Published online Jun 18, 2022. doi: 10.5500/wjt.v12.i6.112
Peer-review started: May 17, 2021
First decision: June 17, 2021
Revised: July 28, 2021
Accepted: May 5, 2022
Article in press: May 5, 2022
Published online: June 18, 2022
Processing time: 394 Days and 0.6 Hours
End-stage kidney failure (ESKD) is a global issue where kidney replacement therapy imposes enormous economic burden to people of developing countries, in addition to the severe limitations to the availability of hemodialysis and peritoneal dialysis technique. The best option of kidney transplantation also requires lifelong combination immunosuppressive medicines, the cost of which is equally comparable to lifelong dialysis. A strategy of achieving transplant tolerance that requires minimum immunosuppressive medicines, although in experimental stage, also requires state-of-art technology with costly medicines and interventions. This is evidently beyond the reach of ESKD patients of developing countries. Hence, globally in developing countries, a need for an innovative but cost-effective tolerance protocol is a burning need for a successful transplant program. In brief, transplant tolerance is defined as a state of donor-specific unresponsiveness to the allograft antigens without the need for ongoing pharmacologic immunosuppression or with a minimal need. Current state-of-art techniques involves: (1) A state of hematological chimera, for complete tolerance; (2) Prope or partial tolerance where immune-reactive T-lymphocytes are inhibited using monoclonal antibodies; and (3) Chimeric antigen receptor for T-regulatory (T-reg) cell therapy using genetically engineered T-reg cells targeting specific T-lymphocyte receptors for inducing anergy. From our real-world experience in transplant management in post-transplant lympho-proliferative disorders (PTLD), we noticed frequently a drastic reduction in the need of immunosuppressive medicines following lympho-ablative therapy for PTLD. We recently published a case study on a real-world experience transplant case where we explained a partial or prope tolerance that developed after lymphocyte ablation therapy, following which the allograft was maintained with low dose dual standard immunosuppressive medicines. Based on this publication, we propose here an innovative tolerance protocol for living related low risk kidney transplantation for developing countries, in this opinion review.
Core Tip: In this opinion review that is based on our recent publication, the core tip concentrates on achieving a partial or prope tolerance in renal allograft through sequential B and T lymphocyte depletion in an approved and in-practice strategy, for living related and low risk kidney transplantation. The allograft would require a half dose dual immunosuppressive therapy subsequently.
- Citation: Suhail SM. Tolerance protocol of living kidney transplant for developing countries through basic strategy of lymphocyte depletion. World J Transplant 2022; 12(6): 112-119
- URL: https://www.wjgnet.com/2220-3230/full/v12/i6/112.htm
- DOI: https://dx.doi.org/10.5500/wjt.v12.i6.112
Renal allograft, unlike autograft or isograft, would invoke rejection process through cellular and humoral immune mechanism by the nonself-antigen mediated alloimmune response. This results in rejection of the grafted organ unless immunosuppressive medicines targeting the donor/recipient T and B lymphocytes are in place. As opposed to the rejection process, tolerance is a state of unresponsiveness to the allograft, where the graft can be maintained without or with minimal immunosuppression. This is achieved by the use of effective innovative and aggressive immunosuppressive protocols[1].
Even though, safe and reliable strategies of achieving transplant tolerance are not in place, anecdotal reports and experimental animal studies targeting T and B lymphocyte ablation, offer hope[2]. However, these need cost and state-of-art infrastructures which are beyond the reach of end-stage renal failure patients in developing countries. Finding an innovative but cost-effective tolerance protocol remains an allusive goal for a successful transplant program for low economic zones.
In real-world experience (RWE) of transplant management when transplanted patients develop post-transplant lympho-proliferative disorders (PTLD), we noticed frequently a drastic reduction in the need of immunosuppressive medicines following lympho-ablative therapy for PTLD. Recently we published a case study of a living kidney transplant who achieved immunologic tolerance requiring low dose calcineurin inhibitor (CNI) with minimal prednisolone after the patient was treated by lympho-ablative therapy for Lymphoma that developed during the post-transplant period[3]. Based on this publication and our RWE with PTLD cases management[3], we would propose in this opinion review a partial or prope tolerance protocol that can be achieved through depletion of lymphocytes pre-emptively in low risk kidney transplant recipients. The added advantages being considered are the reduced requirements of stat-of-the-art technologies and reduced cost that are needed for achieving current desensitization and immunosuppressive protocols required for tolerance.
In anecdotal case reports, complete tolerance was achieved in subsequent renal allograft where bone marrow transplant was done in case of Multiple Myeloma (MM) patients with lymphocyte ablation done by radiation and chemotherapy prior to kidney transplantation from the marrow donor. The grafted kidney did not require immunosuppressive medicines afterward[4]. This is a kind of tolerance obtained because of a form of hematologic chimera thus developed during treatment of MM through allogeneic bone marrow transplant where host immune system was replaced by donor marrow.
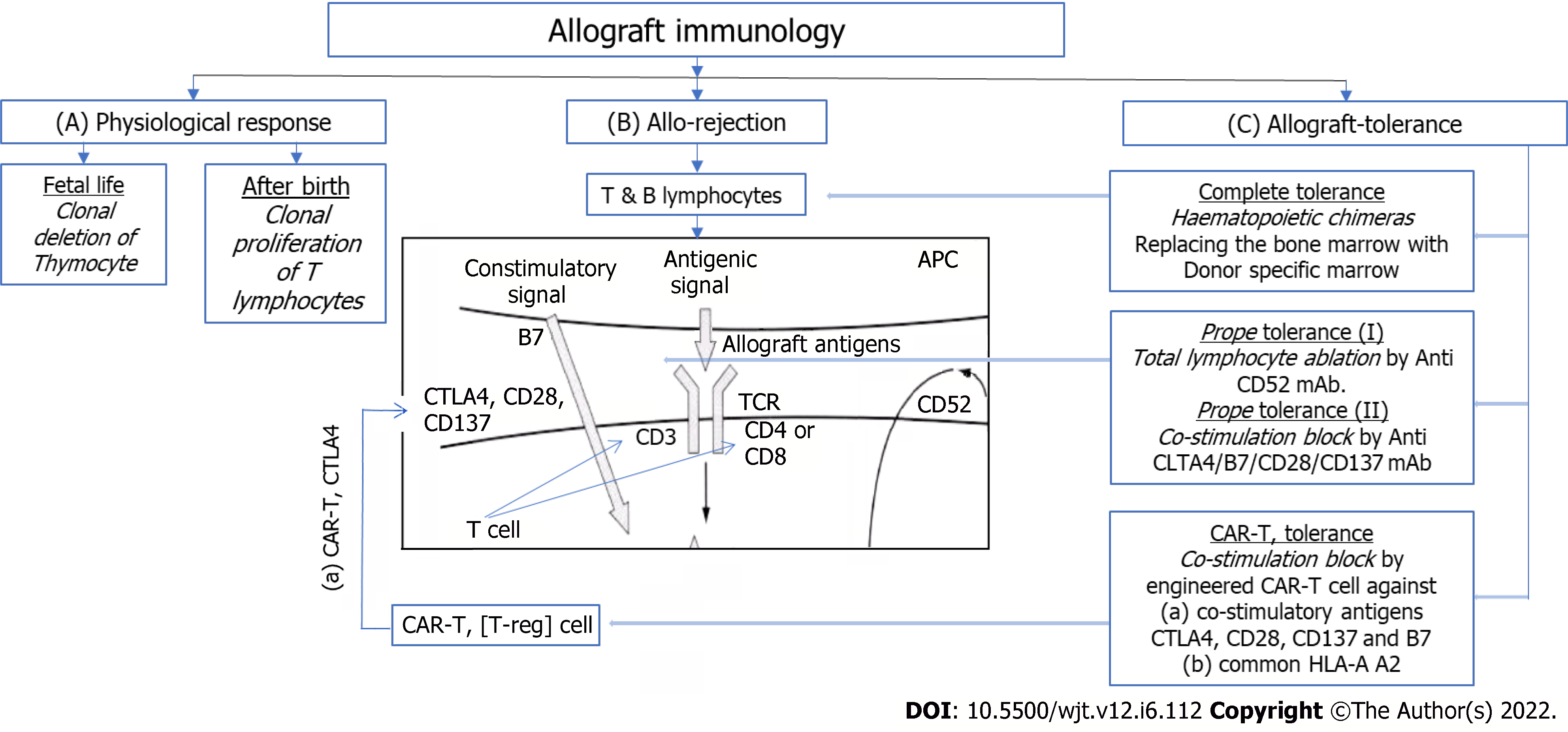
A brief outline of gross immunology physiology in fetal life and life after birth is presented in Figure 1A. Immune reactive cells undergo apoptosis on exposure of fetal self-antigens, thus leaving behind the cells which are naïve to any other foreign antigens. In life after birth, immune response shifts to proliferation and activation state in contrast to fetal state of apoptosis[5].
Thus immune cells show immune response by proliferating and reacting to foreign antigens and allograft, as shown in Figure 1B. This induces T-cell proliferation, and results in cell mediated cytotoxicity and inflammation that results in acute rejection unless immunosuppressive therapies are imposed[6].
Figure 1C summarizes the current research-based adoptable protocols for achieving anergy (tolerance). Firstly, achieving a state of hematologic chimera, in other ward, complete tolerance; Second, a state of partial or prope tolerance, where immunoreactive T-lymphocytes are depleted or suppressed; and third, the newer, CAR-T (Chimeric Antigen Receptor for T-reg therapy). T-reg cells are genetically manipulated to express co-stimulatory receptors on their surfaces, that results in blocking of co-stimulatory signal-2. This causes ablation of T-cell immunoreactivity resulting in anergy or tolerance.
Road to complete tolerance has not opened yet because of lack of available protocols.
Transplantation among monozygotic twins does not require immunosuppressive medications, hence is an example of complete tolerance[7].
Partial or prope tolerance is available using Campath-1H where allograft could be maintained with minimal immunosuppression with Low dose Cyclosporine-A (CSA) alone. CAMPATH-1H is monoclonal antibody (mAb) against CD52 antigen present on surface of all lymphocytes. Anti-CD52 mAb administration causes ablation of all lymphocytes that lasts for long period. The new lymphocytes that are subsequently produced from lymphoreticular tissues are naïve to the grafted kidney, inducing tolerance[8]. This was demonstrated in 3C, INTAC and other studies, showing promising evidences to tolerance[9]. This is costly and requires infrastructures where infections and patient safety protocols can be monitored. In many low economic zones, expected to be not feasible.
Current approach to tolerance is focused on inducing anergy to the reactive host or graft T-lymphocytes by blocking the co-stimulatory signal to CD-3 T-lymphocytes either by unique mAb against receptors for T-lymphocyte co-stimulation [CTLA-4 (cytotoxic T-lymphocyte associated antigen 4), CD28, B7, CD137]—the so called signal-2 co-stimulation, inducing T-lymphocyte anergy, or by CAR-T therapy targeting T-regulatory lymphocyte's CTLA-4 antigen, to block co-stimulation of CD3 T-lymphocytes, inducing tolerance (anergy) for all T-lymphocytes.
BENEFIT study used Belatacept, a selective co-stimulation blocking mAb against CTLA-4 mentioned above for inducing anergy, to show a partial tolerance[10]. But the results were not promising.
Most recently, research on CAR-T therapy targeting CTLA-4 co-stimulatory receptor on the CD-3 T-lymphocytes for induction of T-lymphocyte anergy, produced promising results in pancreatic islet cell graft, as well as cutaneous graft[11,12]. Furthermore, these therapies are exceedingly costly.
