Published online Apr 18, 2022. doi: 10.5500/wjt.v12.i4.65
Peer-review started: March 11, 2021
First decision: June 7, 2021
Revised: July 7, 2021
Accepted: April 3, 2022
Article in press: April 3, 2022
Published online: April 18, 2022
Processing time: 397 Days and 20.8 Hours
Gigantism, characterized by excessive growth and height is due to increased secretion of growth hormone, most commonly from a pituitary adenoma. In addition to the surgical and anesthetic complexity, the extreme stature of these patients presents a unique challenge for kidney transplantation in deciding whether to proceed with a single or dual kidney transplantation. The lack of relevant literature further adds to the dilemma.
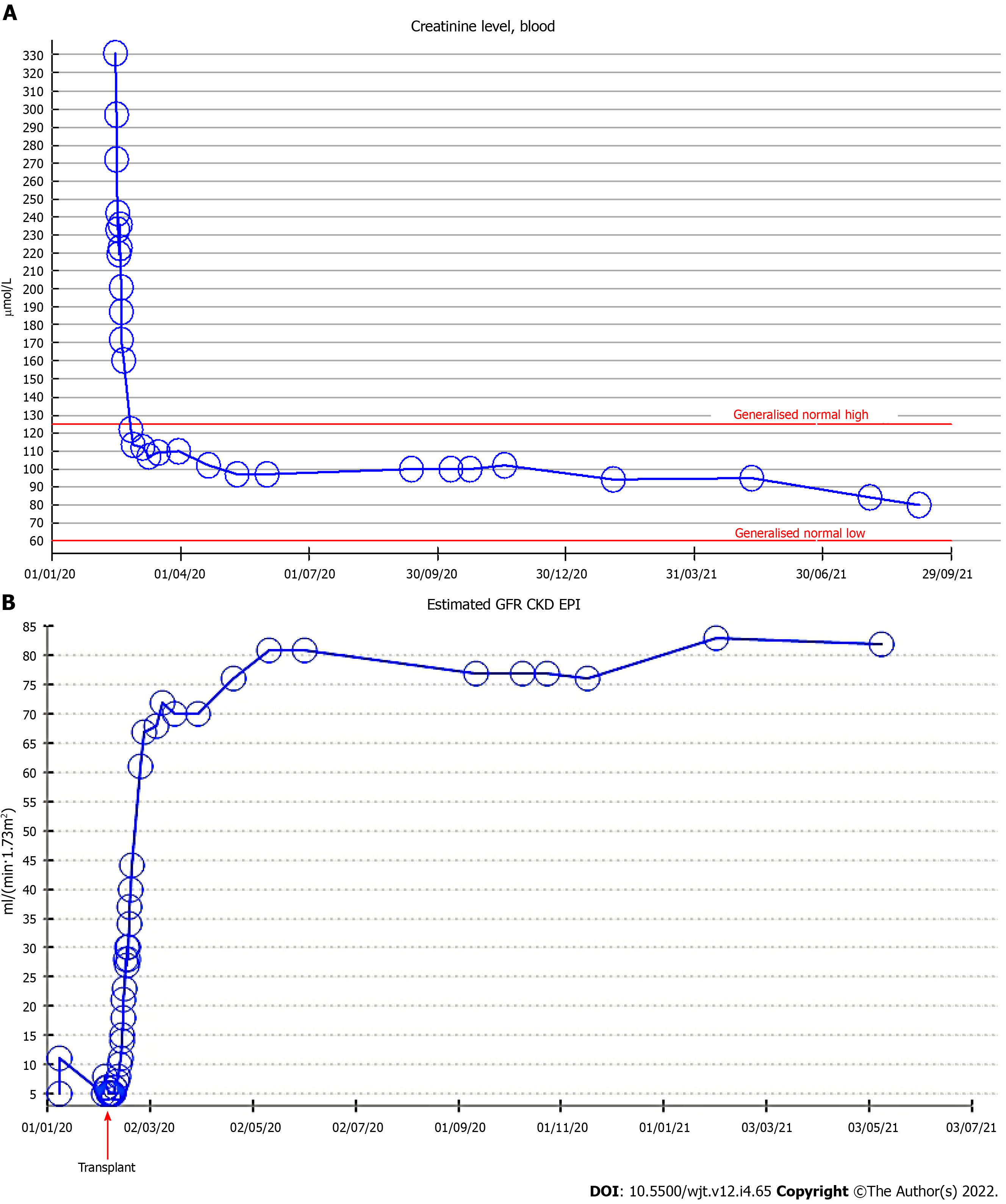
A 45-year-old patient with untreated gigantism and end stage renal failure on renal replacement therapy was waitlisted for a deceased donor dual kidney transplantation due to the extreme physical stature (Height-247 cm and weight-200 kg). He was offered 2 kidneys from a 1-0-1 HLA mismatched 24-year-old DCD donor (Height-179 cm and weight-75 kg), and was planned for a bilateral retroperitoneal implantation into the recipient external iliac vessels. The immunosuppression consisted of alemtuzumab induction (50 mg) and steroid-free maintenance with tacrolimus. The donor’s right kidney was uneventfully implanted extra-peritoneally into the right external iliac vessels. On contralateral exposure, the left common and external iliac arteries were ectatic and frail. A complex vascular reconstruction was not preferred in order to preserve the arterial supply to the left lower limb, to minimise the cold ischemia time and prevent additional warm ischemic insult to the second kidney. Hence, it was decided not to proceed with dual transplantation. Amidst concerns of nephron mass insufficiency, the graft function was remarkable with a serum creatinine of 120 µmol/L within a month from transplantation and 94 µmol/L at 1-year post transplantation, and without proteinuria.
To our knowledge, this is the first case report on kidney transplantation in gigantism. Although it is believed that dual kidney transplantation is ideal, a single kidney transplantation from an appropriately selected donor can provide sufficient functioning nephron mass in patients with gigantism.
Core Tip: We report a patient with untreated pituitary gigantism with end stage renal failure due to IgA nephropathy with secondary focal segmental glomerular sclerosis who underwent a successful deceased donor kidney transplantation. We have described the intra-operative challenges in deciding whether to proceed with a single kidney transplantation or dual kidney transplantation. To the best of our knowledge this is the first case report on kidney transplantation in gigantism.
- Citation: Gopal JP, Charalampidis S, Xiang J, Dor FJMF, Papalois VE. Renal transplantation in gigantism: A case report. World J Transplant 2022; 12(4): 65-71
- URL: https://www.wjgnet.com/2220-3230/full/v12/i4/65.htm
- DOI: https://dx.doi.org/10.5500/wjt.v12.i4.65
Gigantism is a disorder resulting from increased growth hormone secretion before the fusion of growth plate, most often due to a pituitary adenoma and is characterized by excessive growth and height. In addition to the surgical and anesthetic complexity, the extreme stature of these patients presents a particular challenge for kidney transplantation in deciding whether to proceed with a single or dual kidney transplantation. So far there is no literature on kidney transplantation in patients with gigantism. To our knowledge, we are the first to report a patient with untreated pituitary gigantism characterized by uniquely extreme physical size and stature who underwent a successful kidney transplantation for end stage renal failure and discuss the dilemmas involved in his management.
Our patient, a 45-year-old African male presented with end stage renal failure and was awaiting a kidney transplant.
The end stage renal failure was due to IgA nephropathy which was biopsy proven and with secondary focal segmental glomerular sclerosis. He was established on haemodialysis.
He was diagnosed in his early teenage years to have pituitary gigantism but was left untreated.
There were no relevant histories.
His physical stature [Height = 247 cm, weight = 200 kg, body mass index (BMI) was 33 kg/m2, and body surface area (BSA) with the DuBois formula = 3.7 m2], was twice than the normal upper limit.
The laboratory investigations were not relevant apart from deranged kidney function due to end stage renal failure.
A pre-transplant computed tomography (CT) scan of the abdomen and pelvis showed normal iliac vessels bilaterally.
There was a therapeutic dilemma as to whether a single kidney transplantation would be sufficient for a patient of his body surface to alleviate his kidney failure to a degree that would not require further renal replacement therapy. With the above dilemma into consideration the patient was added to the United Kingdom deceased donor kidney transplant waiting list for dual kidney transplantation from a single deceased donor after discussion in our multidisciplinary team (MDT) meeting and approval by the NHS Blood and Transplant’s Kidney Advisory Group.
Less than a year after being waitlisted and after having received a few offers from extended criteria donors that were deemed unsuitable, the patient received and accepted a deceased donor dual kidney transplant offer from a 24-year-old male donation after circulatory death (DCD) donor who suffered irreversible hypoxic brain injury following a road traffic accident. The donor’s past medical history was insignificant and had normal kidney function. His height was 179cm, weight was 75kgs, BMI was 23 kg/m2, and BSA with the DuBois formula was 1.9 m2 (almost half of our prospective recipient). Furthermore, there was a 1-0-1 HLA mismatch between the donor and recipient, the latter of which had a calculated reaction frequency of 0% (and therefore only a virtual crossmatch was performed). Immunosuppression consisted of induction with a depleting monoclonal antibody, alemtuzumab (50 mg) and steroid-free maintenance with tacrolimus as the only immunosuppressant. The organ retrieval in the donor hospital was uneventful.
For the recipient, a customised anaesthetic protocol was implemented based on previous general anaesthetic experience with the patient. He was ventilated using a large (size 6) oropharyngeal airway and a large (size 6) face mask. A long Macintosh blade and size 10 endotracheal tube was used for intubation. Patient was anaesthetized on a hover mattress to enable safe transfer to the operating table, which can take up to 300 kgs body weight. The only issue was the patient’s height; two table extensions were added to the operating table on either side and an instrument trolley was used to support the feet (Figure 1).
A bilateral extraperitoneal implantation in to the recipient’s external iliac vessels was chosen as the preferred implantation technique. Initially the patient’s right external iliac vessels were exposed and an uneventful implantation of the donor’s right kidney was successfully completed with intraoperative urine production from the transplanted kidney. The cold ischemia time was 9 h and 52 min. On subsequent exposure of the recipient’s contralateral iliac fossa, the left common and external iliac arteries were noted to be significantly ectatic and frail, which was not apparent from the pre-operative CT scan. In addition there were abnormal intraoperative Doppler signals (monophasic signals).
Implanting the donor’s left kidney in to the right common/internal iliac vessels was one of the options, but it would involve clamping the right common iliac artery which would potentially add an additional ischemic insult to the transplanted kidney. Implanting the donor’s left kidney into the left internal iliac vessels or intra-peritoneal implantation into the aorta/inferior vena cava were the other options. In order not to further extend the duration of the procedure and the resultant cold ischemia time by performing a complex vascular reconstruction/implantation with potential compromise to the arterial supply of the recipient’s left lower limb and in view of the already completed successful single kidney implantation of the donor’s right kidney, the decision was made to not to proceed with the dual kidney implantation. The donor kidney was of average size without having taken proper measurements. According to the national allocation policy of NHS Blood and Transplant, the donor’s left kidney was subsequently offered to another patient on the waiting list.
The patient was extubated at the end of the operation and was cared in intensive care unit for one day and subsequently stepped down to our high dependency unit. There was primary graft function and following an overall uneventful recovery, he was discharged from the hospital at day 15, with an improving estimated glomerular filtration rate (eGFR) of 44 mL/min/1.73 m2. Following discharge, his serum creatinine continued to improve to 120 µmol/L within a month from the procedure. His serum creatinine remained stable throughout the first year post-transplant and without proteinuria (Figure 2).
Although giants are depicted in literature as individuals with lionized capabilities, the description of patients with gigantism in medical literature is very limited due to the rarity of the condition. Overproduction of growth hormone by a pituitary adenoma or pituitary hyperplasia can lead to pituitary gigantism. They can be either sporadic or can occur as a part of several genetic disorders such as multiple endocrine neoplasia type 1, McCune-Albright syndrome and carney complex[1-5]. There is no bibliographic report of pituitary gigantism patients that required renal transplantation for end stage renal failure.
The underlining challenge in such patients with extreme stature is to ensure that the physiological capacity and the functioning nephron mass of the donor organ can meet the increased metabolic needs of this unique recipients so to alleviate their need for renal replacement therapy and have a significant positive impact on their overall health, quality of life, and life expectancy. Recent studies have confirmed that the graft kidney volume/recipient BSA ratio along with the donor age and recipient’s gender are independent predictors of recipient GFR in the early post-transplant period[4-6]. Considering the above concern, the option of synchronous dual deceased kidney transplantation in such extreme stature patients seems reasonable and needs to be considered at the time of wait listing them for a deceased donor kidney transplant and weighted against depriving the second graft from another potential recipient given the current scarcity of deceased donor organs.
Although there are some variations between jurisdictions in the allocation policy of kidneys for dual kidney transplantation, the common theme is to allocate kidneys from extended criteria donors for dual transplantation[7]. There was a special consideration for our patient due to his body habitus following MDT discussions and discussions in the national kidney advisory group of NHS Blood and Transplant, and hence was listed for dual kidney transplantation and ultimately received an offer from a young donor. Various implantation techniques have been described for dual kidney transplantation[7]. Although it has been reported that the complication rates for bilateral and unilateral placement of kidneys are similar[8,9], a bilateral extraperitoneal approach was chosen based on the operating surgeon’s preference.
Vascular calcifications and atherosclerosis are well established complications of end stage renal failure[10], but it is unusual for isolated arterial aneurysm or ectasia to occur due to renal failure. Despite the intra-operative finding of ectasia and frailty of the left common and external iliac arteries, we still had several options for proceeding with dual kidney transplantation. Implanting the donor’s left kidney in to the right common/internal iliac vessels was one of the options, but it would involve clamping the right common iliac artery which would potentially add an additional ischemic insult to the transplanted kidney. Implanting the donor’s left kidney into the left internal iliac vessels or intra-peritoneal implantation into the aorta/inferior vena cava were the other options. In order not to further extend the duration of the procedure and the resultant cold ischemia time by performing a complex vascular reconstruction/implantation with potential compromise to the arterial supply of the recipient’s left lower limb and in view of the already completed successful single kidney implantation of the donor’s right kidney, the decision was not to proceed with the dual kidney implantation.
Although it has been reported that transplanting small kidneys in to large recipients (donor kidney weight/recipient body weight < 2 g/kg) causes hyperfiltration and results in decreased creatinine clearance and proteinuria 6-months after transplantation[11], in our patient, the single kidney graft has proved sufficient enough to keep him off renal replacement therapy and with normal serum creatinine levels as noted at 1-year post-transplant, and without proteinuria. Additionally, the second kidney from the same deceased donor was eventually implanted to a different recipient in our centre who benefited from an equally good graft function.
Especially in rare cases such that of our patient with extreme physical stature and significant comorbidities one cannot overemphasize the importance of a detailed preoperative assessment and preparation. There are a few general considerations for people with gigantism undergoing transplantation such as a thorough multidisciplinary work up including anaesthetic pre-assessment before wait listing, the design of a customised anaesthesia protocol, modification of the operating table, and arranging an appropriately sized bed post-operatively. Furthermore, every individual organ offer needs to be assessed for suitability in regards to the donor’s past medical history, renal function, age, as well as the body mass index and potentially total kidney volume calculated through any appropriate donor imaging available at the time.
This is the first case report on kidney transplantation in gigantism. Although it is believed that dual kidney transplantation is ideal for such patients based on body surface area, a single kidney transplantation from an appropriately selected donor can provide sufficient functioning nephron mass in patients with gigantism.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Transplantation
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Koukoulaki M, Greece S-Editor: Gong ZM L-Editor: A P-Editor: Gong ZM
| 1. | Xekouki P, Azevedo M, Stratakis CA. Anterior pituitary adenomas: inherited syndromes, novel genes and molecular pathways. Expert Rev Endocrinol Metab. 2010;5:697-709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 2. | Agarwal SK, Ozawa A, Mateo CM, Marx SJ. The MEN1 gene and pituitary tumours. Horm Res. 2009;71 Suppl 2:131-138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Vezzosi D, Vignaux O, Dupin N, Bertherat J. Carney complex: Clinical and genetic 2010 update. Ann Endocrinol (Paris). 2010;71:486-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Lee JH, Won JH, Oh CK. Impact of the ratio of graft kidney volume to recipient body surface area on graft function after live donor kidney transplantation. Clin Transplant. 2011;25:E647-E655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Lee CK, Yoon YE, Choi KH, Yang SC, Lee JS, Joo DJ, Huh KH, Kim YS, Han WK. Clinical implications for graft function of a new equation model for the ratio of living donor kidney volume to recipient body surface area. Korean J Urol. 2013;54:870-875. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Jinfeng L, Jia L, Tao G, Wenjun S, Xinlu P, Yonghua F, Guiwen F. Donor kidney glomerular filtration rate and donor/recipient body surface area ratio influence graft function in living related kidney transplantation. Ren Fail. 2015;37:576-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Snanoudj R, Timsit MO, Rabant M, Tinel C, Lazareth H, Lamhaut L, Martinez F, Legendre C. Dual Kidney Transplantation: Is It Worth It? Transplantation. 2017;101:488-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Rigotti P, Capovilla G, Di Bella C, Silvestre C, Donato P, Baldan N, Furian L. A single-center experience with 200 dual kidney transplantations. Clin Transplant. 2014;28:1433-1440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 9. | Ekser B, Furian L, Broggiato A, Silvestre C, Pierobon ES, Baldan N, Rigotti P. Technical aspects of unilateral dual kidney transplantation from expanded criteria donors: experience of 100 patients. Am J Transplant. 2010;10:2000-2007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 75] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 10. | Campean V, Neureiter D, Varga I, Runk F, Reiman A, Garlichs C, Achenbach S, Nonnast-Daniel B, Amann K. Atherosclerosis and vascular calcification in chronic renal failure. Kidney Blood Press Res. 2005;28:280-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Giral M, Nguyen JM, Karam G, Kessler M, Hurault de Ligny B, Buchler M, Bayle F, Meyer C, Foucher Y, Martin ML, Daguin P, Soulillou JP. Impact of graft mass on the clinical outcome of kidney transplants. J Am Soc Nephrol. 2005;16:261-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 3.2] [Reference Citation Analysis (0)] |