Published online Jul 12, 2014. doi: 10.5499/wjr.v4.i2.14
Revised: June 21, 2014
Accepted: July 5, 2014
Published online: July 12, 2014
Processing time: 156 Days and 13.2 Hours
AIM: To assess: (1) Whether the World Health Organization fracture risk assessment tool (FRAX) can be used for monitoring osteoporosis patients receiving treatment as well as its clinical implications; and (2) The relation between fracture incidence and post-treatment FRAX.
METHODS: Five hundred and seventy-nine osteoporotic women known to be adherent to the prescribed osteoporosis medication, had dual-energy X-ray absorptiometry scan and fracture probability calculated at baseline, 2 and 5-year of osteoporosis treatment. Those patients who responded to treatment and did not sustain a new low trauma fracture during the first 2 years, continued their treatment and were re-assessed 3-year later. The patient subgroup who did not achieve an improvement in their bone mineral density (BMD) or sustain any fracture within the first 2-year, had their osteoporosis treatment changed. Outcome measures included BMD and FRAX assessment calculated 3-year after commencing new osteoporosis treatment.
RESULTS: There was a significant negative correlation between 10-year probability of major osteoporotic and hip fractures and BMD at the total proximal femur at 2-year of treatment (R = -0.449 and -0.479 respectively), and at 5-year (R = -0.489 and -0.594 respectively). At both 2 years and 5 years of treatment, the 10-year fracture probability showed significant correlation with the incidence of fracture (P < 0.01). On comparing fracture probability, there was a significant difference (P < 0.05) between the responders and non-responders to osteoporosis treatment.
CONCLUSION: In women currently or previously treated for osteoporosis, the FRAX tool can be used to predict fracture probability. Osteoporosis treatment does not annul prediction of fractures. FRAX tool may be of value in guiding clinicians towards the need for continuation or withdrawal of treatment.
Core tip: Treatment of osteoporosis should assure that the patient benefits from the treatment without experiencing undue harm. Monitoring of patients treated for osteoporosis has been recommended and so far dual-energy X-ray absorptiometry has been recognized as the tool to monitor osteoporosis therapy by several clinical practice guidelines. Recently, the duration of osteoporosis therapy became a research question with the possibility of having a drug holiday. However, more research is still needed to adequately assess when to stop the osteoporosis therapy and what is the optimal duration of the drug holiday. This work was carried out aiming at determining whether the World Health Organization fracture risk assessment tool can be used as a tool to monitor patients receiving osteoporosis treatment and to evaluate its ability to predict new low trauma fractures.
- Citation: Miedany YE, Gaafary ME, Yassaki AE, Youssef S, Nasr A, Ahmed I. Monitoring osteoporosis therapy: Can FRAX help assessing success or failure in achieving treatment goals? World J Rheumatol 2014; 4(2): 14-21
- URL: https://www.wjgnet.com/2220-3214/full/v4/i2/14.htm
- DOI: https://dx.doi.org/10.5499/wjr.v4.i2.14
Osteoporosis is a prevalent condition that may exist in a silent form, in which increased fracture risk can be detected by measurement of bone mineral density (BMD), or as a symptomatic condition after fragility fracture has occurred[1]. In standard clinical practice, bone mineral density measured by dual-energy X-ray absorptiometry (DXA) is used to diagnose osteoporosis, assess fracture risk, and provide input for the World Health Organization (WHO) fracture risk assessment tool (FRAX)[2]. The conventional wisdom is that patients should be screened for risk factors and those who are at high risk for fracture should be identified by means of BMD testing. Consequently, those who are at sufficiently high risk of fracture should receive pharmacological therapy to reduce the risk of fractures. This has been reported to be the most cost-effective strategy to reduce the huge personal and economic burden of osteoporosis[3]. Treatment of osteoporosis should assure that the patient benefits from the treatment without experiencing undue harm. Monitoring of patients treated for osteoporosis has been also recommended and so far DXA has been recognized as the tool to monitor osteoporosis therapy by several clinical practice guidelines. Recently, the duration of osteoporosis therapy became a research question with the possibility of having a drug holiday. However, more research is still needed to adequately assess when to stop the osteoporosis therapy and what is the optimal duration of the drug holiday.
The FRAX, a diagnostic tool used to evaluate the 10-year probability of bone fracture risk based on the individual’s risk factor profile, was developed by the WHO Collaborating Centre for Metabolic Bone Diseases[4]. FRAX proved to be of great benefit, not only in patients whose DXA revealed osteoporosis, but also in the osteopenic patients. The implementation of 10-year fracture risk probability assessment in day to day clinical practice impacted positively on the physicians’ prescribing pattern[5]. However, when first implemented, it has been recommended that FRAX should be used only for osteoporosis therapy naïve patients and not to be applied for the assessment of the change in fracture risk in individuals who received osteoporosis treatment. This was attributed to the earlier clinical trials’ findings suggesting that the positive anti-fracture effect from osteoporosis therapy is constantly greater than what can be elucidated from the increase in BMD only (which accounts for only a fraction of the anti-fracture effect)[6-8]. On the other hand, in most of the patients, bone mineral density is the only risk factor amongst those encompassed in the FRAX model expected to show a response to treatment. Therefore, the notion of using FRAX as a tool to assess the impact of osteoporosis therapy on fracture prediction might sound logic.
This work was carried out aiming at determining whether FRAX can be used for monitoring patients receiving osteoporosis therapy and to evaluate its ability to predict new low trauma fractures.
This was a retrospective 5-year follow up study of a patients cohort diagnosed to have osteoporosis based on DXA scan measurement of their BMD and having a T-score of ≤ 2.5 at either neck of the femur or spine.
Local ethical and methodological protocols for approval of the study were followed. The study was approved by Ain Shams University research ethics committee. All patients who shared in the study signed an informed consent in keeping with the World Medical Association’s declaration of Helsinki.
Data from consecutive female patients referred for osteoporosis assessment and management were recorded. Inclusion criteria included: time since menopause at least 6 mo and lumbar spine and/or neck of the femur BMD equal to or more than 2.5 SD below the mean for a normal population. Exclusion criteria were: (1) All women younger than 50 years as individuals referred for BMD testing are less representative of the general population; lack of agreed treatment protocols, and treatment rates are usually low; (2) Any patient suffering from diseases, other than primary osteoporosis known to affect bone metabolism; (3) History of hormone replacement therapy, or bisphosphonate therapy within the 6 mo prior to the start of the study; and (4) Low or non-adherent patients to osteoporosis therapy.
The study cohort available for analysis at the time of baseline assessment was 1026 women. All the patients, aged ≥ 50 years were assesses for baseline risk factors as well as BMD measurement and were considered for inclusion in this study. All patients included in this work were treated following the National Osteoporosis Foundation recommendations[9] and screened for adherence to therapy 1-year after commencing their medication. Only the patients who adhered to the prescribed osteoporosis treatment, had 10-year fracture probability calculated and their data recorded for the purpose of this work. At 2 and 5-year of osteoporosis therapy, a follow-up DXA scan was carried out for every patient. On both occasions, risk factors were reassessed and 10-year fracture probabilities were calculated using FRAX. Those patients who did not achieve any improvement in neck of the femur BMD measurement or had a new low trauma fracture within the first 2-year of management, changed had their osteoporosis treatment. BMD and FRAX measurements were carried out after 3-year of commencing new osteoporosis therapy. The patient cohort who showed good response well to treatment and had not sustained any new low trauma fracture within the first 2 years, had their treatment continued with a further assessment at 3-year.
History of previous low trauma fractures as well as other osteoporosis risk factors, necessary for calculating fracture probability with FRAX, were assessed using a new DXA scan referral form[10] completed for every patient before every bone mineral density assessment. The form also included assessment for falls risk using the “Falls Risk Assessment Score (FRAS)” score[11], recent history of low trauma fractures or change in medications. Patients with high falls risk were referred to the local falls clinic. In addition, history of past osteoporosis therapy as well as current medications was included in the referral form. For purposes of the FRAX calculation, prior fragility fracture was considered if it was one of the major osteoporotic fractures (vertebral, hip forearm, or humerus fracture) and was not associated with severe trauma. The patients’ records and radiology investigations were checked to confirm the history of any of these major osteoporotic fractures as well as history of site specific fracture reduction to enhance the diagnostic and temporal specificity of the fracture event. The diagnosis of rheumatoid arthritis and/or smoking over the past 3-year period before DXA scanning as well as prolonged steroid use (regardless of the dose) for more than 3-mo period in the year before the BMD measurement were crosschecked with the patients’ notes.
BMD was measured using a Hologic DXA machine (Hologic Inc., MA, United States). Every patient had a baseline BMD assessment as well as repeat DXA scan at 2 and 5 years post treatment. All the scans were analysed and the femoral neck, total proximal femur and spine BMD. The T scores were calculated using the equation: T score = (BMD value of osteoporotic patient-BMD of healthy premenopausal population)/SD of the premenopausal healthy population. According to WHO classification: Osteoporosis was diagnosed at T-score ≤ -2.5, osteopenia at T-score < -1 to T-score < -2.5, whereas normal BMD was considered at T-score ≥ -1. A quality control program including daily quality controls was conducted throughout the study[12]. The Densitometer showed stable long-term performance (coefficient of variation < 0.5%) and satisfactory in vivo precision. For each subject, the 10-year major osteoporosis as well as the hip fracture probability was calculated for each subject by the WHO Collaborating Centre (http://www.shef.ac.uk/FRAX/tool.aspx?country=21) using the previously defined variables without knowledge of the fracture outcomes. Prior to every DXA assessment every patient had a blood check for bone profile [serum calcium, phosphorus, alkaline phosphatatse and 25 (OH) vitamin D] as well as liver and kidney function tests. Patients who had vitamin D deficiency were treated with high vitamin D therapy. FRAX estimated fracture risk probability was categorized based on fixed cut off points. For major osteoporotic fracture probability cut-off point was > 20% whereas for hip fracture it was > 3%. The patients whose falls risk score was ≥ 3.5 on the FRAS score, were considered at high risk of falls and were referred to specialized falls clinic for further assessment and management.
Osteoporosis therapy was selected from bisphosphonate (oral alendronate/risedronate; intravenous zoledronate), raloxifene, or salmon calcitonin. None of the patients was taking systemic hormone replacement therapy following the Women’s Health Initiative Study[8]. Strontium, Denosumab as well as anabolic therapies were not available at the start of the study, and their use was limited to the patients who did not respond or were intolerant to bisphosphonate therapy. Adherence, as defined by Cramer et al[13], was evaluated using the parameters of compliance and persistence. Compliance was estimated by the medication possession ratio (MPR) and persistence by the time from treatment initiation to discontinuation with no medication refill gap for a period of 30 d or more during the period of interest. MPR was defined as the ratio of actually available doses against the expected doses that the patient should possess over a fixed period of time. Study patients were rated as having good compliance if the annual MPR ≥ 80%. The MPR was calculated in the first year after BMD testing, and medication swapping was considered. Medication use was categorized as: (1) Untreated: no use in the year before or after BMD testing, and < 6-mo lifetime use in earlier years; (2) Low adherence (current user): MPR < 80% in the year following BMD testing; (3) High adherence (current user): MPR ≥ 80% in the year following BMD testing; (4) Past user: any use in the year preceding BMD testing or at least 6 mo lifetime use in earlier years, with no use in the year following BMD testing.
Descriptive analysis was performed using frequency distribution in categorical variables and mean and standard deviation in case of normally distributed continuous variables. Spearman Rank order correlation was used for non-parametric data. Paired student-t test was used for comparing dependent parametric data. For inferential statistics P value was always set at 0.05.
The patients’ age range was from 50 to 79 years (mean 64.3 + 9.4 years). During mean 5.3 years of observation, there were 44/1026 (4.3 %) deaths and 28/1026 (2.7%) changed their address. 376/1026 (36.6%) were excluded as they did not adhere to therapy, therefore, in total 579 women out of 1026 (56.4%) were included in this cohort. Table 1 shows the mean (± SD) proximal femur BMD as well as the mean predicted 10-year major osteoporotic fracture and hip fracture probability estimated for all patients at baseline, 2-year and 5-year of observation. At baseline 49.6% of the patients had documented history of low trauma fracture. In the initial 2-year of the observation period 16.9% individuals had a new major osteoporotic fracture whereas in the following 3 years of observation period, 4.7% of the patients sustained a new low trauma fracture (Table 2). Hip fractures represented 48% of the total low trauma fractures occurring over the 5.3 years study period whereas the remaining 52% had one of the major osteoporosis fractures (spine, forearm or shoulder).
| At baselinemean ± SD | 2-yr of treatmentmean ± SD | 5-yr of treatmentmean ± SD | |
| Total proximal femur BMD | 0.553 ± 0.69 | 0.574 ± 0.06 | 0.579 ± 0.04 |
| T-score | -2.75 ± 0.61 | -2.32 ± 0.45 | -1.46 ± 0.35 |
| FRAX 10-yr major osteoporosis fracture risk | 21.43 ± 7.56 | 14.81 ± 4.79 | 9.67 ± 2.92 |
| FRAX 10-yr hip fracture risk | 7.11 ± 4.55 | 4.4 ± 2.62 | 4.11 ± 4.55 |
| At baseline | At 2-yr | At 5 yr | ||||
| Frequency | Percentage | Frequency | Percentage | Frequency | Percentage | |
| No fracture | 292 | 50.4 | 481 | 83.1 | 552 | 95.3 |
| Low trauma fracture | 287 | 49.36 | 98 | 16.9 | 27 | 4.7 |
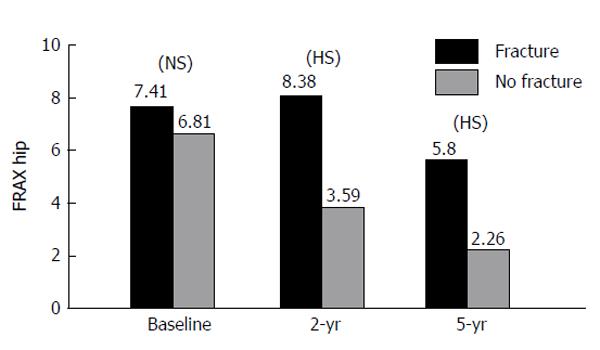
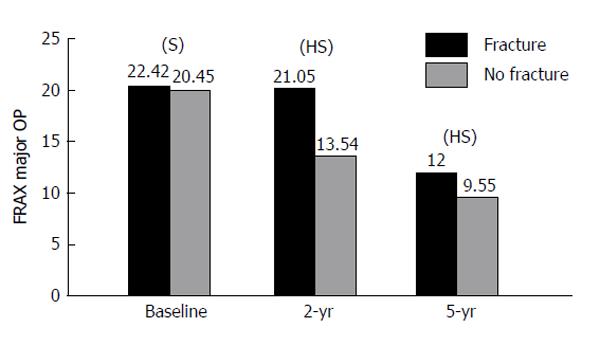
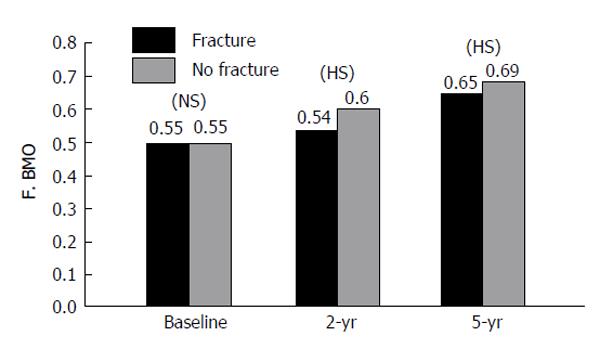
The FRAX stratified major osteoporotic and hip fracture 10-year probability had significant negative correlation with total proximal femur BMD measurement at 2-year as well as at 5-year of therapy (Table 3). After 2 years and 5 years of treatment (Figure 1), there was a significant relation between estimated 10-year predicted fracture probability for major osteoporosis fracture with both femur BMD as well as the fracture (P < 0.01) regardless of the medication used. Similarly, there was a significant relation between the fracture incidence and 10-year predicted fracture probability for hip fracture (P < 0.01) (Figure 2). On assessment of major fracture probability using BMD, there was a significant (P < 0.05) less fracture probability among responders in comparison to non-responders to osteoporosis treatment. Figure 3 shows that, the total proximal femur BMD was a strong predictor to both hip as well as major osteoporotic fractures; this was not affected by medication use (P < 0.05).
| BMD total proximal femur at baseline | BMD total proximal femur at 2-yr | BMD total proximal femur at 5-yr | |
| FRAX 10-yr major osteoporosis fracture risk probability | -0.551b | -0.449b | -0.479b |
| FRAX 10-yr hip fracture risk probability | -0.741b | -0.547b | -0.584b |
| Change in FRAX 10-yr major osteoporosis fracture probability at 2-yr | -0.459b | ||
| Change in FRAX 10-yr hip fracture probability at 2-yr | -0.557b | ||
| Major osteoporosis fracture probability at 5-yr | -0.489b | ||
| Change in FRAX 10-yr hip fracture probability at 5-yr | -0.594b |
The patients who experienced an increase in femoral neck or total proximal femur BMD (expressed as a percentage difference from the baseline BMD) at 2-year treatment had a lower incidence rate of fracture (fracture incidence was 2.5%), compared to treated patients who experienced reduced BMD (fracture incidence was 19%). After controlling for covariates (namely age, steroids intake, smoking), patients with an improvement of at least 3% in femoral neck BMD at 2-year of therapy, were at a lower risk of new fractures development [OR = 0.63 (95%CI: 0.41-0.82)] than patients without such improvement. The results were in the same range when considering changes in total proximal femur BMD [OR = 0.64 (95%CI: 0.48-0.88)]. Changes in spine BMD were not statistically associated with the incidence of vertebral fracture (P = 0.10). For each percentage point increase in femoral neck and total proximal femur BMD observed after 2-year, the risk of sustaining a new vertebral fracture after 2-year decreased by 2.7% (95%CI: 1%-5%) and 1.8% (1%-4%), respectively.
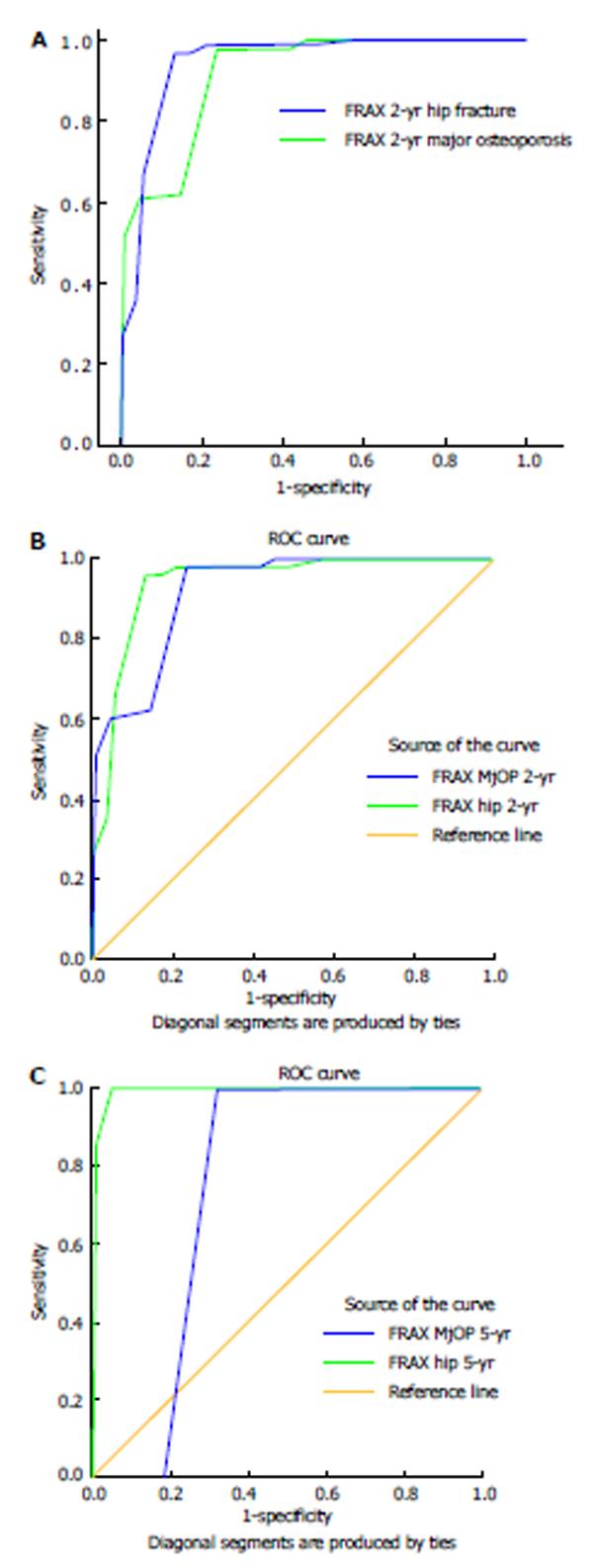
Bisphosphonates were the most common medication used (553/579 patients, 95.5%). Treatment effects were assessed in the 579 patients who showed high adherence to therapy (MPR 0.80) over at least 5 years of bisphosphonate use. The subgroup of patients who did not respond to the treatment and re-measuring of their BMD, at 2-year time, did not reveal significant improvement, had higher incidence of fractures which was significantly higher than predicted. Figure 4A and B is the receiver operating characteristic (ROC) curve is for FRAX major osteoporosis and hip fracture probability at 2 years, whereas Figure 4C is the ROC curve for the 10-year fracture probability both sides at 5-year.
Monitoring the effects of osteoporosis therapy can inform the patient and physician that the drug is having its expected skeletal response. So far, there is no consensus about methods of re-assessment or identifying treatment failures in osteoporosis. Questions have been raised whether the 10-year FRAX can be used to guide treatment decisions. Despite the scientific basis of FRAX and its strengths, the recommendation to the clinicians was not to use the tool in monitoring patients receiving osteoporosis treatment. That was based on the fact that treatment effects were not considered in the model. In turn, this would limit the value of using FRAX to a subgroup of patients whose BMD assessment revealed osteopenia in the presence of other risk factors. This work was carried out aiming at determining whether FRAX can be used as a tool to monitor those patients receiving osteoporosis treatment as well as its clinical implications.
Results of this study revealed that FRAX can be used as a predictor of new post-treatment fracture probability and assess the reduction in the fracture risk in women currently on osteoporosis therapy. FRAX probabilities showed significant correlation with the change in the neck of femur BMD in response to therapy. Besides, FRAX showed a relatively high predicting ability of the incidence of new low trauma fractures. Therefore, FRAX might have a role in guiding the requirement of treatment continuation or withdrawal. These findings are in agreement with the results of a recently published work[14] assessing whether osteoporosis pharmacotherapy invalidates fracture predictions using FRAX. Results of that study[14] revealed that FRAX, showed similar response pattern in untreated, currently treated and previously treated osteoporosis women. In the cohort of women who had high fracture risk with high adherence to at least 5 years of bisphosphonates, hip fracture risk was significantly less than predicted, with a treatment effect that approximated fracture the risk reduction reported in bisphosphonates clinical trials[15-18]. Furthermore, in concordance with the results of this work, there was significant agreement between predicted fracture probability and observed fracture incidence indicating that osteoporosis therapy does not annul using FRAX for fracture prediction. This potentially expands the FRAX clinical role as a tool for persuading patients on their requirement for continued treatment, and whether treatment could possibly be stopped.
So far, BMD measurement using DXA scanning has been a surrogate marker for bone strength and fracture risk. Earlier results showed that stability or a significant increase in BMD is an acceptable response to therapy and is associated with a reduction in fracture risk[3]. Similarly, a significant decrease in BMD suggests a suboptimal response to therapy and may require evaluation for factors contributing to bone loss and possibly changing the osteoporosis medication. In clinical practice, a valid quantitative comparison of BMD measurements requires that measurements be made on the same DXA machine (or different machines that have been cross-calibrated) according to well-established quality standards that include precision assessment and calculation of the least BMD statistically significant change[19]. Results of this study revealed that a statistically significant BMD loss was positively correlated with increased fracture risk probability and a significant predictor of fracture incidence. Furthermore, the timing of follow up BMD testing to monitor osteoporosis treatment has been a matter of debate. In a post-hoc analysis of two randomized, placebo-controlled clinical trials, the concept of regression to the mean was invoked to suggest that treatment should not be changed before one year of therapy[20]. In another post-hoc analysis of a single randomized controlled trial, it was concluded that BMD monitoring is unnecessary in the first three years after starting a potent bisphosphonate[21]. However, the conclusions of both analyses have been challenged[22,23]. Although regression to the mean is a valid statistical concept that is helpful in understanding apparent BMD changes in groups of patients in clinical trials, it does not indicate that serial BMD testing in clinical practice is useless[24]. Results of this study revealed that re-measuring BMD after 2-year of osteoporosis treatment initiation is a valid time interval for BMD changes monitoring.
There are no data providing information on how to monitor osteoporosis patients or guide decision-making about the initiation and termination of “drug holiday”. In the absence of guidance from clinical trials, empiric approaches are necessary. Results of this study highlighted the value of FRAX and BMD measurement in the guidance for the continuation or withdrawal of treatment. Assessment of BMD changes and fracture risk probability 2-year after commencing osteoporosis drug therapy was helpful to identify the non-responders who warranted a change of their medication. Further assessment should be carried out after 5 years of treatment. Persistence of low BMD measures or high fracture risk probability suggests that the patient should continue the osteoporosis medication. On the other hand, drug holiday can be considered for patients whose follow-up BMD reveal normal or osteopenic levels with low fracture risk probability. It can also be suggested that repeat BMD measurement as well as 10-year fracture risk probability assessment be carried out 3-5 years of osteoporosis drug holiday.
Long-term compliance and adherence to osteoporosis therapy has been repeatedly reported as relatively poor. Results of this study revealed that only 56.4% (579/1026) of the patients continued to take their osteoporosis therapy in the first year of management. This agrees with earlier data revealing that only about 50% of patients who begin an osteoporosis drug continue therapy for at least one year[25]. These data, not only highlight the importance of checking the patient’s adherence to therapy in the first year of osteoporosis management, but also stresses on the importance of patient education. Patients should be aware of the relationship between reduction in fracture risk with pharmacologic therapy and BMD increase measured by DXA, and that a combination of BMD and clinical risk factors assessment predict fracture more accurately than BMD or clinical risk factors alone.
A limitation of this study, is the number of osteoporosis therapy agents included in this work. Though the majority of the patients were treated with bisphosphonates, other forms of osteoporosis treatment were also included. These different osteoporosis therapy modalities differ in terms of vertebral and non-vertebral fracture prevention as well as FRAX probability dependency[15,26-28].
In conclusion, this study highlighted the importance of monitoring osteoporosis treatment and its value to the treating clinician in early identification early of non-responders to treatment. This work suggests that the FRAX can be used as a predictor of fracture probability in those women on treatment for osteoporosis, at least on the short term, for guiding the need for continuation, modification or withdrawal of treatment. In general treatment decisions need to be tailored to the patient’s BMD status, fracture risk as well as predisposing risk factors.
We express thanks to all participants, our colleagues, research assistants and nurses for their cooperation and help to bring this research to its final conclusions.
Ten-year fracture risk assessment using the World Health Organization (WHO) fracture risk assessment tool (FRAX) is increasingly used as a guide to osteoporosis management and treatment decisions. However, using FRAX for estimation of fracture probability was recommended for treatment naïve patients only as there was no data published on whether osteoporosis therapy annuls fracture predictions with FRAX. The question of whether FRAX can be used in treated osteoporosis patients is addressed in this study.
There are many differences between subjects in clinical trials and patients being treated in clinical practice. Defining a clinical practice patient as a “non-responder” or “suboptimal responder” to treatment remains a difficult question to answer. Therefore, a pragmatic approach has been suggested considering the evaluation of contributing risk factors and possible treatment changes in patients who have a significant decrease in bone mineral density, or have high fracture risk probability.
This work was carried out aiming at determining whether FRAX can be used as a tool to monitor osteoporotic patients on treatment and to evaluate its ability to predict new low trauma fractures.
This study has positive therapeutic implications as it helps answering the question of whether FRAX works in osteoporotic patients currently on therapy. This work suggests that the FRAX can be used as a predictor of fracture probability in women currently receiving osteoporosis tjerapy.
The WHO FRAX calculator is one of the most thoroughly studied and widely used tools of fracture risk assessment which gained momentum in the past decade. FRAX is mainly used as a guide for clinical decision-making in the patient’s management.
This is a retrospective clinical study to investigate the use of FRAX to monitor the patients receiving osteoporosis therapy and to study the relationship between the post-treatment FRAX and the occurrence of new low trauma fractures.
P- Reviewer: Cheung WH, Gorgey AS, Li JX S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
| 1. | Kleerekoper M, Camacho P. Monitoring osteoporosis therapy. Clin Chem. 2005;51:2227-2228. [PubMed] |
| 2. | Available from: http: //www.sheffield.ac.uk/FRAX/. |
| 3. | Lewiecki EM, Watts NB. Assessing response to osteoporosis therapy. Osteoporos Int. 2008;19:1363-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Kanis JA, Oden A, Johnell O, Johansson H, De Laet C, Brown J, Burckhardt P, Cooper C, Christiansen C, Cummings S. The use of clinical risk factors enhances the performance of BMD in the prediction of hip and osteoporotic fractures in men and women. Osteoporos Int. 2007;18:1033-1046. [PubMed] |
| 5. | Leslie WD, Morin S, Lix LM. A before-and-after study of fracture risk reporting and osteoporosis treatment initiation. Ann Intern Med. 2010;153:580-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Cummings SR, Karpf DB, Harris F, Genant HK, Ensrud K, LaCroix AZ, Black DM. Improvement in spine bone density and reduction in risk of vertebral fractures during treatment with antiresorptive drugs. Am J Med. 2002;112:281-289. [PubMed] |
| 7. | Seeman E. Is a change in bone mineral density a sensitive and specific surrogate of anti-fracture efficacy? Bone. 2007;41:308-317. [PubMed] |
| 8. | Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321-333. [PubMed] |
| 9. | National Osteoporosis Foundation guidelines. Clinician guide to prevention and treatment of osteoporosis. Available from: http: //nof.org/files/nof/public/content/file/950/upload/523.pdf. |
| 10. | El Miedany Y, Youssef S, El Gaafary M, Toth M. Osteoporosis, falls and fractures: 3 confounders in one equation: development and validity of a new form for assessment of patients referred for DXA scanning. Rheumatology (Oxford). 2010;49:80. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | El Miedany Y, El Gaafary M, Toth M. Falls risk assessment score (FRAS): Time to rethink. J Clinical Gerontol and Geriatrics. 2011;2:21-26. [RCA] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Slosman DO, Provvedini DM, Meunier PJ, Delmas PD, Sebert JL, De Vernejoul MC, Tsouderos Y, Reginster JY. The use of different dual X-ray absorptiometry brands in a multicenter clinical trial: consequences and limits. J Clin Densitom. 1999;2:37-44. [PubMed] |
| 13. | Cramer JA, Roy A, Burrell A, Fairchild CJ, Fuldeore MJ, Ollendorf DA, Wong PK. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11:44-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1363] [Cited by in RCA: 1562] [Article Influence: 91.9] [Reference Citation Analysis (0)] |
| 14. | Leslie WD, Lix LM, Johansson H, Oden A, McCloskey E, Kanis JA. Does osteoporosis therapy invalidate FRAX for fracture prediction? J Bone Miner Res. 2012;27:1243-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 98] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 15. | MacLean C, Newberry S, Maglione M, McMahon M, Ranganath V, Suttorp M, Mojica W, Timmer M, Alexander A, McNamara M. Systematic review: comparative effectiveness of treatments to prevent fractures in men and women with low bone density or osteoporosis. Ann Intern Med. 2008;148:197-213. [PubMed] |
| 16. | Papaioannou A, Morin S, Cheung AM, Atkinson S, Brown JP, Feldman S, Hanley DA, Hodsman A, Jamal SA, Kaiser SM. 2010 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada: summary. CMAJ. 2010;182:1864-1873. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 836] [Cited by in RCA: 818] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 17. | Wells GA, Cranney A, Peterson J, Boucher M, Shea B, Robinson V, Coyle D, Tugwell P. Alendronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women. Cochrane Database Syst Rev. 2008;CD001155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 18. | Wells G, Cranney A, Peterson J, Boucher M, Shea B, Robinson V, Coyle D, Tugwell P. Risedronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women. Cochrane Database Syst Rev. 2008;CD004523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Baim S, Binkley N, Bilezikian JP, Kendler DL, Hans DB, Lewiecki EM, Silverman S. Official Positions of the International Society for Clinical Densitometry and executive summary of the 2007 ISCD Position Development Conference. J Clin Densitom. 2003;11:75-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 310] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 20. | Cummings SR, Palermo L, Browner W, Marcus R, Wallace R, Pearson J, Blackwell T, Eckert S, Black D. Monitoring osteoporosis therapy with bone densitometry: misleading changes and regression to the mean. Fracture Intervention Trial Research Group. JAMA. 2000;283:1318-1321. [PubMed] |
| 21. | Bell KJ, Hayen A, Macaskill P, Irwig L, Craig JC, Ensrud K, Bauer DC. Value of routine monitoring of bone mineral density after starting bisphosphonate treatment: secondary analysis of trial data. BMJ. 2009;338:b2266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 22. | Bonnick SL. Monitoring osteoporosis therapy with bone densitometry: a vital tool or regression toward mediocrity? J Clin Endocrinol Metab. 2000;85:3493-3495. [PubMed] |
| 23. | Watts NB, Lewiecki EM, Bonnick SL, Laster AJ, Binkley N, Blank RD, Geusens PP, Miller PD, Petak SM, Recker RR. Clinical value of monitoring BMD in patients treated with bisphosphonates for osteoporosis. J Bone Miner Res. 2009;24:1643-1646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Lewiecki E. Bone Density Testing to Monitor Osteoporosis Therapy in Clinical Practice (Editorial). Available from: http: //www.aafp.org/afp. |
| 25. | Seeman E, Compston J, Adachi J, Brandi ML, Cooper C, Dawson-Hughes B, Jönsson B, Pols H, Cramer JA. Non-compliance: the Achilles’ heel of anti-fracture efficacy. Osteoporos Int. 2007;18:711-719. [PubMed] |
| 26. | Kanis JA, Johansson H, Oden A, McCloskey EV. A meta-analysis of the efficacy of raloxifene on all clinical and vertebral fractures and its dependency on FRAX. Bone. 2010;47:729-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 75] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 27. | Kanis JA, Johansson H, Oden A, McCloskey EV. A meta-analysis of the effect of strontium ranelate on the risk of vertebral and non-vertebral fracture in postmenopausal osteoporosis and the interaction with FRAX(®). Osteoporos Int. 2011;22:2347-2355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 28. | McCloskey EV, Johansson H, Oden A, Austin M, Siris E, Wang A, Lewiecki EM, Lorenc R, Libanati C, Kanis JA. Denosumab reduces the risk of osteoporotic fractures in postmenopausal women, particularly in those with moderate to high fracture risk as assessed with FRAX. J Bone Miner Res. 2012;27:1480-1486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |