Published online Aug 18, 2022. doi: 10.5500/wjt.v12.i8.223
Peer-review started: January 16, 2022
First decision: March 16, 2022
Revised: March 27, 2022
Accepted: August 6, 2022
Article in press: August 6, 2022
Published online: August 18, 2022
Processing time: 213 Days and 23.7 Hours
Acute kidney injury (AKI) incidence is growing rapidly, and AKI is one of the predictors of inpatient mortality. After nephrectomy, all the patients have decreased kidney function with AKI and recover from AKI. However, the characteristic and behavior of AKI is different from usual AKI and compensatory kidney function has been well known in the postoperative setting, especially in living donors. In this review, we have focused on the compensation of kidney function after nephrectomy in living donors. We discuss factors that have been identified as being associated with kidney recovery in donors including age, sex, body mass index, remnant kidney volume, estimated glomerular filtration rate, and various comorbidities.
Core Tip: Acute kidney injury (AKI) incidence is growing rapidly, and AKI is one of the predictors of inpatient mortality. The characteristic and behavior of AKI is different from usual AKI and compensatory kidney function has been well known in the postoperative setting, especially in living donors. In this review, we have focused on the compensation of kidney function after nephrectomy in living donors. We discuss factors of compensation of kidney function after nephrectomy.
- Citation: Okumura K, Grace H, Sogawa H, Yamanaga S. Acute kidney injury and the compensation of kidney function after nephrectomy in living donation. World J Transplant 2022; 12(8): 223-230
- URL: https://www.wjgnet.com/2220-3230/full/v12/i8/223.htm
- DOI: https://dx.doi.org/10.5500/wjt.v12.i8.223
The incidence of acute kidney injury (AKI) is growing rapidly in many situations[1]. Despite advances in medical care, AKI remains an independent predictor of in-hospital mortality[2]. While the nature of kidney is the organ to recover, it is well established that AKI, especially when severe, is a risk factor for incident and progressive chronic kidney disease (CKD) and eventually leading to progressive nephron loss and end-stage renal disease (ESRD)[3,4].
Kidney transplantation has been considered a preferred treatment for patients with ESRD and offers a better quality of life than dialysis[5,6]. While a previous study showed that showed that living donation of kidney is safe in a large cohort, nephrectomy is a major procedure which is associated with potential risks for the donor, including increased cardio-vascular risks and progression to ESRD in the long-term[7]. After donation of the kidney, it has been well known that all patients have hemodynamic changes associated with AKI and have compensated kidney function with the contralateral kidney after donation[6,8-12]. The degree of contralateral kidney function has been reported to be around 60%-70 % on average in previous studies[13,14], however, the degree of compensatory kidney function varies in each donor. In this review, we have discussed the topics related to the clinical factors of compensation and the mechanism of recovery after kidney donation.
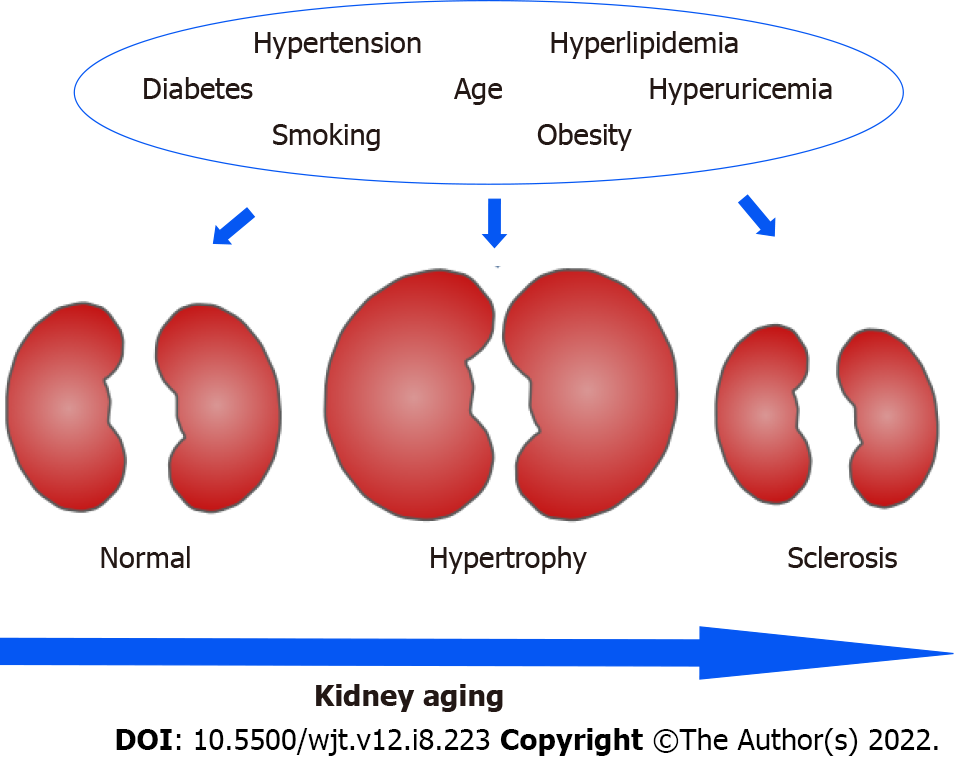
Many variables are involved in the clinical settings for kidney recovery after kidney donation (Table 1, Figure 1). Age is one of the significant factors which affects the extent of recovery. Younger age is associated with favorable outcomes in many studies[6,8,15-19] and this is supported by the facts that aging is associated with underlying abnormalities and structural changes such as nephrosclerosis and nephron hypertrophy[16]. The rate of glomerular density has an inverse correlation with aging[20]. The number of nephrons decreases with aging and affects the function of the kidney[20]. Denic et al[21] investigated the risk factors associated with kidney abnormalities, and they demonstrated that mild hypertension and aging are associated with underlying abnormalities. They showed the changes of the volumes of kidney, cortex and medulla in living kidney donors[22].
| Ref. | Significant factors | ||
| Ohashi et al[17] | Age | Presence of metabolic syndrome | Chronic histological changes |
| Ibrahim et al[8] | Age | Sex | BMI |
| Rook et al[11] | Age | BMI | |
| Denic et al[21] | Age | HTN | |
| Shiraishi et al[15] | Age | Sex | BMI |
| HTN | |||
| Nishida et al[34] | Hyperuricemia | Chronic histological changes | |
| Yakoubi et al[25] | Age | BSA adjusted RKV | Preoperative eGFR |
| Shinoda et al[26] | BMI | RKV/BSA | |
| Okumura et al[6] | Age | Sex | History of HTN |
| RKV/Wt | |||
| Zabor et al[18] | Age | Sex | History of HTN |
| Lee et al[19] | Age | Sex | History of HTN |
| BMI | History of DM | Preoperative eGFR | |
| RKV | |||
| Vaz et al[42] | Age | Sex |
Hypertension is also one of the significant factors which affect the extent of recovery in kidney function[6]. It is known that prevalence of hypertension increases with age. Hypertension was previously regarded as contraindication for living kidney donation, however, living donor donation was reported to be safe if hypertension is under controlled with medication[22]. On understanding of kidney aging, kidney function in people with advanced age have less reserve when they tend to develop CKD and have also higher risk of AKI[23]. As people get old, the prevalence of hypertension also increases, and glomerular hypertrophy has been identified as an integral feature of hypertensive nephropathy and seems to precede rather than to compensate for glomerulosclerosis[24].
Gender is another significant factor for kidney compensation and prognosis. Male gender is associated with poor prognosis in kidney donation[6,8,15], however, this is controversial since many studies showed that gender did not reach to conclusion as one of the independent factors[17,25,26]. This might be more related to the fact that male gender has a higher rate of smoking, which is one of the factors affecting the kidney function and is associated with hypertension.
Metabolic syndrome has been defined by the National Cholesterol Education Program Adult Treatment Panel III if three or more of the following five criteria are met: Waist circumference over 40 inches (men) or 35 inches (women); blood pressure over 130/85 mmHg; fasting triglyceride level over 150 mg/dL; fasting high-density lipoprotein cholesterol level less than 40 mg/dL (men) or 50 mg/dL (women); fasting blood sugar over 100 mg/dL[27]. Metabolic syndrome has been shown to have a negative impact on remnant kidney function after nephrectomy since metabolic syndrome is associated with a high incidence of hypertension, obesity, hyperglycemia, and hyperuricemia[17,28,29].
The impact of serum uric acid level has been an emerging topic on the residual kidney function in living kidney donors. The total 4650 living-donor cohort study showed that donors with post-donation gout had higher risk of developing AKI and progression to CKD[30]. Other living-donor studies from Turkey and Korea also suggested that preoperative hyperuricemia are associated with impaired postoperative renal function at 6 and 12 mo[31-33]. It was also reported that preoperative hyperuricemia was strongly associated with suboptimal renal compensatory function or recovery at one year after renal donation[34]. Furthermore, hyperuricemia had 1.76-fold higher adjusted risk of adverse events within 5 years after donation, such as cardiovascular events, initiation of dialysis, and de novo prescriptions for hypertension, hyperuricemia, diabetes, and dyslipidemia as well as lower estimated glomerular filtration rate (eGFR)[35].
The size of kidney is one of the important factors affecting the donor/recipient outcomes in kidney transplantation[36,37]. Since larger size of the kidney is associated with better renal function, it is recommended to choose the smaller kidney for donation to fulfil the principle of leaving the “better” kidney in donor if there is a more than 10% volume difference between kidneys in donor. The reasons to select suboptimal side of kidneys in donation, were cysts or tumors (46.5%), arterial abnormalities (22.7%), inferior size or function (19.8%), and anatomic abnormalities (11.0%), and those kidneys showed worse long-term overall graft survival regardless of the reasons[38].
Remnant kidney volume (RKV) in living donor is one of the important factors to determine the kidney recovery after donor nephrectomy[6,19]. Shinoda et al[26] showed the ratio of RKV to body surface area (BSA) ratio has an independent factor to predict renal function or compensation after kidney donation. Yakoubi et al[25] also showed BSA adjusted with RKV was an independent predictor of kidney recovery after donation. With respect to recipient outcomes, the ratio of donated kidney volume to body weight (Wt) has been suggested as an important factor related to allograft function[39].
The ratio of RKV to Wt (RKV/Wt) was reported to be one of the significant associated factors in eGFR at 1 year after kidney donation[6]. Although it has been thought that a lower RKV/Wt can cause hyperfiltration and subsequent proteinuria[40], Song et al[41] suggested that a ratio of RKV/Wt less than 2.0 mL/kg did not affect the eGFR in donors but was associated with more severe proteinuria at 1 year after donor nephrectomy. There was no significant difference in the RKV/Wt ratio in the study[41], but they suggested the “deterioration” of kidney function since the donors were associated with presence of proteinuria at 1 year after donation. Thus, a lower RKV/Wt ratio might be associated with hyperfiltration and subsequently decrease “renal reserve”.
Laterality of the donated kidney is another factor to evaluate when considering donor and recipient outcomes in kidney transplantation. Vaz et al[42] studied the outcomes of hand assisted laparoscopic donor nephrectomy (HALDN) of the left and the right kidney among 739 donors. This study concluded that, although most transplant centers and surgeons prefer performing left nephrectomies because of having a longer vein, right HALDN nephrectomy is a safe procedure with similar outcomes to left HALDN. Gunseren et al[43] compared right and left side laparoscopic donor nephrectomy outcomes and found that they had similar intraoperative outcomes. These authors noted, however, that dissection of lymphatic structures during left laparoscopic donor nephrectomy may cause chylous drainage and prolong hospitalization time compared to right-sided nephrectomy. Zeuschner et al[44] evaluated left and right pure laparoscopic donor nephrectomies and found a higher rate of complications for recipients of right grafts, but long-term function and graft survival were equivalent.
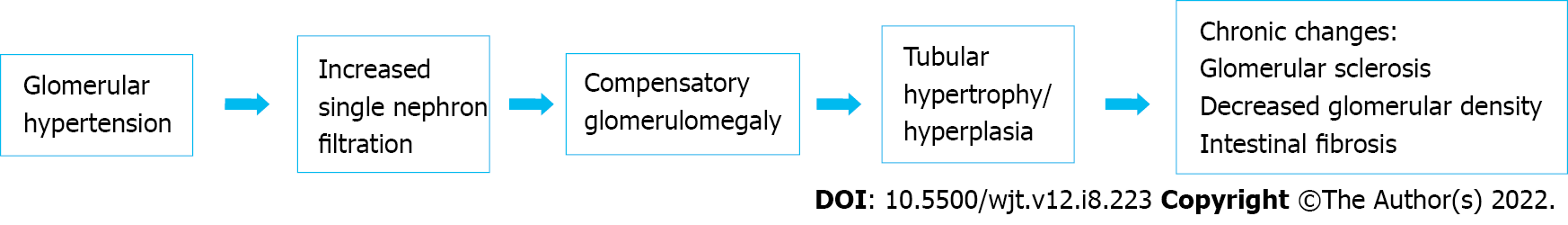
After the nephrectomy, the compensation of contralateral kidney function has been well known. Immediately after nephrectomy, an approximately 40% increase in renal plasma flow and glomerular filtration rate is measured in the remaining kidney[9,45]. This leads to developing glomerular hypertension and increased single-nephron filtration with compensatory glomerulomegaly. The glomerulomegaly from hyperfiltration also occurs in response to nephron loss. In addition to glomerulomegaly, hyperfiltration leads to tubular hypertrophy and hyperplasia. Prolonged hyperfiltration and glomerular hypertension causes glomerular sclerosis and decreased glomerular density (Figure 2).
Once glomerular size reaches a certain threshold, glomerularsclerosis, hypertension, proteinuria, and renal failure may develop[46]. This pathological process was associated with kidney function, blood pressure and metabolic conditions: Metabolic syndrome, hypertension, hyperglycemia and hyperuricemia[17,20,34,47,48]. However, these histological changes might not always be seen in donors since donors were in a relatively good state of health and the unaffected nephrons would respond with compensation[48]. Studies showed that donors who had hyperuricemia, had chronic histological changes such as intestinal fibrosis, tubular atrophy and arterial hyalinosis in the donated kidney[34]. Intestinal fibrosis and tubular atrophy have significant impacts on long term graft function[49]. It is thought that arteriosclerosis has a significant relationship with intestinal fibrosis and tubular atrophy since the chronic ischemic condition caused by arteriosclerosis induces histological changes such as intestinal fibrosis, tubular atrophy and glomerular sclerosis[50].
Rule et al[20] showed that increased GFR, body mass index and uric acid level and a family history of end stage renal disease were independent predictors of decreased glomerular density. The size of individual nephrons can reflect important elements of metabolic regulation. After living kidney donation, donors can develop glomerular hypertension and increased single-nephron filtration with compensatory glomerulomegaly[51-53]. Polichnowski et al[54] showed that contralateral nephrectomy is associated with kidney recovery from ischemic kidney injury and prevent tissue atrophy with capillary repair and tubule redifferentiation. This result supports that remnant kidney is not vulnerable but sustainable after kidney donation. However, we emphasize that the best strategy for AKI is prevention. It is rare to perform living donation in the setting of AKI, however, in deceased donors, Cima et al[55] reported that kidney transplant could be performed from donors with AKI depending on the histological grading score with glomerulosclerosis, tubular atrophy, intestinal fibrosis, vascular damage and acute tubular necrosis[55,56].
At present, the specific mechanism after nephrectomy remain unclear. However, several hypotheses have been proposed and it has shown that endothelial injury and recovery have an important role in the pathogenesis of kidney injury[57]. As discussed above, renal blood flow and GFR significantly increased after nephrectomy. This has been a critical role of upstream factors responsible to recruit dormant nephrons and subsequently to improve in GFR. As renal blood flow increases and renal glomerular filtrate rate increases, it would lead to increase oxygen consumption and cause tissue hypoxia. It induces hypoxia-inducible factor 1 alpha and induces vascular endothelial growth factor. Hypoxia also induces phosphatase and tension homolog in tubules which causes tubule redifferentiation and repair[54].
In another way, renal tubular epithelial cells, which are surviving from ischemic injury, undergo differentiation[58]. These surviving epithelial cells express vimentin (an intermediate filament protein, which is found in undifferentiated mesenchymal cells but not in differentiated kidney cells), and proliferating cells nuclear antigen (a marker of mitogenesis), in contrast, damaged cells do not express either vimentin or proliferating cell nuclear antigen[59]. The molecular drivers in the process of intrinsic repair remain indeterminate, but the transcription factor Sox9 has been shown to be a critical part of the cellular repairing pathway in surviving renal tubular epithelial cells[60].
Oliver et al[60] reported that there are renal specific stem cells, which have been identified in the renal tubules as well as the papilla, however, the contribution of these cells still remains under investigation. Many recent studies have looked into the progenitor cell or bone marrow derived mesenchymal stem cells in renal repair[61]. The mesenchymal stem cell, which are derived from renal specific or bone marrow, may accelerate the process of repairing the injured tubules by direct proliferation or through paracrine effects. In transplant kidney, some studies suggest that the recipient derived cells may repopulate injured tubule[62,63], however, mesenchymal stem cells may predominantly play a role in their beneficial effects via paracrine mechanisms[64]. The mesenchymal stem cells may release microvesicles to communicate between cells and protect renal injury in addition to releasing cytokines[65].
We have performed living donor kidney transplant safely, however, a large cohort study showed that being a donor increased cardiovascular risk and progression to ESRD in the long term[7]. Since the degree of recovery from AKI affects the prognosis of kidney function[66], we believe that it is important to identify the risk of patients without compensation of kidney function of the contralateral kidney to predict the long term risk.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Transplantation
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Eccher A, Italy; Papadopoulos VP, Greece S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Hsu CY, McCulloch CE, Fan D, Ordoñez JD, Chertow GM, Go AS. Community-based incidence of acute renal failure. Kidney Int. 2007;72:208-212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 437] [Cited by in RCA: 403] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 2. | Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365-3370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2256] [Cited by in RCA: 2357] [Article Influence: 117.9] [Reference Citation Analysis (0)] |
| 3. | Chawla LS, Eggers PW, Star RA, Kimmel PL. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med. 2014;371:58-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1210] [Cited by in RCA: 1455] [Article Influence: 132.3] [Reference Citation Analysis (0)] |
| 4. | Evans RG, Ow CP, Bie P. The chronic hypoxia hypothesis: the search for the smoking gun goes on. Am J Physiol Renal Physiol. 2015;308:F101-F102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (2)] |
| 5. | Kasiske BL, Snyder J, Matas A, Collins A. The impact of transplantation on survival with kidney failure. Clin Transpl. 2000;135-143. [PubMed] |
| 6. | Okumura K, Yamanaga S, Tanaka K, Kinoshita K, Kaba A, Fujii M, Ogata M, Hidaka Y, Toyoda M, Uekihara S, Miyata A, Inadome A, Yokomizo H. Prediction model of compensation for contralateral kidney after living-donor donation. BMC Nephrol. 2019;20:283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Muzaale AD, Massie AB, Wang MC, Montgomery RA, McBride MA, Wainright JL, Segev DL. Risk of end-stage renal disease following live kidney donation. JAMA. 2014;311:579-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 660] [Cited by in RCA: 704] [Article Influence: 64.0] [Reference Citation Analysis (0)] |
| 8. | Ibrahim HN, Foley R, Tan L, Rogers T, Bailey RF, Guo H, Gross CR, Matas AJ. Long-term consequences of kidney donation. N Engl J Med. 2009;360:459-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 777] [Cited by in RCA: 752] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 9. | Krohn AG, Ogden DA, Holmes JH. Renal function in 29 healthy adults before and after nephrectomy. JAMA. 1966;196:322-324. [PubMed] |
| 10. | Donadio JV Jr, Farmer CD, Hunt JC, Tauxe WN, Hallenbeck GA, Shorter RG. Renal function in donors and recipients of renal allotransplantation. Radioisotopic measurements. Ann Intern Med. 1967;66:105-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Rook M, Bosma RJ, van Son WJ, Hofker HS, van der Heide JJ, ter Wee PM, Ploeg RJ, Navis GJ. Nephrectomy elicits impact of age and BMI on renal hemodynamics: lower postdonation reserve capacity in older or overweight kidney donors. Am J Transplant. 2008;8:2077-2085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 12. | Hosokawa Y, Tanaka N, Mibu H, Anai S, Torimoto K, Yoneda T, Hirayama A, Yoshida K, Hayashi Y, Hirao Y, Fujimoto K. Follow-up study of unilateral renal function after nephrectomy assessed by glomerular filtration rate per functional renal volume. World J Surg Oncol. 2014;12:59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Kasiske BL, Anderson-Haag T, Israni AK, Kalil RS, Kimmel PL, Kraus ES, Kumar R, Posselt AA, Pesavento TE, Rabb H, Steffes MW, Snyder JJ, Weir MR. A prospective controlled study of living kidney donors: three-year follow-up. Am J Kidney Dis. 2015;66:114-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 136] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 14. | Blantz RC, Steiner RW. Benign hyperfiltration after living kidney donation. J Clin Invest. 2015;125:972-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Shiraishi N, Kitamura K, Kohda Y, Iseki K, Tomita K. Prevalence and risk factor analysis of nephrosclerosis and ischemic nephropathy in the Japanese general population. Clin Exp Nephrol. 2014;18:461-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Denic A, Glassock RJ, Rule AD. Structural and Functional Changes With the Aging Kidney. Adv Chronic Kidney Dis. 2016;23:19-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 499] [Article Influence: 55.4] [Reference Citation Analysis (0)] |
| 17. | Ohashi Y, Thomas G, Nurko S, Stephany B, Fatica R, Chiesa A, Rule AD, Srinivas T, Schold JD, Navaneethan SD, Poggio ED. Association of metabolic syndrome with kidney function and histology in living kidney donors. Am J Transplant. 2013;13:2342-2351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 18. | Zabor EC, Furberg H, Mashni J, Lee B, Jaimes EA, Russo P. Factors Associated with Recovery of Renal Function following Radical Nephrectomy for Kidney Neoplasms. Clin J Am Soc Nephrol. 2016;11:101-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Lee CU, Ryoo H, Chung JH, Song W, Kang M, Sung HH, Jeong BC, Seo SI, Jeon SS, Lee HM, Jeon HG. Preoperative versus Postoperative Compensation of the Contralateral Normal Kidney in Patients Treated with Radical Nephrectomy for Renal Cell Carcinoma. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Rule AD, Semret MH, Amer H, Cornell LD, Taler SJ, Lieske JC, Melton LJ 3rd, Stegall MD, Textor SC, Kremers WK, Lerman LO. Association of kidney function and metabolic risk factors with density of glomeruli on renal biopsy samples from living donors. Mayo Clin Proc. 2011;86:282-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 21. | Denic A, Alexander MP, Kaushik V, Lerman LO, Lieske JC, Stegall MD, Larson JJ, Kremers WK, Vrtiska TJ, Chakkera HA, Poggio ED, Rule AD. Detection and Clinical Patterns of Nephron Hypertrophy and Nephrosclerosis Among Apparently Healthy Adults. Am J Kidney Dis. 2016;68:58-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 22. | Delmonico F; Council of the Transplantation Society. A Report of the Amsterdam Forum On the Care of the Live Kidney Donor: Data and Medical Guidelines. Transplantation. 2005;79:S53-66. [PubMed] |
| 23. | Saito T, Uchida K, Ishida H, Tanabe K, Nitta K. Changes in glomerular filtration rate after donation in living kidney donors: a single-center cohort study. Int Urol Nephrol. 2015;47:397-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Hughson MD, Puelles VG, Hoy WE, Douglas-Denton RN, Mott SA, Bertram JF. Hypertension, glomerular hypertrophy and nephrosclerosis: the effect of race. Nephrol Dial Transplant. 2014;29:1399-1409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 25. | Yakoubi R, Autorino R, Kassab A, Long JA, Haber GP, Kaouk JH. Does preserved kidney volume predict 1 year donor renal function after laparoscopic living donor nephrectomy? Int J Urol. 2013;20:931-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | Shinoda K, Morita S, Akita H, Tamaki S, Takahashi R, Kono H, Asanuma H, Kikuchi E, Jinzaki M, Nakagawa K, Oya M. Pre-donation BMI and preserved kidney volume can predict the cohort with unfavorable renal functional compensation at 1-year after kidney donation. BMC Nephrol. 2019;20:46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143-3421. [PubMed] |
| 28. | Dengel DR, Goldberg AP, Mayuga RS, Kairis GM, Weir MR. Insulin resistance, elevated glomerular filtration fraction, and renal injury. Hypertension. 1996;28:127-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 108] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 29. | Wahba IM, Mak RH. Obesity and obesity-initiated metabolic syndrome: mechanistic links to chronic kidney disease. Clin J Am Soc Nephrol. 2007;2:550-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 381] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 30. | Lam NN, Garg AX, Segev DL, Schnitzler MA, Xiao H, Axelrod D, Brennan DC, Kasiske BL, Tuttle-Newhall JE, Lentine KL. Gout after living kidney donation: correlations with demographic traits and renal complications. Am J Nephrol. 2015;41:231-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Cho A, Lee JE, Jang HR, Huh W, Kim DJ, Oh HY, Kim YG. Association between pre-donation serum uric acid concentration and change in renal function after living kidney donation in women. Intern Med J. 2014;44:1217-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 32. | Kulah E. Pretransplant uric acid levels may be predictive for prognosis of renal transplant donors. Ren Fail. 2016;38:487-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 33. | Bravo RC, Gamo MB, Lee HH, Yoon YE, Han WK. Investigating Serum Uric Acid as a Risk Factor in the Development of Delayed Renal Recovery in Living Kidney Donors. Transplant Proc. 2017;49:930-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 34. | Nishida S, Kinoshida K, Tanaka K, Hidaka Y, Kawabata C, Hamanoue S, Toyoda M, Lnadome A, Uekihara S, Yamanaga S. Factors related to suboptimal recovery of renal function after living donor nephrectomy. Transplantation. 2018;102:S42. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (1)] |
| 35. | Tanaka K, Yamanaga S, Hidaka Y, Nishida S, Kinoshita K, Kaba A, Ishizuka T, Hamanoue S, Okumura K, Kawabata C, Toyoda M, Miyata A, Kashima M, Yokomizo H. Long-term impact of baseline serum uric acid levels on living kidney donors: a retrospective study. BMC Nephrol. 2021;22:89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 36. | Narasimhamurthy M, Smith LM, Machan JT, Reinert SE, Gohh RY, Dworkin LD, Merhi B, Patel N, Beland MD, Hu SL. Does size matter? Clin Kidney J. 2017;10:116-123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 37. | Hugen CM, Polcari AJ, Farooq AV, Fitzgerald MP, Holt DR, Milner JE. Size does matter: donor renal volume predicts recipient function following live donor renal transplantation. J Urol. 2011;185:605-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 38. | Yamanaga S, Freise CE, Stock PG, Rosario A, Fernandez D, Kobayashi T, Tavakol M, Kang SM. Inferior Long-Term Graft Survival of Suboptimal Kidneys After Living Donor Kidney Transplantation. Transplant Proc. 2020;52:1734-1740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 39. | Akoglu H, Yildirim T, Eldem G, Arik G, Yilmaz R, Kutlugun AA, Hazirolan T, Aki FT, Arici M, Erdem Y, Turgan C. Living donor kidney volume as a predictor of graft function: is there a role for proteinuria? Transplant Proc. 2013;45:77-81. [RCA] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 40. | Giral M, Foucher Y, Karam G, Labrune Y, Kessler M, Hurault de Ligny B, Büchler M, Bayle F, Meyer C, Trehet N, Daguin P, Renaudin K, Moreau A, Soulillou JP. Kidney and recipient weight incompatibility reduces long-term graft survival. J Am Soc Nephrol. 2010;21:1022-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 41. | Song T, Rao Z, Qiu Y, Liu J, Huang Z, Wang X, Lin T. Impact of remaining kidney volume to body weight ratio on renal function in living kidney donors. Kaohsiung J Med Sci. 2016;32:185-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 42. | Vaz O, Asderakis A, Sharma V, Moinuddin Z, Shanmugam M, Tavakoli A, van Dellen D, Augustine T. Laterality in laparoscopic hand assisted donor nephrectomy - Does it matter anymore? Surgeon. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 43. | Gunseren KO, Cicek MC, Aydin YM, Ozmerdiven CG, Yavascaoglu I. The Differences Between the Right and Left Side Laparoscopic Donor Nephrectomy Outcomes: A Comparative Analysis of Single-Center Outcomes. Sisli Etfal Hastan Tip Bul. 2021;55:339-343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 44. | Zeuschner P, Stöckle M, Peters R, Miller K, Liefeldt L, Halleck F, Budde K, Hennig L, Friedersdorff F. Does the Side Matter? Urol Int. 2021;105:1076-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 45. | Saxena AB, Myers BD, Derby G, Blouch KL, Yan J, Ho B, Tan JC. Adaptive hyperfiltration in the aging kidney after contralateral nephrectomy. Am J Physiol Renal Physiol. 2006;291:F629-F634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 46. | Hostetter TH, Olson JL, Rennke HG, Venkatachalam MA, Brenner BM. Hyperfiltration in remnant nephrons: a potentially adverse response to renal ablation. Am J Physiol. 1981;241:F85-F93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 316] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 47. | Rule AD, Amer H, Cornell LD, Taler SJ, Cosio FG, Kremers WK, Textor SC, Stegall MD. The association between age and nephrosclerosis on renal biopsy among healthy adults. Ann Intern Med. 2010;152:561-567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 395] [Cited by in RCA: 347] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 48. | Alexander MP, Patel TV, Farag YM, Florez A, Rennke HG, Singh AK. Kidney pathological changes in metabolic syndrome: a cross-sectional study. Am J Kidney Dis. 2009;53:751-759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 73] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 49. | Fahmy LM, Massie AB, Muzaale AD, Bagnasco SM, Orandi BJ, Alejo JL, Boyarsky BJ, Anjum SK, Montgomery RA, Dagher NN, Segev DL. Long-term Renal Function in Living Kidney Donors Who Had Histological Abnormalities at Donation. Transplantation. 2016;100:1294-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 50. | Schelling JR. Tubular atrophy in the pathogenesis of chronic kidney disease progression. Pediatr Nephrol. 2016;31:693-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 114] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 51. | Hoy WE, Hughson MD, Singh GR, Douglas-Denton R, Bertram JF. Reduced nephron number and glomerulomegaly in Australian Aborigines: a group at high risk for renal disease and hypertension. Kidney Int. 2006;70:104-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 192] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 52. | Brenner BM, Garcia DL, Anderson S. Glomeruli and blood pressure. Less of one, more the other? Am J Hypertens. 1988;1:335-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 928] [Cited by in RCA: 893] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 53. | Zandi-Nejad K, Luyckx VA, Brenner BM. Adult hypertension and kidney disease: the role of fetal programming. Hypertension. 2006;47:502-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 226] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 54. | Polichnowski AJ, Griffin KA, Licea-Vargas H, Lan R, Picken MM, Long J, Williamson GA, Rosenberger C, Mathia S, Venkatachalam MA, Bidani AK. Pathophysiology of unilateral ischemia-reperfusion injury: importance of renal counterbalance and implications for the AKI-CKD transition. Am J Physiol Renal Physiol. 2020;318:F1086-F1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 55. | Cima L, Nacchia F, Ghimenton C, Valotto G, Boschiero L, Gobbo S, Zaza G, Neil D, Mescoli C, Vanzo F, D'Errico A, Rugge M, Casartelli-Liviero M, Brunelli M, Novelli L, Eccher A. Histopathology and Long-Term Outcome of Kidneys Transplanted From Donors With Severe Acute Kidney Injury. Prog Transplant. 2019;29:36-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 56. | Koyawala N, Parikh CR. A Review of Donor Acute Kidney Injury and Posttransplant Outcomes. Transplantation. 2020;104:1553-1559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 57. | Sutton TA, Fisher CJ, Molitoris BA. Microvascular endothelial injury and dysfunction during ischemic acute renal failure. Kidney Int. 2002;62:1539-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 384] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 58. | Bonventre JV. Dedifferentiation and proliferation of surviving epithelial cells in acute renal failure. J Am Soc Nephrol. 2003;14 Suppl 1:S55-S61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 439] [Cited by in RCA: 436] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 59. | Witzgall R, Brown D, Schwarz C, Bonventre JV. Localization of proliferating cell nuclear antigen, vimentin, c-Fos, and clusterin in the postischemic kidney. Evidence for a heterogenous genetic response among nephron segments, and a large pool of mitotically active and dedifferentiated cells. J Clin Invest. 1994;93:2175-2188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 486] [Cited by in RCA: 477] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 60. | Oliver JA, Maarouf O, Cheema FH, Martens TP, Al-Awqati Q. The renal papilla is a niche for adult kidney stem cells. J Clin Invest. 2004;114:795-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 323] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 61. | Cantley LG. Adult stem cells in the repair of the injured renal tubule. Nat Clin Pract Nephrol. 2005;1:22-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 76] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 62. | Grimm PC, Nickerson P, Jeffery J, Savani RC, Gough J, McKenna RM, Stern E, Rush DN. Neointimal and tubulointerstitial infiltration by recipient mesenchymal cells in chronic renal-allograft rejection. N Engl J Med. 2001;345:93-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 199] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 63. | Poulsom R, Forbes SJ, Hodivala-Dilke K, Ryan E, Wyles S, Navaratnarasah S, Jeffery R, Hunt T, Alison M, Cook T, Pusey C, Wright NA. Bone marrow contributes to renal parenchymal turnover and regeneration. J Pathol. 2001;195:229-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 434] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 64. | Lin F, Moran A, Igarashi P. Intrarenal cells, not bone marrow-derived cells, are the major source for regeneration in postischemic kidney. J Clin Invest. 2005;115:1756-1764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 284] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 65. | He J, Wang Y, Sun S, Yu M, Wang C, Pei X, Zhu B, Wu J, Zhao W. Bone marrow stem cells-derived microvesicles protect against renal injury in the mouse remnant kidney model. Nephrology (Carlton). 2012;17:493-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 159] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 66. | Pannu N, James M, Hemmelgarn B, Klarenbach S; Alberta Kidney Disease Network. Association between AKI, recovery of renal function, and long-term outcomes after hospital discharge. Clin J Am Soc Nephrol. 2013;8:194-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 238] [Article Influence: 18.3] [Reference Citation Analysis (0)] |