Published online Jan 18, 2021. doi: 10.5500/wjt.v11.i1.1
Peer-review started: June 22, 2020
First decision: November 16, 2020
Revised: December 30, 2020
Accepted: January 8, 2021
Article in press: January 8, 2021
Published online: January 18, 2021
Processing time: 209 Days and 0.3 Hours
To describe the main aspects of back-table surgery in pancreatic graft and the problems arising from poor technique. Back-table surgery for pancreatic graft is a complex, meticulous and laborious technique on which the success of implant surgery and perioperative results depends. The technique can be described in the following steps: Preparation of the sterile table, ex-situ inspection of the pancreas-spleen block, management of the duodenum, identification of the bile duct, preparation of the portal vein, preparation of the own graft arteries and anastomosis to the arterial graft, spleen management and graft preservation prior to implantation in the recipient. A careful inspection of the pancreas-spleen block should be performed. It is important to identify the stump of the main bile duct, the portal vein cuff, and the arrangement of the superior mesenteric artery and splenic artery. The redundant duodenum must be removed. The availability of a good venous cuff facilitates the portal vein anastomosis and the positioning of the graft, two key points to prevent thrombosis. The section line of the arteries must be clean, without atherosclerosis, to prevent arterial thrombosis. The superior and splenic mesenteric arteries are generally separated by dense fibrolymphatic tissue. The artery can be reconstructed by interposing a "Y" graft from the donor iliac artery; or with an end-to-end anastomosis between the splenic artery and the superior mesenteric artery. An exquisite technique of bench work helps to prevent the most feared complications of pancreas transplantation: Thrombosis and graft pancreatitis.
Core Tip: Back-table work in pancreas transplantation is a delicate and complex technique. It consists of numerous steps, all of them aimed at avoiding serious complications in the postoperative period of the transplant. It requires exquisite management of the duodenal remnant, the portal vein of the graft, and the arteries that ensure oxygenation of the gland. It is important to perform a methodical technique by an expert surgical team. A large part of pancreatic transplant failures is due to faulty back-table work.
- Citation: Briceño J, Sánchez-Hidalgo JM, Arjona-Sanchez A. Back-table surgery pancreas allograft for transplantation: Implications in complications. World J Transplant 2021; 11(1): 1-6
- URL: https://www.wjgnet.com/2220-3230/full/v11/i1/1.htm
- DOI: https://dx.doi.org/10.5500/wjt.v11.i1.1
Back-table surgery for pancreatic graft is a complex, meticulous and laborious technique on which the success of implant surgery and perioperative results depends. It is difficult for a defective technique in pancreatic graft surgery to result in a successful transplant. The technique on back bench in pancreatic grafting must be learned diligently and step by step, acquiring the necessary skills to obtain an adequate graft. This involves careful management of the graft duodenum, manipulation of the portal vein and arteries, the performance of anastomosis of the arterial graft, management of intrapancreatic bile duct and manipulation of the gland during the procedure. In this review, we describe all the details of the back bench technique according to the evolution of the pancreas transplant technique.
The back bench technique can be described in the following steps: Preparation of the sterile table, ex situ inspection of the pancreas-spleen block, management of the duodenum, identification of the bile duct, preparation of the portal vein, preparation of the own graft arteries and anastomosis to the arterial graft, spleen management and graft preservation prior to implantation in the recipient.
Before removing the graft from the transport container, it is necessary to make a proper preparation of the worktable. Normally, the previously extracted pancreatic graft is preserved and transported in a preservation solution at 4 °C in cold static conditions. Ice is cheap and easily available. In the United Kingdom – the NHS Blood and Transplant and the National Organ Retrieval Service recommends the use of 3 bowel bags to store and transport pancreatic grafts on ice.
Recently, it is possible to transport the pancreatic graft in homologated systems that maintain constantly the temperature of the medium, and with the possibility of external monitoring by means of devices connected to smartphones. This allows a continuous knowledge of the temperature at which the graft is transported, to record an incident during transport and to warn in case of any adverse event. These devices can be programmed to handle the temperature of the container in a range of ± 2 ºC. It is very important during transport that the pancreatic graft does not directly contact the ice, since there is a danger of graft freezing. Keep in mind that the pancreas is a thin gland and its possibility of freezing is real, unlike other organs with more parenchyma mass such as liver. A good trick is to use a bag with cold preservation liquid in which the pancreatic graft is immersed, surrounded by another bag with sterile cold saline. This creates a liquid chamber interposed between the ice and the graft that prevents its freezing. It is also important to avoid the existence of air chambers in the bags or transport containers since they can avoid maintaining the cold temperature that the organ needs. Instead of ice, Hartmann's or frozen saline can be used to keep the hammer sterile in case the bag breaks.
The table is covered with a sterile sheet, preferably one of single use. A not very deep metal container, between 10 and 15 cm, should be available, usually with a bed of crushed ice, on which a bag with preservation solution is placed. Then the graft is removed from the container and immersed in the preservation solution. During the whole procedure it is important to monitor the temperature of the organ; this can be done by dipping a sterile thermometer into the preservation liquid. It is important to prepare precise dissection and cutting surgical instruments, since sensitive and delicate structures are to be handled. It is also important to have a set of cannulas of different sizes and blunt probes between 2 and 8 millimeters in diameter. They can be useful in the preparation of future arterial anastomoses and in the exploration of the bile duct. It is essential to have 2/0 and 3/0 suture threads, as well as 5/0 and 6/0 polypropylene vascular sutures.
A thorough inspection of the pancreas-spleen block should be performed[1]. Previously, it is important to obtain information from the extractor team about possible incidents during the removal in the donor. This acquires special importance in those cases of superfast in block extraction, where the possibility of injury to the delicate structures of the pancreatic graft is frequent.
Inspection of the graft block starts with a visual inspection of the parenchyma. The gland should have a homogeneous coloration, without areas of ecchymosis and microhemorrhages. Since the duodenum is very sensitive during extraction and preservation, much information can be obtained on how the extraction technique has been by closely observing the duodenum. A duodenum with areas of hematoma or with hemorrhagic dots suggests a difficult or accidental removal. Subsequently, gentle and careful palpation of the gland should be performed to detect hard areas or of different texture to the rest of the gland, which may suggest the presence of areas of freezing or inadvertent tumors. At this time it is important to identify the stump of the main bile duct before beginning its intrapancreatic route, the portal vein cuff and the arrangement of the superior mesenteric artery (SMA) and the splenic artery. It is common for the stumps of the different vascular structures to "bury" in the parenchyma and sometimes it is difficult to identify them. For this it is important that during the extraction they may be identified by a 5/0 stitch of polypropylene with about 2-3 centimeters that will allow its subsequent identification and recovery. Finally, it is important to inspect the spleen attached to the pancreas. This should be complete, with no signs of trauma, lacerations or bruises.
Dissection in back bench starts with the elimination of redundant duodenum that accompanies the graft. Normally, the duodenum is removed along with the first jejunum. Therefore, this portion of the intestine should be removed until a duodenal stump of about 10 cm is achieved. The current tendency is to leave a duodenal graft as small as possible[2]. In general, a small duodenal stump allows all possible types of enteroanastomosis depending on the type of exocrine drainage chosen. Since the duodenum can be the source of postoperative complications, especially pancreatic fistulas, careful manipulation is necessary while separating from the root of the mesentery. Numerous small veins and arteries enter the duodenum and first duodenal portion at this level, so one-to-one ligation of these small vessels is required. A current alternative is to prepare a high energy vessel sealer that can make the procedure faster.
As we move forward in the separation of the first jejunal loop and reach the third and fourth duodenal portions, we approach the entrance of the bile duct into the papilla. It is important to stop the dissection of the duodenum before reaching the ampulla of Vater, since an unnoticed ligation or section of the duct can lead to a subsequent pancreatitis[3] or an injury to the pancreatic duct at its end, with the consequent fistula. To avoid this, a small 3-4 mm caliber probe can be introduced through the bile duct remnant. That allows to externally palpate the papilla position through the wall of the duodenum. The dissection of the duodenum should stop about 3-4 cm from the papilla, this being the place of section of the duodenum. This section should be done with triple stapling suture. In our initial experience, we used a double layer stapler, oversewn with silk suture. Currently, a triple layer stapler without oversewn is our common practice. A matter of controversy is the section of the duodenum at the proximal level. During decades, the pylorus with a distance of 2 centimeters proximal was included. The idea was to use its dense muscular layer to ensure a secure closure of the duodenum with firm stitches. However, this technique involved the duodenum section in a thicker area of the wall and, therefore, with worse stapling and possibility of fistula. Current practice ignores the inclusion of the pylorus and removes this muscle up to approximately 1 cm of the duodenum, where it is sectioned. Once the two ends of the duodenal cuff are sectioned, their cut lines are reinforced with a well-lubricated 3/0 suture.
An alternative technique has been proposed that uses a small circular duodenal patch including the papilla in the center and that is anastomosed to the jejunum or the duodenum of the recipient (button technique). This technique avoids the problems of the duodenal stump by theoretically decreasing its weaknesses (the ends of the stump), and allows a "direct" exocrine drainage in the recipient's intestine[4].
Peripancreatic fat must be removed along the pancreas from the head to the body and the tail peripherally; In particular, the clearance of this fat and also the ligation of small vessels related to the fibrous connective tissue is important.
The preparation of the portal vein is one of the cornerstones of a good result in pancreas transplantation. Vein thrombosis is one of the most fearsome complications that usually leads to graft loss. The availability of a good venous cuff facilitates vein anastomosis and graft positioning, two key points to prevent thrombosis[5].
Normally, the portal vein is cut a sufficient distance from the upper duodenal edge level. It is important to agree with the liver extraction team the sectional level of the portal vein, since an excessively small stump greatly hinders the portal anastomosis in the pancreas implant. Generally, a 1.5 cm stump is enough to perform a subsequent straight anastomosis without twisting, without affecting the needs of the liver team.
The portal vein is referenced in the 3 and 9 position aligning with the longitudinal axis of the pancreas. A small traction is exerted upwards of the vein by the assistant, while the surgeon releases the vein in about two centimeters in length. Generally, the dissection is performed trough an avascular plane, which stops when the first superior pancreatic vein appears, which can be identified at its drainage inside the portal vein lumen[6].
The second important point in back bench after the preparation of the portal vein is the identification and dissection of the SMA and the splenic artery of the graft. Both arteries allow the supply of oxygen to the gland, so the viability of the gland depends on an adequate flow through them. Before starting the dissection, it is important to analyze the appearance of arterial edges. The section line should be clean, without separation of the endothelium from the arterial wall. This situation may be due to poor quality of the artery (atherosclerosis) or may be due to a very traumatic technique in the cutting of the artery. An injury to either of the two arteries generally implies the uselessness of the graft. The use of magnifying glasses for vascular anastomoses and for the preparation of arterial grafts in bench work allows the proper management of these vascular structures, especially when their caliber is small.
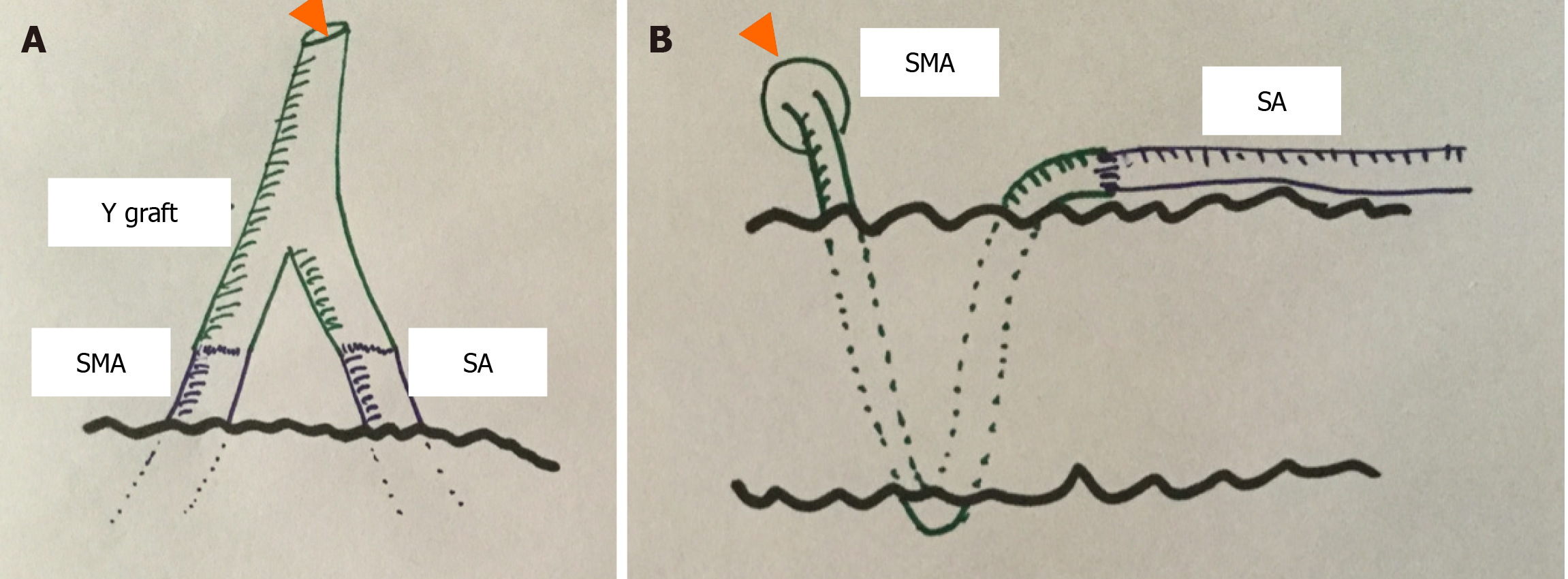
Both arteries are usually separated by a dense fibrolymphatic tissue composed among others by the Stellate ganglion block. There is also a rich lymphatic plexus composed of lymph nodes and lymphatic vessels. A care dissection of the "V" formed between both arteries should be performed until two stumps of sufficient length (approximately 1 cm) are achieved to perform an adequate anastomosis. Once the two arterial cuffs have been prepared, the next step is to perform the anastomosis with the iliac artery graft and thus achieve sufficient length to attach it to the iliac artery of the recipient. There are two technical options (Figure 1): (1) The most widespread is to use a "Y" graft of the iliac artery and anastomose each of the "legs" of the Y to the SMA and the splenic artery of the graft, respectively[7]. For this purpose, a 5/0 polypropylene suture is used, using a single suture point in each of the anastomoses. It is important not to narrow the junction as "an hourglass”, so a probe slightly smaller than the inside diameter of the arteries can be used (Figure 1A); and (2) A second option is to reconstruct the arterial supply of the pancreas with an end-to-end anastomosis between the proximal splenic artery and the distal end of the SMA[8] (Figure 1B).
For some authors, the reconstruction of the gastroduodenal artery is important. Normally, in the classical technique the gastroduodenal artery is ligated and sectioned during the separation of the liver-pancreas block. This would allow to improve the oxygen supply to the head of the pancreas, since this area is irrigated by the gastroduodenal artery and the SMA. Approximately 7% of pancreatic grafts show hypoperfusion of the head of the pancreas if the gastroduodenal artery is ligated[9]. However, reconstruction of the gastroduodenal artery is complex and it is not an extended practice[10].
Another point of controversy is the presence in the donor of an accessory right hepatic artery from the SMA. Generally, the origin of this artery occurs in the first two centimeters from the origin of the SMA, so it is possible to dissect the artery to the origin of the right hepatic artery and then section it after. Another alternative is to section the right hepatic artery in the hepatic hilum and anastomose it to the donor gastroduodenal artery, leaving the cuff of the entire SMA for the pancreas. Today, it cannot be accepted that the presence of an accessory right hepatic artery is a cause of contraindication of pancreatic retrieval.
To avoid injury to the arterial endothelium, the Watson or Cheyne probes may be helpful, as they are blunt and less prone to vessel injury.
At the end of the back bench, the spleen remains attached to the tail of the pancreas. It is important to make a careful separation of both. Through the splenic artery and vein there is an important vascular network that enters the spleen. It is important to carefully ligate each of the arterial and venous branches to this level to avoid significant bleeding at the time of revascularization. Generally, this type of bleeding is very stressful because it is usually a sheet bleeding, difficult to locate and with a position of the pancreatic graft that makes it difficult to dislodge and control the bleeding point.
On the other hand, it is also important not to injure the tail of the pancreas, which may be the origin of future pancreatic fistulas with a difficult control.
Normally, the pancreatic graft should be stored in ice or in a preservation machine until it can be implanted in the recipient. For this purpose, the pancreatic graft is placed in a container or a bag with cold preservation fluid. As during the graft transport that was previously mentioned, it is important not to leave air chambers that can damage the cold preservation, as well as the freezing of the parenchyma by a direct contact with the bed of ice.
As for other abdominal organs, the use of preservation machines for pancreatic grafts has been proposed. However, most of the studies are experimental and their development in the current practice has not been very encouraging. To address this problem, it is necessary to define which preservation protocol is the best for the pancreas (hypo, normo or subnormothermia) and, especially, how the viability and functionality of the graft can be tested. This last aspect is important, if we consider the percentage of grafts discarded due to their fat content or due to edema of the gland[11].
An exquisite technique of bench work helps to prevent the most feared complications of pancreas transplantation: Thrombosis and graft pancreatitis.
Manuscript source: Invited manuscript
Specialty type: Transplantation
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hamaoui K S-Editor: Gao CC L-Editor: A P-Editor: Wang LL
| 1. | Fridell JA, Powelson JA, Sanders CE, Ciancio G, Burke GW 3rd, Stratta RJ. Preparation of the pancreas allograft for transplantation. Clin Transplant. 2011;25:E103-E112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 2. | El-Hennawy H, Stratta RJ, Smith F. Exocrine drainage in vascularized pancreas transplantation in the new millennium. World J Transplant. 2016;6:255-271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 3. | Redfield RR, Rickels MR, Naji A, Odorico JS. Pancreas Transplantation in the Modern Era. Gastroenterol Clin North Am. 2016;45:145-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 4. | Pinchuk A, Dmitriev I, Lazareva K, Storozhev R, Balkarov A, Anisimov Y, Kondrashkin A. Retroperitoneal Pancreas Transplantation With the Use of Duodenal Drainage via "Button Technique": First Clinical Practice (Case Report). Transplant Proc. 2017;49:2347-2351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 5. | Deboudt C, Branchereau J, Luyckx F, Rigaud J, Glemain P, Blancho G, Karam G. [Pancreas transplantation and venous thrombosis: multivariate analysis of risk factors]. Prog Urol. 2012;22:402-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Laftavi MR, Gruessner A, Gruessner R. Surgery of pancreas transplantation. Curr Opin Organ Transplant. 2017;22:389-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 7. | Gill IS, Sindhi R, Jerius JT, Sudan D, Stratta RJ. Bench reconstruction of pancreas for transplantation: experience with 192 cases. Clin Transplant. 1997;11:104-109. [PubMed] |
| 8. | Fernández-Cruz L, Astudillo E, Sanfey H, Llovera JM, Saenz A, López-Boado MA, Bagur C. Combined whole pancreas and liver retrieval: a new technique for arterial reconstruction of the pancreas graft. Br J Surg. 1992;79:239-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Nghiem DD. Revascularization of the gastroepiploic artery in pancreas transplant. Transpl Int. 2008;21:774-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Boggi U, Amorese G, Marchetti P. Surgical techniques for pancreas transplantation. Curr Opin Organ Transplant. 2010;15:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Hamaoui K, Papalois V. Machine Perfusion and the Pancreas: Will It Increase the Donor Pool? Curr Diab Rep. 2019;19:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |