Published online Mar 18, 2024. doi: 10.5500/wjt.v14.i1.89674
Peer-review started: November 8, 2023
First decision: November 29, 2023
Revised: December 4, 2023
Accepted: December 29, 2023
Article in press: December 29, 2023
Published online: March 18, 2024
Processing time: 127 Days and 14.6 Hours
Previous assessments of stem cell therapy for spinal cord injuries (SCI) have encountered challenges and constraints. Current research primarily emphasizes safety in early-phase clinical trials, while systematic reviews prioritize effectiveness, often overlooking safety and translational feasibility. This situation prompts inquiries regarding the readiness for clinical adoption.
To offer an up-to-date systematic literature review of clinical trial results con
A systematic search was conducted across major medical databases [PubMed, Embase, Reference Citation Analysis (RCA), and Cochrane Library] up to October 14, 2023. The search strategy utilized relevant Medical Subject Heading (MeSH) terms and keywords related to "spinal cord", "injury", "clinical trials", "stem cells", "functional outcomes", and "adverse events". Studies included in this review consisted of randomized controlled trials and non-randomized controlled trials reporting on the use of stem cell therapies for the treatment of SCI.
In a comprehensive review of 66 studies on stem cell therapies for SCI, 496 papers were initially identified, with 237 chosen for full-text analysis. Among them, 236 were deemed eligible after excluding 170 for various reasons. These studies encompassed 1086 patients with varying SCI levels, with cervical injuries being the most common (42.2%). Bone marrow stem cells were the predominant stem cell type used (71.1%), with various administration methods. Follow-up durations averaged around 84.4 months. The 32.7% of patients showed functional impro
In the realm of SCI treatment, stem cell-based therapies show promise, but clinical trials reveal potential adverse events and limitations, underscoring the need for meticulous optimization of transplantation conditions and parameters, caution against swift clinical implementation, a deeper understanding of SCI pathophysiology, and addressing ethical, tumorigenicity, immunogenicity, and immunotoxicity concerns before gradual and careful adoption in clinical practice.
Core Tip: In the context of spinal cord injury (SCI) treatment, stem cell-based therapies exhibit promise, as demonstrated in this systematic review of 66 studies. However, the research reveals potential adverse events and limitations, emphasizing the importance of optimizing transplantation conditions, cautious clinical implementation, a deeper understanding of SCI pathophysiology, and addressing ethical, tumorigenicity, immunogenicity, and immunotoxicity concerns before a gradual and careful adoption of stem cell therapy in clinical practice. This underscores the need for further research to ensure the safety and effectiveness of these therapies for SCI patients, while acknowledging their potential for improving functional outcomes.
- Citation: Agosti E, Zeppieri M, Pagnoni A, Fontanella MM, Fiorindi A, Ius T, Panciani PP. Current status and future perspectives on stem cell transplantation for spinal cord injury. World J Transplant 2024; 14(1): 89674
- URL: https://www.wjgnet.com/2220-3230/full/v14/i1/89674.htm
- DOI: https://dx.doi.org/10.5500/wjt.v14.i1.89674
Each year, approximately half a million fresh cases of spinal cord injury (SCI) emerge on a global scale. These instances are predominantly triggered by trauma stemming from car accidents, slips, firearm incidents, or medical/surgical complications. Given the nature of these causative factors, SCI primarily affects younger individuals[1].
The intricate and time-sensitive pathophysiology of SCI renders the exploration of therapeutic targets exceedingly challenging. Following the initial mechanical injury, a cascade of secondary events exacerbates patients' conditions. These events include the inflammatory response, gliosis hyperplasia, the creation of inhibitory environments, and the formation of scars, all of which hinder axonal regeneration and limit the effectiveness of various treatment approaches[2]. These pathophysiological consequences often lead to enduring neurological impairments, including the loss of motor and sensory functions below the injury level, as well as autonomic dysfunction[3].
Present-day clinical approaches prioritize prompt surgical decompression and mechanical stabilization at the location of SCI, bolstered by pharmaceutical measures encompassing methylprednisolone, nimodipine, naloxone, and various others. Subsequent to this crucial stage, patients engage in rehabilitative initiatives geared towards reinstating func
In recent decades, stem cell therapy has emerged as a highly promising avenue within the realm of SCI. After a series of encouraging experimental treatments using diverse stem cell types in animals of various species, clinical trials invo
While prior evaluations of stem cell therapy for SCI have occurred, they have encountered specific challenges and restrictions. Most current investigations consist of single-arm, early-phase clinical trials primarily aimed at gauging the safety of stem cell treatments. In contrast, established systematic appraisals have exclusively featured randomized controlled trials, concentrating solely on the effectiveness of stem cells. Consequently, they have encompassed a limited range of studies and do not provide a comprehensive scrutiny of available data. Furthermore, they overlook critical facets such as the safety and feasibility of translating stem cell therapy from laboratory research to clinical application. Con
This review, in turn, delves into the pathophysiological intricacies of SCI, exploring the potential mechanisms through which various stem cells contribute to the restoration of the spinal cord, and it presents the fundamental characteristics and results of the pertinent clinical trials published.
The systematic review was performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines[8]. Two authors (E.A. and A.P.) performed a systematically comprehensive literature search of the databases PubMed, Web of Science, Cochrane, Embase databases, and Reference Citation Analysis (RCA) (https://www.referencecitationanalysis.com). The first literature search was performed on August 30, 2023, and the search was updated on October 14, 2023. A combination of keyword searches was performed to generate a search strategy. The search keywords, including "spinal cord", "injury", "clinical trials", "stem cells", "functional outcomes", and "adverse events", were used in both AND and OR combinations. Studies were retrieved using the following Medical Subject Heading (MeSH) terms and Boolean operators: ("spinal injury" OR "spinal cord injury") AND ("stem cells" OR "staminal cells") AND ("clinical trials" OR "clinical studies"). Other pertinent articles were identified through reference analysis of selected papers. A search filter was set to show only publications over the designated period, 2010–2023.
The studies were chosen according to the below inclusion criteria: (1) The use of English; (2) clinical trials, such as randomized controlled or non-randomized controlled trials, single-arm or double-arm studies; (3) research on the use of stem cells to treat spinal cord injuries; and (4) research with adverse occurrences or functional results. The subsequent criteria for exclusion were utilized: (1) Publications such as editorials, case reports, case series, cohort studies, literature reviews, and meta-analyses; (2) research with vague methodology and/or findings; (3) research that omits information on adverse occurrences or functional results; (4) study that has been published several times; (5) the complete text is not available; and (6) patients with various significant conditions are included. Duplicates were eliminated from the list of recognized studies before importing it into Endnote X9. E.A. and P.P.P., two independent researchers, examined the data in accordance with the inclusion and exclusion criteria. All differences were settled by M.Z., the third reviewer. After that, full-text screening was applied to the qualifying articles.
We extracted the following data for each study: Authors, year, stage of the clinical trial, number of patients, degree of damage, neurological status prior to treatment, type and origin of stem cells, dosage and mode of administration, dura
Our primary outcomes were: (1) Clinical improvement, evaluated by the American Spinal Cord Injury Association Impairment Scale (ASIA) improvement scale (AIS) (Table 1), or, if not available, with other spinal cord injury scales or reported descriptive clinical data; and (2) adverse events (AEs) pertaining to many systems such as the cardiovascular, neurological, digestive, and musculoskeletal systems.
| A = Complete | No sensory or motor function is preserved in the sacral segments S4–S5 |
| B = Sensory incomplete | Sensory but not motor function is preserved below the neurological level and includes the sacral segments S4-S5 (light touch or pin-prick at S4–S5 or deep anal pressure) AND no motor function is preserved more than three levels below the motor level on either side of the body |
| C = Motor incomplete | Motor function is preserved below the neurological level AND more than half of the key muscle functions below the neurological level of injury have a muscle grade less than 3 (grades 0–2) |
| D = Motor incomplete | Motor function is preserved below the neurological level AND at least half (half or more) of the key muscle functions below the neurological level of injury have a muscle grade ≥ 3 |
| E = Normal | If sensation and motor function as tested with the ISNCSCI are graded as normal in all segments AND the patient has prior deficits, then the AIS grade is E. Someone without an initial SCI does not receive an AIS grade |
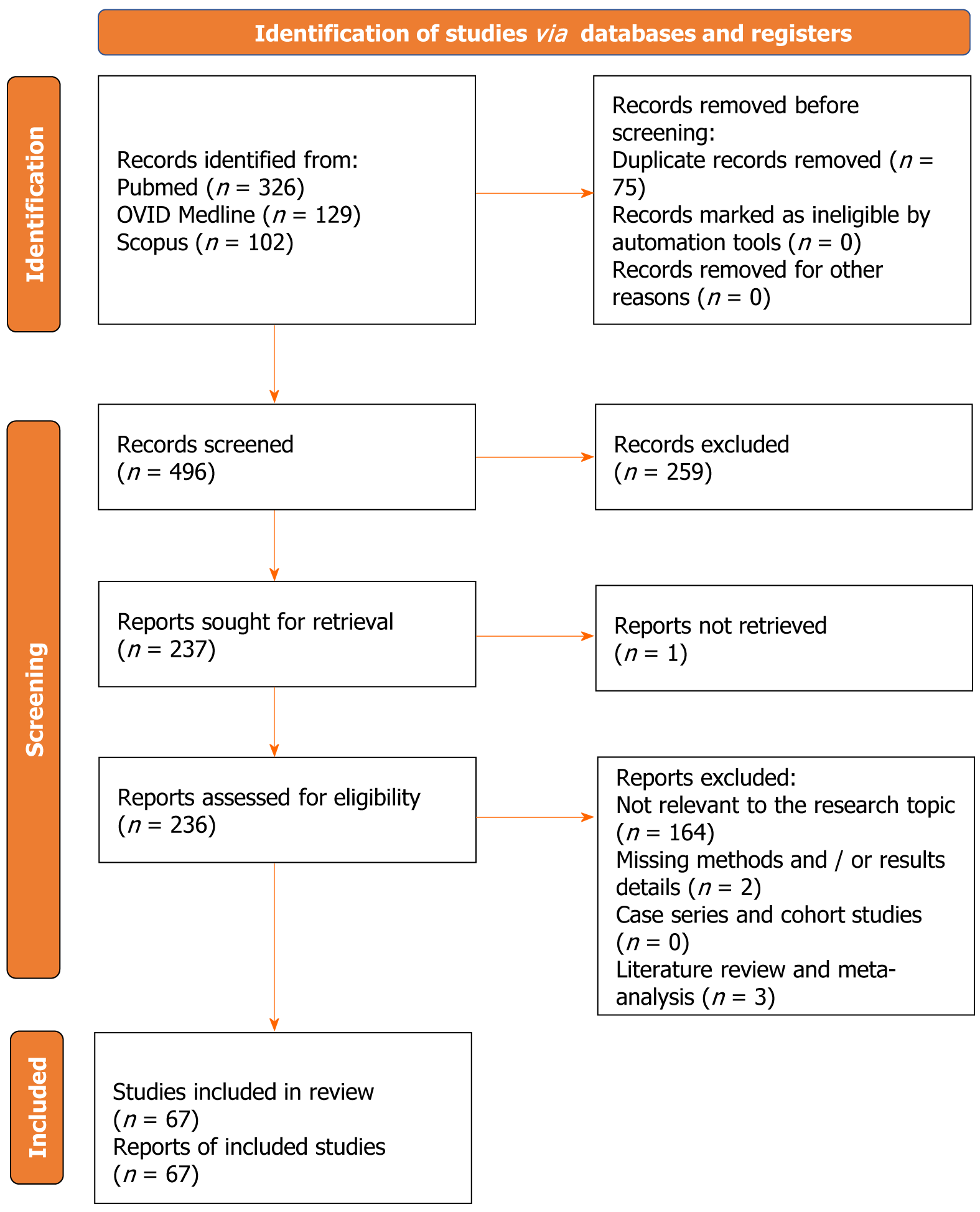
The quality of the included studies was evaluated using the Newcastle-Ottawa Scale[9]. By evaluating the study's comparability, outcome evaluation, and selection criteria, quality assessment was carried out. Nine was the optimal score. Better study quality was reflected by higher ratings. Research that scored seven or above were deemed to be of excellent quality. Independently, E.A. and P.P.P. conducted the quality evaluation. The third author reexamined publications when inconsistencies emerged (Figure 1).
Ranges and percentages were included in the descriptive statistics that were provided. The R statistical software, version 3.4.1, was used for all statistical analyses (http://www.r-project.org).
After duplicates were eliminated, 496 papers in total were found. 237 articles were found for full-text analysis after title and abstract analysis. It was determined who was eligible for 236 articles. The following criteria led to the exclusion of the remaining 169 articles: (1) Unrelated to the study topic (164 articles); (2) lacking methodological and/or outcome information (2 articles); and (3) a systematic review or meta-analysis of the literature (3 articles). For each of the patient groups under consideration, at least one or more outcome measures were available for all of the studies that were part of the analysis. The PRISMA statement's flow chart is depicted in Figure 2. The PRISMA checklist is offered as additional content.
This table presents data from a comprehensive collection of 67 studies that explored the use of stem cell therapies for spinal cord injuries. In total, these studies encompassed 1086 patients with varying injury levels. Cervical injuries were the most prevalent (42.2%), followed by thoracic injuries (32.3%), and lumbar injuries (8.6%). The specific stem cell types used varied across the studies, with bone marrow stem cells (BMSC) being the most common (71.1%), followed by umbilical cord tissue stem cells (UCMSC) in 16%, and others. The treatment approaches included intrathecal administration (61.3%), intramedullary (29.3%), and intravenous or intravenous plus intralesional methods (9.7%).
The follow-up periods for these studies ranged from acute to chronic stages, with an average follow-up duration of approximately 84.4 mo. The outcomes of these treatments were generally positive, with 32.7% of patients showing func
| Ref. | Phase of clinical trial | Patients (n) | Localization of injury | Pre-treatment AIS classification or level of injury | Stem cells | Treatment | Follow up (months) | Outcomes | ||||
| Origin | Type | Dose | Administration route | Time from Injury | Functional improvement | Adverse effects | ||||||
| Park et al[37], 2005 | N/A | 6 | Cervical | AIS A | Autologous (iliac bone marrow) | BMSC | 1.98 × 1010 | Intralesional | N/A | 6-18 | AIS A→C 4, AIS A→B: 1, AIS A=A: 1 | No serious adverse effects |
| Sykova et al[11], 2006 | N/A | 20 | Cervical and thoracic | AIS A: 15; AIS B: 4; AIS C: 1 | Autologous (iliac bone marrow) | BMSC | 104.0 ± 55.3 × 108 | Intravenous + Intraarterial | Subacute or chronic | 24 | AIS A→B: 1, AIS B→D: 1, AIS=: 15 | No serious adverse effects |
| Chernykh et al[12], 2007 | N/A | 18 | Cervical, Thoracic, Lumbar | N/A | Autologous (iliac bone marrow) | BMSC | N/A | Intralesional+ Intravenous | Chronic | 9.4 ± 4.6 | ASIA scale: significant increase in total sensitivity and motor activity score | No serious adverse effects |
| Yoon et al[13], 2007 | I/II | 35 | Cervical (4) and thoracic (4) | N/A | Autologous iliac bone marrow | BMSC | 1 × 108 | Intralesional | Intermediate | 10.4 | AIS grade increased in 30.4% of the acute and subacute treated patients (AIS A→B or A→C) | No serious adverse effects |
| Geffner et al[14], 2008 | N/A | 8 | Thoracic | AIS A: 5, AIS B: 1, AIS C: 2 | Autologous iliac bone marrow | BMSC | 1.2 × 106/kg | Intrathecal | 4 acute and 4 chronic (average 114 months) | 24 | AIS A→C: 4, AIS B→C: 1, AIS C→D: 1 AIS =: 2 | No serious adverse effects |
| Adel et al[38], 2009 | N/A | 43 | Cervical and thoracic | AIS A: 40, AIS C: 3 | Autologous iliac bone marrow | BMSC | 5-10 × 106 | Intrathecal | Chronic (average 43.2 months) | 6 | AIS A→B: 11; AIS A→C: 1; AIS B→C: 3; AIS =: 28 | ADEM: 1/43; Marked increased spasticity: 4/43; Neuropathic pain: 24/43 |
| Kumar et al[39], 2009 | I/II | 297 | N/A | AIS A: 249, AIS B: 12, AIS C: 34, AIS D: 2 | Autologous iliac bone marrow | BMSC | N/A | Intrathecal | N/A | 18.4-20.5 | 32.7% of the ASIA-classified patients showed improvement, in sensory and motor scale | No serious adverse effects. Mild-to-moderate neuropathic pain in few patients |
| Pal et al[40], 2009 | N/A | 30 | Cervical and thoracic | AIS A: 24, AIS C: 6 | Autologous iliac bone marrow | BMSC | 1 × 106/kg | Intrathecal | < 6 months: 20, > 6 months: 30 | 12-36 | No changes in the ASIA scale, SSEP, MEP and NCV | No serious adverse effects. Neuropathic pain in two patients |
| Abdelaziz et al[41], 2010 | N/A | 20 | Thoracic | AIS A: 10, AIS B: 5, AIS C: 5 | Autologous iliac bone marrow | BMSC | 5 × 106/kg | Intrathecal + Intralesional | Chronic (> 6 months) | 12 | AIS A→B: 1, AIS A→C: 2, AIS B→C: 3; AIS=: 14 | No serious adverse effects.Headache (12) and fever (3) |
| Bhanot et al[30], 2011 | N/A | 13 | Cervical and thoracic | AIS A | Autologous | BMSC | 3-6-8 × 106/kg | Intrathecal | Intermediate and chronic (3-132 months, average 28) | 6-38 | AIS A→B: 1, Patchy improvement in sensations below the injured level: 2, Patient subjectively felt improved sense of bladder filling: 1 | No serious adverse effects. Transient increase in spasticity in the lower limbs (50%) |
| Park et al[35], 2012 | N/A | 10 | Cervical | AIS A: 4, AIS B: 6 | Autologous iliac bone marrow | BMSC | 8 × 106 (intralesional) + 4 × 107 (subdural) | Intralesional + Subdural | > 1 months | 6-62 | Improvements in ADL, SSEP, MEP (3/10, all AIS B) | No serious adverse effects |
| Karamouzian et al[18], 2012 | I/II | 11 | Thoracic | AIS A | Autologous iliac bone marrow | BMSC | 0.7-1.2 × 106 | Intrathecal | Acute and intermediate/chronic (max 1.5 months) | 12-33 | AIS A→C: 5, AIS=: 0 | No serious adverse effects |
| Dai et al[28], 2013 | N/A | 20 | Cervical | AIS A, ASIA score: 31.6 ± 9.82 | Autologous iliac bone marrow | BMSC | 2 × 107 | Intralesional | Chronic (51.9 ± 18.3) | 6 | AIS A→B: 9, ASIA score: 43.1 ± 19.32 | No serious adverse effects. Fever (2), Headache and dizziness (1), pain and numbness in spinal cord dominant area (2) |
| Jiang et al[19], 2013 | N/A | 20 | Cervical (4), thoracic (11) and lumbar (5) | AIS A: 8, AIS B: 4, AIS C: 8 | Autologous iliac bone marrow | BMSC | 1 × 108 | Intrathecal | Intermediate and chronic (3-120 months) | 1 | AIS A→B: 3, AIS A→C: 1, →AIS C→D: 8 | No serious adverse effects. Fever and headache |
| Yazdani et al[42], 2013 | I | 8 | Cervical (1) and thoracic (7) | AIS A | Autologous iliac bone marrow | BMSC | 1 × 106 | Intralesional | Chronic (13-63 months) | 26-43 | Although some improvement in light touch and pinprick sensation was observed, no improvement in ASIA classification was seen | No serious adverse effects |
| Amr et al[43], 2014 | N/A | 14 | Thoracic | AIS A | Autologous iliac bone marrow | BMSC | N/A | Scaffold | Intermediate and chronic (5-84 months, average 23 months) | 24 | AIS A→B: 2, AIS A→C: 12 | Haematoma formation (2), Seroma formation (2) |
| Suzuki et al[44], 2014 | N/A | 10 | Cervical and thoracic | AIS A: 5, AIS B:5 | Autologous iliac bone marrow | BMSC | 2.03-8.44 × 108 | Intrathecal | Intermediate and chronic (3 wk-12 months) | 6 | AIS A→B: 1, AIS B→C: 2, AIS B→D: 1; AIS=: 6 | No serious adverse effects. Transient anemia after aspiration of bone-marrow cells (2) |
| Goni et al[45], 2014 | N/A | 9 | Thoracic | AIS A | Autologous iliac bone marrow | BMSC | N/A | Intrathecal | Chronic | 24 | No significant difference in the ASIA score. Statistically significant differences in the Functional Independence Measure and Modified Ashworth Scale | No serious adverse effects. Postoperative temporary neuropathic pain (2) |
| El-kheir et al[10], 2014 | I/II | 50 | Cervical (10) and thoracic (40) | AIS A: 15, AIS B: 35 | Autologous iliac bone marrow | BMSC | 2 × 106/kg | Intrathecal | Chronic (12-36 months, average 18.3 ± 5) | 18 | AIS A→B: 12, AIS A→C: 4, AIS B→C: 18; AIS=: 16 | Temporary mild side effects: Headache, neuropathic pain (30%). No long-term side effects |
| Mendonca et al[46], 2014 | I | 14 | Thoracic and lumbar | AIS A | Autologous iliac bone marrow | BMSC | 5 × 106 | Intralesional | Chronic (18-180 months) | 6 | AIS A→B: 6, AIS A→C: 1; AIS=: 5; Improvements in urologic function (9) and changes in SSEP (1) | One subject developed a postoperatory complication, evolving a cerebrospinal fluid leak that was treated by an additional surgical procedure |
| Shin et al[47], 2015 | I/IIa | 19 | Cervical | AIS A: 17, AIS B: 2 | Human fetal brain | NSC | 1 × 108 | Intralesional | Acute and intermediate | 12 | AIS A→C: 2, AIS A→B: 1, AIS B→D: 2; AIS=: 14. Positive response in SSEP (35.3%) and MEP (58.8%) activities of AIS-A patients below the level of injury | No serious adverse effects |
| Chhabra et al[48], 2016 | I/II | 7 | Thoracic | AIS A, ISCIS total score: 162.6 ± 3.1 | Autologous iliac bone marrow | BMSC | 3.6 × 108 | Intrathecal | Acute | 12 | ISCIS total score: 134.9 ± 2.5 | Liver abscess (1) |
| Oraee-Yazdani et al[49], 2016 | I | 6 | Cervical (1) and thoracic (5) | AIS A | Autologous iliac bone marrow | BMSC | 2 × 106 | Intrathecal | Chronic (38.1 ± 15.3 months average) | 25-36 | AIS A→B: 1. Improvement in sensory level (2), improvement in UDS, especially bladder compliance (1) | No serious adverse effects |
| Oh et al[32], 2016 | III | 16 | Cervical | AIS B | Autologous iliac bone marrow | BMSC | 4.8 × 107 | Subdural | Chronic (24-181 months) | 6 | SEP improvement (4), MEP improvement (6), improvement in motor grade (2) | No serious adverse effects. 8 patients developed mild adverse effects (muscle rigidity, worsened symptoms of tingling sense) |
| Thakkar et al[33], 2016 | N/A | 10 | Thoracic and lumbar | AIS A | Autologous bone marrow + abdominal adipose tissue | BMSC | 1.82 × 108 | Intrathecal | Chronic (30-64.8 months) | 34 | AIS A→B: 6, AIS A→C: 3, AIS A→D: 1 | No serious adverse effects |
| Vaquero et al[27], 2016 | I/II | 12 | Thoracic | AIS A, ASIA score: 165.92 ± 22.83 | Autologous bone marrow | BMSC | 100 × 106 - 230 × 106 | Intralesional | Chronic (38.0-321 months, average 166.3) | 12 | AIS→B: 3, AIS A→C: 1, ASIA score: 213.25 ± 37.19 | 22 adverse events of minor (79.1%) or moderate (20.9%) intensity. |
| Kakabadze et al[25], 2016 | I | 18 | Cervical and thoracic | AIS A: 10, AIS B: 5, AIS C: 3 | Autologous iliac bone marrow | BMSC | 405-964 × 106 | Intrathecal | Intermediate and chronic (max 20 months) | 12 | ASIA scale improvement by one grade: 7/9 (78%) Improvement by two grades: 2/9 (22%) | No serious adverse effects. Transient fever and headache |
| Xiao et al[50], 2016 | N/A | 5 | Cervical (1) and thoracic (4) | AIS A | Autologous iliac bone marrow | BMSC | 1 × 109 | Scaffold | Intermediate and chronic (max 32 months) | 12 | AIS A No improvement also in MEP and SSEP | No serious adverse effects. |
| Chhabra et al[51], 2017 | I/II | 7 | Thoracic | AIS A, ISCIS total score: 172.2 ± 2.3 | Autologous iliac bone marrow | BMSC | 2 × 108 | Intralesional | Acute | 12 | ISCIS total score: 141.7 ± 2.5 | Liver abscess (1) |
| Vaquero et al[52], 2017 | II | 10 | Cervical, thoracic and lumbar | AIS B: 5, AIS C: 5, ASIA total score: 118.2 ±60 | Autologous | BMSC | 30 × 106 × 4 doses | Intratechal | Chronic (29.2-415.1 months, mean 170.5 ± 118.6) | 12 | ASIA total score: 235.5 ± 49.35. Motor and sensory scores, bladder, bowel and sexual functions improved. Spasms (2) and neuropathic pain (2) improved | No serious adverse effects. Transient headache and pain in the area of the lumbar puncture |
| Larocca et al[21], 2017 | I/II | 5 | Thoracic | AIS A | Autologous iliac bone marrow | BMSC | 2 × 107 | Subcutaneous | Chronic (25-111 months) | 6 | AIS A→B: 1, AIS A=: 5; One patient improved AIS A→B but reversed at 6 months. Improvements in SCIM III and FIM scale scores | No serious adverse effects |
| Vaquero et al[20], 2018 | II | 11 | Cervical (4), thoracic (4) and lumbar (3) | AIS A: 3, AIS B: 4, AIS C: 3, AIS D: 1 | Autologous | BMSC | 100 × 106 × 3 doses | Intrathecal | Chronic (mean 163.8 ± 177.5 months) | 10 | AIS improvement in 27% of patients. AIS A→B: 1, AIS B→C: 1; AIS C→D: 1 | No serious adverse effects. Transitory sciatic pain (37.5%), headaches and pain in the area of lumbar puncture |
| Guadalajara et al[53], 2018 | Case report | 1 | Thoracic | AIS A | Autologous iliac bone marrow | BMSC | 300 × 106 × 3 doses (1/months) | Intrathecal | Chronic | 6 | Improvement in functionality and especially in Krogh's; Neurogenic Bowel Dysfunction scale | No serious adverse effects |
| Srivastava et al[54], 2019 | I | 70 | Thoracic and lumbar | AIS A | Autologous iliac bone marrow | BMSC | 2,41 ± 1,198 × 106 | Intrathecal | Acute and intermediate | 12 | AIS A→B: 21, AIS A→C: 29, AIS A→D: 5; AIS=: 15 | No serious adverse effects |
| Phedy et al[55], 2019 | Case report | 1 | Thoracic | AIS A | Autologous iliac bone marrow | BMSC | 10 − 17 × 106 (× 7 times) | Intrathecal ×1 + Intravenous ×6 | Chronic | 60 | AIS A→C. Increase in AIS score: 10→30. Increase in MRC score for L1 and L2 innervated muscles: 0/5→3/5 | No serious adverse effects |
| Chen et al[56], 2020 | I | 7 | Thoracic | AIS A | Autologous iliac bone marrow | BMSC | > 1 × 109 | Scaffold | Acute or intermediate | 36 | All patients showed significant improvements in the FIM and ADL score. No obvious improvement in the ASIA grade, ASIA motor score, motor function, SSEPs, or MEPs was observed | Stress ulcer and lung infection (1), transient hyperthermia (1), shallow wound (1), spasm (4), paraplegic neuralgia (3), pressure ulcers (1), and lower limb amyotrophy (1) |
| Sharma et al[57], 2020 | N/A | 180 | Cervical (63), thoracic and lumbar (117) | AIS A: 138, AIS B: 28, AIS C: 10, AIS D: 3 | Autologous iliac bone marrow | BMSC | 1.06 × 108 | Intrathecal | Intermediate or chronic | 2-16 | FIM and WISCI showed statistically significant improvement | No serious adverse effects |
| Song et al[58], 2020 | N/A | 18 | Cervical, thoracic and lumbar | ASIA score: 59.75 ± 5.22, SCIM-III score: 40.83 ± 6.58 | Autologous iliac bone marrow | BMSC | 1 × 107 | Intrathecal | N/A | 12 | ASIA score: 81.1 ± 3.8, SCIM-III score: 72.5 ± 4.3 | No serious adverse effects |
| Oraee-Yazdani et al[36], 2021 | I/II | 6 | Cervical (1) and thoracic (5) | AIS A, SCIM III score: 28.9 ± 13 | Autologous iliac bone marrow | BMSC | 1 × 106 | Intrathecal | Chronic (max 12 months) | 30 | SCIM III score: 43.1 ± 25.8. Sensory and/or motor improvement was evident in 9 patients according to the AIS assessment | Mild adverse effects: Increase in spasticity, numbness, or tingling sensation, and neuropathic pain |
| Honmou et al[59], 2021 | II | 13 | Cervical | AIS A: 6, AIS B: 2, AIS C: 5 | Autologous | BMSC (auto-serum expanded) | 84−150 × 106 | Intravenous | Subacute | 6 | AIS A→B (3/6 patients), A→C (2/6), B→C (1/2), B→D (1/2), C→D (5/5) | No serious adverse effects |
| Ref. | Phase of clinical trial | Patients (n) | Localization of injury | Pre-treatment AIS classification or level of injury | Stem cells | Treatment | Follow up (months) | Outcomes | ||||
| Origin | Type | Dose | Administration route | Time from Injury | Functional improvement | Adverse effects | ||||||
| Deda et al[60], 2008 | N/A | 9 | Cervical (6) and thoracic (3) | AIS A: 9 | Autologous peripheral blood | HSC | 5 × 106 | Intrathecal | Chronic (6-51 months) | 12 | AIS A→B: 2, AIS A→C: 7 | No serious adverse effects |
| Hammadi et al[61], 2012 | N/A | 277 | Cervical (69) and thoracic (208) | N/A | Autologous peripheral blood | HSC | 1-8 × 108 | Intrathecal | Chronic (6-104 months, average 34.5) | 24 | AIS A→B: 88, AIS A→C: 32, AIS = 157. A subgroup (12 patients) with lesion < 12 months had the best outcome: the percentage improvement reached 50% | No serious adverse effects. Backache and meningism (90%) |
| Al-Zoubi et al[62], 2014 | N/A | 19 | Thoracic | AIS A | Autologous peripheral blood | HSC | 7.6 × 107 | Intrathecal | Chronic (12-48 months) | 60 | AIS A→B: 7. AIS A→C: 2, AIS =: 10 | No serious adverse effects |
| Bryukhovetskiy et al[63], 2015 | I/II | 202 | Cervical (98), thoracic (93) and lumbar (11) | N/A | Autologous peripheral blood | HSC | 5.8 × 106 | Intrathecal | Chronic (> 12 months) | 144 | Restoration of neurologic deficit (54.7%); Repair of the urinary system (47.7%). ASIA score improvement in 23 cases | No serious adverse effects |
| Ref. | Phase of clinical trial | Patients (n) | Localization of injury | Pre-treatment AIS classification or level of injury | Stem cells | Treatment | Follow up (months) | Outcomes | ||||
| Origin | Type | Dose | Administration route | Time from injury | Functional improvement | Adverse effects | ||||||
| Hur et al[26], 2016 | I | 14 | Cervical (6), thoracic (7) and lumbar (1) | AIS A: 12, AIS B: 1, AIS D: 1 | Autologous subcutaneous fat | ADMSC | 9 × 107 | Intrathecal | Intermediate and chronic (max 28 months) | 8 | Improvements in ASIA motor scores (5), voluntary anal contraction (2), ASIA sensory score (10), although degeneration was seen in 1. SSEP median nerve improvement (1) | No serious adverse effects. Transient headache, nausea and vomiting |
| Tien et al[64], 2019 | N/A | 31 | Thoracic | AIS A, Barthel ADL: 3.35 ± 1.35 | Autologous adipose tissue | ADMSC | > 1 × 108 | Intrathecal | Acute | 12 | AIS A→B: 10, AIS A→C: 1, AIS A→D: 2; AIS =: 16 Barthel ADL: 6.48 ± 2.14 | No serious adverse effects |
| Ref. | Phase of clinical trial | Patients (n) | Localization of injury | Pre-treatment AIS classification or level of injury | Stem cells | Treatment | Follow up (months) | Outcomes | ||||
| Origin | Type | Dose | Administration route | Time from injury | Functional improvement | Adverse effects | ||||||
| Shin et al[47], 2015 | I/IIa | 19 | Cervical | AIS A: 17, AIS B: 2 | Human fetal brain | NSC | 1 × 108 | Intralesional | Acute and intermediate | 12 | AIS A→C: 2, AIS A→B: 1, AIS B→D: 2; AIS=: 14. Positive response in SSEP (35.3%) and MEP (58.8%) activities of AIS-A patients below the level of injury | No serious adverse effects |
| Ghobrial et al[65], 2017 | II | 5 | Cervical | AIS A: 1, AIS B: 4 | Allogeneic fetus | huCNSSC® | 15-40 × 106 | Intrathecal | Chronic | 12 | AIS A→B: 1, AIS B→A: 1, AIS=: 3, GRASSP score mean improvement: 14.8 ± 7.8, ISNCSCI score mean improvement: 17.3 ± 16.8 | No serious adverse effects |
| Anderson et al[66], 2017 | I | 6 | Thoracic | N/A | Autologous (sural nerve) | SC | 5, 10 or 15 × 106 | Intramedullary | Subacute | 12 | AIS A→B: 1. Improvement in FIM and SCIM III scores | No serious adverse effects |
| Levi et al[67], 2018 | I/II | 29 | Cervical: 17 (Cohort I: 6, Cohort II: 11) Thoracic: 12 | AIS A: 11, AIS B: 18 | Allogeneic (Stemcells Inc.) | huCNSSC® | 15 − 40 × 106 | Intramedullary | Subacute | Up to 56 | Improvement in AIS motor scores | 15 serious adverse effects in cervical group and 4 in thoracic |
| Curtis et al[68], 2018 | I | 4 | Thoracic | AIS A | Allogeneic (human-spinal-cord-derived neural stem cell) | NSI-566® | 6 injections (Mean number) | Intramedullary | Chronic | 60 | Improved AIS scores, neurological levels and EMG findings. No improvement in QoL | No serious adverse effects |
| Levi et al[69], 2019 | I/II | 17 Cohort I: 6, Cohort II: 11 6/11 monitored | Cervical | AIS A, B | Allogeneic (Stemcells Inc.) | huCNSSC® | 15 + 30 + 40 × 106 (Coh.I) 40 × 106 (Coh.II) | Intramedullary | Intermediate or Chronic (max 24 months) | 12 | Improvement in UEMS score | No serious adverse effects |
| Curt et al[70], 2020 | I/IIa | 12 | Thoracic | AIS A: 7, AIS B: 5 | Allogeneic (Stemcells Inc.) | huCNSSC® | 20 × 106 | Intramedullary | Intermediate or chronic (max 24 months) | 72 | Sensory improvements in 5 out of 12 patients. No motor improvements were observed | N No serious adverse effects |
| Zamani et al[71], 2021 | I | 3 | Thoracic | AIS A | Autologous | OEC+ BMSC | 15 × 106, OEC/BMSC = 1/1 | Intrathecal | Chronic | 24 | AIS A→B: 1 and 6 points improvement in SCIM | Mild adverse effects |
| Gant et al[72], 2022 | I | 8 | Cervical: 4; Thoracic: 4 | N/A | Autologous (sural nerve) | SC | 50 − 200 × 106 | Intramedullary | Chronic | 60 | The neurological level improved by 1 level in 1 patient. Improvement in Sensory score in all patients with thoracic and in 2 patients with cervical lesion | No serious adverse effects |
| Ref. | Phase of clinical trial | Patients (n) | Localization of injury | Pre-treatment AIS classification or level of injury | Stem cells | Treatment | Follow up (months) | Outcomes | ||||
| Origin | Type | Dose | Administration route | Time from injury | Functional improvement | Adverse effects | ||||||
| Dai et al[29], 2013 | N/A | 18 | Cervical and thoracic | AIS A: 12, AIS B: 4, AIS C: 2 | Allogeneic neonatal umbilical cord tissue | UCMSC | 4 × 107 | Intralesional | Chronic (18.67 ± 7.6 months) | 6 | AIS A→B: 7, AIS B→C: 3, AIS=: 8; MEP improvements | No serious adverse effects |
| Liu et al[73], 2013 | N/A | 22 | Cervical (4), cervical + thoracic (2), thoracic + lumbar (2) and lumbar (7) | Motor function: 58.1 ± 22.2. Algesia: 73.2 ± 25.1. Sensory function: 74.2 ± 26.7. ADL: 29.5 ± 12.5 | Allogeneic neonatal umbilical cord tissue | UCMSC | 4 × 106/kg | Intrathecal | Intermediate and chronic (2-204 months) | > 12 | Motor function: 61.5 ± 23.9. Algesia: 77.2 ± 26.1. Sensory function: 77.3 ± 26.1. ADL: 32.7 ± 12.4 | Fever, lumbago, headache, dizziness and other adverse reactions were observed |
| Cheng et al[74], 2014 | N/A | 10 | Thoracic and lumbar | AIS A, Barthel Index: 33.50 ± 6.69 | Allogeneic neonatal umbilical cord tissue | UCMSC | 4 × 107 | Intralesional | Chronic (12-72 months) | 6 | Barthel Index: 41.40 ± 6.42; Muscle strength increased. Muscle tension decreased. Increase in maximum bladder capacity and decrease in maximum detrusor pressure | No serious adverse effects |
| Shroff et al[34], 2016 | N/A | 226 | Cervical and thoracic | AIS A: 153, AIS B: 32, AIS C: 36, AIS D: 5 | Pre-implantation stage fertilized ovum | HESC | 1.6 × 107 + 1-5 × 1.6 × 107 | Intravenous + intralesional | Intermediate and chronic | 6-18 | AIS A: 98, AIS B: 67, AIS C: 126, AIS D: 9, AIS E: 3 | No serious adverse effects. Transient fever and headache |
| Shroff et al[75], 2017 | N/A | 15 | Cervical and thoracic | AIS A: 13, AIS B: 2 | Pre-implantation stage fertilized ovum taken during natural IVF process | HESC | 1.6 × 107 + 1-5 × 1,6 × 107 | Intravenous + intralesional | Acute, intermediate and chronic (6-15 months) | 9 | AIS A: 10, AIS B: 2, AIS C: 3 | No serious adverse effects |
| Zhao et al[76], 2017 | N/A | 8 | Cervical (4) and thoracic (4) | AIS A | Allogeneic neonatal umbilical cord tissue | UCMSC | 4 × 107 | Scaffold | Intermediate and chronic (max 36 months) | 12 | Expansion of sensation level (62.5%) and expansion of the MEP-responsive area (87.5%) but AIS= | No serious adverse effects |
| Xiao et al[77], 2018 | I | 2 | Cervical and thoracic | AIS A | Allogeneic | UCMSC+ Scaffold | 40 × 106 | Intramedullary | Acute | 12 | AIS A→C in both patients | No serious adverse effects |
| Deng et al[72], 2020 | I | 20 | Cervical | AIS A | Allogeneic | UCMSC+ Scaffold | 40 × 106 (Collagen scaffold) | Intramedullary | Acute | 12 | AIS A→B (9 patients), AIS A→C (2 patients). Improvement in ADL scores. Improvement in bowel and bladder function | No serious adverse effects |
| Albu et al[31], 2021 | I/IIa | 10 | Thoracic | AIS A | Allogeneic | WJ-MSC | 10 × 106 | Intrathecal | Chronic | 6 | Significant improvement in pinprick sensation in compared with placebo group. No changes in motor function, independence, QoL, SEPs, MEPs, spasticity or bowel function | No serious adverse effects |
| Yang et al[23], 2021 | I/II | 102 | Cervical, thoracic and lumbar | ASIA score: 158.15 ± 70.93, IANR-SCIFRS total score: 24.54 ± 9.82 | Allogeneic neonatal umbilical cord tissue | UCMSC | 1 × 106/kg | Intrathecal | Intermediate and chronic (max 240 months) | 12 | ASIA score: 183.88 ± 69.76, IANR-SCIFRS total score: 29.49 ± 10.47 | No serious adverse effects. Fever (14.1%), headache (4.2%), transient increase in muscle tension (1.6%) and dizziness (1.3%) |
| Zhao et al[78], 2021 | N/A | 7 | Cervical (3) and thoracic (4) | ASIA pin prick: 55.00 ± 28.46, ASIA light touch: 55.00 ± 28.46, ASIA motor score: 42.00 ± 28.19 | Allogeneic neonatal umbilical cord tissue | UCMSC | 5 × 104 | Intrathecal | Intermediate and chronic (max 60 months) | 6 | ASIA pin prick: 57.06 ± 30.01, ASIA light touch: 58.20 ± 29.36, ASIA motor score: 44.13±27.23 | No serious adverse effects |
| Smirnov et al[16], 2022 | I/IIa | 10 | Cervical, thoracic and lumbar | AIS A: 6, AIS B: 4 | Allogeneic | HUCBC | 14.8 × 106/kg (Total cell number for 4 infusions) | Intravenous | Acute | 12 | AIS A→C: 3, AIS B→D: 2, AIS B→E: 2, AIS A→D: 1 | No serious adverse effects related to therapy |
The number of clinical trials involving stem cells has significantly increased in the last few years. Thousands of registered trials claim to use stem cells in their experimental treatments across the globe[2,4,7,10]. This could imply that stem cell therapy has a strong and established track record in clinical practice. But in actuality, even with some noteworthy breakthroughs, the application of stem cells in medicine is still relatively new.12, 15 Phase I clinical trials, case series, and case reports make up the majority of stem cell clinical research conducted today[2,4,5]. Good randomized controlled trials are hard to come by, and even simple controlled trials are difficult to find. It is therefore difficult to assess the efficacy of stem cells through head-to-head comparisons using meta-analysis. Furthermore, even while differences in patient age, the degree of spinal cord injury, cell kinds, sources, culture conditions, and other variables might make inter-study comparisons more difficult, they are nevertheless essential[5,8,9,11-15].
Our review reveals a general enhancement in patient functionality, encompassing both motor and sensory per
The potential benefits of stem cell therapy for patients remain uncertain, compounded by suboptimal design and execution of clinical trials[12,22]. Rigorously conducted randomized controlled trials, featuring double-blind methodologies and placebo groups, offer the most precise and dependable data, surpassing observational studies or case reports in reliability. Nonetheless, the majority of ongoing investigations consist of observational studies, case series, and similar approaches[15,21]. Clinical trials often suffer from issues such as limited sample sizes and subpar quality[22,23]. Furthermore, a considerable portion of the studies reviewed were phase I clinical trials, typically focused on evaluating stem cell safety. Intriguingly, all of these studies primarily explored and reported on the effectiveness of stem cells while neglecting to document AEs. Consequently, the safety profile of stem cells could potentially be inaccurately elevated[17].
The utmost priority should always be the safety of patients. The safety of stem cell therapy and the occurrence of AEs primarily hinge on the inherent traits of the transplanted stem cells and the transplantation procedure[16,17]. Our review of the studies did not reveal any severe AEs, such as the formation of tumors, further reinforcing the claims of these studies regarding the safety of stem cell therapy. Nevertheless, it's crucial to recognize that the absence of serious AEs doesn't definitively establish the therapy's safety. Many AEs were documented in the 66 research that we looked at. These included effects on the neurological, musculoskeletal, digestive, and cardiovascular systems. Following the proper medical measures, the majority of these AEs were moderate, and the patients recovered well. It would be premature, nevertheless, to declare stem cell treatment safe in all cases. By doing thus, it might unintentionally encourage unjustified trust in the therapy and jeopardize the scientific assessment of its safety and efficacy. Furthermore, Aspinall et al's analysis revealed that only thirty percent of clinical trials sufficiently recorded different AEs during the clinical trial[24]. Consequently, it's plausible that a sizable percentage of studies may have failed to disclose or ignored AEs in an effort to make stem cell treatment appear safer than it actually is.
Among the myriad safety concerns associated with stem cell transplantation, the specter of tumorigenesis looms larger and more ominous than the comparatively milder fever and neuropathic pain stemming from immune or allergic reactions[17,22,23,25]. Stem cell products bear the highest potential for tumorigenesis due to the presence of lingering undifferentiated stem cells, cells carrying malignant transformations or mutations, and genetic instability[26]. Moreover, the expression of foreign genes, such as different growth factors, might result in oncogenic activation, and the danger of insertional mutagenesis in stem cells is introduced by genetically modified viral vectors, such as lentiviruses and retroviruses. It's worth noting that there exists no consensus on a global scale regarding risk assessment strategies for evaluating the tumorigenicity and oncogenicity of stem cells. Curiously, there have been no reports of severe adverse events, including tumorigenesis, in clinical trials thus far. However, this absence of reports might be attributed to the relatively brief follow-up period[16,17,24].
While preclinical studies have indeed established a solid groundwork for stem cell therapy, its translation to clinical practice has encountered significant challenges. The number of newly initiated phase I and II clinical trials experienced steady growth between 2006 and 2012 but has since shown signs of stagnation and decline as of 2018[1-4,17,27]. This trend can be attributed primarily to the underwhelming efficacy of stem cell therapy. The stark contrast between animal studies and patient outcomes is a key contributor to this disparity[28,29]. The goal of animal research is to reduce the number of experimental variables as much as possible, such as the animals' initial features and the precise location and severity of their injuries. But spinal cord injury patients are highly heterogeneous; they include differences in rehabilitation regimens, age, gender, comorbid problems, and the location and degree of the damage[10,12,17,30,31]. Conse
The advancements made in stem cell clinical trials have been nothing short of captivating. However, it's essential to note that the majority of these studies are still situated in the early phase I/II stages, with ongoing data collection[17]. At this juncture, confirming the substantial therapeutic impact of stem cells remains premature. Across various clinical trials, a multitude of disparities and uncertainties surface, spanning the selection of patients, types of cells utilized, timing of intervention, and the dosages and routes employed for stem cell transplantation[35,36]. This necessitates a closer synergy between the preclinical and clinical dimensions of research. Improving trial safety, effectiveness, and repeatability; determining ideal transplant parameters; carefully weighing the advantages and disadvantages of stem cell treatment; and strengthening oversight practices in this area are among the urgent goals[16,17].
Within the realm of SCI treatment, stem cell-based therapies exhibit substantial promise. While rodent models indisputably illustrate the efficacy of stem cells, our exhaustive analysis of clinical trials uncovers a paradox: Despite the considerable potential of stem cells in improving neurological function among SCI patients, their transplantation carries the potential for numerous AEs. Ongoing clinical trials grapple with limitations, encompassing small sample sizes, subpar quality, and the absence of control groups, which collectively hinder the conclusive establishment of stem cell therapy's safety. It is, therefore, imperative to meticulously identify the optimal conditions and parameters for stem cell transplantation to optimize therapeutic outcomes.
Our findings highlight the lack of evidence currently available to justify the broad use of stem cell treatment for spinal cord injury and strongly advise against its immediate introduction into clinical practice. A deeper understanding of the pathophysiological mechanisms at play in SCI is imperative for the creation of treatments that surpass those presently in the investigative stage. Additionally, a range of concerns, encompassing ethical considerations and the assessment of tumorigenicity, immunogenicity, and immunotoxicity associated with diverse stem cell types, demand attention and resolution. The introduction of stem cell therapy into clinical practice should advance gradually and cautiously until well-structured animal experiments and high-caliber clinical studies are executed.
Previous assessments of stem cell therapy for spinal cord injuries (SCI) have encountered challenges and constraints. Current research primarily emphasizes safety in early-phase clinical trials, while systematic reviews prioritize effectiveness, often overlooking safety and translational feasibility.
Current research primarily emphasizes safety in early-phase clinical trials, while systematic reviews prioritize effectiveness, often overlooking safety and translational feasibility.
This study seeks to offer an up-to-date systematic literature review of clinical trial results concerning stem cell therapy for SCI.
A systematic search was conducted across major medical databases.
In a comprehensive review of 66 studies on stem cell therapies for SCI, 496 papers were initially identified, with 237 chosen for full-text analysis. Among them, 236 were deemed eligible after excluding 170 for various reasons.
In the realm of SCI treatment, stem cell-based therapies show promise, but clinical trials reveal potential adverse events and limitations, underscoring the need for meticulous optimization of transplantation conditions and parameters, caution against swift clinical implementation, a deeper understanding of SCI pathophysiology, and addressing ethical, tumorigenicity, immunogenicity, and immunotoxicity concerns before gradual and careful adoption in clinical practice.
There is a need for further research to ensure the safety and effectiveness of these therapies for SCI patients, while ack
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Transplantation
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Wang G, China; Salvadori M, Italy S-Editor: Liu JH L-Editor: A P-Editor: Zhang YL
| 1. | Xia Y, Zhu J, Yang R, Wang H, Li Y, Fu C. Mesenchymal stem cells in the treatment of spinal cord injury: Mechanisms, current advances and future challenges. Front Immunol. 2023;14:1141601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 46] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 2. | Huang L, Fu C, Xiong F, He C, Wei Q. Stem Cell Therapy for Spinal Cord Injury. Cell Transplant. 2021;30:963689721989266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 89] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 3. | Sun X, Huang LY, Pan HX, Li LJ, Wang L, Pei GQ, Wang Y, Zhang Q, Cheng HX, He CQ, Wei Q. Bone marrow mesenchymal stem cells and exercise restore motor function following spinal cord injury by activating PI3K/AKT/mTOR pathway. Neural Regen Res. 2023;18:1067-1075. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 4. | Muthu S, Jeyaraman M, Gulati A, Arora A. Current evidence on mesenchymal stem cell therapy for traumatic spinal cord injury: systematic review and meta-analysis. Cytotherapy. 2021;23:186-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 5. | Montoto-Meijide R, Meijide-Faílde R, Díaz-Prado SM, Montoto-Marqués A. Mesenchymal Stem Cell Therapy in Traumatic Spinal Cord Injury: A Systematic Review. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 6. | Xu P, Yang X. The Efficacy and Safety of Mesenchymal Stem Cell Transplantation for Spinal Cord Injury Patients: A Meta-Analysis and Systematic Review. Cell Transplant. 2019;28:36-46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 7. | Fan X, Wang JZ, Lin XM, Zhang L. Stem cell transplantation for spinal cord injury: a meta-analysis of treatment effectiveness and safety. Neural Regen Res. 2017;12:815-825. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 8. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 40626] [Article Influence: 10156.5] [Reference Citation Analysis (2)] |
| 9. | Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8858] [Cited by in RCA: 12669] [Article Influence: 844.6] [Reference Citation Analysis (0)] |
| 10. | El-Kheir WA, Gabr H, Awad MR, Ghannam O, Barakat Y, Farghali HA, El Maadawi ZM, Ewes I, Sabaawy HE. Autologous bone marrow-derived cell therapy combined with physical therapy induces functional improvement in chronic spinal cord injury patients. Cell Transplant. 2014;23:729-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 112] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 11. | Syková E, Homola A, Mazanec R, Lachmann H, Konrádová SL, Kobylka P, Pádr R, Neuwirth J, Komrska V, Vávra V, Stulík J, Bojar M. Autologous bone marrow transplantation in patients with subacute and chronic spinal cord injury. Cell Transplant. 2006;15:675-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 221] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 12. | Chernykh ER, Stupak VV, Muradov GM, Sizikov MY, Shevela EY, Leplina OY, Tikhonova MA, Kulagin AD, Lisukov IA, Ostanin AA, Kozlov VA. Application of autologous bone marrow stem cells in the therapy of spinal cord injury patients. Bull Exp Biol Med. 2007;143:543-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 13. | Yoon SH, Shim YS, Park YH, Chung JK, Nam JH, Kim MO, Park HC, Park SR, Min BH, Kim EY, Choi BH, Park H, Ha Y. Complete spinal cord injury treatment using autologous bone marrow cell transplantation and bone marrow stimulation with granulocyte macrophage-colony stimulating factor: Phase I/II clinical trial. Stem Cells. 2007;25:2066-2073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 256] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 14. | Geffner LF, Santacruz P, Izurieta M, Flor L, Maldonado B, Auad AH, Montenegro X, Gonzalez R, Silva F. Administration of autologous bone marrow stem cells into spinal cord injury patients via multiple routes is safe and improves their quality of life: comprehensive case studies. Cell Transplant. 2008;17:1277-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 153] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 15. | Deng WS, Ma K, Liang B, Liu XY, Xu HY, Zhang J, Shi HY, Sun HT, Chen XY, Zhang S. Collagen scaffold combined with human umbilical cord-mesenchymal stem cells transplantation for acute complete spinal cord injury. Neural Regen Res. 2020;15:1686-1700. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 16. | Smirnov VA, Radaev SM, Morozova YV, Ryabov SI, Yadgarov MY, Bazanovich SA, Lvov IS, Talypov AE, Grin' AA. Systemic Administration of Allogeneic Cord Blood Mononuclear Cells in Adults with Severe Acute Contusion Spinal Cord Injury: Phase 1/2a Pilot Clinical Study-Safety and Primary Efficacy Evaluation. World Neurosurg. 2022;161:e319-e338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Shang Z, Wang M, Zhang B, Wang X, Wanyan P. Clinical translation of stem cell therapy for spinal cord injury still premature: results from a single-arm meta-analysis based on 62 clinical trials. BMC Med. 2022;20:284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 46] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 18. | Karamouzian S, Nematollahi-Mahani SN, Nakhaee N, Eskandary H. Clinical safety and primary efficacy of bone marrow mesenchymal cell transplantation in subacute spinal cord injured patients. Clin Neurol Neurosurg. 2012;114:935-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 129] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 19. | Jiang PC, Xiong WP, Wang G, Ma C, Yao WQ, Kendell SF, Mehling BM, Yuan XH, Wu DC. A clinical trial report of autologous bone marrow-derived mesenchymal stem cell transplantation in patients with spinal cord injury. Exp Ther Med. 2013;6:140-146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 20. | Vaquero J, Zurita M, Rico MA, Aguayo C, Bonilla C, Marin E, Tapiador N, Sevilla M, Vazquez D, Carballido J, Fernandez C, Rodriguez-Boto G, Ovejero M; Neurological Cell Therapy Group from Puerta de Hierro-Majadahonda Hospital. Intrathecal administration of autologous mesenchymal stromal cells for spinal cord injury: Safety and efficacy of the 100/3 guideline. Cytotherapy. 2018;20:806-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 91] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 21. | Larocca TF, Macêdo CT, Souza BSF, Andrade-Souza YM, Villarreal CF, Matos AC, Silva DN, da Silva KN, de Souza CLEM, Paixão DDS, Bezerra MDR, Alves RL, Soares MBP, Dos Santos RR. Image-guided percutaneous intralesional administration of mesenchymal stromal cells in subjects with chronic complete spinal cord injury: a pilot study. Cytotherapy. 2017;19:1189-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Yang Y, Pang M, Du C, Liu ZY, Chen ZH, Wang NX, Zhang LM, Chen YY, Mo J, Dong JW, Xie PG, Wang QY, Liu B, Rong LM. Repeated subarachnoid administrations of allogeneic human umbilical cord mesenchymal stem cells for spinal cord injury: a phase 1/2 pilot study. Cytotherapy. 2021;23:57-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 23. | Damianakis EI, Benetos IS, Evangelopoulos DS, Kotroni A, Vlamis J, Pneumaticos SG. Stem Cell Therapy for Spinal Cord Injury: A Review of Recent Clinical Trials. Cureus. 2022;14:e24575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Aspinall P, Harrison L, Scheuren P, Cragg JJ, Ferguson AR, Guest JD, Hsieh J, Jones L, Kirshblum S, Lammertse D, Kwon BK, Kramer JLK. A Systematic Review of Safety Reporting in Acute Spinal Cord Injury Clinical Trials: Challenges and Recommendations. J Neurotrauma. 2021;38:2047-2054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Kakabadze Z, Kipshidze N, Mardaleishvili K, Chutkerashvili G, Chelishvili I, Harders A, Loladze G, Shatirishvili G, Chakhunashvili D, Chutkerashvili K. Phase 1 Trial of Autologous Bone Marrow Stem Cell Transplantation in Patients with Spinal Cord Injury. Stem Cells Int. 2016;2016:6768274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 26. | Hur JW, Cho TH, Park DH, Lee JB, Park JY, Chung YG. Intrathecal transplantation of autologous adipose-derived mesenchymal stem cells for treating spinal cord injury: A human trial. J Spinal Cord Med. 2016;39:655-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 117] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 27. | Vaquero J, Zurita M, Rico MA, Bonilla C, Aguayo C, Montilla J, Bustamante S, Carballido J, Marin E, Martinez F, Parajon A, Fernandez C, Reina L; Neurological Cell Therapy Group. An approach to personalized cell therapy in chronic complete paraplegia: The Puerta de Hierro phase I/II clinical trial. Cytotherapy. 2016;18:1025-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 28. | Dai G, Liu X, Zhang Z, Yang Z, Dai Y, Xu R. Transplantation of autologous bone marrow mesenchymal stem cells in the treatment of complete and chronic cervical spinal cord injury. Brain Res. 2013;1533:73-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 108] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 29. | Dai G, Liu X, Zhang Z, Wang X, Li M, Cheng H, Hua R, Shi J, Wang R, Qin C, Gao J, An Y. Comparative analysis of curative effect of CT-guided stem cell transplantation and open surgical transplantation for sequelae of spinal cord injury. J Transl Med. 2013;11:315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Bhanot Y, Rao S, Ghosh D, Balaraju S, Radhika CR, Satish Kumar KV. Autologous mesenchymal stem cells in chronic spinal cord injury. Br J Neurosurg. 2011;25:516-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 31. | Albu S, Kumru H, Coll R, Vives J, Vallés M, Benito-Penalva J, Rodríguez L, Codinach M, Hernández J, Navarro X, Vidal J. Clinical effects of intrathecal administration of expanded Wharton jelly mesenchymal stromal cells in patients with chronic complete spinal cord injury: a randomized controlled study. Cytotherapy. 2021;23:146-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 32. | Oh SK, Choi KH, Yoo JY, Kim DY, Kim SJ, Jeon SR. A Phase III Clinical Trial Showing Limited Efficacy of Autologous Mesenchymal Stem Cell Therapy for Spinal Cord Injury. Neurosurgery. 2016;78:436-47; discussion 447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 127] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 33. | Thakkar UG, Vanikar AV, Trivedi HL, Shah VR, Dave SD, Dixit SB, Tiwari BB, Shah HH. Infusion of autologous adipose tissue derived neuronal differentiated mesenchymal stem cells and hematopoietic stem cells in post-traumatic paraplegia offers a viable therapeutic approach. Adv Biomed Res. 2016;5:51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 34. | Shroff G. Human Embryonic Stem Cell Therapy in Chronic Spinal Cord Injury: A Retrospective Study. Clin Transl Sci. 2016;9:168-175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 35. | Park JH, Kim DY, Sung IY, Choi GH, Jeon MH, Kim KK, Jeon SR. Long-term results of spinal cord injury therapy using mesenchymal stem cells derived from bone marrow in humans. Neurosurgery. 2012;70:1238-47; discussion 1247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 130] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 36. | Oraee-Yazdani S, Akhlaghpasand M, Golmohammadi M, Hafizi M, Zomorrod MS, Kabir NM, Oraee-Yazdani M, Ashrafi F, Zali A, Soleimani M. Combining cell therapy with human autologous Schwann cell and bone marrow-derived mesenchymal stem cell in patients with subacute complete spinal cord injury: safety considerations and possible outcomes. Stem Cell Res Ther. 2021;12:445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 37. | Park HC, Shim YS, Ha Y, Yoon SH, Park SR, Choi BH, Park HS. Treatment of complete spinal cord injury patients by autologous bone marrow cell transplantation and administration of granulocyte-macrophage colony stimulating factor. Tissue Eng. 2005;11:913-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 146] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 38. | Adel N, Gabr H, Hamdy S, Afifi L, Mahmoud H. Stem Cell Therapy in Chronic Spinal Cord Injuries. 2009; 46. |
| 39. | Kumar K, Taylor RS, Jacques L, Eldabe S, Meglio M, Molet J, Thomson S, O'Callaghan J, Eisenberg E, Milbouw G, Buchser E, Fortini G, Richardson J, North RB. The effects of spinal cord stimulation in neuropathic pain are sustained: a 24-month follow-up of the prospective randomized controlled multicenter trial of the effectiveness of spinal cord stimulation. Neurosurgery. 2008;63:762-70; discussion 770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 456] [Cited by in RCA: 461] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 40. | Pal R, Venkataramana NK, Bansal A, Balaraju S, Jan M, Chandra R, Dixit A, Rauthan A, Murgod U, Totey S. Ex vivo-expanded autologous bone marrow-derived mesenchymal stromal cells in human spinal cord injury/paraplegia: a pilot clinical study. Cytotherapy. 2009;11:897-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 171] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 41. | Abdelaziz OS, Marie A, Abbas M, Ibrahim M, Gabr H. Feasibility, Safety, and Efficacy of Directly Transplanting Autologous Adult Bone Marrow Stem Cells in Patients With Chronic Traumatic Dorsal Cord Injury: A Pilot Clinical Study. Neurosurgery Quarterly. 2010;20:216. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 42. | Yazdani SO, Hafizi M, Zali AR, Atashi A, Ashrafi F, Seddighi AS, Soleimani M. Safety and possible outcome assessment of autologous Schwann cell and bone marrow mesenchymal stromal cell co-transplantation for treatment of patients with chronic spinal cord injury. Cytotherapy. 2013;15:782-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 43. | Amr SM, Gouda A, Koptan WT, Galal AA, Abdel-Fattah DS, Rashed LA, Atta HM, Abdel-Aziz MT. Bridging defects in chronic spinal cord injury using peripheral nerve grafts combined with a chitosan-laminin scaffold and enhancing regeneration through them by co-transplantation with bone-marrow-derived mesenchymal stem cells: case series of 14 patients. J Spinal Cord Med. 2014;37:54-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 44. | Suzuki Y, Ishikawa N, Omae K, Hirai T, Ohnishi K, Nakano N, Nishida H, Nakatani T, Fukushima M, Ide C. Bone marrow-derived mononuclear cell transplantation in spinal cord injury patients by lumbar puncture. Restor Neurol Neurosci. 2014;32:473-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 45. | Goni VG, Chhabra R, Gupta A, Marwaha N, Dhillon MS, Pebam S, Gopinathan NR, Bangalore Kantharajanna S. Safety profile, feasibility and early clinical outcome of cotransplantation of olfactory mucosa and bone marrow stem cells in chronic spinal cord injury patients. Asian Spine J. 2014;8:484-490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 46. | Mendonça MV, Larocca TF, de Freitas Souza BS, Villarreal CF, Silva LF, Matos AC, Novaes MA, Bahia CM, de Oliveira Melo Martinez AC, Kaneto CM, Furtado SB, Sampaio GP, Soares MB, dos Santos RR. Safety and neurological assessments after autologous transplantation of bone marrow mesenchymal stem cells in subjects with chronic spinal cord injury. Stem Cell Res Ther. 2014;5:126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 151] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 47. | Shin JC, Kim KN, Yoo J, Kim IS, Yun S, Lee H, Jung K, Hwang K, Kim M, Lee IS, Shin JE, Park KI. Clinical Trial of Human Fetal Brain-Derived Neural Stem/Progenitor Cell Transplantation in Patients with Traumatic Cervical Spinal Cord Injury. Neural Plast. 2015;2015:630932. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 104] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 48. | Chhabra HS, Sarda K, Arora M, Sharawat R, Singh V, Nanda A, Sangodimath GM, Tandon V. Autologous bone marrow cell transplantation in acute spinal cord injury--an Indian pilot study. Spinal Cord. 2016;54:57-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 49. | Oraee-Yazdani S, Hafizi M, Atashi A, Ashrafi F, Seddighi AS, Hashemi SM, Seddighi A, Soleimani M, Zali A. Co-transplantation of autologous bone marrow mesenchymal stem cells and Schwann cells through cerebral spinal fluid for the treatment of patients with chronic spinal cord injury: safety and possible outcome. Spinal Cord. 2016;54:102-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 50. | Xiao Z, Tang F, Tang J, Yang H, Zhao Y, Chen B, Han S, Wang N, Li X, Cheng S, Han G, Zhao C, Yang X, Chen Y, Shi Q, Hou S, Zhang S, Dai J. One-year clinical study of NeuroRegen scaffold implantation following scar resection in complete chronic spinal cord injury patients. Sci China Life Sci. 2016;59:647-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 51. | Chhabra HS, Sarda K. Clinical translation of stem cell based interventions for spinal cord injury - Are we there yet? Adv Drug Deliv Rev. 2017;120:41-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 52. | Vaquero J, Zurita M, Rico MA, Bonilla C, Aguayo C, Fernández C, Tapiador N, Sevilla M, Morejón C, Montilla J, Martínez F, Marín E, Bustamante S, Vázquez D, Carballido J, Rodríguez A, Martínez P, García C, Ovejero M, Fernández MV; Neurological Cell Therapy Group. Repeated subarachnoid administrations of autologous mesenchymal stromal cells supported in autologous plasma improve quality of life in patients suffering incomplete spinal cord injury. Cytotherapy. 2017;19:349-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 99] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 53. | Guadalajara Labajo H, León Arellano M, Vaquero Crespo J, Valverde Núñez I, García-Olmo D. Objective demonstration of improvement of neurogenic bowel dysfunction in a case of spinal cord injury following stem cell therapy. J Surg Case Rep. 2018;2018:rjy300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 54. | Srivastava RN, Agrahari AK, Singh A, Chandra T, Raj S. Effectiveness of bone marrow-derived mononuclear stem cells for neurological recovery in participants with spinal cord injury: A randomized controlled trial. Asian J Transfus Sci. 2019;13:120-128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 55. | Phedy P, Djaja YP, Gatam L, Kusnadi Y, Wirawan RP, Tobing IMS, Subakir N, Mappalilu A, Prawira MA, Yauwenas R, Gatam AR. Motoric Recovery After Transplantation of Bone Marrow Derived Mesenchymal Stem Cells in Chronic Spinal Cord Injury: A Case Report. Am J Case Rep. 2019;20:1299-1304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 56. | Chen W, Zhang Y, Yang S, Sun J, Qiu H, Hu X, Niu X, Xiao Z, Zhao Y, Zhou Y, Dai J, Chu T. NeuroRegen Scaffolds Combined with Autologous Bone Marrow Mononuclear Cells for the Repair of Acute Complete Spinal Cord Injury: A 3-Year Clinical Study. Cell Transplant. 2020;29:963689720950637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 57. | Sharma A, Sane H, Gokulchandran N, Kulkarni P, Jose A, Nair V, Das R, Lakhanpal V, Badhe P. Intrathecal transplantation of autologous bone marrow mononuclear cells in patients with sub-acute and chronic spinal cord injury: An open-label study. Int J Health Sci (Qassim). 2020;14:24-32. [PubMed] |
| 58. | Song H, Suo S, Ning C, Zhang Y, Mu W, Chen S. Bone Marrow Mesenchymal Stem Cells Transplantation on Acute Spinal Cord Injury. J Hard Tissue Biol. 2020;29:91-98. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 59. | Honmou O, Yamashita T, Morita T, Oshigiri T, Hirota R, Iyama S, Kato J, Sasaki Y, Ishiai S, Ito YM, Namioka A, Namioka T, Nakazaki M, Kataoka-Sasaki Y, Onodera R, Oka S, Sasaki M, Waxman SG, Kocsis JD. Intravenous infusion of auto serum-expanded autologous mesenchymal stem cells in spinal cord injury patients: 13 case series. Clin Neurol Neurosurg. 2021;203:106565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 60. | Deda H, Inci MC, Kürekçi AE, Kayihan K, Ozgün E, Ustünsoy GE, Kocabay S. Treatment of chronic spinal cord injured patients with autologous bone marrow-derived hematopoietic stem cell transplantation: 1-year follow-up. Cytotherapy. 2008;10:565-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 87] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 61. | Hammadi AA, Marino A, Farhan S. Clinical response of 277 patients with spinal cord injury to stem cell therapy in iraq. Int J Stem Cells. 2012;5:76-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 62. | Al-Zoubi A, Jafar E, Jamous M, Al-Twal F, Al-Bakheet S, Zalloum M, Khalifeh F, Radi SA, El-Khateeb M, Al-Zoubi Z. Transplantation of purified autologous leukapheresis-derived CD34+ and CD133+ stem cells for patients with chronic spinal cord injuries: long-term evaluation of safety and efficacy. Cell Transplant. 2014;23 Suppl 1:S25-S34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 63. | Bryukhovetskiy AS, Bryukhovetskiy IS. Effectiveness of repeated transplantations of hematopoietic stem cells in spinal cord injury. World J Transplant. 2015;5:110-128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 64. | Tien NLB, Hoa ND, Thanh VV, Thach NV, Ngoc VTN, Dinh TC, Phuong TNT, Toi PL, Chu DT. Autologous Transplantation of Adipose-Derived Stem Cells to Treat Acute Spinal Cord Injury: Evaluation of Clinical Signs, Mental Signs, and Quality of Life. Open Access Maced J Med Sci. 2019;7:4399-4405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 65. | Ghobrial GM, Anderson KD, Dididze M, Martinez-Barrizonte J, Sunn GH, Gant KL, Levi AD. Human Neural Stem Cell Transplantation in Chronic Cervical Spinal Cord Injury: Functional Outcomes at 12 Months in a Phase II Clinical Trial. Neurosurgery. 2017;64:87-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 66. | Anderson KD, Guest JD, Dietrich WD, Bartlett Bunge M, Curiel R, Dididze M, Green BA, Khan A, Pearse DD, Saraf-Lavi E, Widerström-Noga E, Wood P, Levi AD. Safety of Autologous Human Schwann Cell Transplantation in Subacute Thoracic Spinal Cord Injury. J Neurotrauma. 2017;34:2950-2963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 189] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 67. | Levi AD, Okonkwo DO, Park P, Jenkins AL 3rd, Kurpad SN, Parr AM, Ganju A, Aarabi B, Kim D, Casha S, Fehlings MG, Harrop JS, Anderson KD, Gage A, Hsieh J, Huhn S, Curt A, Guzman R. Emerging Safety of Intramedullary Transplantation of Human Neural Stem Cells in Chronic Cervical and Thoracic Spinal Cord Injury. Neurosurgery. 2018;82:562-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 110] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 68. | Curtis E, Martin JR, Gabel B, Sidhu N, Rzesiewicz TK, Mandeville R, Van Gorp S, Leerink M, Tadokoro T, Marsala S, Jamieson C, Marsala M, Ciacci JD. A First-in-Human, Phase I Study of Neural Stem Cell Transplantation for Chronic Spinal Cord Injury. Cell Stem Cell. 2018;22:941-950.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 242] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 69. | Levi AD, Anderson KD, Okonkwo DO, Park P, Bryce TN, Kurpad SN, Aarabi B, Hsieh J, Gant K. Clinical Outcomes from a Multi-Center Study of Human Neural Stem Cell Transplantation in Chronic Cervical Spinal Cord Injury. J Neurotrauma. 2019;36:891-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 115] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 70. | Curt A, Hsieh J, Schubert M, Hupp M, Friedl S, Freund P, Huber E, Pfyffer D, Sutter R, Jutzeler C, Wüthrich RP, Min K, Casha S, Fehlings MG, Guzman R. The Damaged Spinal Cord Is a Suitable Target for Stem Cell Transplantation. Neurorehabil Neural Repair. 2020;34:758-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 71. | Zamani H, Soufizomorrod M, Oraee-Yazdani S, Naviafar D, Akhlaghpasand M, Seddighi A, Soleimani M. Safety and feasibility of autologous olfactory ensheathing cell and bone marrow mesenchymal stem cell co-transplantation in chronic human spinal cord injury: a clinical trial. Spinal Cord. 2022;60:63-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 72. | Gant KL, Guest JD, Palermo AE, Vedantam A, Jimsheleishvili G, Bunge MB, Brooks AE, Anderson KD, Thomas CK, Santamaria AJ, Perez MA, Curiel R, Nash MS, Saraf-Lavi E, Pearse DD, Widerström-Noga E, Khan A, Dietrich WD, Levi AD. Phase 1 Safety Trial of Autologous Human Schwann Cell Transplantation in Chronic Spinal Cord Injury. J Neurotrauma. 2022;39:285-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 63] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 73. | Liu J, Han D, Wang Z, Xue M, Zhu L, Yan H, Zheng X, Guo Z, Wang H. Clinical analysis of the treatment of spinal cord injury with umbilical cord mesenchymal stem cells. Cytotherapy. 2013;15:185-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 74. | Cheng H, Liu X, Hua R, Dai G, Wang X, Gao J, An Y. Clinical observation of umbilical cord mesenchymal stem cell transplantation in treatment for sequelae of thoracolumbar spinal cord injury. J Transl Med. 2014;12:253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 110] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 75. | Shroff G. Magnetic resonance imaging tractography as a diagnostic tool in patients with spinal cord injury treated with human embryonic stem cells. Neuroradiol J. 2017;30:71-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 76. | Zhao Y, Tang F, Xiao Z, Han G, Wang N, Yin N, Chen B, Jiang X, Yun C, Han W, Zhao C, Cheng S, Zhang S, Dai J. Clinical Study of NeuroRegen Scaffold Combined With Human Mesenchymal Stem Cells for the Repair of Chronic Complete Spinal Cord Injury. Cell Transplant. 2017;26:891-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 123] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 77. | Xiao Z, Tang F, Zhao Y, Han G, Yin N, Li X, Chen B, Han S, Jiang X, Yun C, Zhao C, Cheng S, Zhang S, Dai J. Significant Improvement of Acute Complete Spinal Cord Injury Patients Diagnosed by a Combined Criteria Implanted with NeuroRegen Scaffolds and Mesenchymal Stem Cells. Cell Transplant. 2018;27:907-915. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 119] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 78. | Zhao Y, Yao L, Ao L, Ou J, He Y, Shang Y. Study of the Diffusion Tensor Imaging for Preclinical Therapeutic Efficacy of Umbilical Cord Mesenchymal Stem Cell Transplantation in the Treatment of Spinal Cord Injury. Int J Gen Med. 2021;14:9721-9732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |