Published online Jun 18, 2021. doi: 10.5500/wjt.v11.i6.203
Peer-review started: February 7, 2021
First decision: March 17, 2021
Revised: March 17, 2021
Accepted: May 20, 2021
Article in press: May 20, 2021
Published online: June 18, 2021
Processing time: 125 Days and 0.8 Hours
Hyperkalemia is a recognized and potentially life-threatening complication of heart transplantation. In the complex biosystem created by transplantation, recipients are susceptible to multiple mechanisms for hyperkalemia which are discussed in detail in this manuscript. Hyperkalemia in heart transplantation could occur pre-transplant, during the transplant period, or post-transplant. Pre-transplant causes of hyperkalemia include hypothermia, donor heart preservation solutions, conventional cardioplegia, normokalemic cardioplegia, continuous warm reperfusion technique, and ex-vivo heart perfusion. Intra-transplant causes of hyperkalemia include anesthetic medications used during the procedure, heparinization, blood transfusions, and a low output state. Finally, post-transplant causes of hyperkalemia include hemostasis and drug-induced hyperkalemia. Hyperkalemia has been studied in kidney and liver transplant recipients, but there is limited data on the incidence, causes, management, and prevention in heart transplant recipients. Hyperkalemia is associated with an increased risk of hospital mortality and readmission in these patients. This review describes the current literature pertaining to the causes, pathophysiology, and treatment of hyperkalemia in patients undergoing heart transplantation and focuses primarily on post-heart transplantation.
Core Tip: Hyperkalemia is a potentially life-threatening complication of heart transplantation. Recipients of heart transplant are susceptible to hyperkalemia via multiple mechanisms both during transplantation, as well as in the pre- and post-transplant periods. Hyperkalemia has been well studied in kidney and liver transplan
- Citation: Singh J, Kichloo A, Vipparla N, Aljadah M, Albosta M, Jamal S, Ananthaneni S, Parajuli S. Hyperkalemia: Major but still understudied complication among heart transplant recipients. World J Transplant 2021; 11(6): 203-211
- URL: https://www.wjgnet.com/2220-3230/full/v11/i6/203.htm
- DOI: https://dx.doi.org/10.5500/wjt.v11.i6.203
The gold standard for treatment of end-stage heart disease remains orthotopic heart transplantation[1]. Gradually changing approaches to managing perioperative, intraoperative, and postoperative variables for heart transplantation have allowed the survival of primary heart transplant recipients at day 30, 1 year, and 5 years to approach 90.3%, 82.4%, and 69.7%, respectively[2]. These approaches include improved heart preservation techniques, criteria for selection of the donor and recipient, improved surgical techniques, diverse and more potent choices of immuno
Hyperkalemia, specifically, is a recognized and potentially life-threatening compli
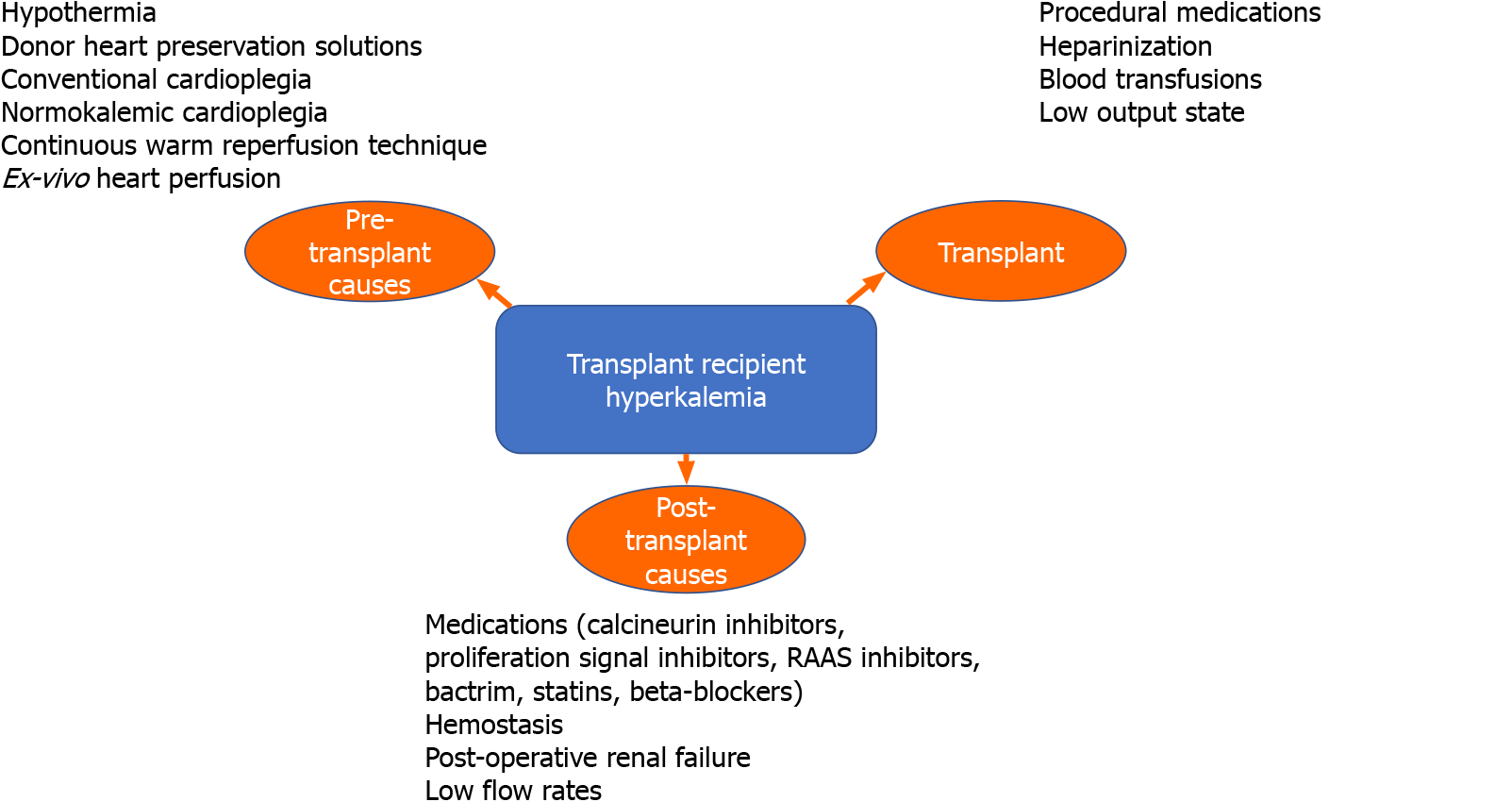
As discussed above, hyperkalemia causes in the setting of heart transplant can be classified into donor and recipient. Donor causes are mainly pre-transplant in origin and include maintaining the heart in a hypothermic state and the use of certain preservative and cardioplegic solutions. Recipient causes can be classified into transplant or post-transplant (Figure 1).
Hypothermia is crucial to preserving donor grafts prior to organ transplantation as it reduces ischemic cellular damage. Decreasing donor organ temperature from 37 °C to 4 °C results in a 12-fold decrease in metabolic demand[5,6]. However, hypothermia can lead to sodium-potassium channel alterations, cellular energy depletion, dysregulation of calcium homeostasis, mitochondrial perturbations, xanthine oxidase accumulation, and increased levels of reactive oxygen species which may impair cellular viability[6]. Therefore, preservative solutions have been implemented for cellular protection. Some of these solutions have high potassium levels which adversely affect the endothelium and membrane transport.
There are over 167 solutions available for preservation of donor grafts[7]. The concentration of potassium in these solutions can range from 10 to 20 mmol/L and can be as high as 140 mmol/L[7-9]. The three major mechanisms of heart endothelium-dependent relaxation that these cardioplegic preservative solutions induce are via cyclooxygenase enzymes, nitric oxide, and endothelium-derived hyperpolarizing factor, which is an aspect of potassium channels. Initially, studies performed on rat hearts showed that infusing hyperkalemic cardioplegic solutions can damage coronary endothelium[10]. However, subsequent studies on porcine and rabbits demonstrated tolerance of coronary endothelium to hyperkalemia in transplant preservation for up to four hours without disruption of endothelium-dependent relaxation[11-13]. Even further studies demonstrated that exposure of porcine coronary arteries to hyper
Conventional cardioplegic solutions rely on hyperkalemia to depolarize the membrane and achieve systolic arrest[7]. The hyperkalemic solution directly contracts the vascular endothelium during cardiac arrest of the donor organ[7]. It also results in an increase in intracellular sodium via non-activating sodium currents that may exacerbate calcium overload during reperfusion[17]. Increase in calcium concentration can also decrease myocardial contractile function, beta adrenergic responsiveness, and active relaxation[18,19].
Normokalemic adenosine-lidocaine cardioplegic solutions contain lidocaine that blocks fast sodium channels, which can cause diastolic arrest. Meanwhile, adenosine maintains a polarized membrane potential. It has been observed that ischemic rat hearts re-perfused with adenosine-lidocaine cardioplegia show improved cardiac function when compared to traditional hyperkalemic cardioplegia. Furthermore, Hamano et al[20] discovered polarized arrests using potassium channel openers minimized calcium overload and improved myocardial function. It was demonstrated that hearts treated with a potassium channel opener had improved transplant outcomes.
In an earlier retrospective study on heart transplantation, researchers found that continuous warm re-perfusion technique during implantation of donor’s heart enhances myocardial preservation. Ischemic time shortened by 31 min, suturing time lasted 12 min longer, ionotropic support duration decreased, length of intensive care and stay in hospital declined, ischemic damage in the first biopsy of the endomyo
Ex-vivo heart perfusion (EVHP) has been used to increase the donor pool by facilitating resuscitation of a donor’s heart after cardiocirculatory death. Cardioprotective EVHP uses a tepid adenosine-lidocaine cardioplegic solution to minimize myocardial injury by maintaining polarized resting membrane potential through diastolic arrest. The cardioprotective properties of EVHP occur by three different mechanisms: (1) by inhibiting apoptosis; (2) by its anti-inflammatory properties; and (3) by minimizing oxidative stress and improving posttransplant function. Ongoing research with Nicorandil, which is an ATP sensitive potassium channel opener and causes an independent outward current shortening the duration of the action potential, has shown to lead to hyperpolarization, reduced myocyte injury caused by ischemia, and long-term cardiac preservation[23].
Several factors such as anesthetic medications used during the procedure, heparinization, blood transfusions, and low output state can trigger hyperkalemia during or immediately after the procedure. Avoiding or limiting the use of medications such as succinylcholine, measures to prevent blood loss, and maintenance of stable vitals during the procedure can all prevent hyperkalemia during an operation.
Hemostasis in the post-heart surgery period can commonly cause hyperkalemia, mainly due to the high potassium levels in cardioplegic solutions along with postoperative renal failure and low flow rates. Drug-induced hyperkalemia is another cause of hyperkalemia after heart transplantation. This can result from angiotensin converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), trimethoprim/sulfamethoxazole, calcineurin inhibitors (CNIs), and other medications that are used after heart transplantation to prevent rejection, as well as for infection prophylaxis.
Recipients undergoing whole organ transplantation who receive CNIs, such as Cyclosporine (CsA) or Tacrolimus (FK506), encounter hyperkalemia as a frequent complication. The incidence of hyperkalemia in kidney transplant recipients receiving CsA varies from 25%-40%. The incidence of hyperkalemia in liver transplant recipients on FK506 is 40%. The mechanism of hyperkalemia in recipients on the above medications is not clear. However, hyperkalemia due to nephrotoxicity is well reported in liver and kidney transplant recipients and only recently in heart transplantation recipients[18,19,24]. With nephrotoxicity, these drugs may cause a syndrome similar to hyporeninemic hypoaldosteronism with decreased release of aldosterone, decreased tubular absorption of potassium by inhibiting sodium-potassium ATPase activity, decreased mineralocorticoid receptor expression, and direct inhibition of potassium channels in collecting tubules[22,24-27]. CsA is also known to activate the sympathetic nervous system, increasing the reabsorption of sodium and other solutes in the renal tubules. This leads to reduced delivery of sodium to the distal tubules, further sodium retention and hypertension, hyperkale
Current management for hyperkalemia in these instances includes reducing the dose of CsA and maintaining a euvolemic state. Thiazide diuretics can also be used to counter the effect of CNIs. Some recipients require dialysis in severe cases. Experimentally, atrial natriuretic peptide has shown promising results in preventing and treating AKI post-heart transplantation through mechanisms requiring further study[7,28,29].
Sirolimus (rapamycin) is a potent immunosuppressant with a different mechanism of action from CNIs. It inhibits activation of T-cells and B-cells by reducing their sensitivity to interleukin-2 through mammalian target of rapamycin (mTOR) inhibition. mTOR is involved in renal potassium excretion and mTOR complex 1 (mTORC1) activation in the collecting ducts which results in features of pseudo-hypoaldosteronism (hyperkalemia, hyperaldosteronism, and metabolic acidosis)[30]. In addition to immunosuppressive properties, proliferation signal inhibitors (PSIs) have important antiproliferative effects. When used as secondary immunosuppressive agents in place of azathioprine or mycophenolate, PSIs prevented cardiac allograft vasculopathy (CAV) progression as well as regression of cardiac hypertrophy and reduced incidence of clinically significant cardiac events. It is important to note that concomitant use of CNIs and PSIs is associated with an increased risk of nephrotoxicity and hyperkalemia.
CAV remains a leading cause of mortality after heart transplantation. The renin angiotensin aldosterone system has also been implicated in the development of native coronary atherosclerosis[31]. ACEIs possess anti-atherogenic properties, which reduce plaque development in the coronary arteries after heart transplant. They accomplish this by decreasing the production of angiotensin-II, reducing oxidative stress, inhibiting smooth muscle proliferation, and improving fibrinolysis[32,33]. Despite the beneficial effects, physicians must be cautious when prescribing ACEIs and ARBs, or even direct renin inhibitors like Aliskerin, because of the serious side effects of hyperkalemia. Potassium levels should be carefully monitored by the physicians, especially in recipients with renal insufficiency, who appear to have a relatively higher incidence of hyperkalemia when compared to recipients with normal renal function on ACEIs. Certain newer medications like patiromer and sodium zirconium cyclosilicate can be used along with ACEIs/ARBs to counteract the effects of hyperkalemia when the benefits outweigh the risks.
Trimethoprim-sulfamethoxazole is a frequently used sulfonamide antibiotic for prophylaxis and treatment of Pneumocystis jiroveci pneumonia in heart transplan
Several case reports have reported that lovastatin can potentiate the nephrotoxic effect of CsA in a dose-dependent fashion, and also increases the risk of hyperkalemia by increasing the efflux of potassium ions from muscle cells that are damaged. While the exact mechanism remains unknown, it has been suggested that CsA affects biliary clearance of lovastatin increasing its levels. It also lowers lipoprotein levels which further increases levels of free CsA, further potentiating its side effects. Thus, it is advised to start statins at a lower dose in heart transplant recipients along with frequent monitoring of CsA levels, liver enzymes, and creatinine kinase (CK) levels. The concomitant use of multiple lipid-lowering drugs should be avoided, and lovastatin should be discontinued in recipients with symptoms of muscle damage or an asymptomatic patient with increased levels of CK.
Lovastatin is not the only statin to cause hyperkalemia. Rhabdomyolysis and acute tubular necrosis are frequent complications in patients undergoing treatment with simvastatin when itraconazole is added. This is especially seen in organ transplant recipients being treated with CsA. It is important to ensure early diagnosis of rhabdomyolysis and withdraw the responsible statin to avoid further muscle damage. Recipients taking simvastatin with a cytochrome inhibitor like itraconazole, keto
Non selective beta-blockers and potassium sparing diuretics used for management of low output states post-heart transplant can also cause hyperkalemia and should be acknowledged in a post-transplant setting. Management should be altered based on the indication for the beta-blocker.
Potassium levels higher than 5.5 mEQ/L can induce life-threatening arrhythmias. Thus, the potassium concentration after cardiac surgery should be maintained at an optimum level of 3.8-4.3 mEQ/L. Hyperkalemia can also lead to myopathy and cause generalized muscle weakness[35]. Treatment of hyperkalemia is based on the severity, presence of symptoms, and electrocardiogram (EKG) changes.
Hyperkalemia can cause EKG changes like peaked T-waves, ST depression, increased PR interval, and QRS widening. These changes should be immediately managed by pharmacological interventions like calcium gluconate/calcium chloride, which stabilizes membrane potential, and insulin with glucose, beta-2 agonists, and sodium bicarbonate that all transiently shift potassium intracellularly. Diuretics like furosemide or thiazides can be used in patients with normal kidney function, as well as kayexalate and hemodialysis to decrease potassium to a safe level.
Many nonselective non-absorbable cation binders are currently available which directly bind to potassium and move it into the cells. Two cation binders include sodium polystyrene sulfonate (SPS), patiromer, and sodium zirconium cyclosilicate (ZS-9).
SPS has a large binding capacity for sodium and works by exchanging sodium for potassium. As it is nonselective, it also binds to magnesium and calcium, leading to possible potassium overcorrection along with hypomagnesemia and hypocalcemia[36]. Although use in the first month after transplant is not ideal as it can lead to bowel necrosis, risk-benefit decisions should be weighed postoperatively in transplant recipients.
Patiromer, another non-selective cation binder, has a more favorable gastrointestinal side-effect profile compared to SPS. It is a sodium free polymer and works by exchanging potassium with calcium. At a starting dose of 8.4 g, it can lower potassium levels within hours of administration, and this decrease continues up to 48 h in a dose-dependent manner[37]. However, due to its nonselective nature, it can also result in hypomagnesemia and hypocalcemia.
ZS-9, another nonselective cation binder, was approved by the Food and Drug Administration in 2018. It acts by exchanging potassium for sodium and hydrogen. Mean serum potassium reduction with a 10 g first dose of ZS-9 was 0.7 mEQ/L at 48 h, with a chance of a further reduction in patients with higher initial potassium levels. ZS-9 should not be given with other pH-dependent drugs for 2 h because of the potential transient increase in gastric pH, which can alter drug solubility and absorption.
Fludrocortisone has been demonstrated to be an effective drug for managing hyperkalemia. It works at the level of the distal convoluted tubule by facilitating resorption of sodium along with promoting potassium excretion. A long list of side effects like fluid retention, hyperglycemia, osteoporosis, hypertension, and hyperna
There are special considerations in the management of hyperkalemia in a heart transplant recipient vs the management of hyperkalemia in a non-transplant patient. If a recipient is being treated with a CNI, particularly Tacrolimus, special care has to be taken when using Patiromer. Patiromer has been shown to increase the level of Tacrolimus over the span of four weeks requiring decreases in dosage[38]. With risk of supratherapeutic Tacrolimus levels, there is risk of insult to the kidney causing acute kidney injury or worsening chronic kidney disease (CKD) that subsequently leads to worsening hyperkalemia. In the setting of persistent hyperkalemia requiring dialysis, this may be a sign of developing CKD or existing CKD progressing to end stage renal disease (ESRD), as the risk of CKD and ESRD increases each year after heart transplantation[39]. The use of dialysis alone in a heart transplant patient confers a 20.3% worse mortality than a recipient without dialysis[39]. While CNIs undoubtedly contribute to the deterioration of the kidneys over time, other risk factors such as hypoperfusion (despite circulatory support) prior to the heart transplant, and other comorbidities such as hypertension or diabetes, can accelerate damage and present mortality risk that is greater than the non-transplant patient. Thus, these comorbidities should be monitored closely and treated appropriately to avoid added risk, if possible.
Low potassium diet, avoiding fasts for long periods, using medications that do not increase potassium, avoiding potassium-sparing diuretics, avoiding succinylcholine and non-steroidal anti-inflammatory drugs can all be used as preventive measures. Recipients with CKD or heart failure who are treated with ACEIs/ARBs should continue these medications if there is mortality benefit. Further preventive measures can decrease the risk of hyperkalemia including close monitoring of serum potassium levels and kidney function, using thiazide or loop diuretics whenever possible to help remove excess potassium from the body, and the use of oral bicarbonate supplements when appropriate[40]. Recent studies have shown that low-dose SPS was found to be safe and effective as a preventive measure for hyperkalemia caused by ACEIs/ARBs in CKD recipients with heart disease[40].
Hyperkalemia is one of the known serious complications following heart transplant. A careful review of the causes, using medications carefully, and employing counteractive measures to manage this complication can ultimately lead to improvement in graft viability. In addition to classic management strategies of hyperkalemia, newer nonselective and non-absorbable cation binders like SPS, Patiromer, ZS-9, and exogenous mineralocorticoids can also be considered for correction and are promising in increasing graft viability. This manuscript provides a complete and concise review for the causes, prevention, and management of hyperkalemia in a heart transplant recipient. This review covers pre-transplant, transplant, and post-transplant causes and should be reviewed when managing such recipients in a hospital or outpatient setting. As new management discoveries are studied, this review will continue to be a compilation of the ground-work of management techniques that have been effective to-date.
Manuscript source: Invited manuscript
Specialty type: Transplantation
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Markic D, Zhou S S-Editor: Gao CC L-Editor: A P-Editor: Yuan YY
| 1. | Hunt SA, Haddad F. The changing face of heart transplantation. J Am Coll Cardiol. 2008;52:587-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 187] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 2. | National Health Service Blood and Transplant. Annual Report on Cardiothoracic Organ Transplantation Report for 2018/2019. [cited 25 January 2021]. In: National Health Service [Internet]. Available from: https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/19874/nhsbt-annual-report-on-cardiothoracic-organ-transplantation-201920.pdf. |
| 3. | Kahan BD. Immunosuppressive therapy with cyclosporine for cardiac transplantation. Circulation. 1987;75:40-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 59] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Cruz DN, Perazella MA. Acute renal failure after cardiac transplantation: a case report and review of the literature. Yale J Biol Med. 1996;69:461-468. [PubMed] |
| 5. | Belzer FO, Southard JH. Principles of solid-organ preservation by cold storage. Transplantation. 1988;45:673-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 986] [Cited by in RCA: 952] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 6. | Guibert EE, Petrenko AY, Balaban CL, Somov AY, Rodriguez JV, Fuller BJ. Organ Preservation: Current Concepts and New Strategies for the Next Decade. Transfus Med Hemother. 2011;38:125-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 208] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 7. | Demmy TL, Biddle JS, Bennett LE, Walls JT, Schmaltz RA, Curtis JJ. Organ preservation solutions in heart transplantation--patterns of usage and related survival. Transplantation. 1997;63:262-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 71] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | George TJ, Arnaoutakis GJ, Baumgartner WA, Shah AS, Conte JV. Organ storage with University of Wisconsin solution is associated with improved outcomes after orthotopic heart transplantation. J Heart Lung Transplant. 2011;30:1033-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Latchana N, Peck JR, Whitson B, Black SM. Preservation solutions for cardiac and pulmonary donor grafts: a review of the current literature. J Thorac Dis. 2014;6:1143-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 10. | Saldanha C, Hearse DJ. Coronary vascular responsiveness to 5-hydroxytryptamine before and after infusion of hyperkalemic crystalloid cardioplegic solution in the rat heart. Possible evidence of endothelial damage. J Thorac Cardiovasc Surg. 1989;98:783-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 71] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Evora PR, Pearson PJ, Schaff HV. Crystalloid cardioplegia and hypothermia do not impair endothelium-dependent relaxation or damage vascular smooth muscle of epicardial coronary arteries. J Thorac Cardiovasc Surg. 1992;104:1365-1374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | He GW, Yang CQ, Wilson GJ, Rebeyka IM. Tolerance of epicardial coronary endothelium and smooth muscle to hyperkalemia. Ann Thorac Surg. 1994;57:682-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | He GW, Yang CQ, Rebeyka IM, Wilson GJ. Effects of hyperkalemia on neonatal endothelium and smooth muscle. J Heart Lung Transplant. 1995;14:92-101. [PubMed] |
| 14. | He GW, Yang CQ. Hyperkalemia alters endothelium-dependent relaxation through non-nitric oxide and noncyclooxygenase pathway: a mechanism for coronary dysfunction due to cardioplegia. Ann Thorac Surg. 1996;61:1394-1399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | He GW, Yang CQ, Yang JA. Depolarizing cardiac arrest and endothelium-derived hyperpolarizing factor-mediated hyperpolarization and relaxation in coronary arteries: the effect and mechanism. J Thorac Cardiovasc Surg. 1997;113:932-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | He GW. Hyperkalemia exposure impairs EDHF-mediated endothelial function in the human coronary artery. Ann Thorac Surg. 1997;63:84-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Snabaitis AK, Shattock MJ, Chambers DJ. Comparison of polarized and depolarized arrest in the isolated rat heart for long-term preservation. Circulation. 1997;96:3148-3156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Marfo K, Glicklich D. Fludrocortisone therapy in renal transplant recipients with persistent hyperkalemia. Case Rep Transplant. 2012;2012:586859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Sahu MK, Singh SP, Das A, Abraham A, Airan B, Alam I, Menon R, Devagourou V, Gupta A. High blood tacrolimus and hyperkalemia in a heart transplant patient. Ann Card Anaesth. 2017;20:270-271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Hamano K, Ohmi M, Esato K, Mohri H. Myocardial tissue blood flow in allotransplanted rat heart with a special reference to acute rejection. J Heart Transplant. 1989;8:48-52. [PubMed] |
| 21. | Pradas G, Cuenca J, Juffé A. Continuous warm reperfusion during heart transplantation. J Thorac Cardiovasc Surg. 1996;111:784-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Aker S, Heering P, Kinne-Saffran E, Deppe C, Grabensee B, Kinne RK. Different effects of cyclosporine a and FK506 on potassium transport systems in MDCK cells. Exp Nephrol. 2001;9:332-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Hachida M, Lu H, Ohkado A, Gu H, Zhang XL, Furukawa H, Nakanishi T, Koyanagi H. Effect of ATP-potassium channel opener nicorandil on long-term cardiac preservation. J Cardiovasc Surg (Torino). 2000;41:533-539. [PubMed] |
| 24. | Tumlin JA, Sands JM. Nephron segment-specific inhibition of Na+/K(+)-ATPase activity by cyclosporin A. Kidney Int. 1993;43:246-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 25. | Heering PJ, Klein-Vehne N, Fehsel K. Decreased mineralocorticoid receptor expression in blood cells of kidney transplant recipients undergoing immunosuppressive treatment: cost efficient determination by quantitative PCR. J Clin Pathol. 2004;57:33-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | White CW, Ali A, Hasanally D, Xiang B, Li Y, Mundt P, Lytwyn M, Colah S, Klein J, Ravandi A, Arora RC, Lee TW, Hryshko L, Large S, Tian G, Freed DH. A cardioprotective preservation strategy employing ex vivo heart perfusion facilitates successful transplant of donor hearts after cardiocirculatory death. J Heart Lung Transplant. 2013;32:734-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 27. | Deppe CE, Heering PJ, Viengchareun S, Grabensee B, Farman N, Lombès M. Cyclosporine a and FK506 inhibit transcriptional activity of the human mineralocorticoid receptor: a cell-based model to investigate partial aldosterone resistance in kidney transplantation. Endocrinology. 2002;143:1932-1941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Wei J, Chang CY, Chuang YC, Su SH, Lee KC, Tung DY, Lee SL, Lee WC. Successful heart transplantation after 13 h of donor heart ischemia with the use of HTK solution: a case report. Transplant Proc. 2005;37:2253-2254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Southard JH, Belzer FO. Organ preservation. Annu Rev Med. 1995;46:235-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 325] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 30. | Chen Z, Dong H, Jia C, Song Q, Chen J, Zhang Y, Lai P, Fan X, Zhou X, Liu M, Lin J, Yang C, Li M, Gao T, Bai X. Activation of mTORC1 in collecting ducts causes hyperkalemia. J Am Soc Nephrol. 2014;25:534-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Fearon WF, Okada K, Kobashigawa JA, Kobayashi Y, Luikart H, Sana S, Daun T, Chmura SA, Sinha S, Cohen G, Honda Y, Pham M, Lewis DB, Bernstein D, Yeung AC, Valantine HA, Khush K. Angiotensin-Converting Enzyme Inhibition Early After Heart Transplantation. J Am Coll Cardiol. 2017;69:2832-2841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 32. | Mehra MR, Ventura HO, Smart FW, Collins TJ, Ramee SR, Stapleton DD. An intravascular ultrasound study of the influence of angiotensin-converting enzyme inhibitors and calcium entry blockers on the development of cardiac allograft vasculopathy. Am J Cardiol. 1995;75:853-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 76] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 33. | Bae JH, Rihal CS, Edwards BS, Kushwaha SS, Mathew V, Prasad A, Holmes DR Jr, Lerman A. Association of angiotensin-converting enzyme inhibitors and serum lipids with plaque regression in cardiac allograft vasculopathy. Transplantation. 2006;82:1108-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 34. | Alejandro DS, Petersen J. Myoglobinuric acute renal failure in a cardiac transplant patient taking lovastatin and cyclosporine. J Am Soc Nephrol. 1994;5:153-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 35. | Mateen FJ, van de Beek D, Kremers WK, Daly RC, Edwards BS, McGregor CG, Wijdicks EF. Neuromuscular diseases after cardiac transplantation. J Heart Lung Transplant. 2009;28:226-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 36. | Scherr L, Ogden DA, Mead AW, Spritz N, Rubin AL. Management of hyperkalemia with a cation-exchange resin. N Engl J Med. 1961;264:115-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 105] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 37. | Bushinsky DA, Williams GH, Pitt B, Weir MR, Freeman MW, Garza D, Stasiv Y, Li E, Berman L, Bakris GL. Patiromer induces rapid and sustained potassium lowering in patients with chronic kidney disease and hyperkalemia. Kidney Int. 2015;88:1427-1433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 38. | Schnelle K, Winters H, Pesavento T, Singh P. Largest Experience of Safety and Efficacy of Patiromer in Solid Organ Transplant. Transplant Direct. 2020;6:e595. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 39. | McCartney SL, Patel C, Del Rio JM. Long-term outcomes and management of the heart transplant recipient. Best Pract Res Clin Anaesthesiol. 2017;31:237-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 40. | Chernin G, Gal-Oz A, Ben-Assa E, Schwartz IF, Weinstein T, Schwartz D, Silverberg DS. Secondary prevention of hyperkalemia with sodium polystyrene sulfonate in cardiac and kidney patients on renin-angiotensin-aldosterone system inhibition therapy. Clin Cardiol. 2012;35:32-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |