Published online Jul 12, 2015. doi: 10.5499/wjr.v5.i2.96
Peer-review started: November 10, 2014
First decision: December 26, 2014
Revised: January 28, 2015
Accepted: March 30, 2015
Article in press: April 4, 2015
Published online: July 12, 2015
Helicobacter pylori (H. pylori) infection is widely prevalent throughout worldwide. H. pylori manage a long-term survival in hostile environment of human stomach leading to peptic ulcer diseases and gastric cancer. But mostly infected person remains asymptomatic. Its chronic interaction with immune system makes H. pylori as an attractive candidate for the researchers to study its association with autoimmune diseases. This article presents a review of the literature on the association of H. pylori infection in selective autoimmune rheumatic diseases (RD). The authors used MeSH terms “Helicobacter pylori” with “rheumatoid arthritis,”“systemic lupus erythematosus,” or “fibromyalgia” to search PubMed database. All relevant studies identified were included. Despite extensive medical advancement many questions on role of H. pylori infection in autoimmune RD still remain unanswered. Further studies are therefore needed to address the role of H. pylori in pathogenesis of RD.
Core tip:Helicobacter pylori (H. pylori) infection is widely prevalent throughout worldwide. Its chronic interaction with immune system makes H. pylori is an attractive candidate for the researchers to study its association with autoimmune disorders. This study presents a review of the literature on the H. pylori association with selective autoimmune rheumatic disorders. Despite extensive medical advancement many questions on the association of H. pylori infection with autoimmune rheumatic disorders still remain unanswered. More studies are therefore required to address the role of H. pylori infection in pathogenesis of rheumatic diseases.
-
Citation: Muhammad JS, Zaidi SF, Ishaq M. Ins and outs of
Helicobacter pylori association with autoimmune rheumatic diseases. World J Rheumatol 2015; 5(2): 96-100 - URL: https://www.wjgnet.com/2220-3214/full/v5/i2/96.htm
- DOI: https://dx.doi.org/10.5499/wjr.v5.i2.96
Rheumatic diseases (RD) include disorders related to joints and connective tissue. Generally these disorders have an autoimmune origin that is associated with progressive disability, systemic complications and early death. Involvement of musculoskeletal system, central and peripheral nervous systems, and other organs such as blood vessels, bone marrow, eye, heart, kidneys, lungs, skin and salivary glands may occurs in more than 40% of patients with RD over a lifetime of disease[1-3].
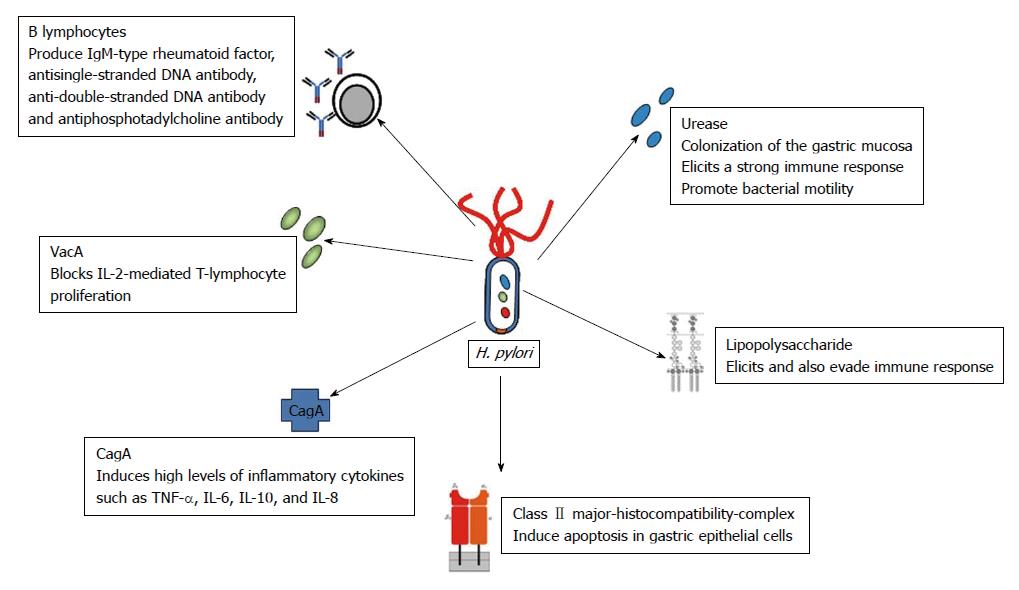
Typically initial Helicobacter pylori (H. pylori) infection is acquired by oral ingestion during the early childhood and H. pylori will persist for life in untreated cases[4]. Frequency of H. pylori infection is approximately 80% in underdeveloped countries compared to 50% in developed parts of the world, correlating the disease prevalence with poor socioeconomic status[5]. Clinically H. pylori infection leads to gastric diseases such as gastric ulcer, mucosa-associated lymphoid tissue lymphoma and gastric cancer[6]. H. pylori infection can induce a chronic immune response in the host cells (Figure 1), suggesting a possible role of H. pylori in the development of autoimmune disorders[7].
Autoimmune RD are thought to depend upon host genetic susceptibility interaction with environmental factors[8]. Amongst various environmental factors, infections agents plays significant role and have been studied extensively[9]. Infectious agents include bacteria, viruses and parasites. Out of all bacterial species implicated in non-organ specific autoimmune disorders, H. pylori have received much attention by researchers[10]. The purpose of this study was to summarize the recent literature on selected RD with autoimmune pathophysiologic mechanisms, which shows positive or negative evidence in relation to H. pylori-associated autoimmune rheumatic disorders.
H. pylori have evolved various survival mechanisms to combat harsh acidic gastric environment and to suppress host immune response. Urease is a key virulence factor of H. pylori which is required for bacterial colonization to gastric mucosa; also it is a potent immunogen that elicits a strong immune response[11]. Urease also serves to promote bacterial motility by decreasing gastric mucous viscosity[12]. In order to evade host innate immune response, the bacterium is also capable of altering its own cell wall antigens rendering antigens to relatively non-antigenic[13].
H. pylori Infection induces a number of immune responses in the host cell by bacterial adhesion to cells and leading to chronic inflammation (Figure 1)[11]. Pathogen can bind to class II major histocompatibility complex present on the cell membrane of gastric epithelial cells leading to apoptosis[14]. CagA translocate inside the gastric epithelial cells to induce high levels of inflammatory cytokines such as IL-6, IL-8, IL-10 and TNF-α[15]. The VacA protein interacts with lymphocytes resulting in blockage of IL-2-mediated T-lymphocyte proliferation[16].
A study by Jackson et al[17] shows elevated C-reactive protein in chronic H. pylori infected patients. Few other reports have demonstrated that chronic H. pylori infection leads to activation and survival of B lymphocytes to produce rheumatoid factor (IgM), antisingle-stranded DNA (anti-ssDNA) and anti-double-stranded DNA (anti-dsDNA) antibody and antiphosphotadylcholine antibody[18,19]. Instead of clearing H. pylori, these antibodies result in the synthesis of anti-H+/K+-ATPase antibodies[20]. These auto-reactive autoantibodies have been involved in the progress of atrophic gastritis. Complex and persistent interaction between host immune system and pathogen might cause immune dysregulation and consequent development of autoimmune RD in susceptible patients.
Rheumatoid arthritis (RA) is an autoimmune chronic inflammatory disorder primarily of unknown origin. The arthritis in RA is symmetrical destructive polyarthritis affecting almost all joints of the body[21]. Various environmental and genetic factors may contribute to disease onset and severity[22]. Search for the role of microbial association with RA dates back to 19th century[23], and several viral and bacterial pathogens such as hepatitis C virus, parvovirus B19, Epstein-Barr virus (EBV), Proteus mirabilis, and Mycobacterium tuberculosis may have a role in its pathogenesis[24]. However the role of H. pylori infection in the pathogenesis of RA is controversial.
A cohort study on RA patients showed 80.4% to be seropositive for H. pylori. However, this was not significantly different from the control group[25]. A study from Japan by Tanaka et al[26] reported 49.3% of RA patients to have H. pylori antibodies, which was lesser compared with the healthy population. Another Japanese study reported a much higher prevalence (61.4%) of H. pylori infection in RA patients[27]. A study by Zentilin et al[28] showed severity of RA in H. pylori seropositive patients and suggested improvement in clinical symptoms after H. pylori eradication.
A direct role of H. pylori infection in RA pathogenesis seems controversial. Besides studies given above, few in vitro studies also suggest association of H. pylori in development of autoimmunity in RA patients. Like Yamanishi et al[18] found chronic stimulation of B cells due to urease produced by H. pylori. This ultimately leads to the generation of rheumatoid factor. But, on the other hand, the clinical evidence for association between RA and H. pylori infection is less substantial and inconclusive. Although RA patients have a high risk of developing peptic ulcer disease (PUD), but the abundant use of non-steroidal anti-inflammatory drugs in the RA patient may also contribute to the risk for PUD development[26]. Furthermore, studies have shown that not only RA patients but also other connective tissue disease patients have a prevalence of H. pylori infection nearly similar to that of control group[25,26]. Hence, the overall data regarding the association of H. pylori infection with RA pathogenesis remains controversial. Further specific in vitro and large scale clinical trials are required to provide clear understanding of this relationship.
Systemic lupus erythematosus (SLE) is an autoimmune chronic inflammatory disease affecting multi-system. Immunologic abnormalities include the production of a number of autoantibodies, such as anti-dsDNA and anti-nuclear antibodies[29]. A number of microorganisms such as parvovirus B19, EBV and cytomegalovirus are associated in the disease pathogenesis[24].
H. pylori prevalence has been studied in SLE patients, but unlike other infectious agents, results vary significantly in published literature. A study by Kalabay et al[30] demonstrated similar frequency of H. pylori infection in SLE patients and control group. Also a study by Showji et al[31] demonstrated that patients with SLE have lesser anti-H. pylori antibodies in contrast to patients with some other connective tissue diseases. However, Yamanashi et al[18] have shown in-vivo induction of anti-single stranded DNA antibodies by H. pylori urease. In contrast to this evidence of SLE related antibody induction by H. pylori, fewer studies have shown protective role of H. pylori-infection in patients with SLE. Such as, Sawalha et al[32] have compared 466 SLE patients to matched control showing lower anti-H. pylori sero-positivity in SLE patients (36.5%:42.9%). Furthermore, in this study African American old age sero-positive females developed SLE more frequently compared to sero-negative females. Hence suggesting that H. pylori-infection have a protective role in the development of SLE is specific to this population group.
Fibromyalgia (FMG), a chronic pain disorder, is associated with widespread musculoskeletal pain, stiffness, fatigue, anxiety, cognitive dysfunction, sleep difficulties and depression. Etiology and pathogenesis of FMG remains unknown[33]. Studies have evaluated association of FMG with bacterial and viral infection, however literature regarding specific role of H. pylori-infection in FMG development is inadequate. Microorganisms might contribute to the development of FMG by activation of inflammatory cytokines leading towards neuroendocrine abnormalities[34].
A study by Malt et al[35] shows that about 33% of the subjects were H. pylori positive in both FMG and control group, therefore they concluded that H. pylori-infection was not associated with psychological changes in both diseased and control subjects. A recent study by Akkaya et al[36] demonstrated an association of H. pylori-infection with FMG patients and compared to similar gender control group. The FMG patients demonstrated higher frequency of an anti-H. pylori antibody (IgG) was seen in when compared to the control group, (30.8% and 17.1% respectively. Further, amongst FMG patients’ depression and anxiety levels were not different between H. pylori-infected FMG patients or un-infected FMG patients.
The unique ability of H. pylori to chronically infect human gastric mucosa to activate inflammation and host immunological response suggests its role in autoimmune diseases. Associations with few autoimmune diseases are strong[7], whereas association of H. pylori infection with autoimmune RD remains controversial. To develop better understanding of H. pylori-association with RD further molecular and clinical research studies with larger sample sizes are warranted.
P- Reviewer: Agarwal V, Cavallasca JA, Enlander D, Martinez-Lostao L, Rothschild BM S- Editor: Tian YL L- Editor: A E- Editor: Jiao XK
| 1. | Guidelines for the initial evaluation of the adult patient with acute musculoskeletal symptoms. American College of Rheumatology Ad Hoc Committee on Clinical Guidelines. Arthritis Rheum. 1996;39:1-8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 91] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 2. | McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365:2205-2219. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3395] [Cited by in F6Publishing: 3471] [Article Influence: 267.0] [Reference Citation Analysis (0)] |
| 3. | Myasoedova E, Crowson CS, Turesson C, Gabriel SE, Matteson EL. Incidence of extraarticular rheumatoid arthritis in Olmsted County, Minnesota, in 1995-2007 versus 1985-1994: a population-based study. J Rheumatol. 2011;38:983-989. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 4. | Everhart JE. Recent developments in the epidemiology of Helicobacter pylori. Gastroenterol Clin North Am. 2000;29:559-578. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 212] [Article Influence: 8.8] [Reference Citation Analysis (1)] |
| 5. | Muhammad JS, Zaidi SF, Sugiyama T. Epidemiological ins and outs of helicobacter pylori: a review. J Pak Med Assoc. 2012;62:955-959. [PubMed] [Cited in This Article: ] |
| 6. | Muhammad JS, Sugiyama T, Zaidi SF. Gastric pathophysiological ins and outs of helicobacter pylori: a review. J Pak Med Assoc. 2013;63:1528-1533. [PubMed] [Cited in This Article: ] |
| 7. | Smyk DS, Koutsoumpas AL, Mytilinaiou MG, Rigopoulou EI, Sakkas LI, Bogdanos DP. Helicobacter pylori and autoimmune disease: cause or bystander. World J Gastroenterol. 2014;20:613-629. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 108] [Cited by in F6Publishing: 100] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 8. | Smyk D, Rigopoulou EI, Baum H, Burroughs AK, Vergani D, Bogdanos DP. Autoimmunity and environment: am I at risk? Clin Rev Allergy Immunol. 2012;42:199-212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 9. | Bogdanos DP, Smyk DS, Invernizzi P, Rigopoulou EI, Blank M, Pouria S, Shoenfeld Y. Infectome: a platform to trace infectious triggers of autoimmunity. Autoimmun Rev. 2013;12:726-740. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 10. | Ram M, Barzilai O, Shapira Y, Anaya JM, Tincani A, Stojanovich L, Bombardieri S, Bizzaro N, Kivity S, Agmon Levin N. Helicobacter pylori serology in autoimmune diseases - fact or fiction? Clin Chem Lab Med. 2013;51:1075-1082. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347:1175-1186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1848] [Cited by in F6Publishing: 1816] [Article Influence: 82.5] [Reference Citation Analysis (3)] |
| 12. | McGee DJ, Mobley HL. Mechanisms of Helicobacter pylori infection: bacterial factors. Curr Top Microbiol Immunol. 1999;241:155-180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Peek RM, Fiske C, Wilson KT. Role of innate immunity in Helicobacter pylori-induced gastric malignancy. Physiol Rev. 2010;90:831-858. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 164] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 14. | Fan X, Gunasena H, Cheng Z, Espejo R, Crowe SE, Ernst PB, Reyes VE. Helicobacter pylori urease binds to class II MHC on gastric epithelial cells and induces their apoptosis. J Immunol. 2000;165:1918-1924. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 119] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 15. | Kim SY, Lee YC, Kim HK, Blaser MJ. Helicobacter pylori CagA transfection of gastric epithelial cells induces interleukin-8. Cell Microbiol. 2006;8:97-106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 96] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 16. | Sundrud MS, Torres VJ, Unutmaz D, Cover TL. Inhibition of primary human T cell proliferation by Helicobacter pylori vacuolating toxin (VacA) is independent of VacA effects on IL-2 secretion. Proc Natl Acad Sci USA. 2004;101:7727-7732. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 202] [Cited by in F6Publishing: 183] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 17. | Jackson L, Britton J, Lewis SA, McKeever TM, Atherton J, Fullerton D, Fogarty AW. A population-based epidemiologic study of Helicobacter pylori infection and its association with systemic inflammation. Helicobacter. 2009;14:108-113. [PubMed] [Cited in This Article: ] |
| 18. | Yamanishi S, Iizumi T, Watanabe E, Shimizu M, Kamiya S, Nagata K, Kumagai Y, Fukunaga Y, Takahashi H. Implications for induction of autoimmunity via activation of B-1 cells by Helicobacter pylori urease. Infect Immun. 2006;74:248-256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 69] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 19. | Kobayashi F, Watanabe E, Nakagawa Y, Yamanishi S, Norose Y, Fukunaga Y, Takahashi H. Production of autoantibodies by murine B-1a cells stimulated with Helicobacter pylori urease through toll-like receptor 2 signaling. Infect Immun. 2011;79:4791-4801. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Amedei A, Bergman MP, Appelmelk BJ, Azzurri A, Benagiano M, Tamburini C, van der Zee R, Telford JL, Vandenbroucke-Grauls CM, D’Elios MM. Molecular mimicry between Helicobacter pylori antigens and H+, K+ --adenosine triphosphatase in human gastric autoimmunity. J Exp Med. 2003;198:1147-1156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 173] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 21. | Lee DM, Weinblatt ME. Rheumatoid arthritis. Lancet. 2001;358:903-911. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 974] [Cited by in F6Publishing: 968] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 22. | Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet. 2010;376:1094-1108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2254] [Cited by in F6Publishing: 2288] [Article Influence: 163.4] [Reference Citation Analysis (0)] |
| 23. | Benedek TG. The history of bacteriologic concepts of rheumatic fever and rheumatoid arthritis. Semin Arthritis Rheum. 2006;36:109-123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Pordeus V, Szyper-Kravitz M, Levy RA, Vaz NM, Shoenfeld Y. Infections and autoimmunity: a panorama. Clin Rev Allergy Immunol. 2008;34:283-299. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 25. | Meron MK, Amital H, Shepshelovich D, Barzilai O, Ram M, Anaya JM, Gerli R, Bizzaro N, Shoenfeld Y. Infectious aspects and the etiopathogenesis of rheumatoid arthritis. Clin Rev Allergy Immunol. 2010;38:287-291. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Tanaka E, Singh G, Saito A, Syouji A, Yamada T, Urano W, Nakajima A, Taniguchi A, Tomatsu T, Hara M. Prevalence of Helicobacter pylori infection and risk of upper gastrointestinal ulcer in patients with rheumatoid arthritis in Japan. Mod Rheumatol. 2005;15:340-345. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Ishikawa N, Fuchigami T, Matsumoto T, Kobayashi H, Sakai Y, Tabata H, Takubo N, Yamamoto S, Nakanishi M, Tomioka K. Helicobacter pylori infection in rheumatoid arthritis: effect of drugs on prevalence and correlation with gastroduodenal lesions. Rheumatology (Oxford). 2002;41:72-77. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Zentilin P, Seriolo B, Dulbecco P, Caratto E, Iiritano E, Fasciolo D, Bilardi C, Mansi C, Testa E, Savarino V. Eradication of Helicobacter pylori may reduce disease severity in rheumatoid arthritis. Aliment Pharmacol Ther. 2002;16:1291-1299. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 49] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 29. | Lisnevskaia L, Murphy G, Isenberg D. Systemic lupus erythematosus. Lancet. 2014;384:1878-1888. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 481] [Cited by in F6Publishing: 484] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 30. | Kalabay L, Fekete B, Czirják L, Horváth L, Daha MR, Veres A, Fónyad G, Horváth A, Viczián A, Singh M. Helicobacter pylori infection in connective tissue disorders is associated with high levels of antibodies to mycobacterial hsp65 but not to human hsp60. Helicobacter. 2002;7:250-256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 31. | Showji Y, Nozawa R, Sato K, Suzuki H. Seroprevalence of Helicobacter pylori infection in patients with connective tissue diseases. Microbiol Immunol. 1996;40:499-503. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 40] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 32. | Sawalha AH, Schmid WR, Binder SR, Bacino DK, Harley JB. Association between systemic lupus erythematosus and Helicobacter pylori seronegativity. J Rheumatol. 2004;31:1546-1550. [PubMed] [Cited in This Article: ] |
| 33. | Goldenberg DL. Diagnosis and differential diagnosis of fibromyalgia. Am J Med. 2009;122:S14-S21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 34. | Goldenberg DL. Do infections trigger fibromyalgia? Arthritis Rheum. 1993;36:1489-1492. [PubMed] [Cited in This Article: ] |
| 35. | Malt EA, Olafsson S, Ursin H. Fibromyalgia a manifestation of Helicobacter pylori infection? Scan J of Rheum. 2004;33:131. [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 36. | Akkaya N, Akkaya S, Polat Y, Turk M, Turk T, Turhal E, Sahin F. Helicobacter pylori seropositivity in fibromyalgia syndrome. Clin Rheumatol. 2011;30:43-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |