Published online Sep 18, 2025. doi: 10.5500/wjt.v15.i3.101496
Revised: December 30, 2024
Accepted: February 6, 2025
Published online: September 18, 2025
Processing time: 213 Days and 15.4 Hours
Liver transplant (LT) candidates face a heightened risk of infection both pre- and post-transplant, owing to immunosuppressive therapy and complications from chronic liver disease. Infections during the pre-transplant period, such as lym
We report the case of a 50-year-old female diagnosed with Hepatitis C virus-related cirrhosis (Child-Pugh C) and recurrent cellulitis due to lymphorrhea in her left lower leg. She suffered repeated episodes of cellulitis over five years, which prevented her from undergoing LT. Initial conservative treatments were unsu
Despite numerous challenges, the patient achieved a successful recovery with satisfactory graft function and was free from lymphorrhea/lymphedema in her left lower limb 3 years post-transplantation. This case underscores the importance of robust infection control during both the pre- and post-transplantation phases and highlights the potential of LVA as a treatment option for managing lymphorrhea and infections in patients with liver cirrhosis.
Core Tip: Lymphorrhea, characterized by significant lymphatic leakage, frequently leads to repeated skin infections that can preclude liver transplantation and increase the risk of dropping out of the waiting list. We present the case of a 50-year-old female patient with Hepatitis C virus-related liver cirrhosis and left lower leg lymphorrhea, which caused repeated episodes of cellulitis. This condition was successfully treated with supermicrosurgical lymphaticovenous anastomoses (LVA), followed by a deceased-donor liver transplantation. This case underscores the importance of stringent infection control both before and after transplantation and highlights LVA as a viable treatment option for managing lymphorrhea and infections in patients with liver cirrhosis.
- Citation: Wu TW, Hou TY, Yang JCS, Wang CC. First report of successful liver transplantation following supermicrosurgical lymphaticovenous anastomoses for lymphorrhea with intractable infection: A case report. World J Transplant 2025; 15(3): 101496
- URL: https://www.wjgnet.com/2220-3230/full/v15/i3/101496.htm
- DOI: https://dx.doi.org/10.5500/wjt.v15.i3.101496
Infection poses a significant challenge in liver transplant (LT) candidates, particularly in those with decompensated cirrhosis. During the post-transplant period, managing infections becomes complicated owing to immunosuppressant use, which impairs the body’s ability to combat pathogens and are associated with poor outcomes. In the pre-transplant period, uncontrolled infections may lead to a temporary suspension of LT and increased mortality while on the waiting list. Lymphorrhea, characterized by prominent lymphatic leakage and persistent infection, represents the most severe form of lymphedema[1]. Approximately 200 million people globally are affected by various degrees of lymphedema. It remains a significant public health issue even in developed Asian countries. For instance, a 2024 statistical report from Korea indicates an annual incidence rate of about 1.85 per 1000 individuals[2]. The lymphatic system, a branching tubular network that extends around blood vessels and into organs, is essential for the human immune and circulatory systems. Notably, the liver produces approximately 25%-50% of the body’s lymph, which recirculates through the bloodstream via the thoracic duct[3]. As cirrhosis progresses, it overwhelms the lymphatic compensatory mechanisms, leading to the development of ascites and edema, which can cause complications such as recurrent soft tissue infections[4]. The incidence of concurrent lymphedema in cases of liver disease is significant. Clinical guidelines recommend that treatment for lymphedema should aim to reduce limb volume and prevent severe infections[5]. Initial treatment should focus on conservative measures such as manual lymphatic drainage, physical therapy, multilayer bandaging, limb elevation, exercise, and skincare. Surgical treatments, which are supported by a lower level of evidence, are recommended only for selected patients, including those with severe deformity from swelling, unsuccessful conservative treatment, poor response to compression therapy, recurrent cellulitis, or intractable pain. In our case of a high-risk patient with lymphorrhea, non-invasive management proved ineffective, leading to continued bouts of recurrent cellulitis. Owing to the high surgical and anesthetic risks associated with liver cirrhosis complications, more invasive options like extirpative reduction and debulking surgery were deemed unsuitable. After careful evaluation, lymphaticovenous anastomoses (LVA) was considered the most suitable treatment option.
Herein, we present a case involving a female patient with Child-Pugh C hepatitis C virus (HCV)-related cirrhosis and lower left leg lymphorrhea accompanied by repeated episodes of cellulitis. This patient was successfully treated with supermicrosurgical LVA and later underwent deceased donor liver transplantation (DDLT). The interplay of severe liver cirrhosis and refractory infections due to lymphorrhea posed significant challenges. This case report not only underscores the importance of stringent infection control but also offers a potential solution for similar clinical scenarios. Additionally, it exemplifies the critical role of interdisciplinary consultation and collaboration in providing timely and effective treatment for patients with complex conditions.
A 50-year-old Asian female patient who presented with anorexia, reduced urine output, and tea-colored urine was referred to our department in July 2014.
The patient had recurrent episodes of biliary tract infection and lower limb cellulitis.
She had a history of hepatitis C infection, hypertension, esophageal varices, and coagulopathy.
No family history of liver disease.
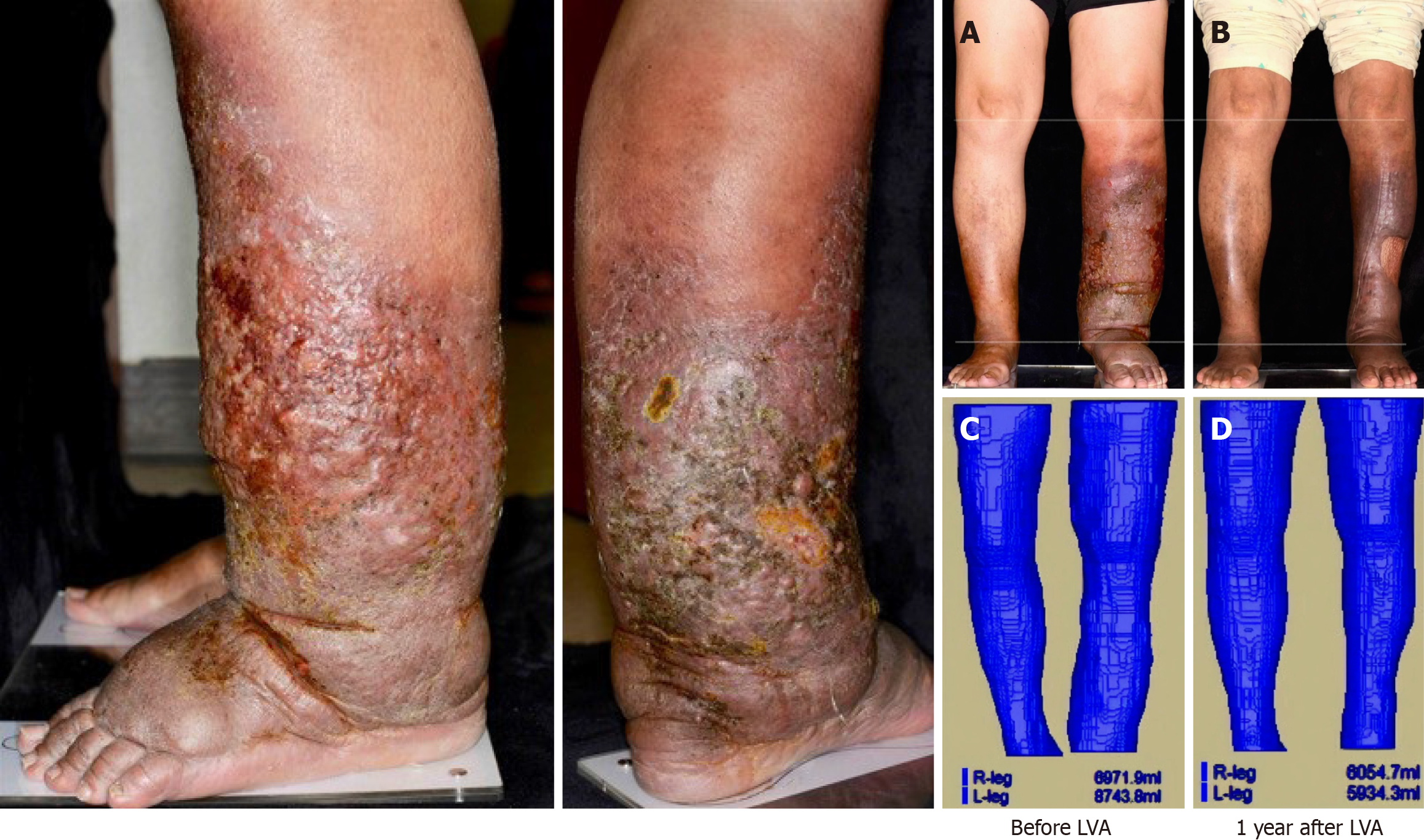
The patient’s vital signs were stable, although she exhibited jaundice, bilateral leg edema, and gradual lymphatic leakage from the skin of her lower left leg. Additional findings included hyperkeratosis and a positive Stemmer sign (Figure 1).
Initial laboratory results revealed the following: Glutamic-oxaloacetic transaminase (GOT): 234 U/L, glutamic-pyruvic transaminase (GPT): 71 U/L, international normalized ratio (INR): 2.06, creatinine: 0.51 mg/dL, sodium: 136 mEq/L, albumin: 2.2 g/dL, total bilirubin: 9.9 mg/dL, and prothrombin time: 22.1 s. The Model for End-Stage Liver Disease (MELD) score was 23.
Abdominal and liver computed tomography scans showed liver cirrhosis with refractory ascites, esophageal varices, and partial portal vein thrombosis. Lymphoscintigraphy and indocyanine green lymphography revealed partial obstruction of lymphatic drainage and diffuse dermal backflow over the lower left leg.
The patient was diagnosed with Child-Pugh C HCV-related cirrhosis and stage 3 lymphedema accompanied by severe lymphorrhea.
The patient was diagnosed with Child-Pugh C HCV-related cirrhosis, characterized by refractory ascites, repeated esophageal varices bleeding, and partial portal vein thrombosis. Owing to her end-stage liver disease and the absence of a compatible living donor, she was placed on the waiting list for a LT. Concurrently, she experienced bilateral leg edema, gradual lymphatic leakage from the skin of her lower left leg, hyperkeratosis, and a positive Stemmer sign (Figure 1), which raised significant concerns regarding her suitability for LT under these conditions[6]. She received conservative treatment for recurrent cellulitis in her lower left leg until February 2019, when she was referred to the Plastic Surgery Department for comprehensive evaluation. Lymphoscintigraphy and indocyanine green lymphography confirmed stage 3 lymphedema with severe lymphorrhea upon results showing partial obstruction of lymphatic drainage and diffuse dermal backflow in her lower left leg[7]. A supermicrosurgical LVA was then performed to alleviate the lymphedema. The procedure involved creating five anastomoses in the dorsal foot, below the knee, and middle thigh areas to facilitate the drainage of stagnant lymph into the recipient’s vein. The lymphatic ducts measured between 1.0-1.5 mm, matching the venule sizes. Nylon 11-0 sutures were used for the LVA anastomoses. Indocyanine green was employed intraoperatively to identify the lymphatic ducts and to evaluate flow and ectasis following the anastomoses. The success of the procedure was evidenced by a significant reduction in limb volume, as verified by post-LVA magnetic resonance volumetry (Figure 1).
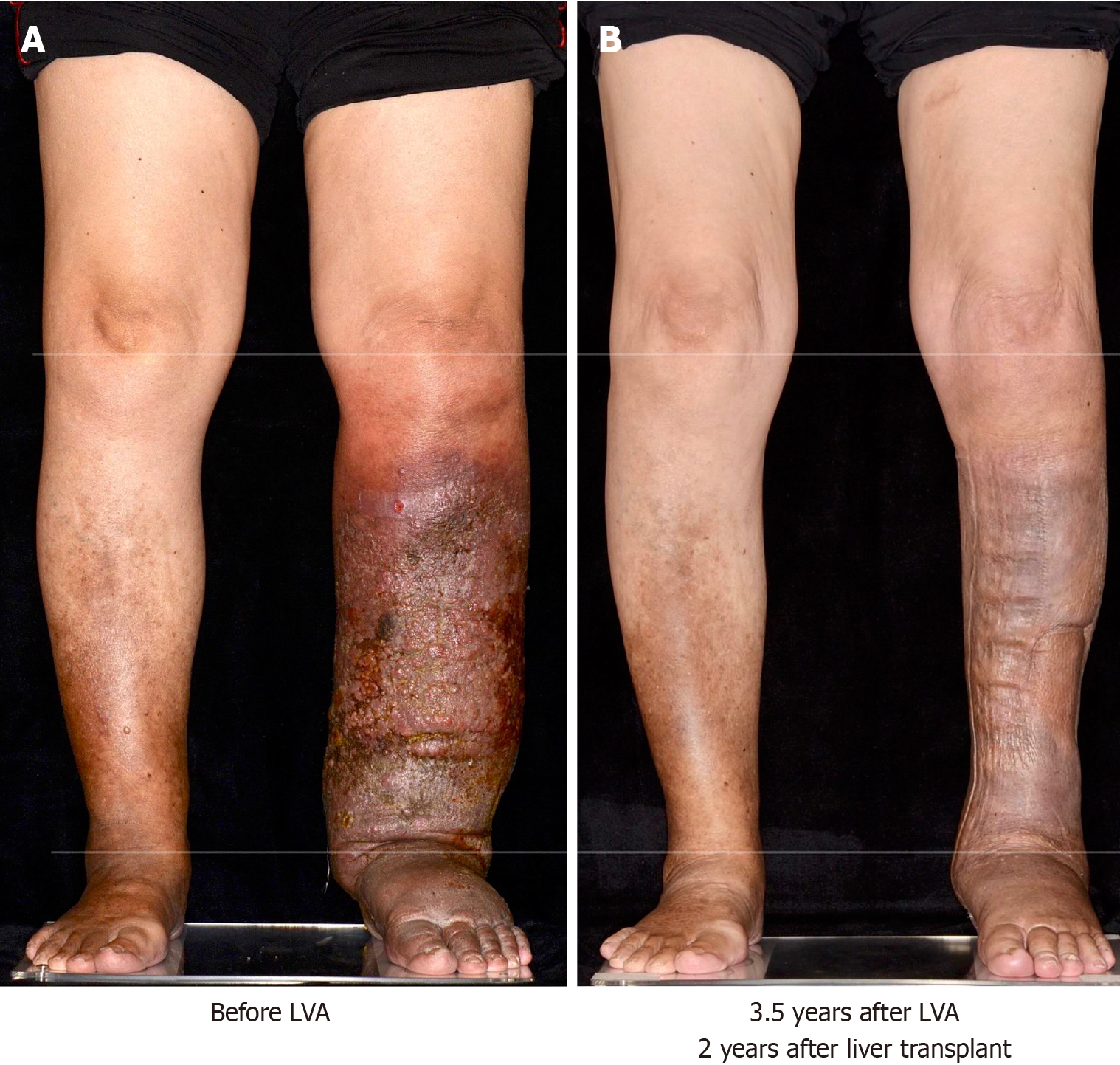
Following the LVA, there was a marked improvement in the condition of the patient’s lower left leg, including the cessation of lymphorrhea and resolution of recurrent cellulitis. After successfully managing the severe lymphedema, the patient was readmitted for an LT evaluation. Laboratory results confirmed Child-Pugh C cirrhosis with a MELD score of 21, characterized by an INR of 1.85, creatinine 0.51 mg/dL, sodium 144 mEq/L, albumin 2.04 g/dL, and total bilirubin 7.4 mg/dL. Computed tomography angiography revealed complete portal vein thrombosis with wall calcification (Yerdel’s grade 4). Eighteen months after the LVA, the patient underwent a DDLT using a right liver graft without the middle hepatic vein. The surgery included a portal vein thrombectomy and stent insertion. The total blood loss was 2800 mL, managed without vasopressors. On postoperative day 1, a complication arose in the form of a surgical wound hematoma, requiring exploratory laparotomy, classified as grade 3B in the Clavien–Dindo classification. The patient was administered mycophenolate mofetil and tacrolimus for post-transplant immunosuppression. Her liver function subsequently normalized, and she was discharged 3 months post-transplantation.
At the 3-year follow-up, the patient’s liver function remained stable, with an INR of 1.31, creatinine: 0.84 mg/dL, albumin: 4.34 g/dL, total bilirubin: 0.7 mg/dL, GOT: 19 U/L, and GPT: 12 U/L. There has been no recurrence of cellulitis (Figure 2).
In this case, recurrent infections delayed the patient’s eligibility for an LT for > 5 years. Following a successful LVA, she underwent a DDLT and experienced a favorable outcome over a 3-year follow-up. Previous reviews indicate that active infections and uncontrolled sepsis are contraindications for LT[8]. Eradicating the source of infection is essential before proceeding with transplantation. Additionally, several studies highlight that peritransplant infections and sepsis can significantly increase 90-day mortality[9]. Another retrospective study involving 24122 LT recipients found that those with preoperative infections were more likely to experience postoperative complications such as effusions, infections, abdominal bleeding, biliary complications, and lower overall survival rates[10]. Conversely, a study including 466 LT cases demonstrated that although pre-transplant infections increased the incidence of postoperative infections and septic shock, they did not significantly impact overall survival rates, suggesting that transplantation should not be delayed if the infection is adequately managed[11]. However, these reviews mostly address infections related to single events. In our case, the infections were recurrent and predictable, with prolonged antibiotic use potentially leading to resistance and increasing post-transplantation risks. Therefore, controlling recurrent cellulitis in this case was mandatory. Although studies on preoperative cellulitis are limited, the necessity for LVA surgery intervention has become increasingly clear.
Lymphedema can be classified into primary and secondary forms, with primary lymphedema typically being con
Although the relationship between cirrhosis and lymphedema remains unclear owing to a lack of extensive studies, an observational study by Arya et al[15], that included 155 cases of cirrhosis with ascites, found that 33.5% of the cases exhibited varying degrees of lymphedema, and 44.5% had lymphatic dysfunction. The liver, as the primary organ for protein synthesis and a crucial component of the portal venous system, significantly influences the distribution of body fluids. As liver function deteriorates and portal pressure increases, more fluid accumulates in the interstitial space, placing a greater burden on the lymphatic system and eventually leading to its damage[16]. This case highlights a feasible strategy for managing the interconnected issues of infection, lymphedema, and cirrhosis.
Despite facing numerous challenges, the patient achieved a successful recovery with satisfactory graft function and was free from lymphedema/lymphorrhea in her lower left limb 3 years after transplantation. This case emphasizes the importance of rigorous infection control during both the pre- and post-transplantation phases and highlights the potential of LVA as a treatment option for lymphorrhea and infection in patients with liver cirrhosis.
| 1. | Yang JC, Wu SC, Lin WC, Chiang MH, Chiang PL, Hsieh CH. Supermicrosurgical Lymphaticovenous Anastomosis as Alternative Treatment Option for Moderate-to-Severe Lower Limb Lymphedema. J Am Coll Surg. 2020;230:216-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 2. | Kim DJ, Kim DS, Yu Y, Chung JH, Yoon ES. Cross-sectional analysis for lymphedema epidemiology in South Korea by HIRA data: An observational study. Medicine (Baltimore). 2024;103:e38779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 3. | Ohtani O, Ohtani Y. Lymph circulation in the liver. Anat Rec (Hoboken). 2008;291:643-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 134] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 4. | Kumar R, Anand U, Priyadarshi RN. Lymphatic dysfunction in advanced cirrhosis: Contextual perspective and clinical implications. World J Hepatol. 2021;13:300-314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 5. | O'Donnell TF Jr, Allison GM, Iafrati MD. A systematic review of guidelines for lymphedema and the need for contemporary intersocietal guidelines for the management of lymphedema. J Vasc Surg Venous Lymphat Disord. 2020;8:676-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 6. | Martin P, DiMartini A, Feng S, Brown R Jr, Fallon M. Evaluation for liver transplantation in adults: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Hepatology. 2014;59:1144-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 702] [Article Influence: 63.8] [Reference Citation Analysis (1)] |
| 7. | Executive Committee. The Diagnosis and Treatment of Peripheral Lymphedema: 2016 Consensus Document of the International Society of Lymphology. Lymphology. 2016;49:170-184. [PubMed] |
| 8. | Farkas S, Hackl C, Schlitt HJ. Overview of the indications and contraindications for liver transplantation. Cold Spring Harb Perspect Med. 2014;4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 9. | Martin Mateos R, Albillos A. Sepsis in Patients With Cirrhosis Awaiting Liver Transplantation: New Trends and Management. Liver Transpl. 2019;25:1700-1709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Xiang Z, Song Y, Liu J, Xu C, Zhou Z, Li J, Su R, Shu W, Lu Z, Wei X, Yang J, Yang Y, Zheng S, Xu X. Impact of preoperative infection on the outcomes of liver transplant recipients: a national propensity score-matched retrospective cohort study in China. Int J Surg. 2024;110:2196-2206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 11. | Incicco S, Tonon M, Zeni N, Gambino C, Gagliardi R, Calvino V, Barone A, Zilio G, Feltracco P, Burra P, Cillo U, Angeli P, Piano S. Impact of bacterial infections prior to liver transplantation on post-transplant outcomes in patients with cirrhosis. JHEP Rep. 2023;5:100808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 12. | Connell FC, Gordon K, Brice G, Keeley V, Jeffery S, Mortimer PS, Mansour S, Ostergaard P. The classification and diagnostic algorithm for primary lymphatic dysplasia: an update from 2010 to include molecular findings. Clin Genet. 2013;84:303-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 109] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 13. | Scaglioni MF, Fontein DBY, Arvanitakis M, Giovanoli P. Systematic review of lymphovenous anastomosis (LVA) for the treatment of lymphedema. Microsurgery. 2017;37:947-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 163] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 14. | Thomas M, Pike C, Humphreys I, Bragg T, Ghattaura A. Impact and outcomes after lymphaticovenous anastomosis for 150 cases of lymphoedema followed up over 24 months. J Plast Reconstr Aesthet Surg. 2023;85:104-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 15. | Arya R, Kumar R, Kumar T, Kumar S, Anand U, Priyadarshi RN, Maji T. Prevalence and risk factors of lymphatic dysfunction in cirrhosis patients with refractory ascites: An often unconsidered mechanism. World J Hepatol. 2023;15:1140-1152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Jeong J, Tanaka M, Iwakiri Y. Hepatic lymphatic vascular system in health and disease. J Hepatol. 2022;77:206-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |