Published online Jun 24, 2015. doi: 10.5500/wjt.v5.i2.52
Peer-review started: November 28, 2014
First decision: December 26, 2014
Revised: January 16, 2015
Accepted: April 27, 2015
Article in press: April 29, 2015
Published online: June 24, 2015
Processing time: 203 Days and 21.1 Hours
Ischemia/reperfusion injury is an unavoidable relevant consequence after kidney transplantation and influences short term as well as long-term graft outcome. Clinically ischemia/reperfusion injury is associated with delayed graft function, graft rejection, chronic rejection and chronic graft dysfunction. Ischemia/reperfusion affects many regulatory systems at the cellular level as well as in the renal tissue that result in a distinct inflammatory reaction of the kidney graft. Underlying factors of ischemia reperfusion include energy metabolism, cellular changes of the mitochondria and cellular membranes, initiation of different forms of cell death-like apoptosis and necrosis together with a recently discovered mixed form termed necroptosis. Chemokines and cytokines together with other factors promote the inflammatory response leading to activation of the innate immune system as well as the adaptive immune system. If the inflammatory reaction continues within the graft tissue, a progressive interstitial fibrosis develops that impacts long-term graft outcome. It is of particular importance in kidney transplantation to understand the underlying mechanisms and effects of ischemia/reperfusion on the graft as this knowledge also opens strategies to prevent or treat ischemia/reperfusion injury after transplantation in order to improve graft outcome.
Core tip: In kidney transplantation the ischemia reperfusion injury is a severe unavoidable consequence that may impact the graft outcome. The underlying mechanisms are not completely understood and new findings are continuously being discovered. These involve the biological cellular mechanisms and the gene related response to injury as ischemia and reperfusion. Therapeutically, is extremely important to control this severe complication. Several drugs and strategies are now available and a number of international trials are ongoing. In addition future therapies are now in the pipeline and will be described in this manuscript.
- Citation: Salvadori M, Rosso G, Bertoni E. Update on ischemia-reperfusion injury in kidney transplantation: Pathogenesis and treatment. World J Transplant 2015; 5(2): 52-67
- URL: https://www.wjgnet.com/2220-3230/full/v5/i2/52.htm
- DOI: https://dx.doi.org/10.5500/wjt.v5.i2.52
Ischemia reperfusion injury (IRI) is a relevant factor in determining high morbidity and mortality in several diseases among which, the myocardial infarction, the ischemic stroke, the acute kidney injury (AKI) and trauma. In organ transplantation, as well as in major surgery IRI is a relevant challenge, that importantly influences the clinical outcome (Table 1). A reduced metabolic supply with respect to the demand within an ischemic organ, causes a severe hypoxia associated with micro vascular dysfunction[1,2]. Paradoxically, the subsequent reperfusion does not restore the normal conditions, but further enhances the damage activating several mechanisms, among which the innate and the adaptive immune response and the cell death programs[3]. Recently, important advances in understanding the basis at molecular level of the ischemia and reperfusion have been made. This new relevant knowledge probably will lead to new therapeutic strategies for treating patients affected by ischemia and reperfusion-associated tissue inflammation. This will have a particular relevance in the field of organ transplantation[4].
| Affected organ and surgicalprocedures | Example of clinical manifestation |
| Heart | Acute coronary syndrome |
| Kidney | Acute kidney injury |
| Intestine | Intestinal ischemia and reperfusion |
| Brain | Stroke |
| Cardiac surgery | Acute heart failure after cardiopulmonary bypass |
| Thoracic surgery | Acute lung injury |
| Peripheral vascular surgery | Compartment syndrome of extremity |
| Major vascular surgery | Acute kidney injury |
| Solid organ transplantation | Acute graft failure; early graft rejection |
In this paper the main consequences of IRI that may influence the course of the transplanted kidney will be examined. After analyzing the main clinical factors that affect IRI and the clinical consequences, the biologic mechanisms at the basis of IRI will be discussed. Finally new exciting and promising therapeutic strategies will be described.
IRI is a step frequently occurring during kidney transplantation and is principally caused by blood flow disturbances. Impairment of blood flow starts with the brain death and is due to severe hemodynamic disturbances in the cadaveric donors. These disturbances already causes in the donor activation of complement cascade and of the innate immune system. The clamping of renal artery causes a short, but severe renal ischemia during the harvesting operation. In addition, the cold ischemia during allograft kidney storage may also cause a further ischemic damage[5-7]. The allograft kidney transplantation from living related donors is also subjected to warm ischemia, but in such condition disturbances related to brain death are not present and cold ischemia is also shorter: indeed IRI is less frequent and less severe in transplantation from living donors.
The final and biologically more severe stage of the injury occurs during the reperfusion as a consequence of the blood flow reconstruction[8].
The delayed graft function (DGF) is one of the more frequent early complications after the deceased-donor kidney transplantation and is primarily a consequence of post-ischemic acute tubular necrosis caused by IRI[9]. As aforementioned the degree of IRI is related to several factors that may happen in the donor, during transplantation and later in the recipient[10]. DGF is a severe complication that frequently occurs in the initial post-transplant period. In addition to the acute complications related to the renal failure and the associated costs of prolonged hospitalization, several studies document an association between the occurrence of DGF and the subsequent acute and chronic allograft dysfunction. However is not clear whether the DGF directly affects the long-term graft survival[11,12].
The IRI determines a two-step injury in the transplanted kidney. The first step that happens immediately after transplantation is related to the ischemic damage, while the second step occurs later and is linked to the IRI related activation of the innate and adaptive immune response and may cause either antibody-mediated rejection (ABMR)[13] and cell mediated rejection[14].
Recently, Curci et al[15] documented that IRI might also cause renal fibrosis due to the endothelial-to-mesenchimal transition (EndMT) mediated by the complement anaphylatoxins and by the Akt pathway. Due to the relevance of the consequences of IRI, the Food and Drug Administration (FDA) held an open workshop to summarize the current status of knowledge related to IRI upon the outcomes in kidney transplantation[16].
The workshop identified the following factors as relevant causes affecting IRI and DGF: (1) donor factors: Relevant donor-related factors that increase the risk of DGF are the donor age, the biopsy findings at the implantation[17] and the cardiac or brain death[18]; (2) recipient factors: Most relevant recipient-related factors that influence the incidence and severity of IRI and DGF are the male gender, the African American race, body mass index greater than 30 and high panel reactive antibodies[19]; and (3) storage preservation.
The duration of storage and cold ischemia time correlate with DGF. An adequate preservation of renal allograft during the cold storage is also important to prevent the DGF. Recently also the pulsatile hypothermic machine perfusion has been documented by several authors to significantly reduce the DGF, even if a meta-analysis comparing static cold storage and hypothermic machine perfusion did not document a different influence on long-term outcomes[20,21].
Similarly the FDA workshop and further studies[22] documented the clinical consequences of IRI on the kidney graft function and survival rate. Clinically, IRI is associated with the DGF, the graft rejection and the chronic graft dysfunction with a progressive interstitial fibrosis: (1) delayed graft function; The DGF is a result of IRI related ischemic graft damage that impacts upon the short-term and the long-term outcome of the kidney graft [12,23]. However, due to the lack of clarity of the DGF definition, the impact of the DGF on the long-term graft survival is controversial[12]. Clearly, if DGF determines an impaired graft function at discharge, this represents an independent predictor of a poorer long-term graft outcome[24]; (2) graft rejection: The inflammatory response that follows the IRI after the kidney transplantation causes also an increased immunogenicity of the graft[25]. In addition, the IRI may amplify the humoral immune response to antigens. This amplification is also favored by a facilitated cross-talk between T and B cells. The consequence is an increased ABMR rates. In addition, the facilitated antigen presentation by the dendritic cells to the naive T cells may further enhance the immunogenicity of the graft leading to the T cell-mediated rejections[26]; (3) chronic graft dysfunction: The IRI results in progressive interstitial fibrosis of the kidney graft in experimental kidney transplantation models[15,27]. In the humans, the development of interstitial fibrosis/tubular atrophy is also associated with IRI. However, is not clear whether in a specific graft transplantation the severity of the chronic damage should be related to the severity of the IRI itself or to a genetic predisposition of the graft[22].
The physiopathology of the ischemia reperfusion (I/R) should be distinguished from the physiopathology of the injury caused by the ischemia-reperfusion injury (IRI).
The I/R occurs when the blood flow supply is either interrupted or severely disturbed. During the process of transplantation the organs are subjected to hypoxic and ischemic injury during the procurement, the preservation and after the reperfusion. This principally occurs for the kidneys retrieved from brain dead donors. A recent study comparing kidneys retrieved from living donors and deceased donors (DD) documented that immediately after retrieval from DD there is a high increase of pro-inflammatory genes as interleukin-1 beta (IL-1β), IL-6, P-selectin and monocyte chemotactic protein 1 (MCP-1)[28].
The I/R is a pathological condition characterized by an initial reduction of the blood supply to an organ followed by the subsequent perfusion with consequent re-oxygenation. In any organ the blood flow reduction leads from one hand to the reduction in oxygen and nutrient deliveries, from the other hand to the reduction of waste product removal[29].
Ischemia is an event always associated to the kidney transplantation. Ischemia begins already in the donor with the brain death, principally when is associated with severe hemodynamic disturbances. In addition, the ischemic tissue injury is increased by hypothermic kidney storage. The final stage of the ischemia injury occurs in the reperfusion stage, during which the repair and regeneration processes occur, together with the cellular death[30].
At cellular level two phases should be distinguished: the damage occurring during the ischemia and the damage occurring after the reperfusion. The vast majority of the studies concerning the aforementioned processes have been conducted on the heart, but the same phenomena occur also in the kidney.
The first change induced by the ischemia is the decrease in the oxygen delivery. This will induce a switch from the aerobic to the anaerobic metabolism[30]. The anaerobic metabolism does not meet the demand of aerobic tissues and, as a consequence, the intracellular ATP levels rapidly fall. In addition, the intracellular acidosis may be enhanced by lactic acid that increases because of the lactate-dependent ATP production.
These processes lead to (1) the destabilization of lysosome membrane with the leakage of lysosome enzymes and the breakdown of the cell structure[31]; and (2) the inhibition of the membrane-bound Na+-K+-ATPase activity[32,33]. The latter process causes a large intracellular increase of Na+ ions and water, with consequent edema[30]. Along with Na+ ions accumulation into the cell, the intracellular Ca2+ levels are also increased because of the stop of pumping Ca2+ out of the cells[34] and because ATP depletion inhibits the Ca2+ re-uptake[35]. The calcium overload causes the activation of calcium dependent proteases such as calpains. Calpains remain inactive because of the acid environment, but may damage the cells after pH normalization at the reperfusion[36]. Another effect of Ca2+ overload is the generation of reactive oxigen species (ROS) at mitochondrial level during the ischemia. This causes the opening of the mitochondrial transition pore (mPTP) after reperfusion, with apoptosis and cell death[37,38].
During the hypoxia phase, only exiguous amounts of ROS are produced because of redox-reduction of the cytochromes[39], nitric oxide (NO) synthases[40], xanthine oxidase and NADPH oxidase activations[41,42].
Despite all the aforementioned processes, during the ischemia only a small quantity of cells is lost with respect to the reperfusion phase. In a study in vitro on cardiomyocytes[43] 4% and 17% of cardiomyocytes viability were lost after 1 and 4 h of ischemia in comparison to 73% of viability loss after 3 h of reperfusion.
Upon reperfusion, we observe both an increase in oxygen levels and extracellular pH normalization. This normalization is dangerous for cells previously undergone the ischemia. Indeed, after reperfusion there is a further increase of cytoplasm and mitochondrial calcium overloads that activate the calpains, which cause the cell structure impairment and the cell death. The return to normoxia causes a large production of ROS and a reduction in antioxidant capacity level[41,44]. ROS contribute to damage membranes and cytoskeleton[45]. Together, the ROS increase and the increased mitochondrial calcium content cause the mPTP opening. Once opened the mPTP lead to cell death through different mechanisms as apoptosis, necrosis and autophagy[45,46].
A recently described and relevant factor is the hypoxia-inducible factor (HIF) that might defense cells against I/R[47]. HIF is now considered to be the principal mechanism of defense, controlling the cellular response to hypoxia and regulating several genes involved in the metabolic cell cycle. The HIF pathway is to date the topic of many researches as a possible target for many clinical conditions as I/R.
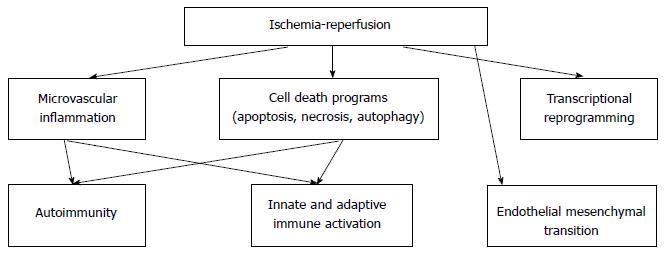
Ischemia-reperfusion injury may cause cell damage through several pathways (Figure 1).
The ischemia-reperfusion activates different programs of cell death, which may be categorized in necrosis, apoptosis, or autophagy associated cell-death.
The necrosis, characterized by the cell swelling with subsequent rupture of surface membranes[48], is a frequent consequence of the I/R. The necrotic cells stimulate the immune system and lead to tissue infiltration of inflammatory-cells with consequent cytokine release. In contrast, the apoptosis activating a complex caspase signaling cascade induces a self-limiting program of cell death. Generally the apoptosis process was considered as less immunostimulating than the necrosis process[49]; however recent data have documented that the extracellular release of ATP from the apoptotic cells may attract phagocytes[50,51]. Programmed cell death has been a synonymous of apoptosis until recently, when new pathways of regulated necrosis (RN) have been described. The best studied RN pathway is the necroptosis that is activated by disturbances of the caspase-8-mediated apoptosis and is the consequence of an interaction between the protein kinases 1 and 3 (RIPK1/RIPK3) and their receptors[52,53]. In this condition the necroptosome is formed, which is able to promote the inflammatory injury and to activate the innate and adaptive immunity[54]. In addition, Goncalves-Primo et al[55] recently found that the apoptosis-related gene expression levels (BAX, BCL2) in pre-implantation biopsies are predictors of kidney DGF.
Finally, in response to the ischemic injury, the cells may maintain their metabolic functions and avoid the death. A recent review highlights that the autophagy is one of the principal tool adopted by the injured cells to maintain their viability[56]. According to this review, the autophagy may be regarded as a protective response to pathological injuries and its stimulation may therefore improve the graft outcome[57]. However, other studies[58] highlight that the stimulation of autophagy may not necessarily protect the graft.
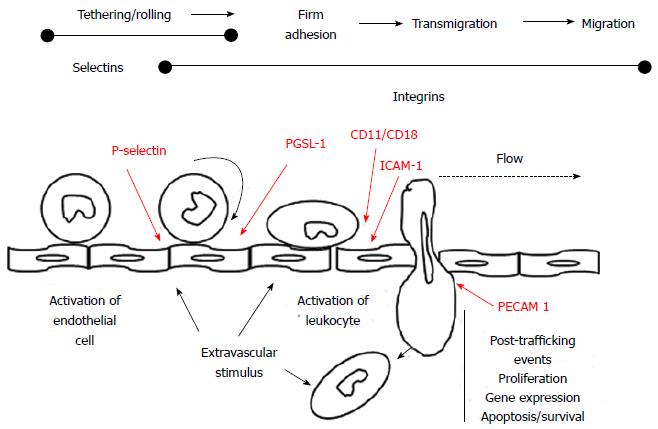
The ischemia and reperfusion are associated with a vascular dysfunction with increased vascular permeability and endothelial cell inflammation. In addition, the recruitment of polymorphonucleates (PMN) and other cells, and the activation of coagulation and the complement system cause further injury. At vascular level, the I/R leads to endothelial cell swelling, loss of glycocalyx, breakdown of the actin cytoskeleton. This leads to lose of the endothelial cell-cell contacts and, as a consequence of the increased micro vascular permeability, there is a fluid loss in the interstitium[59]. Furthermore, the I/R promotes vasoconstriction by inducing the endothelial productions of vasoconstrictor substances (platelet derived growth factor-B and Endothelin-1)[60]. The increased vascular permeability induced by hypoxia may also be generated by the production of several adenosine receptors, among which the A2BAR. Recent studies have documented that the repression of the A2BAR also selectively increases the endothelial leak in response to hypoxia in vitro[61]. The IRI is characterized by leukocyte activation, chemotaxis, leukocyte-endothelial cell adhesion and transmigration[62]. The leukocytes interact with the vascular endothelium in different steps. First we have the leukocyte “rolling” on the endothelium, then the firm adherence of leukocytes to the endothelium and, finally, the endothelium transmigration of the leukocytes[63] (Figure 2).
The leukocyte rolling is induced by the increase of endothelial P-selectin (CD62P) surface expression, which interacts with P-selectin glycoprotein 1 (PSGL-1) located on the leukocytes. A firm leukocyte adherence is a consequence of the interaction of the leukocyte beta 2 integrins CD11a/CD18 and CD11b/CD18 with the endothelial intercellular adhesion molecule 1. The leukocyte transmigration into the interstitial compartment is then facilitated by the platelet-endothelial cell adhesion molecule 1. Later on, in the interstitial compartment the activated leukocytes release toxic ROS, proteases and elastases, so causing several further injuries as an increased micro vascular permeability, edema, thrombosis and parenchyma cell death[62]. The PMN accumulation in the extra vascular compartment is also facilitated by the IL-8 releases by the hypoxic tissues. Indeed IL-8 realizes a chemotactic gradient that facilitates the neutrophils moving from the intravascular space towards the hypoxic interstitium[64].
The vasoconstriction is increased by a reduced NO production in the reperfusion phase, associated with a reduction in the production of the eNOS protein and other vasodilator substances, which are no more produced by the damaged endothelium[65]. In addition, the vasoconstriction is intensified by increased arterioles reactivity to vasoconstrictor substances such as angiotensin II, thromboxane A2, prostaglandin H2, leukotrienes C4 and D4 and adenosine[1,66].
After reperfusion, the activated endothelial cells produce the vascular adhesion molecule 1 as well as the P and E selectins on the endothelial membranes[67]. Mechanistically, the E-selectin activation by E-selectin ligand 1 induces the polarized, activated αMβ2 integrin clusters at the leading edge of crawling neutrophils, so inducing the increased adherence of circulating erythrocytes and platelets[68].
The attenuated vascular relaxation, after reperfusion, in addition to a sustained pericyte contraction[69] may result in a “no reflow phenomenon” characterized by an increased impedance of micro vascular blood flow after the restoration of the normal conditions.
The transcriptional reprogramming is a consequence of the I/R that should be regarded as a defense mechanism and not as an injury. This phenomenon has been principally studied in the I/R of organs as liver, brain or heart.
The ischemic period is associated with significant alterations in the transcription control of the gene expression. The ischemia is associated with an inhibition of the oxygen-sensing prolylhydroxylase (PHD) enzymes that require oxygen as a co-factor. Hypoxia-associated inhibition of the PHD enzymes leads to the post-translational activation of hypoxia and of the inflammatory signaling cascades, which control the stability of the transcription factors HIF and nuclear factor-κB (NF-κB), respectively[70]. In particular in hypoxic conditions, the HIFs move to the nucleus, where, binding to a hypoxia response promoter element (HRE), induce the transcription of numerous genes, among which the genes that induce NF-κB and toll-like receptors (TLRs). This represents an additional attempt to restore oxygenation and to help the tissue to adapt to the hypoxia[71].
Recently, it has also been found that the protective phenotype in response to the ischemia depends on an integrated response at the genomic, molecular, and cellular and tissue levels. This finding has been called “genomic reprogramming” following ischemic preconditioning[72].
The innate immune system is an overlapping response to conditions of disturbed tissue integrity as happens in IRI. Numerous cells and mechanisms are involved in the innate immunity.
Cells: Following reperfusion, the neutrophils adhere to the endothelium and migrate into the tissue. The neutrophils react to any unspecific injuries and release proteases, ROS and pro-inflammatory cytokines as IL-4, IL-6, interferon-γ, tumor necrosis factor-α[73]. Similarly, also the macrophages produce proinflammatory cytokines and may be found in the damaged tissues since the early stages of the IRI[74]. The natural killer (NK) cells play a central role in the renal IRI and the perforin dependent killing of tubular cells by the NK cells is a major mechanism of the renal IRI[75]. The dendritic cells (DCs) represent an essential step in the pathogenesis of the IRI. Indeed DCs undergo an antigen-independent maturation process induced by damage-associated or pathogen-associated molecular proteins (DAMPs, PAMPs). In addition, the DCs represent the connecting bridge between the innate and the adaptive immune activation. In renal transplantation, where the deceased donor undergoes an oxidative stress induced by brain death, the donor DCs are activated favoring the subsequent activation of the recipient T cells[76].
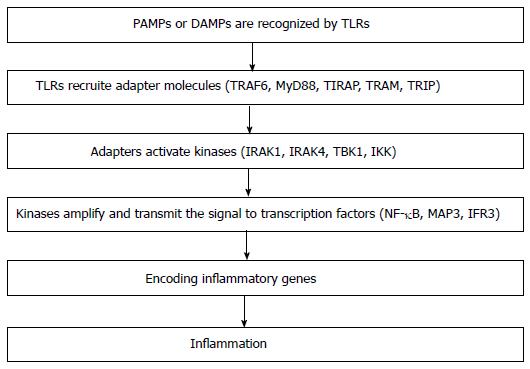
TLRs: The TLRs are small proteins, located on cell membranes or into the cytoplasm that are able to recognize the pathogen-associated molecules. Once activated, the TLRs recruit adapter molecules within the cytoplasm able to generate several kinases that, on turn activate transcription factors, as NFκB. The transcription factors may induce an inflammatory response[77]. In addition to the microbial-associated molecular patterns, the TLRs may be also activated by the endogenous molecules called DAMPs. Several DAMPs are able to activate TLRs and might be associated to IRI. Among them only the nuclear protein High Mobility Group Box 1 (HMGB-1) has been documented to be linked to the pathogenesis of the IRI[78,79]. HMGB-1 binds the DNA and regulates the transcription and the chromatin modeling. In deceased-donor kidneys where the IRI is more frequent and more severe, the TLR-4 has been found to be up-regulated and tubular HMGB-1 is detectable[80]. The TLR-4 exerts a crucial role in the IRI. Indeed, the activation of TLR-4 on the leukocytes, the vascular endothelial cells and the tubular epithelial cells leads to an increased production of pro-inflammatory cytokines and adhesion molecules, which realize an inflammatory response in both the renal microvasculature and the interstitial space. This intensifies the kidney damage already initiated during the ischemic phase through a massive leukocyte infiltrations and generating further cytotocicity. The increased endothelial and epithelial cell damage accelerates the antigen processing and presenting. Therefore the immunogenicity is increased and an immune reaction is generated. The tubules and vasculature severely damaged might promote fibrosis, and all these molecular events may predispose to chronic allograft failure[81].
Strictly connected with the TLRs are the inflammasomes. The inflammasomes are multiprotein complexes present in the cells of the kidney. The inflammasomes respond to DAMPs and may be activated by any cellular damage. For example, the NOD leucine-rich repeat pyrin domain containing NLRP, named NLRP1, activates the caspase-1 cascade producing pro-inflammatory cytokines. Other inflammasome like NLRP3 seems to exert a protective effect in mice[82].
Complement: A central role of the innate immunity is exerted by the complement. The complement is involved in the IRI. The DAMPs may activate all the three complement pathways, binding either to C1q, or to C3 or to mannose-lectin[12]. When the complement pathways are activated the anaphylatoxins C3a and C5a are released and the MAC (C5b-9) is formed. As a result, chemokines are induced and a neutrophil activation and infiltration occur leading to cell injury, apoptosis and necrosis[83].
It has been recently documented that in the complex setting of the IRI, there is a close cross-talk between the complement and the TLRs, another component of innate immunity[84].
The complement may be activated by the brain death and the complement component C5a, generated by the donor brain death, acts directly on the C5a receptor which is also expressed on the DCs, resulting in the cell activation and subsequently enhances its capacity for the allo-specific T cell stimulation[85]. Li et al[86] suggest that the donor epithelium bound C3 may up-regulate the alloimmune response. It is postulated that the surface bound C3 interacts with the complement receptors on the alloreactive T cells or on the antigen presenting cells to increase the allo-immune stimulation.
Finally, it should be considered that the majority of transplanted kidneys are retrieved from cadaveric donors. In such kidneys C3 may be present in the organ already before retrieval because of donor suffering. Damman et al[84] found higher gene expression of C3 and increased deposition of C3d in kidney biopsies obtained from graft from deceased donors. It has been documented that the complement component C3 is capable of modulating the rejection of the renal allograft in vivo and of regulating the T-cell responses in vivo and in vitro[14,87].
While the activation of the innate immune system takes places within minutes, the adaptive immune response is generated after a longer period. The T-cells involved in either antigen-specific or antigen-unspecific responses play a key role in the kidney IRI[88].
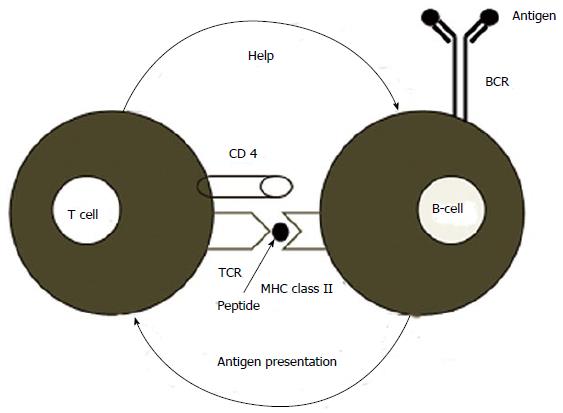
Summarizing the chain of the events that happen as a consequence of the I/R and the consequent activation of the immune system, two steps should be distinguished: (1) activation of the innate system: The recognition receptors of the innate immunity are principally the TLRs (both intra and extracellular), the intracellular receptors, NOD-like receptors and retinoic acid-inducible gene 1 receptor. TLRs are essential in recognizing the PAMPs or DAMPs. The TLRs activate a number of kinases [IL-1-receptor-associated kinase 1 (IRAK1), IL-1-receptor-associated kinase 4 (IRAK4), TANK binding kinase 1, inhibitor of NFκB kinase] recruiting in the cytoplasm adaptor molecules [myeloid differentiation 88 (MyD88), Toll/IL receptor containing adaptor protein, TIR domain-containing adaptor inducing interferon (TRIF) and TRIF-related adaptor molecule]. The kinases amplify and transmit the signal to the transcription factors NFκB, MAP3 kinase (MAP3) and interferon regulatory factor 3. Finally the transcription factors encode the genes regulating the inflammatory cells[12] (Figure 3); and (2) activation of the adaptive system: In tissues affected by innflammation, the DCs become mature, bind the antigen and migrate to the lymph nodes where they may present the antigen to the T cells. The activation of T cell is mediated by signals generated by the T cell receptor and the co-stimulation molecules. The strict interaction between T and B cells may generate an alloimmune response (Figure 4). Recently, has also been documented that the renal IRI may amplify the humoral immune response generating an antibody mediated rejection (ABMR)[13]. Indeed, following the I/R an amplified IgG response, antigen specific, may be generated in the presence of functional alternative pathway of the complement.
Autoimmunity is principally referred to the adaptive immune system. However several studies reveal that also the innate immune system, under specific circumstances may be self-reactive and may initiate the reaction against self-tissues similarly as occurs with pathogens. This specific event is referred as “innate autoimmunity”[89]. Several studies have linked the reperfusion injury to the occurrence of the so-called “natural” antibodies, leading to the activation of the complement system. These natural antibodies are produced in the absence of any immunization and are principally composed of IgM and, in some cases, IgG[90].
In mouse models, non-muscle myosin and heavy chain type II A and C have been identified as a self-target for natural IgM in the initiation of reperfusion injury[91]. More recently, additional neoepitopes have been identified as the soluble cytosolic protein annexin IV[90]. These studies indicate that these neoepitopes generated by the ischemic tissue may become the targets for the natural antibodies principally during the reperfusion phase, thus causing complement activation, neutrophil recruitment and tissue injury.
EndMT has been recently described in different human diseases[92]. During the EndMT, the endothelial cells (ECs) acquire a mesenchimal phenotype characterized by the loss of specific endothelial markers and by the gain of mesenchymal markers, such as the fibroblast specific protein 1, the neuronal cadherin (N-cadherin) and the alpha-smooth muscle actin (α-SMA). Under these conditions, the ECs move from their normal organized cell layer and invade the underlying tissue inducing interstitial fibrosis and favoring the development of chronic kidney disease[93,94].
To date, we are aware of the possible role of the EndMT in the renal IRI but little is known about the pathogenetic factors regulating its development after IRI at renal level. In a recent study, Carney[95] documented that, during the IRI, the activation of the classical and the lectin pathways of the complement system occur primarily at the endothelial cell level. These authors analyzed in large mammals the role of complement in the induction of EndMT by using recombinant C1 inhibitor in vivo. Their data documented that the activation of the serine/threonine-specific protein kinase (Akt) was essential to induce EndMT in vitro. In accordance, inhibition of complement in vivo abrogated the Akt signaling, with inhibition of EndMT and of tissue fibrosis. These data document for the first time that the process of EndMT and the vascular rarefaction at the renal level are activated by the IRI through the priming of the complement system and the subsequent activation of the Akt pathway leading to renal fibrosis[15].
Medical products that limit the short term deleterious effects of the IRI and improve the long term allograft survival are urgently needed.
To date 34 clinical trials are ongoing over this issue[96]. The targets, as we have documented may be quite different.
An optimal management of the deceased donor is essential to reduce the risk and the consequences of the IRI, as well as an accurate surgical technique, a reduced cold ischemia time, and an optimal allograft perfusion.
The ischemic preconditioning implies a first period of organ ischemia “tolerizing” the graft to a subsequent second ischemia period. In this period, the administration of thymoglobulin (rATG) to rats with brain death reduced the expression of pro-inflammatory cytokines and ameliorated the renal damage[97]. The supplementation of Klotho, a transmembrane protein with pleiotropic functions, may protect from the IRI and may suppress the fibrosis[98]. The ischemic preconditioning in a recent systematic review on kidney animal models has been effective in reducing the IRI[99]; however it did not translate by now into clinical transplantation.
Historically, the cold static preservation has been the standard preservation method, principally for kidney transplantation but hypothermic machine perfusion is now used more frequently. A large trial has demonstrated that the use in machine perfusion results in better outcomes principally in the case of deceased donor kidneys, with reduced rates and intensity of DGF and improved outcomes[100,101]. These studies were recently confirmed by Gill et al[102].
Several therapeutic gases have been used for the treatment of the I/R, among which hydrogen (H2), NO, hydrogen sulfide (H2S) and carbon monoxide[4]. The best studied is NO because this gas is also synthesized in the endothelial cells by the endothelial NO synthase. NO principally acts on the endothelial function; in addition, contributes to maintain the blood oxygenation through hypoxic pulmonary vasoconstriction. Patients inhaling NO during liver transplantation had an improved liver function also related to a reduced apoptosis of the hepatocytes[103]. Similarly, the administration of nitrites stimulating NO signaling attenuated the IRI in a rat kidney transplant model[104].
During the ischemia phase, the energy metabolism switches from fatty acid oxidation to glycolysis, allowing the tissues to remain viable. This switch is controlled and improved by the HIF transcription factor whose stability is regulated by the oxygen-sensing PHD enzymes. The treatment with pharmacological doses of PHD inhibitors results in an increased tolerance of the kidneys to the ischemia[105]. In addition, the inhibition of PHD2 has been documented to be able to restore the tumor oxygenation and inhibit metastasis via endothelial normalization[106].
The erythropoietin (EPO) has also been tested in the prevention of the renal IRI. A study by Imamura[107] documented that EPO increases the HIF-1α and attenuates the tubular hypoxia. The protective effect of heme oxygenase 1 (HO-1) in the renal IRI has also been tested. In a mice transplant model, HO-1 induction in the donor attenuated the consequences of donor brain death and increased graft survival rate[108].
Adenosine is a well-known anti-inflammatory molecule. Activation of the adenosine receptor A2ABR expressed on the DCs leads to the inhibition of NFkB. Recently it has been documented that the administration of the selective A2ABR agonist (BAY 60-6583), attenuates the renal IRI via a tolerizing effect on the DCs[61,109].
The enzyme superoxide dismutase (SOD) scavenges the superoxide anions on free radicals produced during the tissue injury and catalyzes the dismutation of superoxide into oxygen and hydrogen peroxide[110]. The SOD administered intravenously during transplantation, significantly reduced the incidence of acute rejections and improved the long term outcomes of renal transplanted patients. These results were reviewed ten years later and the beneficial effects of SOD were confirmed. In a small trial, renal recipients were assigned to receive treatment with N-acetylcysteine or to receive a control solution. DGF incidence rate was significantly lower among the treated group as well as the markers of oxidative stress[111].
Manipulation of the dendritic cells: The DCs have a relevant role in the immune response as they may operate as a link between the innate and adaptive immunity. The rATG inhibits the DCs function[112]. In addition, in a primate model of IRI, the rATG administered prior to the reperfusion, resulted in a reduced expression of ICAM-1, platelet endothelial cell adhesion molecules, CD11b and E selectin[113].
A recent study documented a more powerful protection against the renal IRI by the T-cell-specific NFkB inhibition[114].
TLRs: Experimental studies showed that the prevention of the activation of the innate immunity may be achieved by inhibiting TLR2, which is expressed on the tubular epithelial cells together with the TLR4. The inhibition of the TLR2 with a new monoclonal antibody might significantly reduce the IRI consequences in models of myocardial IRI[115]. After a successful phase I study in man[116], a placebo-controlled study to evaluate the safety and efficacy of OPN-305, the monoclonal antibody anti TLR2, in preventing DGF, is now ongoing (Identifier: NCT01794663). Another possible target is the TLR4[81]. To date, only one study has been performed to inhibit TLR4 in renal IRI. It has been documented that the blockade of TLR4 by “eritoran” reduced the renal IRI in terms of renal function and histology[117]. Other possible targets are the adaptive molecule MyD88[118], the natural killers and the inflammasomes[10]. More recently, Kondo et al[119] reported his experience with the use of a novel IRAK-4 inhibitor. The IRAK-4 inhibitor, in addition to block the toll like receptor pathway, was able to attenuate the progression of the chronic kidney disease[120].
Complement inhibition: Several molecules are currently tested in clinical trials attempting to inhibit the complement that is a relevant component in the innate immune response[83] (Table 2).
| Complementinhibitor | Target | Major mechanism of action |
| Eculizumab | C5 | Inhibit the formation of C5b-9 and C5a |
| rhC1-INH | C1r, C1s, Plasmin, C3b, Kallikrein, Xia, XIIa, MASP1, MASP2 | Regulatory effect on coagulation Inhibition of the alternative pathway Control of the release of bradykinin |
| sCR1 | C3b, C4b | Inactivation of C3 and C5 convertase |
Eculizumab is a humanized monoclonal antibody directed against the C5 component of the complement cascade, already used to treat the atypical hemolytic uremic syndrome (aHUS) and the ABMR. Renal damage due to complement activation occurs in two phases after transplantation: during reperfusion after that the donor kidney has undergone significant period of ischemia and during the acute rejection once the innate and adaptive immune system has recognized the donor antigens. In both conditions the complement may play a relevant role. C5 cleavage is a key step in the pathogenesis of IRI and its block could be an effective prophylactic tool to prevent acute kidney injury (AKI). The eculizumab might be used to prevent IRI. Four clinical trials to evaluate eculizumab in the prevention and treatment of the IRI in kidney allograft are currently ongoing[121].
The beneficial effect of recombinant C1 inhibitor (C1-INH) on the IRI has been widely studied by Castellano et al[122]. Purified or recombinant C1-INH is a host serine protease inhibitor that is able to block the complement cascade acting either at level of classical or lectin pathway[123].
To date, a trial with C1-INH was started (NCT02134314) to prevent DGF in patients receiving deceased donor kidney transplant. In addition, the use of C1-INH to inhibit the Akt pathway has been documented to be effective on the EndMT[15].
The soluble CR1 is among the proteins that regulate the C3 convertase. CR1 is a cell-surface glycoprotein, expressed on several cells, among which monocytes, APCs, T and B cells and podocytes. As a consequence, soluble complement receptor 1 (sCR1) may modulate the complement cascade at multiple levels on all cells expressing on their surface CR1[124].
In normal conditions only small quantities of sCR1 are in circulation. Li et al[125] administered high sCR1 in patients undergoing cardiopulmonary by-pass to inhibit complement activity. sCR1 has been recently used in renal diseases and in renal transplantation.
The effect of Mirocept (APT070) (sCR1) has been widely described by Sacks et al[126] and is currently the subject of a large scale study in kidney transplantation to test the superiority of Mirocept in the prevention of the IRI in cadaveric renal allograft[127].
In addition, administration or targeting of other complement regulator proteins such as CD59, CD55 or CD46 might be a potential way to reduce renal injury during renal transplantation, but to date these molecules are not yet object of clinical trials in the IRI[84].
A recent paper by Columbia University Medical Center reviewed the novel therapies in managing IRI[128].
Diannexin: Phosphatidylserine is a phospholipid normally absent from the endothelial cell surface. The IRI and the consequent ATP depletion cause the translocation of phosphatidylserine to the endothelial cell surface[129]. Once expressed, the phophatidylserine binds leukocytes and platelets. A recombinant annexin A5, Diannexin, binds with higher affinity to phosphatidylserine with respect to the endogenous annexin and is able to reduce the IRI as documented in a study on mice[130]. To date a phase II/III clinical trial is ongoing to assess the efficacy and safety of diannexin in de novo renal transplant recipients[131].
Recombinant P selectin glycoprotein ligand Ig fusion protein (rPSGL-Ig): The rPSGL-Ig efficiently binds P and E-selectin and prevents the granulocyte adhesion and the sequestration to the site of injury. Two multicenter, randomized, placebo-controlled phase I/II studies (YSL0001) were performed to clinically evaluate the possible use of YSPSL in the prevention of the IRI in deceased-donor renal transplant recipients[132,133]. No differences in the DGF rate were found, but treated patients had a significantly lower serum creatinine. Cheadle et al[134] documented that the prereperfusion intravenous YSPSL, significantly reduced the induction of both MCP-1 and tumor growth factor beta.
15NP: The inhibition of p53 after cell damage causes a delayed cell death. Experiments in animal models have documented that the p53 inhibition causes a significant protection on proximal tubule cells[135]. 15NP is a synthetic small interfering ribonucleic acid (siRNA) designed to inhibit the p53 (RNAi) pathway[136]. After preclinical studies in rats, a double blind, multicenter, placebo-controlled trial is ongoing to assess the safety and efficacy of 15NP in men[137].
IAC: The ROS production is an important cause of I/R. A non-peptidyl low molecular weight radical scavenger (IAC) has documented to have anti-oxidant properties in different mice and human models of induced ischemia[138]. A preliminary study on mice documented an IAC protective effect over IRI[139].
Heat shock protein 70: Despite the evidence that heat shock protein 70 (Hsp70) induction can mediate renal protection after the IRI[140], current researches in this area did not document how to enhance the protective Hsp expression strategies in the recovering from the renal IRI. A better understanding on the recovery phase therapy may arise from better understanding of how Hsp70 induction acts on the cells involved in the renal IRI.
After transplantation the recipient circulation carries continuously inflammatory cells to the kidney. These cells are possible treatment targets because of their capacity to either maintain or resolve tissue inflammation[1]. The induction of Hsp70 often may occur in immune cells far from the kidney after heat shock and might have a relevant role in increasing Treg responses in the renal IRI[141,142].
Future anticomplement drugs: Compstatin is an agent that prevents cleavage and activation of the complement protein C3. The drug is to date studied for the treatment of paroxysmal nocturnal hemoglobinuria (PNH) in humans[143]. Its major limitations are the instability and the short plasma half-life. Chen et al[83] are now testing the compstatin efficacy in renal allotransplant monkey models to investigate the effect on the ABMR. No clinical trial is ongoing to test the efficacy on the IRI.
Yunnan-cobra venom factor (Y-CVF) acts as a more stable C3 convertase, causes consumption of C3 and its eventual depletion. The drug has been used to enable renal allograft accommodation in presensitized non-human primates[144]. Major concerns are its potential toxicity, its immunogenicity and its capacity to generate anaphylatoxins. No clinical trial is ongoing to test its efficacy in the IRI.
Vaccinia virus complement control protein prevents the activated C3 (C3b) and C4 (C4b) from triggering further steps in the complement cascade. An improvement in kidney structure and function in rats after IRI has been documented[145,146]. Also for this molecule no clinical trial is to date ongoing for the human IRI.
Ischemia-reperfusion injury is a frequent and severe consequence of both major surgery and organ transplantation. In the case of renal transplantation the IRI occurs principally with kidneys from a deceased donor. Indeed, the impairment of blood flow starts with brain death and is related to the severe hemodynamic disturbances. Warm ischemia after kidney vessel clamping and the cold ischemia after refrigeration also reduce oxygen and nutrients supply to the tissues. The reperfusion further aggravates the state of oxidation and inflammation created by the ischemia.
The principal causes of the IRI are related to the donor and recipient factors and the storage preservation.
The principal clinical consequences of the IRI in clinical transplantation are the DGF, due to tubular dysfunction, the graft rejection, related to enhanced graft immunogenicity and the chronic graft dysfunction related both to the chronic rejection and to endothelial mesenchymal transition.
Ischemia-reperfusion injury may cause cell damage through several pathways as cell death, micro vascular dysfunction, transcriptional reprogramming, activation of innate and adaptive immune system, autoimmunity and EndMT.
The distinction of the above mentioned pathways is relevant for the different therapeutical approaches.
These include an optimal management of donor and recipient, anti-inflammatory strategies and antioxidant therapies with L-arginine and N-acetylcysteine.
The activation of the innate and adaptive immune system has a central role in the pathogenesis of the IRI. Indeed the danger signals released by the dying cells alarm the Toll-like receptors which encode the genes regulating the inflammatory cells and the mediators. In the inflammatory environment the DCs intercept the antigen, migrate to lymph nodes and present the antigen to immunocompetent cells, so activating the adaptive immunity and favoring the rejection. As a consequence, the interference with the signals leading to activation of innate immunity, the inactivation of complement or the manipulation of DCs are promising therapeutic options for the next future.
Finally the pipeline is filled with possible future therapies. Many of them are the object of current ongoing clinical trials or are in preclinical phases.
P- Reviewer: Cantarovich F, Rydzewski A S- Editor: Tian YL L- Editor: A E- Editor: Wu HL
| 1. | Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest. 2011;121:4210-4221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1196] [Cited by in RCA: 1473] [Article Influence: 105.2] [Reference Citation Analysis (0)] |
| 2. | Munshi R, Hsu C, Himmelfarb J. Advances in understanding ischemic acute kidney injury. BMC Med. 2011;9:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 111] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 3. | Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2577] [Cited by in RCA: 2846] [Article Influence: 158.1] [Reference Citation Analysis (0)] |
| 4. | Eltzschig HK, Eckle T. Ischemia and reperfusion--from mechanism to translation. Nat Med. 2011;17:1391-1401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1886] [Cited by in RCA: 2509] [Article Influence: 179.2] [Reference Citation Analysis (0)] |
| 5. | Southard JH, Rice MJ, Ametani MS, Belzer FO. Effects of short-term hypothermic perfusion and cold storage on function of the isolated-perfused dog kidney. Cryobiology. 1985;22:147-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Ploeg RJ, Vreugdenhil P, Goossens D, McAnulty JF, Southard JH, Belzer FO. Effect of pharmacologic agents on the function of the hypothermically preserved dog kidney during normothermic reperfusion. Surgery. 1988;103:676-683. [PubMed] |
| 7. | Dong VM, Tilney NL. Reduction of ischemia/reperfusion injury in organ transplants by cytoprotective strategies. Current Opinion in Organ Transplantation. 2001;6:69-74. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Gulec B. Ischemia Reperfusion Injury in Kidney Transplantation. In: Trzcinska M. Kidney Transplantation - New Perspectives. INTECH Open Access Publishe 2011; Available from: http://cdn.intechopen.com/pdfs/18118/InTech-Ischemia_reperfusion_injury_in_kidney_transplantation.pdf. |
| 9. | Schröppel B, Legendre C. Delayed kidney graft function: from mechanism to translation. Kidney Int. 2014;86:251-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 167] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 10. | Cheung KP, Kasimsetty SG, McKay DB. Innate immunity in donor procurement. Curr Opin Organ Transplant. 2013;18:154-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Yarlagadda SG, Coca SG, Formica RN, Poggio ED, Parikh CR. Association between delayed graft function and allograft and patient survival: a systematic review and meta-analysis. Nephrol Dial Transplant. 2009;24:1039-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 501] [Cited by in RCA: 567] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 12. | Ponticelli C. Ischaemia-reperfusion injury: a major protagonist in kidney transplantation. Nephrol Dial Transplant. 2014;29:1134-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 219] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 13. | Fuquay R, Renner B, Kulik L, McCullough JW, Amura C, Strassheim D, Pelanda R, Torres R, Thurman JM. Renal ischemia-reperfusion injury amplifies the humoral immune response. J Am Soc Nephrol. 2013;24:1063-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 14. | Pratt JR, Basheer SA, Sacks SH. Local synthesis of complement component C3 regulates acute renal transplant rejection. Nat Med. 2002;8:582-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 400] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 15. | Curci C, Castellano G, Stasi A, Divella C, Loverre A, Gigante M, Simone S, Cariello M, Montinaro V, Lucarelli G. Endothelial-to-mesenchymal transition and renal fibrosis in ischaemia/reperfusion injury are mediated by complement anaphylatoxins and Akt pathway. Nephrol Dial Transplant. 2014;29:799-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 88] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 16. | Cavaillé-Coll M, Bala S, Velidedeoglu E, Hernandez A, Archdeacon P, Gonzalez G, Neuland C, Meyer J, Albrecht R. Summary of FDA workshop on ischemia reperfusion injury in kidney transplantation. Am J Transplant. 2013;13:1134-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 115] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 17. | Matignon M, Desvaux D, Noël LH, Roudot-Thoraval F, Thervet E, Audard V, Dahan K, Lang P, Grimbert P. Arteriolar hyalinization predicts delayed graft function in deceased donor renal transplantation. Transplantation. 2008;86:1002-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Locke JE, Segev DL, Warren DS, Dominici F, Simpkins CE, Montgomery RA. Outcomes of kidneys from donors after cardiac death: implications for allocation and preservation. Am J Transplant. 2007;7:1797-1807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 200] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 19. | Doshi MD, Garg N, Reese PP, Parikh CR. Recipient risk factors associated with delayed graft function: a paired kidney analysis. Transplantation. 2011;91:666-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 20. | Kayler LK, Magliocca J, Zendejas I, Srinivas TR, Schold JD. Impact of cold ischemia time on graft survival among ECD transplant recipients: a paired kidney analysis. Am J Transplant. 2011;11:2647-2656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 21. | O’Callaghan JM, Morgan RD, Knight SR, Morris PJ. Systematic review and meta-analysis of hypothermic machine perfusion versus static cold storage of kidney allografts on transplant outcomes. Br J Surg. 2013;100:991-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 112] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 22. | Menke J, Sollinger D, Schamberger B, Heemann U, Lutz J. The effect of ischemia/reperfusion on the kidney graft. Curr Opin Organ Transplant. 2014;19:395-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 23. | Cooper JE, Wiseman AC. Acute kidney injury in kidney transplantation. Curr Opin Nephrol Hypertens. 2013;22:698-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 24. | Ortiz F, Paavonen T, Törnroth T, Koskinen P, Finne P, Salmela K, Kyllönen L, Grönhagen-Riska C, Honkanen E. Predictors of renal allograft histologic damage progression. J Am Soc Nephrol. 2005;16:817-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 25. | Erpicum P, Detry O, Weekers L, Bonvoisin C, Lechanteur C, Briquet A, Beguin Y, Krzesinski JM, Jouret F. Mesenchymal stromal cell therapy in conditions of renal ischaemia/reperfusion. Nephrol Dial Transplant. 2014;29:1487-1493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 26. | Denecke C, Tullius SG. Innate and adaptive immune responses subsequent to ischemia-reperfusion injury in the kidney. Prog Urol. 2014;24 Suppl 1:S13-S19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 27. | Gueler F, Gwinner W, Schwarz A, Haller H. Long-term effects of acute ischemia and reperfusion injury. Kidney Int. 2004;66:523-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 152] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 28. | Saat TC, Susa D, Roest HP, Kok NF, van den Engel S, Ijzermans JN, de Bruin RW. A comparison of inflammatory, cytoprotective and injury gene expression profiles in kidneys from brain death and cardiac death donors. Transplantation. 2014;98:15-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 29. | Chatauret N, Badet L, Barrou B, Hauet T. Ischemia-reperfusion: From cell biology to acute kidney injury. Prog Urol. 2014;24 Suppl 1:S4-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 30. | Kosieradzki M, Rowiński W. Ischemia/reperfusion injury in kidney transplantation: mechanisms and prevention. Transplant Proc. 2008;40:3279-3288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 217] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 31. | Sugiyama S, Hanaki Y, Ogawa T, Hieda N, Taki K, Ozawa T. The effects of SUN 1165, a novel sodium channel blocker, on ischemia-induced mitochondrial dysfunction and leakage of lysosomal enzymes in canine hearts. Biochem Biophys Res Commun. 1988;157:433-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 32. | Kako K, Kato M, Matsuoka T, Mustapha A. Depression of membrane-bound Na+-K+-ATPase activity induced by free radicals and by ischemia of kidney. Am J Physiol. 1988;254:C330-C337. [PubMed] |
| 33. | Kato M, Kako KJ. Effects of N-(2-mercaptopropionyl)glycine on ischemic-reperfused dog kidney in vivo and membrane preparation in vitro. Mol Cell Biochem. 1987;78:151-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 34. | Roberts BN, Christini DJ. NHE inhibition does not improve Na(+) or Ca(2+) overload during reperfusion: using modeling to illuminate the mechanisms underlying a therapeutic failure. PLoS Comput Biol. 2011;7:e1002241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 35. | Sanada S, Komuro I, Kitakaze M. Pathophysiology of myocardial reperfusion injury: preconditioning, postconditioning, and translational aspects of protective measures. Am J Physiol Heart Circ Physiol. 2011;301:H1723-H1741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 277] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 36. | Inserte J, Hernando V, Garcia-Dorado D. Contribution of calpains to myocardial ischaemia/reperfusion injury. Cardiovasc Res. 2012;96:23-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 117] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 37. | Peng TI, Jou MJ. Oxidative stress caused by mitochondrial calcium overload. Ann N Y Acad Sci. 2010;1201:183-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 404] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 38. | Gustafsson AB, Gottlieb RA. Heart mitochondria: gates of life and death. Cardiovasc Res. 2008;77:334-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 304] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 39. | Becker LB. New concepts in reactive oxygen species and cardiovascular reperfusion physiology. Cardiovasc Res. 2004;61:461-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 501] [Cited by in RCA: 494] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 40. | Alkaitis MS, Crabtree MJ. Recoupling the cardiac nitric oxide synthases: tetrahydrobiopterin synthesis and recycling. Curr Heart Fail Rep. 2012;9:200-210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 41. | Li C, Jackson RM. Reactive species mechanisms of cellular hypoxia-reoxygenation injury. Am J Physiol Cell Physiol. 2002;282:C227-C241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 755] [Cited by in RCA: 753] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 42. | Simone S, Rascio F, Castellano G, Divella C, Chieti A, Ditonno P, Battaglia M, Crovace A, Staffieri F, Oortwijn B. Complement-dependent NADPH oxidase enzyme activation in renal ischemia/reperfusion injury. Free Radic Biol Med. 2014;74:263-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 43. | Vanden Hoek TL, Shao Z, Li C, Zak R, Schumacker PT, Becker LB. Reperfusion injury on cardiac myocytes after simulated ischemia. Am J Physiol. 1996;270:H1334-H1341. [PubMed] |
| 44. | Bayrak O, Bavbek N, Karatas OF, Bayrak R, Catal F, Cimentepe E, Akbas A, Yildirim E, Unal D, Akcay A. Nigella sativa protects against ischaemia/reperfusion injury in rat kidneys. Nephrol Dial Transplant. 2008;23:2206-2212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 45. | Kalogeris T, Baines CP, Krenz M, Korthuis RJ. Cell biology of ischemia/reperfusion injury. Int Rev Cell Mol Biol. 2012;298:229-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1400] [Cited by in RCA: 1522] [Article Influence: 117.1] [Reference Citation Analysis (0)] |
| 46. | Gottlieb RA. Cell death pathways in acute ischemia/reperfusion injury. J Cardiovasc Pharmacol Ther. 2011;16:233-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 126] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 47. | Akhtar MZ, Sutherland AI, Huang H, Ploeg RJ, Pugh CW. The role of hypoxia-inducible factors in organ donation and transplantation: the current perspective and future opportunities. Am J Transplant. 2014;14:1481-1487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 48. | Hotchkiss RS, Strasser A, McDunn JE, Swanson PE. Cell death. N Engl J Med. 2009;361:1570-1583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 828] [Cited by in RCA: 907] [Article Influence: 56.7] [Reference Citation Analysis (0)] |
| 49. | Chen GY, Nuñez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10:826-837. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2408] [Cited by in RCA: 2257] [Article Influence: 150.5] [Reference Citation Analysis (0)] |
| 50. | Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SF, Park D, Woodson RI, Ostankovich M, Sharma P. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 2009;461:282-286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1331] [Cited by in RCA: 1273] [Article Influence: 79.6] [Reference Citation Analysis (0)] |
| 51. | Chekeni FB, Elliott MR, Sandilos JK, Walk SF, Kinchen JM, Lazarowski ER, Armstrong AJ, Penuela S, Laird DW, Salvesen GS. Pannexin 1 channels mediate ‘find-me’ signal release and membrane permeability during apoptosis. Nature. 2010;467:863-867. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 930] [Cited by in RCA: 891] [Article Influence: 59.4] [Reference Citation Analysis (0)] |
| 52. | Linkermann A, Hackl MJ, Kunzendorf U, Walczak H, Krautwald S, Jevnikar AM. Necroptosis in immunity and ischemia-reperfusion injury. Am J Transplant. 2013;13:2797-2804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 148] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 53. | Mannon RB. Necroptosis in solid organ transplantation: a missing link to immune activation. Am J Transplant. 2013;13:2785-2786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 54. | Lau A, Wang S, Jiang J, Haig A, Pavlosky A, Linkermann A, Zhang ZX, Jevnikar AM. RIPK3-mediated necroptosis promotes donor kidney inflammatory injury and reduces allograft survival. Am J Transplant. 2013;13:2805-2818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 260] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 55. | Goncalves-Primo A, Mourão TB, Andrade-Oliveira V, Campos EF, Medina-Pestana JO, Tedesco-Silva H, Gerbase-DeLima M. Investigation of apoptosis-related gene expression levels in preimplantation biopsies as predictors of delayed kidney graft function. Transplantation. 2014;97:1260-1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 56. | Pallet N, Livingston M, Dong Z. Emerging functions of autophagy in kidney transplantation. Am J Transplant. 2014;14:13-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 57. | Jiang M, Wei Q, Dong G, Komatsu M, Su Y, Dong Z. Autophagy in proximal tubules protects against acute kidney injury. Kidney Int. 2012;82:1271-1283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 330] [Cited by in RCA: 405] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 58. | Decuypere JP, Pirenne J, Jochmans I. Autophagy in renal ischemia-reperfusion injury: friend or foe. Am J Transplant. 2014;14:1464-1465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 59. | Basile DP, Friedrich JL, Spahic J, Knipe N, Mang H, Leonard EC, Changizi-Ashtiyani S, Bacallao RL, Molitoris BA, Sutton TA. Impaired endothelial proliferation and mesenchymal transition contribute to vascular rarefaction following acute kidney injury. Am J Physiol Renal Physiol. 2011;300:F721-F733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 240] [Cited by in RCA: 234] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 60. | Faller DV. Endothelial cell responses to hypoxic stress. Clin Exp Pharmacol Physiol. 1999;26:74-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 253] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 61. | Eckle T, Faigle M, Grenz A, Laucher S, Thompson LF, Eltzschig HK. A2B adenosine receptor dampens hypoxia-induced vascular leak. Blood. 2008;111:2024-2035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 251] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 62. | Carden DL, Granger DN. Pathophysiology of ischaemia-reperfusion injury. J Pathol. 2000;190:255-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 63. | Eltzschig HK, Collard CD. Vascular ischaemia and reperfusion injury. Br Med Bull. 2004;70:71-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 310] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 64. | Colgan SP, Dzus AL, Parkos CA. Epithelial exposure to hypoxia modulates neutrophil transepithelial migration. J Exp Med. 1996;184:1003-1015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 78] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 65. | Kwon O, Hong SM, Ramesh G. Diminished NO generation by injured endothelium and loss of macula densa nNOS may contribute to sustained acute kidney injury after ischemia-reperfusion. Am J Physiol Renal Physiol. 2009;296:F25-F33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 66. | Legrand M, Mik EG, Johannes T, Payen D, Ince C. Renal hypoxia and dysoxia after reperfusion of the ischemic kidney. Mol Med. 2008;14:502-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 67. | Molitoris BA, Sutton TA. Endothelial injury and dysfunction: role in the extension phase of acute renal failure. Kidney Int. 2004;66:496-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 245] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 68. | Hidalgo A, Chang J, Jang JE, Peired AJ, Chiang EY, Frenette PS. Heterotypic interactions enabled by polarized neutrophil microdomains mediate thromboinflammatory injury. Nat Med. 2009;15:384-391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 290] [Cited by in RCA: 279] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 69. | Yemisci M, Gursoy-Ozdemir Y, Vural A, Can A, Topalkara K, Dalkara T. Pericyte contraction induced by oxidative-nitrative stress impairs capillary reflow despite successful opening of an occluded cerebral artery. Nat Med. 2009;15:1031-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 559] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 70. | Eltzschig HK, Carmeliet P. Hypoxia and inflammation. N Engl J Med. 2011;364:656-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1400] [Cited by in RCA: 1561] [Article Influence: 111.5] [Reference Citation Analysis (0)] |
| 71. | Semenza GL. Life with oxygen. Science. 2007;318:62-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 511] [Cited by in RCA: 516] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 72. | Della-Morte D, Guadagni F, Palmirotta R, Ferroni P, Testa G, Cacciatore F, Abete P, Rengo F, Perez-Pinzon MA, Sacco RL. Genetics and genomics of ischemic tolerance: focus on cardiac and cerebral ischemic preconditioning. Pharmacogenomics. 2012;13:1741-1757. [PubMed] |
| 73. | Awad AS, Rouse M, Huang L, Vergis AL, Reutershan J, Cathro HP, Linden J, Okusa MD. Compartmentalization of neutrophils in the kidney and lung following acute ischemic kidney injury. Kidney Int. 2009;75:689-698. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 192] [Cited by in RCA: 180] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 74. | Li L, Huang L, Sung SS, Vergis AL, Rosin DL, Rose CE, Lobo PI, Okusa MD. The chemokine receptors CCR2 and CX3CR1 mediate monocyte/macrophage trafficking in kidney ischemia-reperfusion injury. Kidney Int. 2008;74:1526-1537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 317] [Cited by in RCA: 309] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 75. | Zhang ZX, Wang S, Huang X, Min WP, Sun H, Liu W, Garcia B, Jevnikar AM. NK cells induce apoptosis in tubular epithelial cells and contribute to renal ischemia-reperfusion injury. J Immunol. 2008;181:7489-7498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 126] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 76. | Land WG. The role of postischemic reperfusion injury and other nonantigen-dependent inflammatory pathways in transplantation. Transplantation. 2005;79:505-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 192] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 77. | Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6034] [Cited by in RCA: 6316] [Article Influence: 300.8] [Reference Citation Analysis (0)] |
| 78. | Leventhal JS, Schröppel B. Toll-like receptors in transplantation: sensing and reacting to injury. Kidney Int. 2012;81:826-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 79. | Li J, Gong Q, Zhong S, Wang L, Guo H, Xiang Y, Ichim TE, Wang CY, Chen S, Gong F. Neutralization of the extracellular HMGB1 released by ischaemic damaged renal cells protects against renal ischaemia-reperfusion injury. Nephrol Dial Transplant. 2011;26:469-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (1)] |
| 80. | Krüger B, Krick S, Dhillon N, Lerner SM, Ames S, Bromberg JS, Lin M, Walsh L, Vella J, Fischereder M. Donor Toll-like receptor 4 contributes to ischemia and reperfusion injury following human kidney transplantation. Proc Natl Acad Sci USA. 2009;106:3390-3395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 270] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 81. | Zhao H, Perez JS, Lu K, George AJ, Ma D. Role of Toll-like receptor-4 in renal graft ischemia-reperfusion injury. Am J Physiol Renal Physiol. 2014;306:F801-F811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 82. | Iyer SS, Pulskens WP, Sadler JJ, Butter LM, Teske GJ, Ulland TK, Eisenbarth SC, Florquin S, Flavell RA, Leemans JC. Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome. Proc Natl Acad Sci USA. 2009;106:20388-20393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 575] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 83. | Chen G, Chen S, Chen X. Role of complement and perspectives for intervention in transplantation. Immunobiology. 2013;218:817-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 84. | Damman J, Daha MR, van Son WJ, Leuvenink HG, Ploeg RJ, Seelen MA. Crosstalk between complement and Toll-like receptor activation in relation to donor brain death and renal ischemia-reperfusion injury. Am J Transplant. 2011;11:660-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 85. | van der Touw W, Cravedi P, Kwan WH, Paz-Artal E, Merad M, Heeger PS. Cutting edge: Receptors for C3a and C5a modulate stability of alloantigen-reactive induced regulatory T cells. J Immunol. 2013;190:5921-5925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 105] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 86. | Li K, Patel H, Farrar CA, Hargreaves RE, Sacks SH, Zhou W. Complement activation regulates the capacity of proximal tubular epithelial cell to stimulate alloreactive T cell response. J Am Soc Nephrol. 2004;15:2414-2422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 87. | Pratt JR, Abe K, Miyazaki M, Zhou W, Sacks SH. In situ localization of C3 synthesis in experimental acute renal allograft rejection. Am J Pathol. 2000;157:825-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 68] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 88. | Satpute SR, Park JM, Jang HR, Agreda P, Liu M, Gandolfo MT, Racusen L, Rabb H. The role for T cell repertoire/antigen-specific interactions in experimental kidney ischemia reperfusion injury. J Immunol. 2009;183:984-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 89. | Carroll MC, Holers VM. Innate autoimmunity. Adv Immunol. 2005;86:137-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 74] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 90. | Kulik L, Fleming SD, Moratz C, Reuter JW, Novikov A, Chen K, Andrews KA, Markaryan A, Quigg RJ, Silverman GJ. Pathogenic natural antibodies recognizing annexin IV are required to develop intestinal ischemia-reperfusion injury. J Immunol. 2009;182:5363-5373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 102] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 91. | Zhang M, Alicot EM, Chiu I, Li J, Verna N, Vorup-Jensen T, Kessler B, Shimaoka M, Chan R, Friend D. Identification of the target self-antigens in reperfusion injury. J Exp Med. 2006;203:141-152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 185] [Cited by in RCA: 188] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 92. | Potenta S, Zeisberg E, Kalluri R. The role of endothelial-to-mesenchymal transition in cancer progression. Br J Cancer. 2008;99:1375-1379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 366] [Cited by in RCA: 433] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 93. | Kizu A, Medici D, Kalluri R. Endothelial-mesenchymal transition as a novel mechanism for generating myofibroblasts during diabetic nephropathy. Am J Pathol. 2009;175:1371-1373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 83] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 94. | Li J, Qu X, Bertram JF. Endothelial-myofibroblast transition contributes to the early development of diabetic renal interstitial fibrosis in streptozotocin-induced diabetic mice. Am J Pathol. 2009;175:1380-1388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 263] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 95. | Carney EF. Acute kidney injury: critical role of complement in EndMT. Nat Rev Nephrol. 2014;10:183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 96. | Ischemia reperfusion injury in Renal Transplantation. Accessed October 5th 2014. Available from: http://clinicaltrial.gov/. |
| 97. | Cicora F, Roberti J, Lausada N, González P, Guerrieri D, Stringa P, Cicora P, Vásquez D, González I, Palti G. Donor preconditioning with rabbit anti-rat thymocyte immunoglobulin ameliorates ischemia reperfusion injury in rat kidney transplantation. Transpl Immunol. 2012;27:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 98. | Hu MC, Shi M, Zhang J, Quiñones H, Kuro-o M, Moe OW. Klotho deficiency is an early biomarker of renal ischemia-reperfusion injury and its replacement is protective. Kidney Int. 2010;78:1240-1251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 311] [Cited by in RCA: 300] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 99. | Wever KE, Menting TP, Rovers M, van der Vliet JA, Rongen GA, Masereeuw R, Ritskes-Hoitinga M, Hooijmans CR, Warlé M. Ischemic preconditioning in the animal kidney, a systematic review and meta-analysis. PLoS One. 2012;7:e32296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 145] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 100. | Moers C, Smits JM, Maathuis MH, Treckmann J, van Gelder F, Napieralski BP, van Kasterop-Kutz M, van der Heide JJ, Squifflet JP, van Heurn E. Machine perfusion or cold storage in deceased-donor kidney transplantation. N Engl J Med. 2009;360:7-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 758] [Cited by in RCA: 781] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 101. | Moers C, Pirenne J, Paul A, Ploeg RJ. Machine perfusion or cold storage in deceased-donor kidney transplantation. N Engl J Med. 2012;366:770-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 203] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 102. | Gill J, Dong J, Eng M, Landsberg D, Gill JS. Pulsatile Perfusion Reduces the Risk of Delayed Graft Function in Deceased Donor Kidney Transplants, Irrespective of Donor Type and Cold Ischemic Time. Transplantation. 2014;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 103. | Lang JD, Teng X, Chumley P, Crawford JH, Isbell TS, Chacko BK, Liu Y, Jhala N, Crowe DR, Smith AB. Inhaled NO accelerates restoration of liver function in adults following orthotopic liver transplantation. J Clin Invest. 2007;117:2583-2591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 177] [Article Influence: 9.8] [Reference Citation Analysis (1)] |
| 104. | Kelpke SS, Chen B, Bradley KM, Teng X, Chumley P, Brandon A, Yancey B, Moore B, Head H, Viera L. Sodium nitrite protects against kidney injury induced by brain death and improves post-transplant function. Kidney Int. 2012;82:304-313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 105. | Hill P, Shukla D, Tran MG, Aragones J, Cook HT, Carmeliet P, Maxwell PH. Inhibition of hypoxia inducible factor hydroxylases protects against renal ischemia-reperfusion injury. J Am Soc Nephrol. 2008;19:39-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 233] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 106. | Mazzone M, Dettori D, Leite de Oliveira R, Loges S, Schmidt T, Jonckx B, Tian YM, Lanahan AA, Pollard P, Ruiz de Almodovar C. Heterozygous deficiency of PHD2 restores tumor oxygenation and inhibits metastasis via endothelial normalization. Cell. 2009;136:839-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 614] [Cited by in RCA: 621] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 107. | Imamura R, Moriyama T, Isaka Y, Namba Y, Ichimaru N, Takahara S, Okuyama A. Erythropoietin protects the kidneys against ischemia reperfusion injury by activating hypoxia inducible factor-1alpha. Transplantation. 2007;83:1371-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 108. | Kotsch K, Francuski M, Pascher A, Klemz R, Seifert M, Mittler J, Schumacher G, Buelow R, Volk HD, Tullius SG. Improved long-term graft survival after HO-1 induction in brain-dead donors. Am J Transplant. 2006;6:477-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 109. | Grenz A, Osswald H, Eckle T, Yang D, Zhang H, Tran ZV, Klingel K, Ravid K, Eltzschig HK. The reno-vascular A2B adenosine receptor protects the kidney from ischemia. PLoS Med. 2008;5:e137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 176] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 110. | Land W, Schneeberger H, Schleibner S, Illner WD, Abendroth D, Rutili G, Arfors KE, Messmer K. The beneficial effect of human recombinant superoxide dismutase on acute and chronic rejection events in recipients of cadaveric renal transplants. Transplantation. 1994;57:211-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 301] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 111. | Danilovic A, Lucon AM, Srougi M, Shimizu MH, Ianhez LE, Nahas WC, Seguro AC. Protective effect of N-acetylcysteine on early outcomes of deceased renal transplantation. Transplant Proc. 2011;43:1443-1449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 112. | Naujokat C, Berges C, Fuchs D, Sadeghi M, Opelz G, Daniel V. Antithymocyte globulins suppress dendritic cell function by multiple mechanisms. Transplantation. 2007;83:485-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 113. | Beiras-Fernandez A, Chappell D, Hammer C, Beiras A, Reichart B, Thein E. Impact of polyclonal anti-thymocyte globulins on the expression of adhesion and inflammation molecules after ischemia-reperfusion injury. Transpl Immunol. 2009;20:224-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 114. | Xue C, Liu Y, Li C, Li Y, Yang T, Xie L, Zhou P. Powerful protection against renal ischemia reperfusion injury by T cell-specific NF-κB inhibition. Transplantation. 2014;97:391-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 115. | Lepper PM, Bals R. On the edge: targeting Toll-like receptor 2 in ischemia/reperfusion injury. Circ Cardiovasc Interv. 2012;5:146-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 116. | Reilly M, Miller RM, Thomson MH, Patris V, Ryle P, McLoughlin L, Mutch P, Gilboy P, Miller C, Broekema M. Randomized, double-blind, placebo-controlled, dose-escalating phase I, healthy subjects study of intravenous OPN-305, a humanized anti-TLR2 antibody. Clin Pharmacol Ther. 2013;94:593-600. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 103] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 117. | Gueler F, Rong S, Gwinner W, Mengel M, Bröcker V, Schön S, Greten TF, Hawlisch H, Polakowski T, Schnatbaum K. Complement 5a receptor inhibition improves renal allograft survival. J Am Soc Nephrol. 2008;19:2302-2312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 103] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 118. | Rivas MN, Koh YT, Chen A, Nguyen A, Lee YH, Lawson G, Chatila TA. MyD88 is critically involved in immune tolerance breakdown at environmental interfaces of Foxp3-deficient mice. J Clin Invest. 2012;122:1933-1947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 119. | Kondo M, Tahara A, Hayashi K, Abe M, Inami H, Ishikawa T, Ito H, Tomura Y. Renoprotective effects of novel interleukin-1 receptor-associated kinase 4 inhibitor AS2444697 through anti-inflammatory action in 5/6 nephrectomized rats. Naunyn Schmiedebergs Arch Pharmacol. 2014;387:909-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 120. | Schmaderer C, Heemann U. Blocking innate immunity to slow the progression of chronic kidney disease. Naunyn Schmiedebergs Arch Pharmacol. 2014;387:905-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 121. | Eculizumab for prevention and treatment of IRI. Accessed November 13th, 2014. Available from: http://clinicaltrial.gov/. |
| 122. | Castellano G, Melchiorre R, Loverre A, Ditonno P, Montinaro V, Rossini M, Divella C, Battaglia M, Lucarelli G, Annunziata G. Therapeutic targeting of classical and lectin pathways of complement protects from ischemia-reperfusion-induced renal damage. Am J Pathol. 2010;176:1648-1659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 126] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 123. | Ricklin D, Lambris JD. Complement in immune and inflammatory disorders: therapeutic interventions. J Immunol. 2013;190:3839-3847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 181] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 124. | Zhang Y, Nester CM, Holanda DG, Marsh HC, Hammond RA, Thomas LJ, Meyer NC, Hunsicker LG, Sethi S, Smith RJ. Soluble CR1 therapy improves complement regulation in C3 glomerulopathy. J Am Soc Nephrol. 2013;24:1820-1829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 125. | Li JS, Sanders SP, Perry AE, Stinnett SS, Jaggers J, Bokesch P, Reynolds L, Nassar R, Anderson PA. Pharmacokinetics and safety of TP10, soluble complement receptor 1, in infants undergoing cardiopulmonary bypass. Am Heart J. 2004;147:173-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 126. | Sacks S, Karegli J, Farrar CA, Asgari E, Schwaeble W, Zhou W, Smith RA. Targeting complement at the time of transplantation. Adv Exp Med Biol. 2013;735:247-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 127. | MRC Centre for Transplantation. An investigation into the treatment of the donor kidney to see if this improves the recovery of the kidney after transplantation. [accessed. In: Trzcinska M. Kidney Transplantation - New Perspectives. INTECH Open Access Publishe 2012; August 3] Available from: http://www.isrctn.com/ISRCTN49958194. |
| 128. | Powell JT, Tsapepas DS, Martin ST, Hardy MA, Ratner LE. Managing renal transplant ischemia reperfusion injury: novel therapies in the pipeline. Clin Transplant. 2013;27:484-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 129. | Ran S, Downes A, Thorpe PE. Increased exposure of anionic phospholipids on the surface of tumor blood vessels. Cancer Res. 2002;62:6132-6140. [PubMed] |
| 130. | Wever KE, Wagener FA, Frielink C, Boerman OC, Scheffer GJ, Allison A, Masereeuw R, Rongen GA. Diannexin protects against renal ischemia reperfusion injury and targets phosphatidylserines in ischemic tissue. PLoS One. 2011;6:e24276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 131. | Astellas Pharma Inc. A Study to Evaluate the Effect of ASP8597 in Adult Kidney Transplant Patients. gov [Internet]. Bethesda (MD): National Library of Medicine (US). [cited 2014; June 17] Available from: https://clinicaltrials.gov/ct2/show/NCT01442337 NLM Identifier: NCT01442337. |
| 132. | Y’s Therapeutics, Inc . YSPSL for Prevention of Delayed Graft Function Part A. gov [Internet]. Bethesda (MD): National Library of Medicine (US). [cited 2008; Jan 24] Available from: https://clinicaltrials.gov/ct2/show/NCT00298181 NLM Identifier: NCT00298181. |
| 133. | Gaber AO, Mulgaonkar S, Kahan BD, Woodle ES, Alloway R, Bajjoka I, Jensik S, Klintmalm GB, Patton PR, Wiseman A. YSPSL (rPSGL-Ig) for improvement of early renal allograft function: a double-blind, placebo-controlled, multi-center Phase IIa study. Clin Transplant. 2011;25:523-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 134. | Cheadle C, Watkins T, Ehrlich E, Barnes K, Gaber AO, Hemmerich S, Rabb H. Effects of anti-adhesive therapy on kidney biomarkers of ischemia reperfusion injury in human deceased donor kidney allografts. Clin Transplant. 2011;25:766-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 135. | Kelly KJ, Plotkin Z, Vulgamott SL, Dagher PC. P53 mediates the apoptotic response to GTP depletion after renal ischemia-reperfusion: protective role of a p53 inhibitor. J Am Soc Nephrol. 2003;14:128-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 168] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 136. | Vincenti F, Kirk AD. What’s next in the pipeline. Am J Transplant. 2008;8:1972-1981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 72] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 137. | Quark Pharmaceuticals. I5NP for Prophylaxis of Delayed Graft Function in Kidney Transplantation. gov [Internet]. Bethesda (MD): National Library of Medicine (US). [cited 2014; Nov 14] Available from: https//clinicaltrials.gov/ct2/show/NCT00802347 NLM Identifier: NCT00802347. |
| 138. | Corsi L, Zavatti M, Geminiani E, Zanoli P, Baraldi M. Anti-inflammatory activity of the non-peptidyl low molecular weight radical scavenger IAC in carrageenan-induced oedema in rats. J Pharm Pharmacol. 2011;63:417-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 139. | Bassi R, Vergani A, D’Addio F, Ben Nasr M, Mocci A, Rastaldi MP, Ichimura T, Bonventre JV, Fiorina P. Positive effects of a novel non-peptidyl low molecular weight radical scavenger in renal ischemia/reperfusion: a preliminary report. Springerplus. 2014;3:158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 140. | Wang Z, Gall JM, Bonegio RG, Havasi A, Hunt CR, Sherman MY, Schwartz JH, Borkan SC. Induction of heat shock protein 70 inhibits ischemic renal injury. Kidney Int. 2011;79:861-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 141. | Kim MG, Jung Cho E, Won Lee J, Sook Ko Y, Young Lee H, Jo SK, Cho WY, Kim HK. The heat-shock protein-70-induced renoprotective effect is partially mediated by CD4+ CD25+ Foxp3 + regulatory T cells in ischemia/reperfusion-induced acute kidney injury. Kidney Int. 2014;85:62-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 142. | O’Neill S, Hughes J. Heat-shock protein-70 and regulatory T cell-mediated protection from ischemic injury. Kidney Int. 2014;85:5-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 143. | Risitano AM, Ricklin D, Huang Y, Reis ES, Chen H, Ricci P, Lin Z, Pascariello C, Raia M, Sica M. Peptide inhibitors of C3 activation as a novel strategy of complement inhibition for the treatment of paroxysmal nocturnal hemoglobinuria. Blood. 2014;123:2094-2101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 157] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 144. | Chen Song S, Zhong S, Xiang Y, Li JH, Guo H, Wang WY, Xiong YL, Li XC, Chen Shi S, Chen XP. Complement inhibition enables renal allograft accommodation and long-term engraftment in presensitized nonhuman primates. Am J Transplant. 2011;11:2057-2066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 145. | Hong SD, Ha MY, Balachandar S. Static and dynamic contact angles of water droplet on a solid surface using molecular dynamics simulation. J Colloid Interface Sci. 2009;339:187-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 146. | Pushpakumar SB, Perez-Abadia G, Soni C, Wan R, Todnem N, Patibandla PK, Fensterer T, Zhang Q, Barker JH, Maldonado C. Enhancing complement control on endothelial barrier reduces renal post-ischemia dysfunction. J Surg Res. 2011;170:e263-e270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |