Published online Mar 18, 2025. doi: 10.5500/wjt.v15.i1.96696
Revised: October 2, 2024
Accepted: October 15, 2024
Published online: March 18, 2025
Processing time: 197 Days and 5.6 Hours
Coronavirus disease 2019 (COVID-19) pneumonia with severe septic shock and acute respiratory distress syndrome (ARDS) are critical illnesses for patients following transplant. Intravenous immunoglobulin (IVIG) plays a role in both immune support and inflammation control, especially in immunocompromised patients. This case report describes the first successful experience using IVIG and pulse steroids to manage this critical condition following lung transplantation.
A 65-year-old male patient reported a history of chronic obstructive pulmonary disease and poor lung function and received bilateral sequential lung transplantations. Postoperatively, he developed COVID-19 pneumonia, severe septic shock, and ARDS. He recovered from this critical condition after empirical antibiotics administration and veno-venous extracorporeal membrane oxygenation, in addition to IVIG and pulse steroids.
IVIG is a valuable adjunct in managing severe sepsis in lung transplant recipients after COVID-19 infection. We aim, for the first time, to report the success of such a management approach for COVID-19 ARDS and sepsis in the post-lung tran
Core Tip: The first successful experience indicated intravenous immunoglobulin as a valuable adjunct in managing severe coronavirus disease 2019 infection complicated with severe acute respiratory distress syndrome and septic shock in a lung transplant recipient.
- Citation: Kuo YS, Lin KH, Chen YY, Tsai YM, Wu TH, Huang HK, Huang TW. Success of intravenous immunoglobulin and steroids in managing severe COVID-19 following lung transplantation: A case report. World J Transplant 2025; 15(1): 96696
- URL: https://www.wjgnet.com/2220-3230/full/v15/i1/96696.htm
- DOI: https://dx.doi.org/10.5500/wjt.v15.i1.96696
Patients with transplanted organs, who experience chronic immunosuppression, may demonstrate a heightened susceptibility to coronavirus disease 2019 (COVID-19) pneumonia caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) compared with the general population[1]. Managing this condition, particularly in patients with exacerbation post-cytokine storm, with or without concurrent allograft deterioration, poses a significant challenge in the clinical setting, primarily because of the lack of targeted antiviral agents[2]. Intravenous immunoglobulin (IVIG) and corticosteroids have been explored for treating severe COVID-19, with varying results based on timing, dosing, and patient conditions[3-5]. This report emphasizes the efficacy of a combined therapeutic regimen that involved high-dose IVIG and corticosteroid pulses administered in a critical case of COVID-19 pneumonia with severe acute respiratory distress syndrome (ARDS) and septic shock in a patient who had undergone bilateral lung transplantation. The patient, requiring mechanical ventilation and extracorporeal membrane oxygenation (ECMO) support due to COVID-19 pneumonia, exhibited marked clinical improvement and progressive recovery of allograft function after IVIG and methylprednisolone therapy. This resulted in the patient’s discharge 114 days after treatment initiation. The synergistic immunomodulatory action of IVIG and the anti-inflammatory and immunosuppressive capabilities of corticosteroids may provide a viable therapeutic approach for managing severe COVID-19 pneumonia, particularly in patients with transplanted lung grafts experiencing an uncontrolled inflammatory response.
A 65-year-old man was admitted to our chest surgery ward for preparation for lung transplantation due to the end-stage lung disease caused by chronic obstructive pulmonary disease (COPD) since 2015.
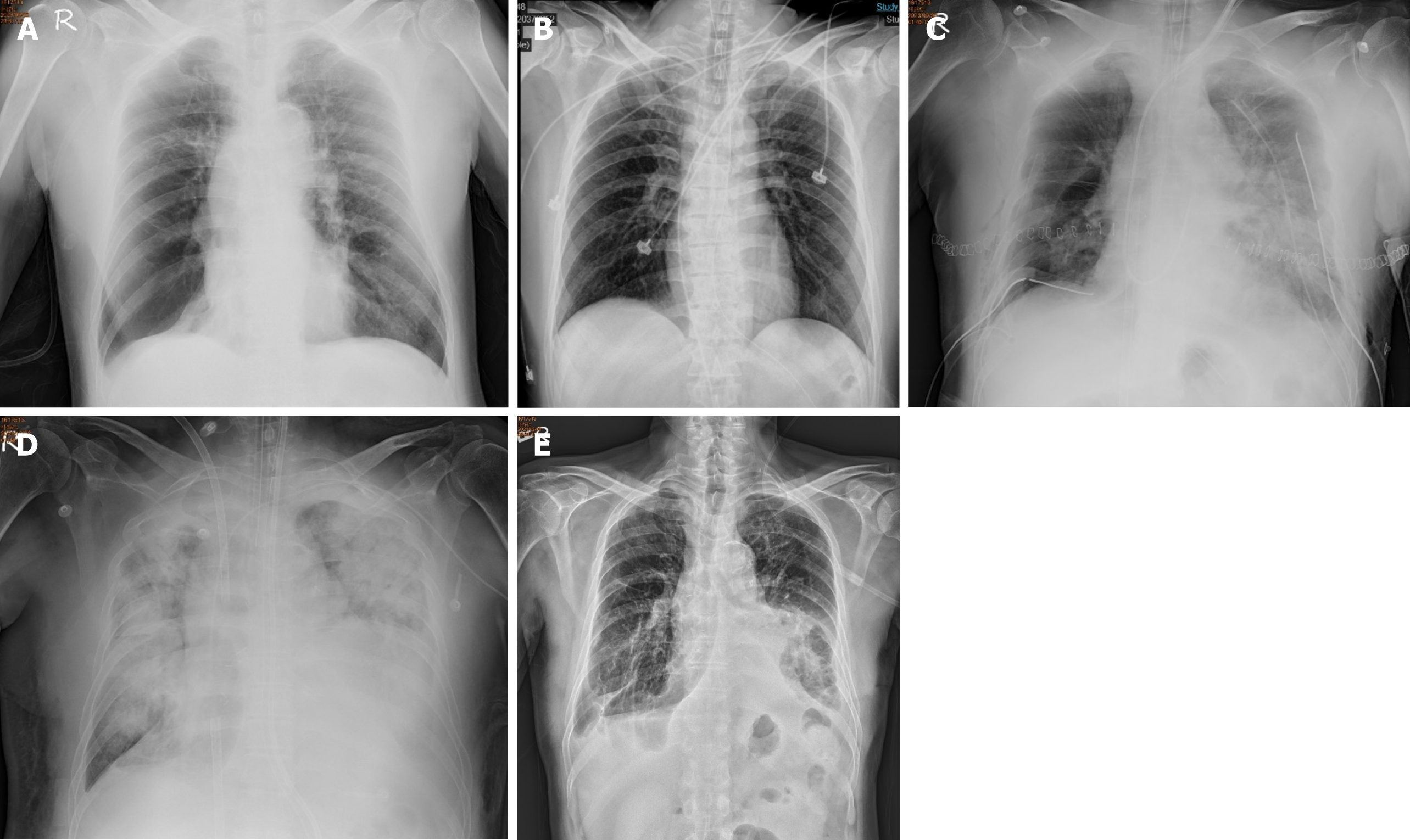
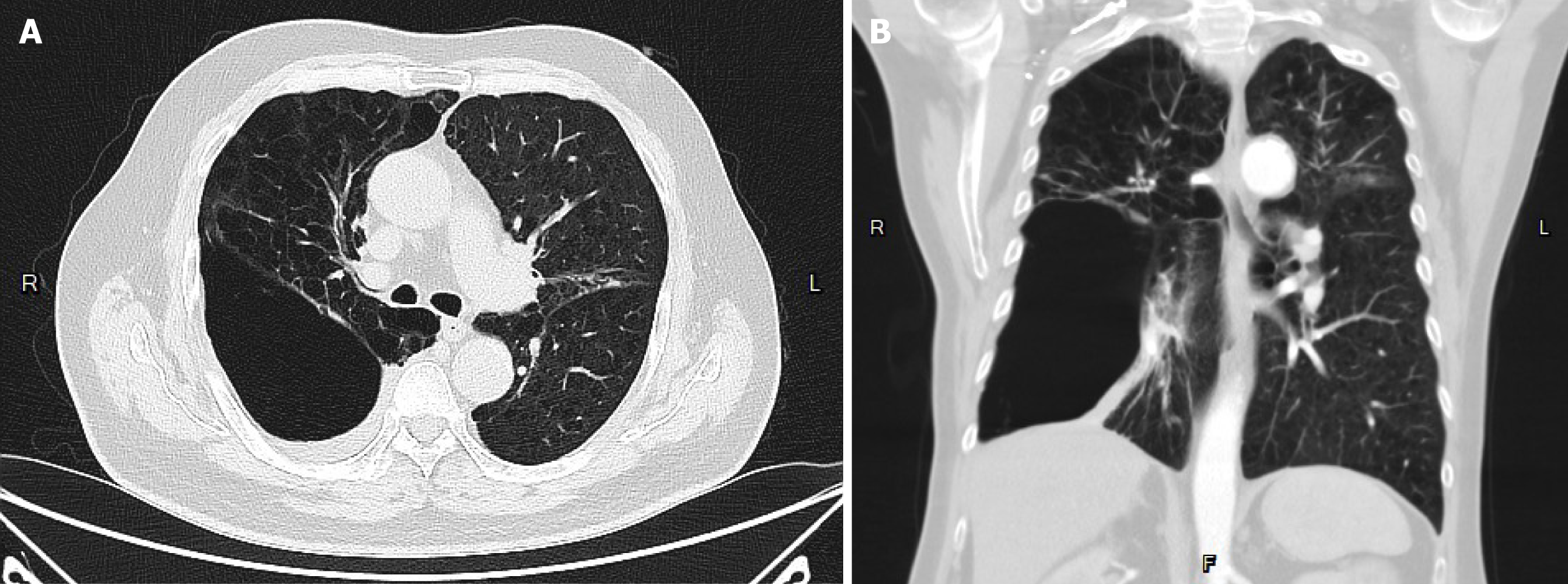
The patient suffered from COPD since 2015. He had episodes of acute COPD exacerbations with hospitalization. Recently, he demonstrated forced expiratory volume in 1 second of 14% of predicted values and severely limited 6-minute walking distance (154 m; 23.5% of predicted values). He received long-term oxygen support since 2017. Currently, he requires oxygen supplementation with oxygen of 2 L/minute for 24 hours a day to meet oxygen demand. He was listed as a recipient of lung transplantation in April 2023 because of progressive disease. The pretransplant chest plain film revealed hyperinflation and increased opacification of both lung fields except for the right lower lung field which demonstrated hypertranslucency (Figure 1A). The pretransplant chest computed tomography (CT) revealed a diffuse emphysematous change of bilateral lungs, with marked right lung destruction (Figure 2). He underwent sequential double lung transplantation on September 29, 2023. The donor was a 39-year-old male patient with diffuse subarachnoid hematoma without cardiopulmonary resuscitation. The donor’s arterial blood gas analysis indicated an arterial oxygen partial pressure and fractional inspired oxygen (PaO2/FiO2) ratio of 591, and the chest plain film detected a bilateral clear lung field (Figure 1B). The donor’s and recipient’s human leukocyte antigen panel reactive antibody were both 0%. They were both 175 and 165 cm in height, respectively.
The sequential double lung transplantation took approximately 10 hours. The right-side lung transplantation was first conducted, followed by left-side lung graft implantation. Pleural adhesion lysis was performed after entering the bilateral pleural cavity. A thoracic surgeon performed an end-to-end anastomosis of the bronchus. The membranous portions of the donor and recipient bronchi were approximated and anastomosed with a running suture, whereas the cartilaginous layers were anastomosed with interrupted sutures. A cardiovascular surgeon performed the end-to-end anastomosis of pulmonary arteries and veins. The patient exhibited an unstable hemodynamic status during the left-side lung transplantation, which may be caused by the cardiac traction and compression. Venoarterial (V-A) ECMO with cannulation at the left femoral vein to the right femoral artery was implanted.
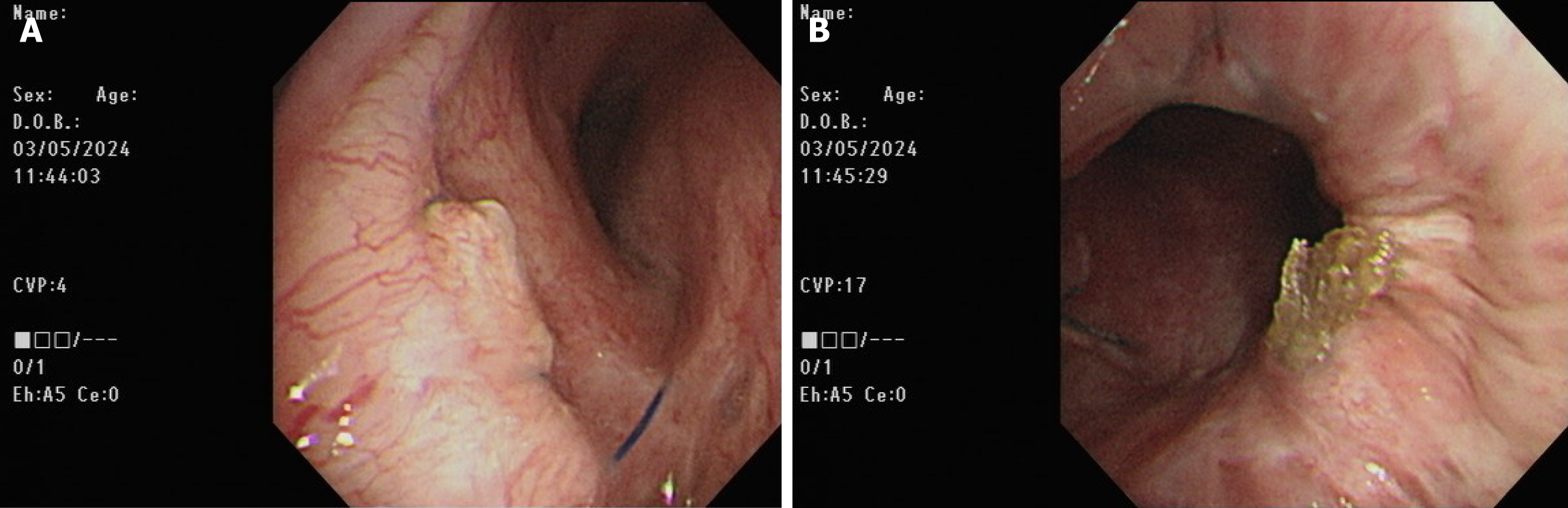
Postoperatively, the patient was transferred to the surgical intensive care unit. Primary graft dysfunction manifested itself as hypoxemia and alveolar infiltration on the chest radiograph (Figure 1C). Additionally, the COVID-19 rapid screen test was positive on postoperative day (POD) 1. Daily intravenous remdesivir of 100 mg was intravenously administered to reduce the viral load. V-A ECMO was shifted to veno-venous (V-V) ECMO with cannulation at the right internal jugular vein and right femoral vein on POD 7 under stable hemodynamic status to reduce the incidence of V-A ECMO complications. However, severe ARDS and septic shock subsequently occurred on POD 8 (Figure 1D). The patient’s oxygenation and hemodynamic status rapidly deteriorated, ultimately requiring the high ventilator settings and ECMO and vasopressor support within 5 days of IgG-enriched IVIG therapy plus steroids (intravenous Privigen of 5000 mg daily plus Medason of 125 mg every 12 hours), and the patient recovered gradually. The serum C-reactive protein level decreased from 16.09 mg/dL at the start of IVIG therapy to 4.11 mg/dL 5 days thereafter. Flexible bronchoscopy was performed at 3-day intervals for airway secretion clearance to assess the condition of the bronchial anastomosis (Figure 3). A bronchial complication was not observed. The V-V ECMO was removed on POD 35. A tracheostomy was conducted on POD 43. The patient was weaned off the ventilator on POD 50. The tracheostomy tube was removed on POD 110. The patient was discharged on POD 121 after the multidisciplinary team care and pulmonary rehabilitation. The follow-up chest plain film demonstrated the remission of pneumonia patches and a stable condition (Figure 1E).
The timeline of the events we mentioned was summarized as Table 1.
| Timeline of events | |
| 2023/9/28 | Admission |
| 2023/9/29 | Sequential double lung transplantation; VA ECMO; inhale NO support |
| 2023/9/30 | COVID-19 (+) > remdisivir |
| 2023/10/3 | Turn off the inotropic agent |
| 2023/10/7 | ARDS and severe septic shock |
| 2023/10/7-2023/10/12 | IVIG plus steroids |
| 2023/10/10 | Turn off the inotropic agent |
| 2023/10/27 | Bedside rehabilitation |
| 2023/11/5 | Remove ECMO |
| 2023/11/13 | Tracheostomy |
| 2023/12/5 | Transfer to ordinary ward |
| 2024/2/5 | Discharge |
The patient was diagnosed with COPD in 2015. He had frequent episodes of secondary pneumothorax and COPD with acute exacerbations from 2015 to 2023.
The patient, an ex-smoker, denied any family cancer history.
The physical examination showed a barrel-shaped chest and decreased bilateral chest sounds.
The respiratory panel for virus screening was positive for COVID-19 on POD 1. Laboratory data on POD 8 (the day before the administration of IVIG and steroids) were as follows:
The results of the arterial blood gas analysis were as follows: PH, 7.471-7.488; PaCO, 39.7-35.4 mmHg; PaO2, 73-77.8 mmHg; HCO3-, 29.2-27.1 mmol/L; SO2, 95.4%-96.4%; PiO2/FiO2, 112.3-119.7 mmHg, under V-V ECMO (pump speed, 2490 RPM; blood flow, 2.46 L/minute; gas flow, 2.5; FiO2, 100%) and APRV MODE with T high, 4 seconds; T low, 0.4 seconds; P high, 26; P low, 0; P mean, 24; TVi, 290-285 mL; RR, 13-14 BPM; MV, 4.9-3.8 L/minute; SpO2, 88%-94%.
The patient's blood examination revealed an elevated leukocyte count of 18020/L, with 92.5% neutrophils, a low hemoglobin level at 8.8 g/dL, and a high procalcitonin level at 6.06 ng/mL.
The first positive sputum culture was reported on POD 10, which indicated the growth of Stenotrophomonas maltophilia and Acinetobacter nosocomialis, which were sensitive to tigecycline and minocycline.
The postoperative chest CT film revealed infiltration over the left lower lung field, which indicated primary graft dysfunction of the left lung. Chest plain film on POD 3 revealed patch opacities of bilateral lungs, indicating COVID-19 with pulmonary involvement.
An infection disease specialist and a rheumatologist recommended standard antibiotic and antiviral therapy in co
The final diagnosis was post-lung transplantation COVID-19 complicated with ARDS and septic shock.
IVIG infusion plus steroid administration, empiric antibiotic administration, and V-V ECMO support.
After IVIG infusion, empiric antibiotic administration, and V-V ECMO support, the patient recovered from ARDS and septic shock. Finally, the patient was weaned off the ventilator and was discharged POD 121. The patient remained stable during the outpatient follow-up.
Individuals who have undergone lung transplantation are at high risk for severe COVID-19, primarily because of a weakened immune system. The virus triggers an excessive immune reaction, also known as a cytokine storm, which is a severe hyperinflammatory state in such patients. This condition may cause septic shock, which is marked by severe circulation, cellular function, and metabolism disruptions, thereby substantially increasing the risk for mortality[6].
Messika et al[7] investigated 35 lung transplant recipients across 11 French transplant centers who contracted COVID-19 during the study period. The diagnosis was confirmed through positive reverse-transcription polymerase chain reaction tests for SARS-CoV-2 in 30 (85.7%) patients, with a strong suspicion in the remaining 5 (14.3%). Of the 13 patients with critical illness, 7 (53.9%) underwent invasive mechanical ventilation. The overall survival rate was 85.7%, following a median follow-up of 50 (range, 41.0-56.5) days. Among the non-survivors, four experienced bronchial complications or an increase in immunosuppression before contracting the virus. Lung transplant recipients with COVID-19 are at greater risk of critical conditions, such as septic shock, and are more likely to require intensive care unit admission and mechanical ventilation, causing higher mortality rates compared with the general population.
Septic shock incidence among lung transplant recipients with COVID-19 significantly varies across different studies, a variation that likely reflects differences in patient demographics, transplant-specific factors, and COVID-19 timing following transplant. Managing COVID-19 in these patients requires a nuanced approach that balances antiviral treatments, supportive care, and adjustments to immunosuppressive therapies. Early intervention is crucial, as is vigilant monitoring for sepsis signs and robust supportive care. The therapeutic regimen, including steroids, antiviral agents, such as remdesivir, and immunomodulatory drug administration, must be carefully customized to each patient’s unique circumstances to reduce the risk of progression to septic shock[8].
IVIG is selected for its comprehensive range of antibodies, which neutralize a diverse array of pathogens. It provides passive immunity to recipients, improving the immune system’s capacity to combat infections. Additionally, IVIG possesses anti-inflammatory properties, which play a crucial role in managing the systemic inflammatory response syndrome frequently associated with sepsis. This makes IVIG a valuable tool in both immune support and inflammation control.
IVIG administration is indicated for several purposes in organ transplantation and severe sepsis as follows:
IVIG is administered to prevent infections during high-risk periods, particularly just after an organ transplant, to improve patient outcomes by boosting immune defenses.
IVIG serves as an adjunct to standard antimicrobial therapy. It is valuable when dealing with infections related to multidrug-resistant organisms or suspected polymicrobial infections, thereby improving treatment regimen efficacy.
IVIG helps in reducing the severity of the sepsis-associated inflammatory response. This immune system modulation decreases the risk of advancing to septic shock, thereby improving patient management and survival[9].
Few studies have discussed issues on IVIG and lung transplantations. IVIG was used for the desensitization protocol for recipients after lung transplantations.
The non-IVIG group demonstrated a higher incidence of donor-specific antibody development (58.8% vs 33.3%), with an odds ratio of 2.80 (95% confidence interval: 0.77-10.79, P = 0.13), compared with the IVIG group. The median time to antibody development was 9 (Q1, Q3: 7, 19) and 28 (Q1, Q3: 7, 58) days for the non-IVIG and IVIG groups, respectively. No significant differences were observed between the groups in terms of primary graft dysfunction 72 hours following transplant or the incidence of acute cellular rejection, antibody-mediated rejection, and chronic lung allograft dysfunction at 12 months[10].
A systematic review and meta-analysis demonstrated sufficient evidence to support that IVIG reduces sepsis mortality. However, fewer articles have revealed the role of IVIG in controlling sepsis in solid-organ transplantation, particularly in recipients after lung transplantation.
The limitation of the present study includes unavailable data on serum immunoglobulin levels that could represent the inflammation status.
To the best of our knowledge, this report is the first to address the successful experience and potential therapeutic value of IVIG in treating severe COVID-19 following lung transplantation[11].
IVIG represents a valuable adjunct in treating severe sepsis in lung transplant recipients after COVID-19. Its use, both prophylactically and therapeutically, supports the immune system’s capacity to combat infections, thereby improving patient outcomes. This warrants investigation moving forward to determine the ideal management for such a patient population. This would consequently pave the way for establishing guidelines for managing such complex patients in the post-lung transplant setting.
We thank the infectious disease specialist, Wang YC and the rheumatologist, Lu CC for the suggestion of administration of intravenous immunoglobulin during the period of severe septic shock of the patient.
| 1. | Azzi Y, Bartash R, Scalea J, Loarte-Campos P, Akalin E. COVID-19 and Solid Organ Transplantation: A Review Article. Transplantation. 2021;105:37-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 236] [Article Influence: 59.0] [Reference Citation Analysis (0)] |
| 2. | Castelli V, Cimini A, Ferri C. Cytokine Storm in COVID-19: "When You Come Out of the Storm, You Won't Be the Same Person Who Walked in". Front Immunol. 2020;11:2132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 91] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 3. | Kwapisz D, Bogusławska J. Intravenous immunoglobulins (IVIG) in severe/critical COVID-19 adult patients. Biomed Pharmacother. 2023;163:114851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 4. | Ali HS, Elshafei MS, Saad MO, Mitwally HA, Al Wraidat M, Aroos A, Shaikh N, Ananthegowda DC, Abdelaty MA, George S, Nashwan AJ, Mohamed AS, Khatib MY. Clinical outcomes of intravenous immunoglobulin therapy in COVID-19 related acute respiratory distress syndrome: a retrospective cohort study. BMC Pulm Med. 2021;21:354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Liu X, Cao W, Li T. High-Dose Intravenous Immunoglobulins in the Treatment of Severe Acute Viral Pneumonia: The Known Mechanisms and Clinical Effects. Front Immunol. 2020;11:1660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 6. | Gusev E, Sarapultsev A, Solomatina L, Chereshnev V. SARS-CoV-2-Specific Immune Response and the Pathogenesis of COVID-19. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 158] [Article Influence: 52.7] [Reference Citation Analysis (0)] |
| 7. | Messika J, Eloy P, Roux A, Hirschi S, Nieves A, Le Pavec J, Sénéchal A, Saint Raymond C, Carlier N, Demant X, Le Borgne A, Tissot A, Debray MP, Beaumont L, Renaud-Picard B, Reynaud-Gaubert M, Mornex JF, Falque L, Boussaud V, Jougon J, Mussot S, Mal H; French Group of Lung Transplantation. COVID-19 in Lung Transplant Recipients. Transplantation. 2021;105:177-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 89] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 8. | Zimmermann J, Glueck OM, Fertmann JM, Sienel WG, Yavuz G, Damirov F, Kovács JR, Tufman A, Irlbeck M, Kneidinger N, Michel S, Kauke T, Hatz RA, Schneider CP. COVID-19 in Recent Lung Transplant Recipients: Clinical Outcomes and Management Strategies. Transplant Proc. 2022;54:1504-1516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Perez EE, Orange JS, Bonilla F, Chinen J, Chinn IK, Dorsey M, El-Gamal Y, Harville TO, Hossny E, Mazer B, Nelson R, Secord E, Jordan SC, Stiehm ER, Vo AA, Ballow M. Update on the use of immunoglobulin in human disease: A review of evidence. J Allergy Clin Immunol. 2017;139:S1-S46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 421] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 10. | Goldsby J, Beermann K, Frankel C, Parish A, Stauffer N, Schandert A, Erkanli A, Reynolds JM. Preemptive immune globulin therapy in sensitized lung transplant recipients. Transpl Immunol. 2023;80:101904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 11. | Pan B, Sun P, Pei R, Lin F, Cao H. Efficacy of IVIG therapy for patients with sepsis: a systematic review and meta-analysis. J Transl Med. 2023;21:765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Reference Citation Analysis (0)] |