmapheresis (DFPP) (1-2 plasma volume exchange). The aim was to achieve an antibody titer of IgG ≤ 1:16 at the day of transplantation (Day 0). A haemagglutination titration technique was used for the measurement of anti-A/B abs. One day prior to surgery intravenous immunoglobulin (IVIG) 0.5 g/kg was administered.
Postoperatively three apheresis sessions were routinely performed every other day. In cases of persistently elevated antibody titers, additional sessions were delivered. Antibody rebound was defined as a rise in the antibody titer equal to 1:32 or higher.
In ABOc kidney transplant recipients immunosuppression was initiated ten days before transplantation with TAC (through level 6 to 8 ng/mL) or ciclosporin (2 h post-dose level 700-900 ng/mL). MPA (MMF 1000 mg bid or mycophenolate sodium 720 mg bid) was administered one day prior to transplantation.
Induction therapy consisted of either Basiliximab (20 mg on days 0 and 4 of transplantation) or Daclizumab (1 mg/kg on days 0, 15, 30, 45 post-transplantation). Eleven patients (36.7%) received Basiliximab and 63.3% Daclizumab. Methylprednisolone was administered at a dose of 500 mg during surgery followed by 20 mg/d postoperatively.
Three months post-transplantation TAC trough levels were reduced to 5-7 ng/mL, whereas ciclosporine target was maintained at a level of 600-800 ng/mL, 2 h postdose. Methylprednisolone was tapered to a dose of 4 mg/d until the third month post-transplantation.
Antiviral prophylaxis for cytomegalovirus (valgancyclovir) was administrated to all ABOi kidney transplant recipients for six months. ABOc kidney transplant recipients received cytomegalovirus (CMV) prophylaxis for three and six months according to donor and recipient CMV status. Pneumocystis jirovecii pneumonia prophylaxis (trimethoprim/sulfamethoxazole) was also administered postoperatively for three and six months in ABOc and ABOi kidney transplant recipients respectively.
All kidney biopsies that were performed under clinical indication during the follow-up period were interpreted by an experienced nephropathologist. Histological findings were graded and recorded according to Banff Congresses grading system[5]. Diagnoses in patients with more than one biopsy were documented according to the predominant histological feature based on Banff guidelines.
Two tissue samples were provided for pathological examination. Formalin fixed paraffin embedded tissue sections were processed for light microscopy examination. Three Hematoxyline and Eosin stains as well as PAS, Silver and Masson histochemical stains were available. A small part of cortex from each biopsy was processed for indirect immunofluorescence in frozen sections and a second small tissue sample, in selected cases, was processed in glutarhaldeyde for electron microscopy examination. Adequate samples were included at least seven gomeruli and cut sections of at least one artery according to Banff criteria. Immunohistochemical assay for C4d detection was applied in all tissue samples.
As bacterial infections identified only those led to hospitalization. Polyoma (BK) virus infection was diagnosed when BK-DNA levels were elevated and/or BK was histological proven. CMV disease was defined as elevated CMV-DNA levels in context with the presence of clinical symptoms.
Statistical analysis was performed using SPSS 17.0 software. Comparisons between the two groups were made with Fisher’s exact test and independent-samples t-test. Patient and graft survival were determined using the Kaplan-Meier method. P < 0.05 was considered statistically significant.
Baseline patient characteristics are shown in Table 1. No significant difference in the age and gender of recipients and donors, the number of HLA mismatches and panel reactive antibody (PRA) was recorded between the ABOi and ABOc groups. Pre-transplant dialysis time was significantly higher in the ABOi group. All patients had negative CDC T-cell crossmatch and a negative flow cytometry crossmatch. None of them was hypersensitized (PRA > 75%) and none had received a prior kidney transplant.
| ABOi (n = 30) | ABOc (n = 30) | P-value | |
| Recipient age (yr) | 39 ± 11 (16-60) | 37 ± 11 (19-64) | ns |
| Donor age (yr) | 59 ± 9 (42-77) | 61 ± 11 (41-78) | ns |
| Recipient gender female/male | 8/22 | 7/23 | ns |
| Donor gender female/male | 24/6 | 19/11 | ns |
| HLA mismatches | 2.7 ± 1.2 | 2.4 ± 1.2 | ns |
| PRAs > 10% | 3 | 3 | ns |
| Time on dialysis (mo) | 37 ± 34 (4-132) | 19 ± 18 (0-82) | 0.014a |
| ABOi | |||
| A-O | 12 (40%) | ||
| B-O | 4 (13.3%) | ||
| A-B | 2 (6.7%) | ||
| B-A | 1 (3.3%) | ||
| AB-A | 5 (16.7%) | ||
| AB-B | 6 (20%) | ||
| Anti-A/B IgG titer at referral (median) | 1:64 (1:1-1:128) | ||
| No. of pretransplant IA | 5.1 ± 3.1 (0-14) | ||
| No. of posttrasplant IA | 3.3 ± 1.4 (1-7) | ||
| Cause of renal failure | |||
| Polycystic disease | 6 (20%) | 1 (3.3%) | |
| Hypertension | 1 (3.3%) | 1 (3.3%) | |
| Glumerulonephritis | 8 (26.7%) | 5 (16.7%) | |
| Genetic disorder | 3 (10%) | 4 (13.3%) | |
| Diabetes | 3 (10%) | 1 (3.3%) | |
| Unknown | 6 (20%) | 16 (53.4%) | |
| Other | 3 (10%) | 2 (6.7%) |
Half of the recipients (52%) were blood group O (Table 1). The highest initial titer of anti-A or anti-B IgG abs was 1:128, while the median titer was 1:64 (1:1-1:128).
A mean number of 5.0 ± 3.0 (range 0-14) pre-transplantation apheresis sessions were required in order to reach the target titer of 1:16. Before transplantation, we did not perform IA in two patients with a titer of anti-A/B IgG abs equal or lower to 1:4. In the first 24 ABOi patients we performed immunoadsorptions using the antigen-specific carbohydrate column (Glycosorb A/B®), according to the Swedish protocol. Then, due to its high cost we switched to the protein A adsorption column (Immunosorba®). In some cases we also used DFPP alone or in combination with Immunosorba®. Following the same protocol for the number of apheresis sessions, we achieved the necessary ant-A/anti-B abs titer prior to transplantation, regardless of the apheresis method that was used.
Post-transplantation a mean number of 3.3 ± 1.4/patient (range 1-7) apheresis sessions were performed. Seven patients underwent only 1-2 apheresis sessions due to a very low titer of anti-A/B IgG abs (≤ 1:4) immediately post-transplantation. Rebound of anti-A/anti-B abs was not observed post-transplantation.
The mean follow-up period was 74 mo (range 14-114) in the ABOi transplant recipients vs 78 mo (13-116) in the ABOc patients (P = ns). Patient survival in ABOi in comparison to ABOc group at 1, 3, 5 and 8 years did not differ significantly (100% vs 100%, 96% vs 100%, 92% vs 100% and 92% vs 100%, P = ns) (Figure 1). Two deaths with a functioning graft occurred during the study period in the ABOi group. The first patient died 37 mo post-transplantation due to acute liver failure of unidentified viral infection. The second patient died because of acute myocardial infarction at 43 mo post-transplant.
Death-censored graft survival was similar in the two groups at any time point (100% vs 100%, 96% vs 96%, 92% vs 96% and 81% vs 92%, P = ns) (Figure 2).
Delayed graft function was not recorded in any of the two groups of patients. Serum creatinine at 1, 3, 5 and 8 years did not differ significantly between the ABOi and the ABOc group at any time point (Table 2). Furthermore, estimated glomerular filtration rate calculated using the modification of diet in renal disease formula at 1, 3, 5 and 8 years was similar between the two groups (Table 2).
| ABOi (n = 30) | ABOc (n = 30) | P-value | |
| Mean follow-up (mo) | 74 (14-114) | 78 (13-116) | ns |
| Serum creatinine (mg/dL) | |||
| 1 yr after KTx | 1.56 ± 0.34 | 1.53 ± 0.46 | ns |
| 3 yr after KTx | 1.53 ± 0.37 | 1.5 ± 0.43 | ns |
| 5 yr after KTx | 1.6 ± 0.48 | 1.53 ± 0.55 | ns |
| 8 yr after KTx | 1.78 ± 0.57 | 1.76 ± 0.58 | ns |
| eGFR by MDRD (mL/min per 1.73 m2) | |||
| 1 yr after KTx | 56.1 ± 13.4 | 56.3 ± 16.8 | ns |
| 3 yr after KTx | 51.5 ± 17.1 | 56.1 ± 16.1 | ns |
| 5 yr after KTx | 53.1 ± 17 | 54.5 ± 17.3 | ns |
| 8 yr after KTx | 47.3 ± 20.5 | 44.4 ± 16.4 | ns |
| Rejections | |||
| Acute cellular rejection | 4 (13.3%) | 3 (10%) | ns |
| Acute antibody - mediated rejection | 0 (0%) | 0 (0%) | ns |
A total of 39 biopsies were performed in 18 ABOi kidney transplant recipients and 29 biopsies in 13 ABOc recipients. Histological diagnoses and findings per patient are summarized in Table 3.
| Patients with biopsies | ABOi (n = 18) | ABOc (n = 13) | P-value |
| Acute tubular injury | 2 (11) | 3 (23) | ns |
| Acute cellular rejection | 4 (22) | 3 (23) | ns |
| Endarteritis | 1 (6) | 0 (0) | ns |
| Acute cellular rejection < 1 yr post transplantation | 4 (22) | 2 (15) | ns |
| Acute cellular rejection > 1 yr post transplantation | 0 (0) | 1 (8) | ns |
| Acute antibody - mediated rejection with histological signs | 0 (0) | 0 (0) | ns |
| Chronic antibody - mediated rejection, C4d (+) | 0 (0) | 2 (15) | ns |
| Chronic antibody - mediated rejection, C4d (+) < 1 yr post transplantation without TGL | 0 (0) | 1 (8) | ns |
| Chronic antibody - mediated rejection, C4d (+) > 1 yr post transplantation with TGL | 0 (0) | 1 (8) | ns |
| C4d+ in peritubular capillaries | 9 (50) | 2 (15) | ns |
| CNI toxicity | 1 (6) | 1 (8) | ns |
| BK nephropathy | 4 (22) | 1 (8) | ns |
| Primary disease recurrence | 1 (6) | 2 (15) | ns |
| IF/TA with no evidence of any specific etiology | 2 (11) | 1 (8) | ns |
| No findings | 4 (22) | 2 (15) | ns |
Acute cellular rejection occurred in 13.3% (4/30) and 10% (3/30) of patients in ABOi and ABOc group respectively (P = ns) (Table 2). No acute or chronic antibody-mediated rejection (AMR) was identified in ABOi group. Two cases of chronic AMR were revealed in ABOc group, one associated with transplant glomerulopathy. Interestingly, histological evidence of primary disease recurrence accompanied chronic AMR in these two patients. The first patient had IgA nephropathy while the second had membranous nephropathy. Importantly, C4d staining along peritubular capillaries walls was more often encountered in ABOi group, although no statistical difference was demonstrated (50% vs 15%, P = 0.06, ns).
Histological proven BK nephropathy was more frequent in the ABOi group (22% vs 8%, P > 0.05, ns) eventhough statistically insignificant. Both findings are probably attributed to the small number of patients. All other histological parameters between the two groups, including chronic lesions (glomerular sclerosis, interstitial fibrosis/tubular atrophy, arteriolar hyalinosis and arteriosclerosis) were similar.
Graft loss due to a major surgical complication was not recorded. There was no significant difference in the incidence of minor surgical complications between the two groups (Table 4).
| ABOi | ABOc | P-value | |
| (n = 30) | (n = 30) | ||
| Surgical complications | |||
| Lemphocele | 4 (13.3) | 1 (3.3) | ns |
| Other | 2 (6.7) | 2 (6.7) | ns |
| Infectious complications | |||
| Bacterial (requiring hospitalization) | 15 (50) | 17 (56.7) | ns |
| Bacteraemia | 5 (16.7) | 4 (13.3) | ns |
| CMV | 1 (3.3) | 1 (3.3) | ns |
| BKV | 5 (16.7) | 2 (6.7) | ns |
Infectious complications were similar between the two groups (Table 4). Urinary tract infections were the most common bacterial infections. Interestingly, among viral infections, polyoma BK virus was recorded as the most frequent cause. In the ABOi group 5 cases (16.7%) of BK virus infection have been diagnosed. The incidence of the infection was greater during the first 6 mo post-transplantation.
During the last decade, the expansion of new, safe and potent immunosuppressants enabled us to perform ABOi kidney transplantations in many centers worldwide[2,6-9]. The use of rituximab instead of splenectomy and the excellent short term results of ABOi kidney transplantation in combination with the organ shortage in our country, forced us to expand our living donor pool. We started the ABOi program in 2005, nevertheless we are still the only transplant center performing ABOi transplantations in Greece. Our results show that ABOi kidney transplantation is a safe and effective therapeutic strategy. The long term patient and graft survival rates are excellent and do not differ significantly from the control group. Our findings are consistent with the long term results of ABOi kidney transplantations in Japan and in the United States[10,11].
For recipient preconditioning, we adopted the slightly modified Swedish protocol. We substituted MMF with EVR in the majority of our ABOi patients. To our knowledge this is the first report with the use of de novo everolimus in ABOi kidney transplantation. Uchida et al[12] showed the safety of switching from MMF to EVR one year post-transplantion in ABOi kidney transplant recipients. Everolimus is an inhibitor of the mammalian target of rapamycin (mTORi). It has inhibitory effects on cell proliferation and differentiation in the early stage of B-cell differentiation into plasma cell. Also, in vitro studies showed that EVR inhibits the differentiation into plasmablasts even in the middle phase[13]. The combination of an mTOR inhibitor with a calcineurin inhibitor (CNI) has shown to be safe and effective in a number of previous reported studies in renal transplantation[14-16]. The combination of EVR with TAC could be used as an alternative to MMF plus TAC in ABOi kidney transplant recipients. No acute or chronic antibody-mediated rejection was seen in the ABOi group. Studies comprising mainly recipients from deceased donors show that concerns about prolonged delayed graft function (DGF) with de novo everolimus seem to be unjustified[17,18]. None of the recipients in our ABOi group experienced DGF.
The choice of everolimus initially was based on the consideration that the combination of an mTORi with a CNI could prove to be more potent in preventing acute rejection episodes compared to CNI plus MPA for this high immunological risk patient group[19,20]. Thus for the first trimester, the combination of mTOR with CNI at low doses to avoid toxicity was used. After three months, we switched to the immunosuppresive regimen which is the standard of care in most centers including ours, namely CNI plus MPA in order to avoid nephrotoxicity in the long term. It is already well known that after the period of “accommodation”, ABOi recipients have not significantly higher immunologic risk than their ABOc counterparts[2].
Some reports indicate that recipients with blood group O have a higher incidence of acute AMR[21]. Most of our ABOi recipients (52%) were blood group O but no association with AMR was found. Similar findings have been reported from centers in the United States and Japan. They showed excellent results across all donor and recipient blood groups[2,10].
In agreement with previous reports, C4d staining along peritubular capillary walls was found more often in the ABOi group compared to ABOc group of patients. However, this finding was not accompanied by histological findings of AMR[6,22-24]. No other significant differences were found in histological parameters on kidney biopsies between ABOi and ABOc patients.
In our center we mainly used immunoadsorption for the removal of isoagglutinins. At the beginning, we used antigen-specific carbohydrate columns (Glycosorb A/B®), according to the Swedish protocol[25]. Then, due to the high cost, we switched to the protein A adsorption column (Immunosorba®)[26]. Our target titer for IgG anti-A/B abs prior to transplantation was ≤ 1:16. Independently of the method used for the removal of isoagglutinins, we reached our target titer with a relatively low number of apheresis sessions (mean number 5 ± 3, range 0-14) before transplantation and without experiencing a rebound of ABO abs during the post-transplantation period. It is worth mentioning that our patients’ highest initial anti-A/B IgG abs titer was equal to 1:128. Chung et al[27] showed that patients with a higher baseline ABO ab titer (≥ 1:256) had a higher tendency of antibody rebound and risk for acute rejection.
An issue of special interest in ABOi transplantation is the concern about over-immunosuppression and the incidence of infectious complications long term. Our preconditioning protocol included routine administration of rituximab, IA, IVIG and initiation of the combination of CNI plus mTORi before transplantation. After transplantation, we performed a standard number of three apheresis sessions indicated by the protocol and maintained medium to high levels of CNIs and mTORis. Three months after transplantation, mTORi was switched to standard dose MPA. Long term maintenance immunosuppression did not differ between the ABOi and the ABOc control group (data not shown). We did not observe any significant differences neither in bacterial nor in viral infections in comparison to the control group. Our results are in agreement with other studies that used similar protocols[28,29]. However there are others, who report an increased incidence of infections with the use of rituximab[23]. We indeed observed a numerically higher incidence of BK virus infections in the ABOi group (5 patients, 16.7% vs 2 patients, 6.7%), as well as biopsy proven BK nephropathy in the ABOi group (4 patients, 22%) compared to 1 patient (8%) in the ABOc group, but the difference was not statistically significant. The optimum dosage of rituximab is still an issue that needs investigation. Lower doses have been proven efficacious in Asians[30]; however it is difficult to extrapolate the results for Caucasians. Therefore, we decided to administer the dosages generally applied in Europe which have been proven efficacious in depleting B-cells.
The relatively small sample size is a limitation of our study. Another important issue in ABOi transplantation is the immunological risk, which is best reflected by biopsy proven acute rejection episodes (BPAR), especially early, i.e., during the first year post-transplant. We performed biopsies in about 50% of our patients (n = 18 in the ABOi and n = 13 in the ABOc group) at a minimum level of clinical indication. We had no episode of ABMR, during the first year, while acute cellular rejection episodes occurred in 22% (n = 4 patients) in the ABOi and 15% (n = 2 patients) in the ABOc group, respectively. Though numerically higher in the ABOi recipients, biopsy proven acute rejection episodes did not differ statistically. BPAR episodes did not differ statistically. Moreover, all but one - in the ABOi group- were mild and easily reversible. On the other hand, it is a very homogenous group of patients, with long term follow up at one center.
The most important point in our study - indeed in accordance with others who perform ABOi transplantations - are the excellent results long term. With no risks of over-immunosuppression long-term and comparable BPAR episodes and infectious complications, patient and graft survival reaches 92% and 81% at 8 years respectively.
This strongly supports the evidence, that especially in a small country like Greece with unacceptably long deceased-donor waiting lists and no possibility to support a paired-exchange donation program, it is crucial to continue the effort to perform ABO incompatible kidney transplantations.
Shortage of available transplant organs worldwide has implemented renal ABO-incompatible (ABOi) kidney transplantation as a potential therapeutic strategy for end-stage renal disease patients.
A combination of various immunosuppressants including Everolimus could potentially improve long-term results in ABOi kidney transplantation.
Optimal immunosuppression is the key for the excellent long-term results in ABOi kidney transplantation.
ABOi kidney transplantation contributes to the enlargement of the living donor pool, especially in countries with organ shortage.
ABOi kidney transplantation (ABOi kidney transplantation is a method of transplantation regardless of ABO blood type with the use of an appropriate desensitization protocol).
This is a useful paper that adds to the knowledge of ABOi transplantation outcome.
P- Reviewer: Hatakeyama S, Milford DV
S- Editor: Qiu S L- Editor: A E- Editor: Li D
| 1. | Becker LE, Süsal C, Morath C. Kidney transplantation across HLA and ABO antibody barriers. Curr Opin Organ Transplant. 2013;18:445-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 2. | Takahashi K, Saito K, Takahara S, Okuyama A, Tanabe K, Toma H, Uchida K, Hasegawa A, Yoshimura N, Kamiryo Y. Excellent long-term outcome of ABO-incompatible living donor kidney transplantation in Japan. Am J Transplant. 2004;4:1089-1096. [PubMed] |
| 3. | Muramatsu M, Gonzalez HD, Cacciola R, Aikawa A, Yaqoob MM, Puliatti C. ABO incompatible renal transplants: Good or bad? World J Transplant. 2014;4:18-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 40] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (2)] |
| 4. | Tydén G, Kumlien G, Genberg H, Sandberg J, Lundgren T, Fehrman I. ABO incompatible kidney transplantations without splenectomy, using antigen-specific immunoadsorption and rituximab. Am J Transplant. 2005;5:145-148. [PubMed] |
| 5. | Solez K, Colvin RB, Racusen LC, Haas M, Sis B, Mengel M, Halloran PF, Baldwin W, Banfi G, Collins AB. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant. 2008;8:753-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1514] [Cited by in RCA: 1496] [Article Influence: 88.0] [Reference Citation Analysis (0)] |
| 6. | Zschiedrich S, Kramer-Zucker A, Jänigen B, Seidl M, Emmerich F, Pisarski P, Huber TB. An update on ABO-incompatible kidney transplantation. Transpl Int. 2015;28:387-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Montgomery RA, Locke JE, King KE, Segev DL, Warren DS, Kraus ES, Cooper M, Simpkins CE, Singer AL, Stewart ZA. ABO incompatible renal transplantation: a paradigm ready for broad implementation. Transplantation. 2009;87:1246-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 181] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 8. | Flint SM, Walker RG, Hogan C, Haeusler MN, Robertson A, Francis DM, Millar R, Finlay M, Landgren A, Cohney SJ. Successful ABO-incompatible kidney transplantation with antibody removal and standard immunosuppression. Am J Transplant. 2011;11:1016-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 9. | Genberg H, Kumlien G, Wennberg L, Tydén G. Long-term results of ABO-incompatible kidney transplantation with antigen-specific immunoadsorption and rituximab. Transplantation. 2007;84:S44-S47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Montgomery JR, Berger JC, Warren DS, James NT, Montgomery RA, Segev DL. Outcomes of ABO-incompatible kidney transplantation in the United States. Transplantation. 2012;93:603-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 190] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 11. | Takahashi K, Saito K. ABO-incompatible kidney transplantation. Transplant Rev (Orlando). 2013;27:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 102] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 12. | Uchida J, Machida Y, Iwai T, Kuwabara N, Kabei K, Naganuma T, Kumada N, Kawashima H, Nakatani T. Conversion of stable ABO-incompatible kidney transplant recipients from mycophenolate mofetil with standard exposure calcineurin inhibitors (CNIs) to everolimus with very low exposure CNIs-a short-term pilot study. Clin Transplant. 2014;28:80-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Haneda M, Owaki M, Kuzuya T, Iwasaki K, Miwa Y, Kobayashi T. Comparative analysis of drug action on B-cell proliferation and differentiation for mycophenolic acid, everolimus, and prednisolone. Transplantation. 2014;97:405-412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Tedesco Silva H, Cibrik D, Johnston T, Lackova E, Mange K, Panis C, Walker R, Wang Z, Zibari G, Kim YS. Everolimus plus reduced-exposure CsA versus mycophenolic acid plus standard-exposure CsA in renal-transplant recipients. Am J Transplant. 2010;10:1401-1413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 216] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 15. | Eisen HJ, Tuzcu EM, Dorent R, Kobashigawa J, Mancini D, Valantine-von Kaeppler HA, Starling RC, Sørensen K, Hummel M, Lind JM. Everolimus for the prevention of allograft rejection and vasculopathy in cardiac-transplant recipients. N Engl J Med. 2003;349:847-858. [PubMed] |
| 16. | Cibrik D, Silva HT, Vathsala A, Lackova E, Cornu-Artis C, Walker RG, Wang Z, Zibari GB, Shihab F, Kim YS. Randomized trial of everolimus-facilitated calcineurin inhibitor minimization over 24 months in renal transplantation. Transplantation. 2013;95:933-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 17. | Albano L, Berthoux F, Moal MC, Rostaing L, Legendre C, Genin R, Toupance O, Moulin B, Merville P, Rerolle JP. Incidence of delayed graft function and wound healing complications after deceased-donor kidney transplantation is not affected by de novo everolimus. Transplantation. 2009;88:69-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 18. | Dantal J, Berthoux F, Moal MC, Rostaing L, Legendre C, Genin R, Toupance O, Moulin B, Merville P, Rerolle JP. Efficacy and safety of de novo or early everolimus with low cyclosporine in deceased-donor kidney transplant recipients at specified risk of delayed graft function: 12-month results of a randomized, multicenter trial. Transpl Int. 2010;23:1084-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 19. | Lorber MI, Mulgaonkar S, Butt KM, Elkhammas E, Mendez R, Rajagopalan PR, Kahan B, Sollinger H, Li Y, Cretin N. Everolimus versus mycophenolate mofetil in the prevention of rejection in de novo renal transplant recipients: a 3-year randomized, multicenter, phase III study. Transplantation. 2005;80:244-252. [PubMed] |
| 20. | Vítko S, Margreiter R, Weimar W, Dantal J, Kuypers D, Winkler M, Øyen O, Viljoen HG, Filiptsev P, Sadek S. Three-year efficacy and safety results from a study of everolimus versus mycophenolate mofetil in de novo renal transplant patients. Am J Transplant. 2005;5:2521-2530. [PubMed] |
| 21. | Toki D, Ishida H, Horita S, Yamaguchi Y, Tanabe K. Blood group O recipients associated with early graft deterioration in living ABO-incompatible kidney transplantation. Transplantation. 2009;88:1186-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Ushigome H, Okamoto M, Koshino K, Nobori S, Okajima H, Masuzawa N, Urasaki K, Yoshimura N. Findings of graft biopsy specimens within 90 days after ABO blood group incompatible living donor kidney transplantation compared with ABO-identical and non-identical transplantation. Clin Transplant. 2010;24 Suppl 22:16-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Habicht A, Bröker V, Blume C, Lorenzen J, Schiffer M, Richter N, Klempnauer J, Haller H, Lehner F, Schwarz A. Increase of infectious complications in ABO-incompatible kidney transplant recipients--a single centre experience. Nephrol Dial Transplant. 2011;26:4124-4131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 110] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 24. | Haas M, Segev DL, Racusen LC, Bagnasco SM, Locke JE, Warren DS, Simpkins CE, Lepley D, King KE, Kraus ES. C4d deposition without rejection correlates with reduced early scarring in ABO-incompatible renal allografts. J Am Soc Nephrol. 2009;20:197-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 71] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 25. | Genberg H, Kumlien G, Wennberg L, Tyden G. The efficacy of antigen-specific immunoadsorption and rebound of anti-A/B antibodies in ABO-incompatible kidney transplantation. Nephrol Dial Transplant. 2011;26:2394-2400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 26. | Tanabe K, Takahashi K, Agishi T, Toma H, Ota K. Removal of anti-A/B antibodies for successful kidney transplantation between ABO blood type incompatible couples. Transfus Sci. 1996;17:455-462. [PubMed] |
| 27. | Chung BH, Lim JU, Kim Y, Kim JI, Moon IS, Choi BS, Park CW, Kim YS, Yang CW. Impact of the baseline anti-A/B antibody titer on the clinical outcome in ABO-incompatible kidney transplantation. Nephron Clin Pract. 2013;124:79-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Wilpert J, Fischer KG, Pisarski P, Wiech T, Daskalakis M, Ziegler A, Neumann-Haefelin E, Drognitz O, Emmerich F, Walz G. Long-term outcome of ABO-incompatible living donor kidney transplantation based on antigen-specific desensitization. An observational comparative analysis. Nephrol Dial Transplant. 2010;25:3778-3786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 90] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 29. | Genberg H, Kumlien G, Wennberg L, Berg U, Tydén G. ABO-incompatible kidney transplantation using antigen-specific immunoadsorption and rituximab: a 3-year follow-up. Transplantation. 2008;85:1745-1754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 134] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 30. | Toki D, Ishida H, Horita S, Setoguchi K, Yamaguchi Y, Tanabe K. Impact of low-dose rituximab on splenic B cells in ABO-incompatible renal transplant recipients. Transpl Int. 2009;22:447-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 69] [Article Influence: 4.1] [Reference Citation Analysis (0)] |