Highly sensitized recipients and marginal donors would impact the outcome of immunosuppression and concepts of tolerance.
A higher immunosuppressive protocol for graft survival is required for recipients with preformed antibodies against donor antigens that includes pre-transplant desensitization[13]. ABO incompatible recipient and recipient with donor specific antibodies requires desensitization protocol. Recipients with multiple blood transfusion recipients, multigravida, cases of repeat transplant, are highly immunogenic showing frequent cross-match positive results for both B and T-lymphocytes[14]. Consequently, tolerance protocols may not be appropriate for these groups of highly immunogenic recipients.
Organ donors with high immunogenicity are ABO incompatible and HLA mismatch donors, deceased donors, and harvested kidney with long cold ischemia time. These require increased immunosuppression[15,16]. In addition, may require desensitization protocol with cascade plasmapheresis and immuno-adsorbtion techniques. This is combined with use of various anti-lymphocyte antibodies and combination of potent immunosuppressive medicines. These protocols are available to be practised in targeted high risk kidney transplantation. Obviously achieving a successful protocol of tolerance could be a matter of ingenuity here.
The objectives of tolerance protocol are: (1) Minimum acute rejections; (2) minimum use of immunosuppressive medicines; (3) normal graft function; and (4) reduced short term and long term complications.
Shift to tolerance from conventional immunosuppression should be planned for minimally and normally immunogenic kidney donors and recipients, as described above. ABO compatible, better HLA matching, closer family members and matching body parameters are important considerations. All other donor recipient relationships are not appropriate for any tolerance protocol.
Available protocols for partial tolerance involve depletion of lymphocytes at the initial period of transplant surgery. The examples are, 3C, INTAC studies, where lymphocyte depletion was achieved using CAMPATH-1H mAb[8,9]. Sadly, lack of generalization and limiting factors of higher incidences of sepsis and malignancy limit their application[10]. Use of CAR-T therapy against T-lymphocyte receptors is also in infancy for renal transplantation[11,12]. For low socio-economic zones, nonetheless, they are irrelevant.
In RWE cases of PTLD, the point to note is depletion of lymphocytes with use of R-CHOP cycles for PTLD as mentioned in earlier sections. Profound lymphocytopenia and neutropenia that resulted from these R-CHOP therapy, required withdrawal of some immunosuppression like Mycophenolate Mofetil (MMF). The grafted kidney was subsequently maintained with a small dose of prednisone and a low dose of CSA[3].
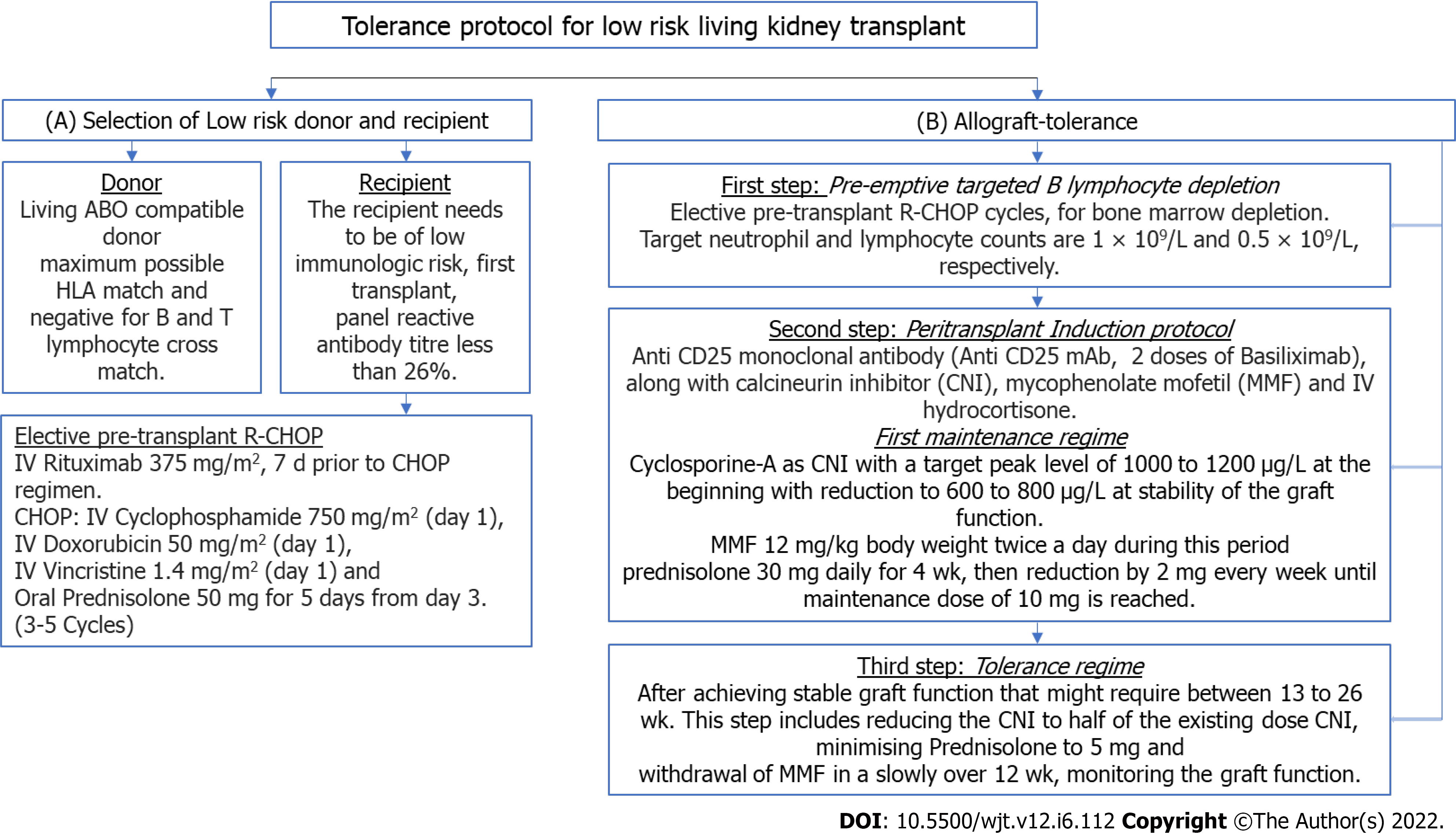
Thus we summarize the protocol in Figure 2 as follows: The protocol starts with selection of donor and recipient, as shown in Figure 2A—the donor would be living ABO compatible donor with maximum possible HLA match and negative for B and T-lymphocyte cross match. The recipient needs to be of low immunologic risk with Panel Reactive Antibody titer less than 26%.
The subsequent steps are shown in Figure 2B as follows: First step is elective bone marrow suppression with a few R-CHOP cycles as described, each cycle consisted of IV Rituximab, IV Cyclophosphamide, IV Doxorubicin and IV Vincristine. This is followed by oral Prednisolone 50 mg daily for 5 days. This cycle is repeated 3 to 6 times till the desired depletion of Lymphocytes is achieved as mentioned earlier[3].
Second step: For low risk renal transplant, induction with Anti-CD25 mAb along with MMF, CNI and IV Hydrocortisone (or Solumedrol) at standard doses till stable graft function is achieved. We used 2 doses of IV Basiliximab as anti-CD25 mAb 20 mg IV at interval of 4 d at induction. We used CSA as CNI with a target Peak level of 1000 to 1200 μg/L at the beginning with reduction to 600 to 800 μg/L at stability of the graft function. MMF was used at 12 mg/kg body weight twice a day during this period. We used Prednisolone 30 mg daily for 4 wk, then reduction by 2 mg every week until maintenance dose of 10 mg is reached.
Third step: After achieving stable graft function that might require between 13 to 26 wk, to reduce CNI to half of the existing dose (target peak level and trough levels, 300 and 50 μg/L respectively). Over time, Prednisolone to be reduced to 5 mg daily and MMF to be withdrawn slowly over 12 wk, monitoring the graft function[17].
Firstly, the use of R-CHOP therapy is validated as B-lymphocyte depleting treatment in Lympho-proliferative diseases as a standard therapy[3]. This was used in the RWE scenario for treating the PTLD that developed later. Subsequently, the allograft was maintained with low dose dual immunosuppression with stable graft function for long time. Following this practical experience, use of this B-lymphocyte depletion regime is aimed to achieve predominant B-lymphocyte depletion prior to transplant surgery. Subsequently following the transplant of the allograft, the recipient’s marrow would produce B-lymphocytes (now new host B-lymphocytes) that are naïve to the renal allograft antigens (resident antigens). Consequently, as the new host B-lymphocytes are naïve to the grafted resident antigens, it would not display humoral immune response against the graft tissue.
Secondly, the validity for using MMF and CNI at the beginning is to avoid incidence of acute cellular rejection by depleting resident and host T-lymphocytes at the engraftment period post-transplant[18]. New batch of T-lymphocytes are produced by lymphoreticular system that are naïve to the renal graft. Thus, the newer lymphocytes (host T-lymphocytes), appear to take the allograft antigens (resident antigens) as self, thus do not cause cellular immune rejection.
Thirdly, B-lymphocyte depletion in a sequential manner as above before transplant surgery followed by immediate post-transplant T-lymphocyte depletion by anti CD25 mAb with CSA and MMF, enables the host acquire a state of prope tolerance to the renal allograft that was observed in the RWE scenario. The dual immunosuppressive medicines at lower dose maintain the graft and avoids long and short term complications of currently used medicines[19].
Lastly, risk of infection post-lymphocyte depletion, as described, would be similar to current existing strategies used in high risk renal transplant programs as well as same as lymphocyte ablative therapies used in Lymphoma. Paradoxically, the risk of infection would be rather reduced following the cycle of lymphocyte depletion strategy as mentioned, because the strategy is time limited. This therapy would be followed by rather a reduced and dual immunosuppressive low CNI trough level therapy to maintain the renal graft. In practical situations of Lymphoma treatment, infection and recurrent malignancies are rather infrequent. In the RWE case and several other similar situations, recurrent malignancies and infections were not of frequent impediments.
Current transplant protocols with newer monoclonal antibodies, desensitization procedures and newer drugs, may impact disastrously in many programs of transplantation[18]. Nevertheless, kidney transplant is considered best renal replacement therapy in End-stage kidney failure (ESKD).
For a sustainable transplant program guideline-based immunosuppressive regimens and opinion based protocols are required for highly immunogenic donor-recipient relationship. The parody lies in the disparity of the economics and infrastructures for provision, and extent of ESKD cases in developing regions. In such situation, an alternative approach may be considered.
This tolerance protocol could be suitable and applicable in RWE situations for low risk transplant scenario. In developing countries ethics committee may contribute to the feasibility of low risk living renal transplantation for maintaining a reasonable transplant program to reduce the burden of ESKD at lower cost and feasible infrastructures.
We aimed at a sequential lymphocyte depletion therapy rather than an ablative therapy. The sequence starts with B lymphocyte depletion with cycles of R-CHOP therapy to achieve the target Neutrophil and lymphocyte levels, pre-transplant. Following living kidney donation (LKD) transplant with a low immunogenic donor-recipient risk-relation, standard triple immunosuppressive protocol with CNI, MMF and prednisolone will resume for achieving stable graft function. This will be followed by step wise and monitored reduction of immunosuppression to a half trough level CNI and minimum alternate day Prednisolone regimen. Thus, episodes of immediate acute rejections are minimized and a prope or partial tolerance with low dose dual immunosuppressive strategy is achieved.
The strategy of CNI half trough level as described, and alternate day low dose prednisolone is described as prope or partial tolerance. The monitoring of this tolerance would be the regular monitoring of graft function by serum creatinine levels and hematuria and proteinuria levels. In essence, it is the equivalent monitoring of a standard graft kidney.
This strategy to induce partial or prope tolerance, even though is meant for facilitating low risk LKD transplant in developing countries for reasons explained in the epilog, in fact, it will benefit the recipients world-wide. I would rather think that developed countries are better equipped with ancillary supportive infrastructure to consider this proposed protocol.
In the abstract, a detailed background introduction was mentioned in order to simplify the understanding of issues related to scope of transplant needs, especially in developing countries with marked limitations in infrastructure, finance, and scarcity of dialysis facilities for an increasing population of ESKD. To maintain a universal understanding of different stakeholders of chronic kidney disease, the article did a little elaboration before focusing on the strategy of partial tolerance.
In our recent publication[3], we discussed the real world experience scenario renal transplant case who achieved prope or partial tolerance requiring a low dose dual immunosuppression following B lymphocyte depletion therapy for PTLD. In this opinion review, we extrapolate that B lymphocyte depletion protocol to living kidney transplant of low immunogenic risk. Considering the impact of ESKD burden in developing nations, respective transplant societies with their corresponding ethics committee, would consider this proposed protocol for low risk living kidney transplant program.
We felt impulse for attracting relevant transplant organizations in particular, in the developing nations, where discrepancy in the availability of infrastructure for state-of-the-art technology for immunosuppressive protocols and the ESKD burden, makes a successful transplant program, difficult. With that view in mind we progressed to this opinion review based on our recent publication on this subject[3]. The opinion and conclusion of this opinion review are those of the author only.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Transplantation
Country/Territory of origin: Singapore
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gong N, China; Gong N, China; Ietto G, Italy S-Editor: Chang KL L-Editor: A P-Editor: Chang KL
| 1. | Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523-532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2593] [Cited by in RCA: 2470] [Article Influence: 145.3] [Reference Citation Analysis (0)] |
| 2. | Calne R, Moffatt SD, Friend PJ, Jamieson NV, Bradley JA, Hale G, Firth J, Bradley J, Smith KG, Waldmann H. Campath IH allows low-dose cyclosporine monotherapy in 31 cadaveric renal allograft recipients. Transplantation. 1999;68:1613-1616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 228] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 3. | Suhail SM, Woo KT. Paradigm Shift to Tolerance from Conventional Immunosuppression in Renal Transplant: A Basic Strategy through Lymphocyte Depletion. J Nephrol Ren Dis. 2020;4:1. [DOI] [Full Text] |
| 4. | Bühler LH, Spitzer TR, Sykes M, Sachs DH, Delmonico FL, Tolkoff-Rubin N, Saidman SL, Sackstein R, McAfee S, Dey B, Colby C, Cosimi AB. Induction of kidney allograft tolerance after transient lymphohematopoietic chimerism in patients with multiple myeloma and end-stage renal disease. Transplantation 2002; 74: 1405-1409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 202] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 5. | Ander SE, Diamond MS, Coyne CB. Immune responses at the maternal-fetal interface. Sci Immunol. 2019;4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 415] [Article Influence: 69.2] [Reference Citation Analysis (0)] |
| 6. | Ciancio G, Burke GW, Gaynor JJ, Carreno MR, Cirocco RE, Mathew JM, Mattiazzi A, Cordovilla T, Roth D, Kupin W, Rosen A, Esquenazi V, Tzakis AG, Miller J. A randomized trial of three renal transplant induction antibodies: early comparison of tacrolimus, mycophenolate mofetil, and steroid dosing, and newer immune-monitoring. Transplantation. 2005;80:457-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 165] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 7. | Niederhuber J, Feller I. Permanent skin homografting in identical twins. Arch Surg. 1970;100:126-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 8. | Wang H, Dong J, Zuo L, Liu J, Zhu W, Li Y, Gu L, Zhao J, Zhang L, Gong J, Zhang W, Li N, Li J. Anti-mouse CD52 monoclonal antibody ameliorates iron-deficient anaemia in IL-10 knockout mice. Br J Nutr. 2014;111:987-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Hanaway MJ, Woodle ES, Mulgaonkar S, Peddi VR, Kaufman DB, First MR, Croy R, Holman J; INTAC Study Group. Alemtuzumab induction in renal transplantation. N Engl J Med. 2011;364:1909-1919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 268] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 10. | Vincenti F, Charpentier B, Vanrenterghem Y, Rostaing L, Bresnahan B, Darji P, Massari P, Mondragon-Ramirez GA, Agarwal M, Di Russo G, Lin CS, Garg P, Larsen CP. A phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study). Am J Transplant. 2010;10:535-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 722] [Cited by in RCA: 720] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 11. | Boardman DA, Philippeos C, Fruhwirth GO, Ibrahim MA, Hannen RF, Cooper D, Marelli-Berg FM, Watt FM, Lechler RI, Maher J, Smyth LA, Lombardi G. Expression of a Chimeric Antigen Receptor Specific for Donor HLA Class I Enhances the Potency of Human Regulatory T Cells in Preventing Human Skin Transplant Rejection. Am J Transplant. 2017;17:931-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 244] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 12. | Salomon B, Bluestone JA. Complexities of CD28/B7: CTLA-4 costimulatory pathways in autoimmunity and transplantation. Annu Rev Immunol. 2001;19:225-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 817] [Cited by in RCA: 801] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 13. | Amico P, Hönger G, Mayr M, Steiger J, Hopfer H, Schaub S. Clinical relevance of pretransplant donor-specific HLA antibodies detected by single-antigen flow-beads. Transplantation. 2009;87:1681-1688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 207] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 14. | Couzi L, Araujo C, Guidicelli G, Bachelet T, Moreau K, Morel D, Robert G, Wallerand H, Moreau JF, Taupin JL, Merville P. Interpretation of positive flow cytometric crossmatch in the era of the single-antigen bead assay. Transplantation. 2011;91:527-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Stuart F. Overview of living and deceased organ donors, immunosuppression, and outcomes. In: Stuart F, Abecassis M, Kaufman D. Organ transplantation. Georgetown: Landes Bioscience, 2000: 52-53. [DOI] [Full Text] |
| 16. | Al Meshari K, Pall A, Chaballout A, El Gamal H, Al Mana H, Humaidan H, Alzayer F, Al Talhi M. Outcome of desensitization in human leukocyte antigen- and ABO-incompatible living donor kidney transplantation: a single-center experience in more than 100 patients. Transplant Proc. 2013;45:1423-1426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Marfo K, Lu A, Ling M, Akalin E. Desensitization protocols and their outcome. Clin J Am Soc Nephrol. 2011;6:922-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 185] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 18. | Halloran P, Mathew T, Tomlanovich S, Groth C, Hooftman L, Barker C. Mycophenolate mofetil in renal allograft recipients: a pooled efficacy analysis of three randomized, double-blind, clinical studies in prevention of rejection. The International Mycophenolate Mofetil Renal Transplant Study Groups. Transplantation. 1997;63:39-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 456] [Cited by in RCA: 426] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 19. | Vincenti F, Ramos E, Brattstrom C, Cho S, Ekberg H, Grinyo J, Johnson R, Kuypers D, Stuart F, Khanna A, Navarro M, Nashan B. Multicenter trial exploring calcineurin inhibitors avoidance in renal transplantation. Transplantation. 2001;71:1282-1287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 207] [Article Influence: 8.6] [Reference Citation Analysis (0)] |