Published online Mar 24, 2015. doi: 10.5500/wjt.v5.i1.26
Peer-review started: October 27, 2014
First decision: January 8, 2015
Revised: February 10, 2015
Accepted: March 5, 2015
Article in press: March 9, 2015
Published online: March 24, 2015
Processing time: 150 Days and 23.7 Hours
AIM: To compare prevalence of chronic renal dysfunction (CRD) according to serum creatinine (sCr) vs estimated glomerular filtration rate (eGFR) among maintenance liver transplant patients.
METHODS: The ICEBERG study was an observational, retrospective, cross-sectional, and multicenter study. Consecutive adult patients (aged 18 years or older) with liver transplantation (LT) performed at least two years previously were recruited. Multi-organ transplant recipients were excluded. Chronic renal dysfunction was defined according to sCr based criteria in routine clinical practice (≥ 2 mg/dL) and eGFR using MDRD-4 equation (< 60 mL/min per 1.73 m2). Agreement between sCr definition and eGFR assessment was evaluated using the Kappa index. Cox regression analysis was applied to identify predictive factors for developing CRD after LT.
RESULTS: A total of 402 patients were analyzed (71.6% males). Mean ± SD age at transplant was 52.4 ± 9.8 years. Alcoholic cirrhosis without hepatocellular carcinoma was the most common reason for LT (32.8%). Mean time since LT was 6.9 ± 3.9 years. Based on sCr assessment, 35.3% of patients (95%CI: 30.6-40.0) had CRD; 50.2% (95%CI: 45.3-55.1) according to eGFR. In 32.2% of cases, sCr assessment had underestimated CRD. Multivariate analysis showed the following factors associated with developing CRD: eGFR < 60 mL/min per 1.73 m2 at three months post-transplant [hazard ratio (HR) = 4.76; 95%CI: 2.78-8.33; P < 0.0001]; calcineurin inhibitor use (HR = 2.31; 95%CI: 1.05-5.07; P = 0.0371); male gender (HR = 1.98; 95%CI: 1.09-3.60; P = 0.0260); and ≥ 10 years post-transplantation (HR = 1.95; 95%CI: 1.08-3.54; P = 0.0279).
CONCLUSION: Seven years after LT, CRD affected half our patients, which was underestimated by sCr. An eGFR < 60 mL/min per 1.73 m2 three months post-LT was predictive of subsequent CRD.
Core tip: We aimed to compare the prevalence of chronic renal dysfunction (CRD) according to serum creatinine (sCr) vs that based on estimated glomerular filtration rate (eGFR) among maintenance liver transplant patients. According to eGFR assessment, after seven years of post-transplant follow-up, half of patients have CRD, suggesting that the occurrence of renal dysfunction is significantly under-estimated by sCr assessment in routine practice. The study outlines the importance of early CRD detection using more sensitive tools. In this sense, eGFR at 3-mo post-transplantation provides a powerful independent predictive factor for the development of CRD in liver transplant recipients.
- Citation: Varo E, Bañares R, Guilera M. Underestimation of chronic renal dysfunction after liver transplantation: ICEBERG study. World J Transplant 2015; 5(1): 26-33
- URL: https://www.wjgnet.com/2220-3230/full/v5/i1/26.htm
- DOI: https://dx.doi.org/10.5500/wjt.v5.i1.26
Chronic renal dysfunction (CRD) is a common and dangerous complication following liver transplantation (LT)[1-3]. The majority of liver transplant recipients who survive beyond the first six months post-transplant develop CRD[4,5]. The reported incidence varies widely, from 20% to 80%[5-7], depending on the definition of CRD and the methodology used in studies[8].
The key causative factor for renal disorders in nonrenal transplant recipients has been attributed to calcineurin inhibitor (CNI) nephrotoxicity[2,9]. Nevertheless, other risk factors-including older age, hepatitis C virus (HCV) infection, the presence of diabetes mellitus or hypertension before transplantation, and pre-transplant renal dysfunction-are known to be independent predictors of CRD after LT[10-16].
Development of CRD after nonrenal organ transplantation is associated with a greater than 4-fold increase in the risk of death[12]. Therefore, early detection of CRD following LT is essential to delay the progression of renal disease and reduce its associated morbidity/mortality.
Serum creatinine (sCr) is the most established tool for estimating renal function. However, sCr alone may not be an accurate indicator of the degree of renal dysfunction. Not only it is a delayed marker of decreased kidney function[17], but it is also influenced by such nonrenal factors as gender, age, race, weight or protein intake and, additionally, is significantly decreased in patients with chronic liver disease[9,17]. Consequently, estimated glomerular filtration rate (eGFR) using a prediction equation that takes into account the sCr level and some of these independent factors has been recommended as a method for measuring renal function in these patients[18]. A number of creatinine-based equations have been developed for estimating GFR[19-23]. In adults, the modification of diet in renal disease (MDRD) equation[20] provides a clinically useful estimate of GFR[18].
This is a descriptive study primarily aiming to evaluate a national cohort of liver transplant patients still alive after a median follow-up of seven years and to assess CRD prevalence by comparing two measurements currently employed in routine practice: sCr and GFR estimated by MDRD-4. Secondary objectives were to analyze how renal function evolved, identify potential risk factors for developing CRD and assess to what extent the clinical diagnosis of CRD leads to a change in immunosuppressive therapy.
The ICEBERG study was an observational, retrospective, cross-sectional, multicenter study conducted in 21 LT outpatient clinics in Spain. Patients eligible for inclusion were consecutive patients seen at the clinic aged 18 years or older at transplantation, with at least two years of post-transplant data on renal function to better ensure stable renal function. Multi-organ transplant recipients were excluded. The study was approved by the ethics committee at Hospital Clinic of Barcelona (Spain). Signed informed consent was obtained from all patients prior to their inclusion.
Patients fulfilling the selection criteria were consecutively enrolled by the participating investigators, resulting in the inclusion of 409 patients between September and November 2009. Patient profiles consisted of current clinical and analytical data and medical records.
CRD diagnosis was recorded based on sCr and, alternatively, estimating GFR using the abbreviated MDRD-4 equation[20,21]: estimated GFR (mL/min per 1.73 m2) = 186 × (serum creatinine)-1.154× (age)-0.203× (0.742 if female) × (1.210 if African-American). The cut-off point to define CRD was ≥ 2 mg/dL for sCr and < 60 mL/min per 1.73 m2 for eGFR based on Kidney Disease Outcome Quality Initiative (K-DOQI) guidelines[18,24].
McNemar’s test was used to compare frequencies between subgroups for qualitative variables. Agreement between sCr definition and eGFR assessment was evaluated using the Kappa index. Cox regression analysis was applied to determine the predictors of CRD after LT. A P-value < 0.05 was considered significant. Statistical analyses were performed with SPSS (version 12.0, SPSS Inc., Chicago, Illinois, United States).
The statistical methods of this study were reviewed by Daniel Mosteiro (Senior Biostatistician) from TFS.
A total of 402 patients were included in the analysis. Seven patients with missing values for sCr were excluded. Table 1 shows the main characteristics of the study sample. The vast majority of patients were male Caucasians, with a mean age of 52.4 ± 9.8 years at transplant and an average Model for End-Stage Liver Disease (MELD) score during the transplant evaluation of 15.9 ± 6.1 (125 patients were lacking data). Mean time post-transplantation was 6.9 ± 3.9 years (range: 2-20 years). At the time of transplantation, 17.7% of patients had diabetes mellitus and 9.0% hypertension. The main indication for LT was alcoholic cirrhosis without hepatocellular carcinoma (32.8%), while hepatocellular carcinoma was the impetus for transplantation in 22.9% of patients. Antibody induction therapy was used in 16.9% of patients (mainly anti-CD25). At the time of discharge, the most commonly used immunosuppressants were CNI (either cyclosporine or tacrolimus), prescribed as monotherapy (8.5%) or in combination with other immunosuppressive treatments (91.5%). Biopsy-confirmed acute rejection was diagnosed in 94 patients (23.4%) and, during the maintenance phase, diabetes and hypertension were diagnosed in 135 (33.6%) and 208 (51.7%) patients respectively. Additionally, 48 patients (11.9%) developed a malignancy following transplantation.
| Variables | n (%) |
| Age at transplant (yr), mean ± SD | 52.4 ± 9.8 |
| Gender (male) | 288 (71.6) |
| Ethnicity (Caucasian) | 400 (99.5) |
| Donor age, mean ± SD | 47.0 ± 18.9 |
| Time since transplantation (yr), mean ± SD | 6.9 ± 3.9 |
| Pre-transplant comorbidities | |
| Diabetes mellitus | 71 (17.7) |
| Hypertension | 36 (9.0) |
| Dyslipidemia | 13 (3.2) |
| Coronary heart disease | 8 (2.0) |
| Reason for transplantation | |
| Alcoholic cirrhosis without hepatocellular carcinoma | 132 (32.8) |
| Hepatocellular carcinoma (in HCV or HBV-related liver cirrhosis, alcoholic cirrhosis or non-cirrhotic liver) | 92 (22.9) |
| HCV-related liver cirrhosis without hepatocellular carcinoma | 74 (18.4) |
| Cholestatic liver disease | 24 (6.0) |
| HBV-related liver cirrhosis without hepatocellular carcinoma | 23 (5.7) |
| Acute liver failure | 9 (2.2) |
| Others | 45 (11.2) |
| Induction therapy | 68 (16.9) |
| Immunosuppressive treatment (at discharge) | |
| Monotherapy | 34 (8.5) |
| Cyclosporine | 14 (3.5)1 |
| Tacrolimus | 20 (5.0)1 |
| Combined therapies | 368 (91.5) |
| Cyclosporine-based | 155 (38.6)1 |
| Tacrolimus-based | 149 (37.1)1 |
| mTOR inhibitor-based | 63 (15.7)1 |
| Others | 1 (0.3)1 |
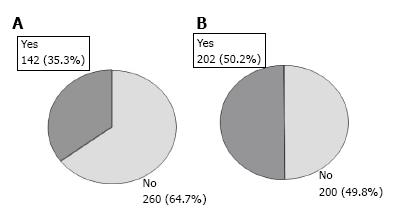
Based on sCr, CRD was diagnosed in 142 out of 402 patients (35.3%, 95%CI: 30.6 to 40.0) whereas, according to MDRD-4, CRD was diagnosed in 202 patients (50.2%, 95%CI: 45.3 to 55.1; P < 0.0001) (Figure 1). Of the 202 patients with eGFR below 60 mL/min per 1.73 m2, 63 (31.2%) had creatinine levels ≥ 2 mg/dL but 139 (68.8%) had creatinine < 2 mg/dL (Table 2). When examining the concordance between the sCr-based definition and eGFR, diagnosis of CRD according to the former was established in 98.4% of patients with laboratory values of sCr ≥ 2 mg/dL and eGFR < 60 mL/min per 1.73 m2. However, 46.0% of CRD patients with sCr < 2 mg/dL and eGFR < 60 mL/min per 1.73 m2 were not correctly diagnosed. In this patient subgroup, 56.3% of patients had creatinine values above 1.25 mg/dL but below 2 mg/dL (Table 2). Among 31 patients with creatinine < 1.25 mg/dL and eGFR < 60 mL/min per 1.73 m2, only 3 cases (4%) were adequately diagnosed by the sCr. In summary, there was moderate agreement between the two definitions Kappa coefficient: 0.65 (95%CI: 0.58-0.72); with 32.2% of patients with eGFR < 60 mL underdiagnosed using the sCr based assessment (Table 3).
| CRD diagnosis according to serum creatinine definition | ||||
| Yes | No | Total | ||
| CRD diagnosis according to eGFR (MDRD-4) | Creatinine ≥ 2 mg/dL and eGFR < 60 mL/min per 1.73 m2 | 62 (98.4)1 | 1 (1.6)1 | 63 (31.2)2 |
| Creatinine < 2 mg/dL and eGFR < 60 mL/min per 1.73 m2 | 75 (54.0)1 | 64 (46.0)1 | 139 (68.8)2 | |
| Creatinine < 1.25 mg/dL | 3 (4.0)3 | 28 (43.8)3 | ||
| Creatinine 1.25 - < 1.50 mg/dL | 19 (25.3)3 | 30 (46.9)3 | ||
| Creatinine 1.50 - < 2.0 mg/dL | 53 (70.7)3 | 6 (9.4)3 | ||
| Total | 137 (67.8)2 | 65 (32.2)2 | 202 (100) | |
The mean time point when CRD was clinically diagnosed according to sCr was 2.5 ± 3.7 years after transplantation; the time from transplantation to CRD diagnosis was less than 2 years in 62.7% of patients, from 2-5 years in 20.4% and over 6 years later in 16.9% of patients.
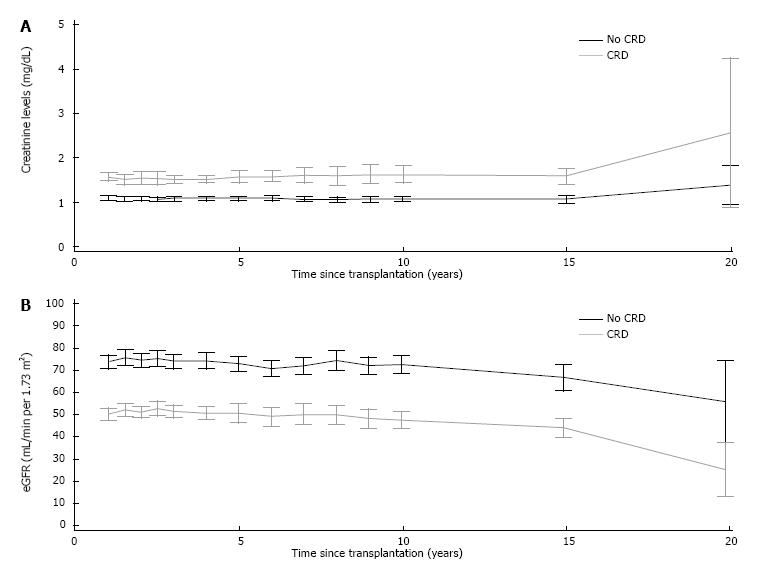
Figure 2 shows the changes in sCr levels and eGFR in liver recipients with and without clinical diagnosis of CRD one year post-transplant. Thereafter, patients with diagnosis of CRD showed higher levels of sCr and lower estimated GFR compared to those patients without CRD.
Multivariate Cox regression analysis showed that the following factors were associated with an increased risk of CRD: an eGFR value below 60 mL/min per 1.73 m2 at 3 mo post-transplant; CNI-based immunosuppressive therapy at discharge; recipient male gender; and time since transplantation (Table 4).
| Variables | HR (95%CI) | P-value |
| Three months post-transplant eGFR (< 60 vs≥ 60 mL/min per 1.73 m2) | 4.76 (2.78-8.33) | < 0.0001 |
| CNI treatment at discharge (CNI vs non-CNI) | 2.31 (1.05-5.07) | 0.0371 |
| Recipient gender (male vs female) | 1.98 (1.09-3.60) | 0.026 |
| Year of transplantation ( ≤ 1999 vs > 1999) | 1.95 (1.08-3.54) | 0.0279 |
Following a diagnosis of CRD based on sCr, renal biopsy was performed in only four patients (2.8%). Renoprotective treatment [angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptor blockers] was introduced in 43 out of 142 patients (30.3%), while 7 patients (4.9%) needed renal replacement therapy (5 hemodyalisis, 1 renal transplant and 1 both). When CRD was diagnosed, changes in immunosuppressive therapy were initiated in 128 out of 142 patients (90.1%). All such changes were based on a reduction in CNI therapy. In addition, modifications to mycophenolic acid (MPA) therapy or introduction of mammalian target of rapamycin (mTOR) inhibitor therapy were undertaken in 45.1% and 12.0% of patients with CRD respectively.
Early identification of renal dysfunction after LT is essential to delay the progression of chronic kidney disease and improving long-term patient health[11,16]. In our study of LT patients, CRD was a common post-transplant complication with a prevalence ranging from 35.3% to 50.2% depending on the criteria applied. It is worth noting that the study shows how CRD is markedly underestimated by the sCr based assessment still used in clinical practice. Three out of ten patients with criteria for CRD based on eGFR using the MDRD-4 equation[21] had been underdiagnosed. It is important to note that sCr values from 1.25 to 2 mg/dL can frequently be misinterpreted despite concomitant abnormal eGFR values. Our results indicate that clinical diagnosis of renal dysfunction in routine clinical practice relies frequently on the less sensitive measurement of increased sCr concentration when, in fact, the eGFR may provide a better tool for detecting early renal dysfunction[24]. However, our findings are within the range of CRD prevalence reported by previous studies that had already shown CRD to be a common post-LT complication[5-7,12]. For instance, in the adult Finnish LT population, almost 40% of patients had an eGFR below 60 mL/min at three years post-transplantation[7], and according to Gayowski et al[6], 28% of liver transplant recipients developed late-onset renal failure, defined as sCr levels persistently exceeding 2.0 mg/dL six months post-transplantation. However the lack of a standard definition for CRD explains differences in prevalence among studies.
We performed a retrospective analysis of liver recipients to identify risk factors for the development of CRD. Several studies have reported that eGFR, either at the time of LT or during the early stages following transplantation, is an independent predictor of post-transplant chronic kidney disease[10,11,25-28]. Our study validated these results and showed that a low eGFR three months post-transplant was associated with an increased risk of CRD [hazard ratio (HR) = 4.76 for eGFR < 60 mL/min per 1.73 m2vs eGFR ≥ 60 mL/min per 1.73 m2]. This finding is particularly interesting since it suggests that it may be possible to identify those patients at high risk of developing CRD within the first three months after transplantation with an easy-to-use tool such as the MDRD-4 equation.
After examining a variety of demographic and clinical variables, in contrast to previous studies, we found that male gender was a predictive factor of CRD (HR = 1.98 for male vs female). Curiously, other studies have reported just the opposite, with female gender being associated with a higher risk for developing CRD after LT[10,12,29]. However, Ojo et al[12] defined chronic renal failure as an eGFR ≤ 29 mL/min per 1.73 m2, instead of using a CRD cut-off point (eGFR < 60 mL/min per 1.73 m2), which could explain the different outcomes discussed above.
In the current study, time since transplantation was also significantly associated with the risk of developing CRD (HR = 1.95 for transplantations performed prior to 1999 vs those carried out after that date), as had previously been reported by other authors[12]. In fact, this might largely be explained by the more persistent nephrotoxic effects of immunosuppression in those patients with better survival rates and longer follow-up available[9].
In contrast to previous studies[12,15,16], we found that such comorbidities as hypertension or diabetes mellitus prior to transplantation were not predictors of CRD. HCV-related disease has also been reported to be a risk factor affecting renal function[10,12], though this did not prove significant in our study. Differences in comorbidity profile and therapeutic management among different patient cohorts may account for the disparities in the results.
The introduction of ACE inhibitors and angiotensin receptor blockers may be of particular benefit in liver transplant recipients due to the renoprotective effects they confer[30,31]. Nevertheless, based on routine clinical practice criteria, the introduction of renoprotective treatment after clinical diagnosis of CRD was moderately low (approximately 30% of patients). Moreover, renal biopsy was performed in a low percentage of patients (2.8%) and few patients (4.9%) required renal replacement therapy, similar to what has been previously reported[32].
CNI-associated chronic nephrotoxicity has been widely reported[9,30,31] and CNI-based regimens at discharge have already been identified as independent predictors of CRD following transplantation[12,15], which is consistent with our own results (HR = 2.31 for CNI vs non-CNI). Moreover, CNI reduction in combination with MMF has been shown to improve eGFR in de novo LT, as well as in patients with moderately impaired renal function[33-35]. In our study, a strategy based on the reduction or withdrawal of CNI therapy was carried out in approximately 80% of liver recipients with diagnosis of CRD based on the sCr definition, while MPA therapy modification was undertaken in nearly half of them.
The present study has several strengths. Firstly, the relatively large sample size of a country-based cohort and secondly, the patients are representative of routine clinical practice in Spain. Several indicators, such us the high percentage of changes in immunosuppressive therapy and the low percentage of patients requiring renal replacement therapy among patients with CRD, demonstrate adequate clinical management in current practice. Thirdly, patients were enrolled by consecutive sampling. All this should outweigh the limitations inherent to retrospective studies which can lead to patient selection bias and inaccurate data collection. Moreover, we were able to compile data over a prolonged time period (almost 20 years), which allowed us to examine long-term changes in renal function. However, the laboratory criteria used to define CRD were arbitrarily established using a cut-off point of 2 mg/dL that has been used in other studies in solid organ transplantation[36]. Furthermore, local creatinine assessment techniques were not analized. Thus, heterogeneity in diagnosis cannot be ruled out. In addition, the use of creatinine secretion inhibitors was not an exclusion criterion. Also, the use of a simplified MDRD equation for GFR estimation also carries some limitations[37,38] although it has been validated in liver transplant patients[39]. Additionally, the study focused only on CRD defined two years after liver transplant and did not differentiate between other common functional renal disorders such as hepatorenal syndrome. Nevertheless, we have been able to provide detailed independent data on eGFR and creatinine in order to better understand the interpretation of these parameters in the clinic-based liver transplant setting. Another constraint worth mentioning is the lack of MELD scores, which have been used since 2002, in a third of the patients. Consequently, we were not able to evaluate how the introduction of these prioritization criteria might have influenced worsening of renal function in these patients[40]. Lastly, data on the effects of immunosuppression could only be analyzed on the basis of drug class; once the CRD diagnosis according to sCr was established, we could not assess whether or not these therapeutic interventions had any effects on renal function.
In conclusion, our study corroborates that CRD is a prevalent condition following LT and that the occurrence of renal dysfunction is significantly under-assessed in routine practice. The significant divergence between a currently used sCr based definition and an eGFR assessment of CRD may stem from the absence of broadly accepted criteria among physicians, thus hindering their ability to accurately identify the disorder. In this sense, estimated GFR at 3-mo post-transplantation provides a powerful and independent predictive factor for the development of CRD in LT patients. The use of more accurate diagnostic measurements will not only permit earlier detection of renal dysfunction, but also facilitate appropriate therapeutic intervention, which could yield significant benefits for long-term renal function and patient survival.
We are grateful to Eva Mateu of TFS for editorial assistance.
Chronic renal dysfunction (CRD) is a common complication following liver transplantation. Serum creatinine is the most established tool for estimating renal function. However, serum creatinine alone may not be an accurate indicator of the degree of renal dysfunction. The abbreviated modification of diet in renal disease equation could provide a clinically useful estimate of glomerular filtration rate.
Serum creatinine not only is a delayed marker of decreased renal function, but it is also influenced by nonrenal factors. Consequently, estimated glomerular filtration rate (eGFR) using a prediction equation that takes into account the serum creatinine level and some of these independent factors, such as gender, age or race, has been recommended as a method for measuring renal function.
The study results suggest that there is a significant divergence between the diagnosis of CRD based on a serum creatinine assessment and the eGFR, under daily practice conditions. According to eGFR assesment, CRD is present in almost half percent of liver recipients after approximately seven years of post-transplant follow-up. However, the rate of CRD is significantly under-estimated according to serum creatinine assessment in daily practice.
Overall, this study outlines the importance of early CRD detection among liver transplant recipients via the use of more sensitive tools. In this sense, eGFR at 3-mo post-transplantation is a powerful independent predictive factor for the development of CRD in liver transplant recipients.
Chronic renal dysfunction is defined as kidney damage or glomerular filtration rate < 60 mL/min per 1.73 m2 for three months or more, irrespective of the cause.
The data provided show that CRD is more prevalent than expected in liver transplants, and that a change from calcineurin Inhibitors to mammalian target of rapamycin inhibiting drugs may alleviate the renal damage.
P- Reviewer: Poltronieri P, Stavroulopoulos A, Watanabe T S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Åberg F, Isoniemi H, Höckerstedt K. Long-term results of liver transplantation. Scand J Surg. 2011;100:14-21. [PubMed] |
| 2. | Ojo AO. Renal disease in recipients of nonrenal solid organ transplantation. Semin Nephrol. 2007;27:498-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 88] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 3. | Pham PT, Pham PC, Wilkinson AH. Management of renal dysfunction in the liver transplant recipient. Curr Opin Organ Transplant. 2009;14:231-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Schmitz V, Laudi S, Moeckel F, Puhl G, Stockmann M, Tran ZV, Kahl A, Neumann U, Neuhaus P. Chronic renal dysfunction following liver transplantation. Clin Transplant. 2008;22:333-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Fisher NC, Nightingale PG, Gunson BK, Lipkin GW, Neuberger JM. Chronic renal failure following liver transplantation: a retrospective analysis. Transplantation. 1998;66:59-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 248] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 6. | Gayowski T, Singh N, Keyes L, Wannstedt CF, Wagener MM, Vargas H, Laskus T, Rakela J, Fung JJ, Marino IR. Late-onset renal failure after liver transplantation: role of posttransplant alcohol use. Transplantation. 2000;69:383-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Aberg F, Koivusalo AM, Höckerstedt K, Isoniemi H. Renal dysfunction in liver transplant patients: comparing patients transplanted for liver tumor or acute or chronic disease. Transpl Int. 2007;20:591-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Fabrizi F, Dixit V, Martin P, Messa P. Chronic kidney disease after liver transplantation: Recent evidence. Int J Artif Organs. 2010;33:803-811. [PubMed] |
| 9. | Charlton MR, Wall WJ, Ojo AO, Ginès P, Textor S, Shihab FS, Marotta P, Cantarovich M, Eason JD, Wiesner RH. Report of the first international liver transplantation society expert panel consensus conference on renal insufficiency in liver transplantation. Liver Transpl. 2009;15:S1-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 106] [Article Influence: 6.6] [Reference Citation Analysis (1)] |
| 10. | Burra P, Senzolo M, Masier A, Prestele H, Jones R, Samuel D, Villamil F. Factors influencing renal function after liver transplantation. Results from the MOST, an international observational study. Dig Liver Dis. 2009;41:350-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 11. | Herlenius G, Fistouris J, Olausson M, Felldin M, Bäckman L, Friman S. Early renal function post-liver transplantation is predictive of progressive chronic kidney disease. Scand J Gastroenterol. 2008;43:344-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Ojo AO, Held PJ, Port FK, Wolfe RA, Leichtman AB, Young EW, Arndorfer J, Christensen L, Merion RM. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349:931-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1703] [Cited by in RCA: 1636] [Article Influence: 74.4] [Reference Citation Analysis (0)] |
| 13. | Dehghani SM, Derakhshan A, Taghavi SA, Gholami S, Jalaeian H, Malek-Hosseini SA. Prevalence and risk factors of renal dysfunction after liver transplant: a single-center experience. Exp Clin Transplant. 2008;6:25-29. [PubMed] |
| 14. | Sezer S, Karakan S, Erişmiş B, Çolak T, Haberal M. Risk factors for kidney impairment and differential impact of liver transplantation on renal function. Transplant Proc. 2011;43:609-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Paramesh AS, Roayaie S, Doan Y, Schwartz ME, Emre S, Fishbein T, Florman S, Gondolesi GE, Krieger N, Ames S. Post-liver transplant acute renal failure: factors predicting development of end-stage renal disease. Clin Transplant. 2004;18:94-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 111] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 16. | Cantarovich M, Tchervenkov J, Paraskevas S, Ghali P, Wong P, Deschênes M, Chaudhury P, Hassanain M, Vrochides D, Metrakos P. Early changes in kidney function predict long-term chronic kidney disease and mortality in patients after liver transplantation. Transplantation. 2011;92:1358-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Duncan L, Heathcote J, Djurdjev O, Levin A. Screening for renal disease using serum creatinine: who are we missing? Nephrol Dial Transplant. 2001;16:1042-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 164] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 18. | K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1-266. [PubMed] |
| 19. | Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10672] [Cited by in RCA: 10998] [Article Influence: 224.4] [Reference Citation Analysis (1)] |
| 20. | Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11183] [Cited by in RCA: 11814] [Article Influence: 454.4] [Reference Citation Analysis (0)] |
| 21. | Levey AS, Greene T, Kusek JW, Beck GJ, Group MS. A simplified equation to predict glomerular filtration rate from serum creatinine. J Am Soc Nephrol. 2000;11:155A (A0828). |
| 22. | Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604-612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20319] [Cited by in RCA: 20032] [Article Influence: 1252.0] [Reference Citation Analysis (0)] |
| 23. | Nankivell BJ, Gruenewald SM, Allen RD, Chapman JR. Predicting glomerular filtration rate after kidney transplantation. Transplantation. 1995;59:1683-1689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 233] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 24. | Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, Hogg RJ, Perrone RD, Lau J, Eknoyan G. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3156] [Cited by in RCA: 3205] [Article Influence: 145.7] [Reference Citation Analysis (0)] |
| 25. | Sharma P, Welch K, Eikstadt R, Marrero JA, Fontana RJ, Lok AS. Renal outcomes after liver transplantation in the model for end-stage liver disease era. Liver Transpl. 2009;15:1142-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 131] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 26. | Fabrizi F, Dixit V, Martin P, Messa P. Pre-transplant kidney function predicts chronic kidney disease after liver transplant: meta-analysis of observational studies. Dig Dis Sci. 2011;56:1282-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Giusto M, Berenguer M, Merkel C, Aguilera V, Rubin A, Ginanni Corradini S, Mennini G, Rossi M, Prieto M, Merli M. Chronic kidney disease after liver transplantation: pretransplantation risk factors and predictors during follow-up. Transplantation. 2013;95:1148-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Sato K, Kawagishi N, Fujimori K, Ohuchi N, Satomi S. Renal function status in liver transplant patients in the first month post-transplant is associated with progressive chronic kidney disease. Hepatol Res. 2015;45:220-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 29. | Fussner LA, Charlton MR, Heimbach JK, Fan C, Dierkhising R, Coss E, Watt KD. The impact of gender and NASH on chronic kidney disease before and after liver transplantation. Liver Int. 2014;34:1259-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 30. | Bloom RD, Reese PP. Chronic kidney disease after nonrenal solid-organ transplantation. J Am Soc Nephrol. 2007;18:3031-3041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 144] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 31. | Bahirwani R, Reddy KR. Outcomes after liver transplantation: chronic kidney disease. Liver Transpl. 2009;15 Suppl 2:S70-S74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 32. | Sethi A, Estrella MM, Ugarte R, Atta MG. Kidney function and mortality post-liver transplant in the Model for End-Stage Liver Disease era. Int J Nephrol Renovasc Dis. 2011;4:139-144. [PubMed] |
| 33. | Neuberger JM, Mamelok RD, Neuhaus P, Pirenne J, Samuel D, Isoniemi H, Rostaing L, Rimola A, Marshall S, Mayer AD. Delayed introduction of reduced-dose tacrolimus, and renal function in liver transplantation: the ‘ReSpECT’ study. Am J Transplant. 2009;9:327-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 209] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 34. | Gerhardt T, Terjung B, Knipper P, Palmedo H, Woitas RP, Kalff J, Sauerbruch T, Spengler U. Renal impairment after liver transplantation - a pilot trial of calcineurin inhibitor-free vs. calcineurin inhibitor sparing immunosuppression in patients with mildly impaired renal function after liver transplantation. Eur J Med Res. 2009;14:210-215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 35. | Saner FH, Cicinnati VR, Sotiropoulos G, Beckebaum S. Strategies to prevent or reduce acute and chronic kidney injury in liver transplantation. Liver Int. 2012;32:179-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 36. | Grinyo JM, Saval N, Campistol JM. Clinical assessment and determinants of chronic allograft nephropathy in maintenance renal transplant patients. Nephrol Dial Transplant. 2011;26:3750-3755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 37. | Gonwa TA, Jennings L, Mai ML, Stark PC, Levey AS, Klintmalm GB. Estimation of glomerular filtration rates before and after orthotopic liver transplantation: evaluation of current equations. Liver Transpl. 2004;10:301-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 258] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 38. | Rule AD, Larson TS, Bergstralh EJ, Slezak JM, Jacobsen SJ, Cosio FG. Using serum creatinine to estimate glomerular filtration rate: accuracy in good health and in chronic kidney disease. Ann Intern Med. 2004;141:929-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 736] [Cited by in RCA: 774] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 39. | De Simone P, Nevens F, De Carlis L, Metselaar HJ, Beckebaum S, Saliba F, Jonas S, Sudan D, Fung J, Fischer L. Everolimus with reduced tacrolimus improves renal function in de novo liver transplant recipients: a randomized controlled trial. Am J Transplant. 2012;12:3008-3020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 260] [Cited by in RCA: 253] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 40. | Sharma P, Schaubel DE, Guidinger MK, Goodrich NP, Ojo AO, Merion RM. Impact of MELD-based allocation on end-stage renal disease after liver transplantation. Am J Transplant. 2011;11:2372-2378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 101] [Article Influence: 7.2] [Reference Citation Analysis (0)] |