Revised: December 23, 2011
Accepted: July 5, 2012
Published online: October 3, 2012
Pulmonary arterial hypertension (PAH) represents a devastating vascular complication of systemic sclerosis (SSc) and is found in 10%-15% of cases carrying a severe prognosis. PAH has a dramatic impact on the clinical course and overall survival, being the single most common cause of death in patients with this entity. The clinical course and aggressive progression of PAH has led clinicians to perform annual screening for it, since early detection and diagnosis are the cornerstone of a prompt therapeutic intervention. The diagnosis of PAH can be challenging to clinicians, particularly in its early stages, since in the context of SSc, the multiple causes of dyspnea need to be assessed. Doppler echocardiography represents the best initial screening tool, however, right heart catheterization remains the gold standard and definitive diagnostic means. Remarkable advances have been achieved in elucidating the pathogenesis of PAH in the past two decades, leading to the development of disease-specific targeted therapies: prostacyclin analogues, endothelin receptor antagonists and inhibitors of five phosphodiesterase pathways. However, the clinical response to these therapies in SSc-associated PAH has not been as great as the one seen with idiopathic PAH. This review also focuses on the diagnosis and novel therapies that are currently available for PAH, as well as potential future therapeutic developments based on newly acquired knowledge of diverse pathogenic mechanisms.
- Citation: Vera-Lastra O, Porres-Aguilar M. Pulmonary arterial hypertension associated with systemic sclerosis: Current diagnostic approach and therapeutic strategies. World J Rheumatol 2012; 2(2): 12-20
- URL: https://www.wjgnet.com/2220-3214/full/v2/i2/12.htm
- DOI: https://dx.doi.org/10.5499/wjr.v2.i2.12
Pulmonary arterial hypertension (PAH), hemodynamically defined as a mean pulmonary arterial pressure (mPAP) greater than 25 mmHg, a mean pulmonary capillary wedge pressur < 15 mmHg, and pulmonary vascular resistance greater than 3 Wood units, represents a progressive syndrome of the pulmonary vasculature that leads to progressive right ventricular failure, long-term disability and often death if left untreated within 2-2.5 years[1,2]. Systemic sclerosis (SSc) is defined as a heterogeneous disorder characterized by endothelial dysfunction, dysregulation of fibroblasts resulting in excessive production of collagen, and abnormalities in the immune system[3,4]. These processes lead to progressive fibrosis of the skin and internal organs resulting in premature organ failure and death. Pulmonary involvement in SSc include interstitial lung disease (ILD) and PAH, which are the most common pulmonary manifestations nowadays and are now the leading causes of death in SSc[5]. Typically, SSc-associated PAH (SSc-PAH) will develop in patients with a limited form of SSc after 10-15 years of evolution of the disease[6,7].
The frequency of SSc-PAH is about 8%-15% depending on the diagnostic method used. The following methods have been recommended for its diagnosis, treatment-follow-up and prognosis: Doppler transthoracic echocardiogram (TTE), complete pulmonary function tests (PFTs) or spirometry including carbon monoxide diffusing capacity (DLCO), the 6 min walk test (6MWT), and biological markers: n-terminal pro-brain natriuretic peptide (NT-pro-BNP). The right heart catheterization (RHC) remains the gold standard for definitive diagnosis of PAH[8-10].
Remarkable advances have been achieved in elucidating the pathogenesis of PAH over the past two decades, leading to the rapid development of disease-specific therapies. However, despite these achievements, the response to therapies is often divergent and suboptimal in the subgroup of patients with SSc-PAH, since survival remains poor, particularly when compared with idiopathic PAH (IPAH)[11].
Endothelial dysfunction plays an essential role in the pathogenesis of SSc-PAH, which histopathologically is characterized by intimal hyperplasia, medial hypertrophy, and adventitial fibrosis. These changes lead to the development of concentric obliterative arteriolar vasculopathy with angioproliferative plexiform lesions; however, there are fewer plexiform lesions, increased intimal fibrosis, and more heterogeneity when compared with lesions in IPAH[12]. Two recent histopathological studies have demonstrated the presence of pulmonary veno-occlusive disease characterized by fibrotic remodeling of post-capillary venules and preseptal veins, however, this needs to be confirmed in larger studies[12].
Autoimmunity appears to play a central role in pulmonary vascular remodeling. These include endothelial cell apoptosis and activation with expression of cell adhesion molecules, inflammatory cellular recruitment, hypercoagulable state, and intimal proliferation and adventitial fibrotic changes leading to obliterative arteriolopathy[13,14]. Several studies have demonstrated increased circulating factors like the soluble vascular cell adhesion molecule, consistent with endothelial cell injury[15]. Dysregulated angiogenesis may play also an important role in the development of SSc-PAH, reflected by increased levels of circulating vascular endothelial growth factor (VEGF)[16].
Autoantibodies are often associated with the development of certain phenotypes in SSc with the subsequent development of PAH. Antifibrillarin antibodies are frequently found in SSc-PAH patients and the antiendothelial cell antibodies correlate with digital ischemia and infarcts, and could display distinct reactivity profiles against antigens from the micro and macrovascular beds[17,18].
Given the importance of the concept of vasoproliferation and endothelial dysfunction described in different forms of PAH[19,20], it has also been hypothesized in SSc-PAH: an imbalance of vasomediators leading to vasoconstriction, endothelial damage leading to further vascular remodeling, proliferation of the endothelium and vascular smooth muscle cells, along with in situ thrombosis[19,20]. Increased levels of endothelin type-1 (ET-1), a potent selective pulmonary vasoconstrictor produced in the pulmonary vascular endothelium, has been shown to play a prominent role in the pathobiology of PAH[20]. Both serotonin and ET-1 are dual-action potent pulmonary vascoconstrictors that may induce significant pulmonary vascular remodeling change as well as mitogenic changes in the pulmonary arterioles[21,22]. At the same time, synthesis of vasodilators such as nitric oxide (NO) and prostacyclin may be decreased in different forms of PAH, facilitating further the vascular remodeling and the proliferative response. Importantly, prostacyclin synthase levels have been demonstrated to be down-regulated in patients with PAH[23].
Typically, patients with SSc-PAH are predominantly women, have limited SSc, and tend to be older. Clinical symptoms in PAH tend to be nonspecific, and dyspnea on exertion is the most common initial complaint[1,19-22]. Other common symptoms include fatigue, generalized weakness, light-headedness, and orthopnea. Physical examination may show elevated jugular venous pressure in the neck, an accentuated pulmonic component of the second heart sound, a systolic murmur that could be consistent with tricuspid regurgitation or a murmur of pulmonic insufficiency (Graham-Steele murmur), as well as a pulsatile liver, suggestive of hepatic congestion. Dependent bilateral lower extremity edema may be a sign of right ventricular dysfunction and PAH[20]. In addition, since other organs could be commonly affected in SSc, including myocardial, pericardial, or generalized vascular and musculoskeletal organs, causing also the above mentioned myriad of symptoms, the initial diagnostic approach represents a complete challenge for the clinician. Furthermore, patients with SSc-PAH more commonly present with pericardial effusion when compared with IPAH, although it remains unclear whether the effusions are due to progressive right ventricular (RV) dysfunction or due to the underlying autoimmune process.
Patients with SSc have an advantage over IPAH patients, since SSc patients (both limited and diffuse SSc) are identified as a population at high risk to develop PAH overtime. Therefore, we recommend close clinical surveillance as well as annual screening by useful tools that we will discuss in the latter section of this review, particularly screening for pulmonary complications like ILD and/or PAH. This constitutes an annual or biannual Doppler TTE, complete PFTs or spirometry including DLCO and the 6MWT[24]. A recently published consensus statement from the American College of Cardiology, American Heart Association in conjunction with the American College of Chest Physicians (ACCP), American Thoracic Society and the Pulmonary Hypertension Association strongly recommends yearly TTE for patients with SSc to screen for PAH[24].
PFTs abnormalities, such as progressive decline in DLCO, alone or in combination of a forced vital capacity (FVC)%/DLCO% ratio > 1.4 may identify SSc patients that could be developing PAH, however, this strategy may not be routinely performed by clinicians[6,25,26]. Hormonal and humoral dysfunction is also common in PAH, as evidenced by signs of neurohormonal activation by elevated levels of NT-pro-BNP, a neuropeptide released in response to right ventricular stretch and stress, is frequently elevated in SSc-PAH and appears significantly higher than in patients with IPAH despite similar hemodynamic alterations[27]. Simultaneously, hyponatremia, a marker of neurohormonal activation, is also very common in SSc-PAH, and portends a poor prognosis[28].
The 6MWT is employed as a simple, reproducible, and valid measure of submaximal cardiopulmonary exercise capacity. The utility of the test as a predictor of prognosis, and measure of response to pharmacological therapy has been well studied and validated. The test has also been used as a surrogate to predict survival and utilized as a primary outcome in pharmacological PAH clinical trials and has also been well studied and prospectively validated in the IPAH subgroup[29]. However, its value in the evaluation of submaximal exercise capacity in SSc-PAH has become a great matter of debate, since in this subset of patients their functional status is not only affected by the cardiorespiratory status, but also arthropathy, myopathy, musculoskeletal dysfunction or lower extremity digital ischemia associated with SSc[30]. Hence, the 6MWT does not always represent a reliable tool when evaluating the cardiopulmonary capacity in SSc-PAH patients, limiting its utility[31].
NT-pro-BNP represents an acceptable serum marker for severity, prognosis, and response to therapy in patients with different forms of PAH[32]. However, in SSc, subclinical myocardial involvement is common and NT-pro-BNP can be elevated in patients with early myocardial involvement, as well as in SSc-PAH[33]; moreover, elevated NT-pro-BNP does not help differentiate left heart disease from right ventricular systolic or diastolic dysfunction in the setting of SSc, especially when both pathophysiological entities coexist[33].
TTE represents an essential, probably the best non-invasive method of choice for the initial assessment and screening tools in the diagnostic approach for diverse forms of PAH[1,8,19-22]. In a large multicenter French study, patients with SSc and non severely depressed FVC by PFTs, were screened using Doppler TTE; those with a tricuspid regurgitation velocity (TRV) jet > 3 m/s, or 2.5-3 m/s accompanied by unexplained dyspnea, underwent RHC to confirm PAH[7]. This study supported the idea that proper screening may help identify patients at an early stage of their disease. TTE is useful because it can help in the differential diagnosis of pulmonary hypertension, identifying elevated pulmonary arterial pressures due to systolic and diastolic left ventricular dysfunction. Several indices of right ventricular function, such as the tricuspid annular plane systolic excursion (TAPSE) and the right ventricular systolic performance index (Tei index), can also be determined by this technique[34,35]. Acknowledging the limitations of TTE for the definitive diagnosis of PAH, the echocardiographic estimation of the likelihood of PAH is among the key elements in the decision-making process, related to the need and timing of RHC in patients with suspected PAH. A retrospective analysis assessed 137 SSc patients regardless of the presence or absence of ILD. The cut off from the estimated right ventricular systolic pressure (RVSP) of 35 mmHg and the TRV jet of 2.75 m/s had an 88% sensitivity and 42% specificity for the diagnosis of PAH[36]. A cut off of RVSP > 50 mmHg and TRV jet > 3.3 m/s had 97% specificity but only 47% of sensitivity for the diagnosis of PAH[36].
Frea et al[37] prospectively studied 38 patients with SSc without PAH during a 12 mo period including TTE evaluation, calculating RV function and morphology, TRV jets, RVSP, TAPSE, Tei index, and pulmonary flow acceleration time (AcT), as well as RV outflow tract time-velocity integral (TVI), and found that four patients developed PAH. Only TRV/AcT and TRV/TVI ratios significantly predicted the development of PAH, showing good diagnostic power (TRV/TVI ratio with 75% sensitivity and 95% specificity and TRV/AcT ratio with 75% sensitivity and 71% specificity). The multicenter pulmonary hypertension assessment and recognition of outcomes in scleroderma registry prospectively follows patients with SSc at high risk or with incidental PAH. Analyses of this multinational registry will allow identification of risk factors for the development of PAH among SSc patients and enhance understanding of the course of SSc-PAH[38].
The ongoing detecting early tumors enables cancer therapy study in SSc patients is currently evaluating prospectively the role of TTE against RHC for sensitivity, specificity, predictive value in identifying patients with PAH[39].
RHC assessing cardiopulmonary hemodynamics represents the gold standard and is necessary for the definitive diagnosis of PAH[1,2,8]. Mean right atrial pressure, decreased cardiac index (CI), and increased mPAP are predictors of death or need for lung transplantation in IPAH[24]. However, although these data have been prospectively validated in the IPAH subgroup of patients, they remain of unclear usefulness in SSc-associated PAH patients. In a retrospective analysis comparing baseline hemodynamic parameters between IPAH and SSc-PAH patients, patients with SSc had significantly lower mPAP and pulmonary vascular resistance by RHC and equally depressed CI compared with IPAH patients; however, follow-up demonstrated that SSc patients were four times more likely to die when compared with IPAH patients despite comparable therapy[7]. These paradoxical findings suggest that the RV may have a reduced ability to adapt to increased mPAP, perhaps related in part due to myocardial involvement in SSc.
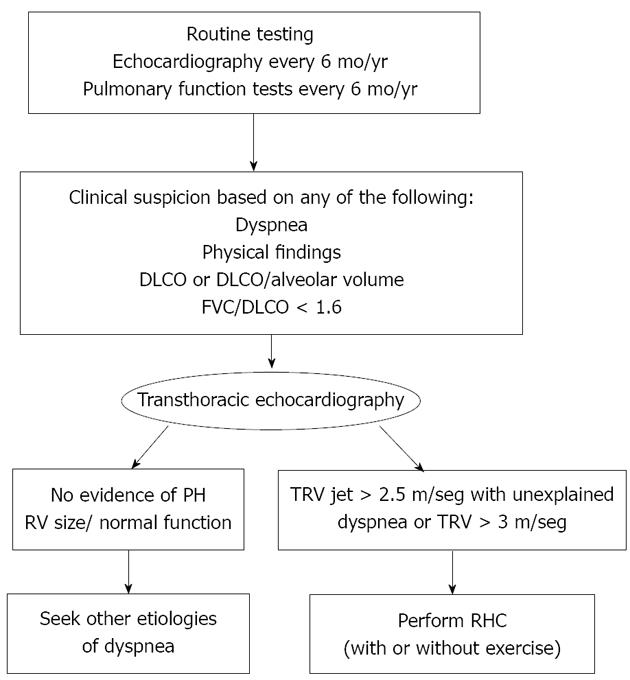
The Johns Hopkins Pulmonary Hypertension Center of Excellence has recently developed and proposed a diagnostic algorithm for routine clinical tests and tools in patients with SSc, which may allow early detection of PAH, as well as other potential causes of dyspnea such as myocardial involvement (left ventricular dysfunction), as well as diffuse parenchymal lung disease such as ILD[40] (Figure 1).
A better understanding of the pathophysiologic mechanisms in PAH, has allowed clinicians to develop new and effective therapeutic targets for this devastating disease[41-43]. Currently, three main classes of drugs for the treatment of PAH exist: prostacyclin analogues, endothelin receptor antagonists (ETRA) and phosphodiesterase type 5 inhibitors (PDEI-5)[44].
Prostacyclin and its analogues are metabolites of the arachidonic acid that are produced by the vascular endothelium. They exhibit potent vasodilatory, antithrombotic, antiproliferative and anti-inflammatory properties. The vasoconstriction, thrombosis, proliferation and the lack of endogenous prostaglandin I-2 (PGI-2) associated with PAH may contribute substantially to this condition[45]. PAH shows low levels of PGI-2 thus, several analogues have been developed for its management.
Intravenous epoprostenol was the first approved drug for the treatment of PAH, especially for patients with functional class IV and advanced right ventricular failure. Treatment with epoprostenol was associated with improvement in exercise capacity, hemodynamic measures and quality of life not only in patients with IPAH, but also in patients with PAH-SSc[46,47]. Intravenous epoprostenol has been approved by the Food and Drug Administration for the treatment of severe IPAH, supported by the results of randomized controlled trials (RCT) which have documented significant improvement in the survival of these patients[48,49]. Therefore, it is recommended for the treatment of IPAH as well as for severe SSc-PAH[50].
Treprostinil (subcutaneous, intravenous or inhalation), iloprost (intravenous or inhaled) and beraprost (oral) are other PGI-2 analogues with longer half-life which were developed later, and can be administered by different routes and have also proved effective in the treatment of PAH. Subcutaneous treprostenil has been studied in a large RCT of 470 patients with PAH, which included patients with connective tissue disease (CTD-PAH), where it was found to improve exercise capacity 6WMT, hemodynamics and clinical events[51]. A post-hoc analysis of data from 90 patients with CTD-PAH including SSc-PAH demonstrated that continuous subcutaneous infusion of treprostenil improved exercise capacity, symptoms of PAH and pulmonary hemodynamic parameters[52].
Studies suggested that inhaled iloprost, a stable PA, promotes selective pulmonary vasodilatation, improves hemodynamics and exercise capacity in patients with PAH. This medication was investigated in 203 patients, 17 of whom had CTD-PAH and it was concluded that there was an improvement in 6WMT in patients who received inhaled iloprost vs deterioration in those who received placebo[53]. Inhaled iloprost is an effective therapy for patients with severe PAH. An uncontrolled study in SSc-PAH patients treated with aerosolized iloprost showed it is potentially useful as a treatment for these patients[54].
ETRA have proven useful in patients with IPAH and with CTD-PAH, especially SSc. PAH is characterized by excess production of endothelin-1 (ET-1), therefore blocking the effects of ET-1 via antagonism of the ETA and ETB receptors is an important therapeutic strategy[41]. Three molecules are currently available for the treatment of PAH. Bosentan, which non-selectively blocks both ETA and ETB receptors, sitaxsentan and ambrisentan, which selectively blocks the ETA receptor[41,55]. Two RCTs demonstrated that bosentan improves exercise capacity, functional class and some hemodynamic measures in PAH[56,57].
Denton et al[58] published a subgroup analysis on the use of bosentan in the treatment of severe CTD-PAH including SSc-PAH. This study found that short-term treatment with bosentan seemed to have a favorable effect compared with placebo.
The long-term follow-up of these patients suggests that bosentan, plus other PAH treatments, if required, is safe for long-term treatment and may have a positive effect on patient outcome. The 92% estimate for survival at 48 wk is a significant achievement in this patient population[59]. A retrospective study showed that bosentan in patients with SSc-PAH and IPAH with a follow up of at least 6 mo was associated with long term improvement in functional class and good survival in patients with functional class III IPAH. However, most SSc-PAH patients experienced stability and some showed impairment in functional class who tended to have a higher mortality[60]. Analysis of the two RTC and their long-term extension studies suggested that bosentan may improve survival in SSc-PAH in comparison with historic controls[61,62]. Based on the results of RCT, bosentan was recommended in the current guidelines of the ACCP[42].
The new selective ETRA sitaxsentan and ambrisentan have also shown to be efficacious in the treatment of PAH, resulting in small gains in 6MWT and other clinical markers[63]. Studies with these agents which included patients wich SSc-PAH, revealed their efficacy in the treatment of PAH[64]. Sitaxsentan has been studied in two RCT of which STRIDE-2 is the most important. In this study which included 74 patients with CTD-PAH, treatment with sitaxsentan led to improvement in 6WMT over the 18 wk treatment period[64], with a low incidence of hepatic toxicity. Supported by two RCT studies, results indicate that sitaxentan improved exercise capacity, functional class and some hemodynamic measures in PAH. At present, sitaxentan may also be considered in the treatment of SSc-PAH[62,64,65]. Ambrisentan was evaluated in two double-blind studies in 64 patients with IPAH or CTD-PAH, during 12 wk. Their results appeared to improve exercise capacity, symptoms, and hemodynamics in patients with PAH and the incidence and severity of liver enzyme abnormalities was also low[66].
NO works via the cyclic guanosine monophosphate (cGMP) pathway to mediate vasodilation and antiproliferation. In PAH there is impaired NO production. Sildenafil inhibits phosphodiesterase type 5 (an enzyme that metabolizes cGMP), thereby enhancing the cGMP mediated relaxation and growth inhibition of vascular smooth-muscle cells, including those in the lung. In a post-hoc subgroup analysis of 84 patients with CTD-PAH in sildenafil use in pulmonary arterial hypertension-1 (45% of the patients had SSc), sildenafil revealed improvement in exercise capacity, hemodynamic and functional class after 12 wk of treatment. Side effects of sildenafil included headache, flushing and heartburn, among the most common[67,68]. Tadalafil is another PDEI-5 that should be used in the treatment of PAH. In patients with PAH, tadalafil 40 mg was given orally and was well tolerated and improved exercise capacity and quality of life measures and reduced clinical worsening[41,69] although further studies are necessary for the treatment of SSc-PAH.
Based on current knowledge regarding the complex pathobiology involved in the development of PAH, it has been proposed that combined therapy (CT) can provide synergistic effects on the pulmonary vasculature. Presently, CT has been used in treating patients whose response to monotherapy was very poor. The best results can be achieved by either the simultaneous administration of two or more agents or either by the sequential addition of one or more agents to ongoing therapy[70]. Despite the encouraging results in the treatment of IPAH, there is scant information about its use in patients with SSc-PAH[41].
Adding inhaled iloprost to patients receiving bosentan has shown to be beneficial in a small RCT study. In this study, CT was well tolerated and led to an improvement in New York Heart Association (NYHA) functional class, functional class, mPAP and delayed time to clinical worsening[71].
Another study showed that the addition of oral sildenafil to intravenous epoprostenol improved exercise capacity, hemodynamic measurements, time to clinical worsening, and quality of life, but not Borg dyspnea score. Increased rates of headache and dyspepsia occurred in the add-on arm. However, this study excluded patients with SSc-PAH[72]. These results have been more encouraging in the treatment of IPAH than SSc-PAH.
On the other hand, the addition of sildenafil after bosentan monotherapy failed to improve NYHA functional class and 6MWT in IPAH and SSc-PAH. Further studies are necessary to evaluate the tolerability, efficacy and safety CT in patients with SSc-PAH[73].
CT of PDEI with ETRA is currently being evaluated. Therapy of PAH is usually started with oral monotherapy, frequently using an ETRA. When the first line therapy is not tolerated, ETRA is substituted by a PDEI. If treatment goals are not achieved with monotherapy, CT could be used. Treatment of SSc-PAH follows the same algorithms as in IPAH[74].
The PAH is characterized by an aberrant proliferation of endothelial, smooth muscle cell, and increased expression of secreted growth factors such as the VEGF and the platelet derived grow factor (PDGF). These pivotal discoveries have changed the views in the treatment of PAH. Two strategies that are presently tested: disruption of PDGF and VEGF signaling pathways Imatinib whose mechanism is to inhibit the Bcr-Abl kinase is the prototypical PDGF receptor signaling inhibitor currently under clinical investigation. Sorafenib is the other drug currently being tested. Their efficacy is due to their dual inhibition of VEGF and PDGF signaling pathways. In experimental models of PAH, imatinib has been tested and shown to be effective[40,75,76]. Some reports have indicated its utility including one in patients with SSc-PAH[77-79]. A Phase II study evaluating safety, tolerability and efficacy of imatinib in PAH has been completed. Although the study failed to demonstrate improvement in 6MWD there were statistically significant improvements in hemodynamic measurements. Post hoc subgroup analyses indicate that patients with more hemodynamic impairment may respond better than patients with less impairment[80]. If these new antineoplastic drugs with anti-tyrosine kinase activity can play a role in SSc-HAP or in IPAH remains to be proven[40,75].
Based on the potential role of autoimmunity in SSc-PAH, other therapeutic strategies are being studied such as rituximab, an anti CD 20 therapy that depletes B cell lineages. Lately the transcription factor Fos-related antigen-2 (Fra-2), a member of the activator protein 1 family implicated in transforming growth factor-β and PDGF signaling has been found to be up-regulated in patients with SSc. Due to the fact that Fra-2 causes fibrosis and vascular disease, this factor can be a potential therapeutic target[81].
Lung transplantation (LT) is the last option for patients with PAH who fail to respond to medical management. Although, SSc is not an absolute contraindication to LT, these patients often have associated comorbidities and multiorgan involvement, placing them at a high risk for LT with a two-year survival rates in cases of SSc patients adequately screened and detected to have PAH comparable to IPAH patients[40,75,82,83].
The European league against rheumatism and scleroderma trial and research group has recently published the following recommendations for the management of SSc-PAH (Table 1).
| Type of drugs | Drugs and doses | Study | Results | Strength of recommendation | Ref. |
| Endothelin receptor antagonists | Bosentan, 62.5 mg twice daily for 4 wk, followed 125 twice daily for 12 wk | 2 RCT | Improves exercise capacity, functional class and some hemodynamic measures | A/B | [56,57] |
| Sitaxentan, 100 mg/d for 18 wk | STRIDE-2 study group STRIDE-1 study group | Improves exercise capacity, functional class and some hemodynamic measures | A/B | [64,66] | |
| PDEI-5 | Sildenafil, 20, 40, 80 mg three times daily for 12 wk | SUPER study group | Improves exercise capacity, functional class and some hemodynamic measures | A/B | [67] |
| Prostacyclin analogues | Intravenous epoprostenol at the start usually < 2 ng/kg of body weight per minute (infused continuously by infusion pump); during 12 wk study, doses were adjusted with mean epoprostenol infusion rate of 11 ng/kg per minute | RCT | Improves exercise capacity, functional class and hemodynamic measures | A/B | [46] |
The SSc-PAH is a devastating complication of SSc, deserving adequate periodic screening and prompt diagnosis that will lead to an early treatment. Currently, despite the advances in the knowledge of the pathophysiologic mechanisms of PAH, treatment with PA, ETRA and PDEI have not been as successful as with IPAH. A better understanding of the pathophysiologic mechanisms of the pulmonary vascular remodeling and its impact on the heart and other vital organs in SSc is of paramount importance and cornerstone in order to develop novel therapies.
Peer reviewer: Dr. Allison B Reiss, Department of Medicine, Vascular Biology Institute, Winthrop University Hospital, 222 Station Plaza, North Mineola, NY 11501, United States
S- Editor Wu X L- Editor A E- Editor Wu X
| 1. | Elliott CG, Barst RJ, Seeger W, Porres-Aguilar M, Brown LM, Zamanian RT, Rubin LJ. Worldwide physician education and training in pulmonary hypertension: pulmonary vascular disease: the global perspective. Chest. 2010;137:85S-94S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | Humbert M. Update in pulmonary hypertension 2008. Am J Respir Crit Care Med. 2009;179:650-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Jimenez SA, Derk CT. Following the molecular pathways toward an understanding of the pathogenesis of systemic sclerosis. Ann Intern Med. 2004;140:37-50. [PubMed] |
| 4. | LeRoy EC, Black C, Fleischmajer R, Jablonska S, Krieg T, Medsger TA, Rowell N, Wollheim F. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol. 1988;15:202-205. [PubMed] |
| 5. | Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis, 1972-2002. Ann Rheum Dis. 2007;66:940-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1233] [Cited by in RCA: 1125] [Article Influence: 62.5] [Reference Citation Analysis (0)] |
| 6. | Steen V, Medsger TA. Predictors of isolated pulmonary hypertension in patients with systemic sclerosis and limited cutaneous involvement. Arthritis Rheum. 2003;48:516-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 334] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 7. | Steen V. Predictors of end stage lung disease in systemic sclerosis. Ann Rheum Dis. 2003;62:97-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 90] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 8. | Hachulla E, Gressin V, Guillevin L, Carpentier P, Diot E, Sibilia J, Kahan A, Cabane J, Francès C, Launay D. Early detection of pulmonary arterial hypertension in systemic sclerosis: a French nationwide prospective multicenter study. Arthritis Rheum. 2005;52:3792-3800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 504] [Cited by in RCA: 466] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 9. | Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, Yaici A, Weitzenblum E, Cordier JF, Chabot F. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med. 2006;173:1023-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1271] [Cited by in RCA: 1288] [Article Influence: 67.8] [Reference Citation Analysis (0)] |
| 10. | Hachulla E, de Groote P, Gressin V, Sibilia J, Diot E, Carpentier P, Mouthon L, Hatron PY, Jego P, Allanore Y. The three-year incidence of pulmonary arterial hypertension associated with systemic sclerosis in a multicenter nationwide longitudinal study in France. Arthritis Rheum. 2009;60:1831-1839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 133] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 11. | Fisher MR, Mathai SC, Champion HC, Girgis RE, Housten-Harris T, Hummers L, Krishnan JA, Wigley F, Hassoun PM. Clinical differences between idiopathic and scleroderma-related pulmonary hypertension. Arthritis Rheum. 2006;54:3043-3050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 254] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 12. | Overbeek MJ, Vonk MC, Boonstra A, Voskuyl AE, Vonk-Noordegraaf A, Smit EF, Dijkmans BA, Postmus PE, Mooi WJ, Heijdra Y. Pulmonary arterial hypertension in limited cutaneous systemic sclerosis: a distinctive vasculopathy. Eur Respir J. 2009;34:371-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 118] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 13. | Sgonc R, Gruschwitz MS, Boeck G, Sepp N, Gruber J, Wick G. Endothelial cell apoptosis in systemic sclerosis is induced by antibody-dependent cell-mediated cytotoxicity via CD95. Arthritis Rheum. 2000;43:2550-2562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Cerinic MM, Valentini G, Sorano GG, D'Angelo S, Cuomo G, Fenu L, Generini S, Cinotti S, Morfini M, Pignone A. Blood coagulation, fibrinolysis, and markers of endothelial dysfunction in systemic sclerosis. Semin Arthritis Rheum. 2003;32:285-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 101] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 15. | Denton CP, Bickerstaff MC, Shiwen X, Carulli MT, Haskard DO, Dubois RM, Black CM. Serial circulating adhesion molecule levels reflect disease severity in systemic sclerosis. Br J Rheumatol. 1995;34:1048-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 87] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 16. | Hummers LK, Hall A, Wigley FM, Simons M. Abnormalities in the regulators of angiogenesis in patients with scleroderma. J Rheumatol. 2009;36:576-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 86] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 17. | Okano Y, Steen VD, Medsger TA Jr. Autoantibody to U3 nucleolar ribonucleoprotein (fibrillarin) in patients with systemic sclerosis. Arthritis Rheum. 1992;35:95-100. |
| 18. | Tamby MC, Chanseaud Y, Humbert M, Fermanian J, Guilpain P, Garcia-de-la-Peña-Lefebvre P, Brunet S, Servettaz A, Weill B, Simonneau G. Anti-endothelial cell antibodies in idiopathic and systemic sclerosis associated pulmonary arterial hypertension. Thorax. 2005;60:765-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 113] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 19. | Porres-Aguilar M, Anaya-Ayala JE, Porres-Muñoz M, Bracamontes F. [Pulmonary thromboendarterectomy in patients with chronic thromboembolic pulmonary hypertension]. Cir Cir. 2007;75:131-138. [PubMed] |
| 20. | Porres-Aguilar M, Zuckerman MJ, Figueroa-Casas JB, Krowka MJ. Portopulmonary hypertension: state of the art. Ann Hepatol. 2008;7:321-330. [PubMed] |
| 21. | Rabinovitch M. Pathobiology of pulmonary hypertension. Annu Rev Pathol. 2007;2:369-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 95] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 22. | Porres-Aguilar M, Porres-Munoz M. Progress in the pharmacological management of pulmonary arterial hypertension. Med Int Mex. 2004;20:208-220. |
| 23. | Tuder RM, Cool CD, Geraci MW, Wang J, Abman SH, Wright L, Badesch D, Voelkel NF. Prostacyclin synthase expression is decreased in lungs from patients with severe pulmonary hypertension. Am J Respir Crit Care Med. 1999;159:1925-1932. [PubMed] |
| 24. | McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, Mathier MA, McGoon MD, Park MH, Rosenson RS. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association. Circulation. 2009;119:2250-2294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 768] [Cited by in RCA: 771] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 25. | Goldberg A. Pulmonary arterial hypertension in connective tissue diseases. Cardiol Rev. 2010;18:85-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Proudman SM, Stevens WM, Sahhar J, Celermajer D. Pulmonary arterial hypertension in systemic sclerosis: the need for early detection and treatment. Intern Med J. 2007;37:485-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 85] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 27. | Mathai SC, Bueso M, Hummers LK, Boyce D, Lechtzin N, Le Pavec J, Campo A, Champion HC, Housten T, Forfia PR. Disproportionate elevation of N-terminal pro-brain natriuretic peptide in scleroderma-related pulmonary hypertension. Eur Respir J. 2010;35:95-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 99] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 28. | Forfia PR, Mathai SC, Fisher MR, Housten-Harris T, Hemnes AR, Champion HC, Girgis RE, Hassoun PM. Hyponatremia predicts right heart failure and poor survival in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2008;177:1364-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 121] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 29. | Rasekaba T, Lee AL, Naughton MT, Williams TJ, Holland AE. The six-minute walk test: a useful metric for the cardiopulmonary patient. Intern Med J. 2009;39:495-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 262] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 30. | Garin MC, Highland KB, Silver RM, Strange C. Limitations to the 6-minute walk test in interstitial lung disease and pulmonary hypertension in scleroderma. J Rheumatol. 2009;36:330-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 85] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 31. | Pamidi S, Mehta S. Six-minute walk test in scleroderma-associated pulmonary arterial hypertension: are we counting what counts? J Rheumatol. 2009;36:216-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Warwick G, Thomas PS, Yates DH. Biomarkers in pulmonary hypertension. Eur Respir J. 2008;32:503-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 113] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 33. | Allanore Y, Wahbi K, Borderie D, Weber S, Kahan A, Meune C. N-terminal pro-brain natriuretic peptide in systemic sclerosis: a new cornerstone of cardiovascular assessment? Ann Rheum Dis. 2009;68:1885-1889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 34. | Forfia PR, Fisher MR, Mathai SC, Housten-Harris T, Hemnes AR, Borlaug BA, Chamera E, Corretti MC, Champion HC, Abraham TP. Tricuspid annular displacement predicts survival in pulmonary hypertension. Am J Respir Crit Care Med. 2006;174:1034-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 765] [Cited by in RCA: 747] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 35. | Vachiéry JL, Coghlan G. Screening for pulmonary arterial hypertension in systemic sclerosis. Eur Respir Rev. 2009;18:162-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 36. | Mukerjee D, St George D, Knight C, Davar J, Wells AU, Du Bois RM, Black CM, Coghlan JG. Echocardiography and pulmonary function as screening tests for pulmonary arterial hypertension in systemic sclerosis. Rheumatology (Oxford). 2004;43:461-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 248] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 37. | Frea S, Capriolo M, Marra WG, Cannillo M, Fusaro E, Libertucci D, Morello M, Gaita F. Echo Doppler predictors of pulmonary artery hypertension in patients with systemic sclerosis. Echocardiography. 2011;28:860-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 38. | Hinchcliff M, Fischer A, Schiopu E, Steen VD. Pulmonary Hypertension Assessment and Recognition of Outcomes in Scleroderma (PHAROS): baseline characteristics and description of study population. J Rheumatol. 2011;38:2172-2179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 39. | Vonk M, Coghlan G, Bonderman D, Denton C, Distler O, Grunig E, Khanna D, McLaughlin V, Muller-Ladner U, Pope J. The DETECT study: a two-staged, prospective, observational, cohort study in scleroderma patients to evaluate screening tests and the incidence of pulmonary arterial hypertension and pulmonary hypertension. Clin Exp Rheumatol. 2010;28:Suppl: 55. |
| 40. | Hassoun PM. Therapies for scleroderma-related pulmonary arterial hypertension. Expert Rev Respir Med. 2009;3:187-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 41. | McLaughlin V, Humbert M, Coghlan G, Nash P, Steen V. Pulmonary arterial hypertension: the most devastating vascular complication of systemic sclerosis. Rheumatology (Oxford). 2009;48 Suppl 3:iii25-iii31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 42. | Badesch DB, Abman SH, Simonneau G, Rubin LJ, McLaughlin VV. Medical therapy for pulmonary arterial hypertension: updated ACCP evidence-based clinical practice guidelines. Chest. 2007;131:1917-1928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 344] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 43. | Badesch DB, Abman SH, Ahearn GS, Barst RJ, McCrory DC, Simonneau G, McLaughlin VV. Medical therapy for pulmonary arterial hypertension: ACCP evidence-based clinical practice guidelines. Chest. 2004;126:35S-62S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 298] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 44. | Humbert M, Sitbon O, Simonneau G. Treatment of pulmonary arterial hypertension. N Engl J Med. 2004;351:1425-1436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1235] [Cited by in RCA: 1154] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 45. | Gomberg-Maitland M, Olschewski H. Prostacyclin therapies for the treatment of pulmonary arterial hypertension. Eur Respir J. 2008;31:891-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 186] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 46. | Badesch DB, Tapson VF, McGoon MD, Brundage BH, Rubin LJ, Wigley FM, Rich S, Barst RJ, Barrett PS, Kral KM. Continuous intravenous epoprostenol for pulmonary hypertension due to the scleroderma spectrum of disease. A randomized, controlled trial. Ann Intern Med. 2000;132:425-434. [PubMed] |
| 47. | Humbert M, Sanchez O, Fartoukh M, Jagot JL, Le Gall C, Sitbon O, Parent F, Simonneau G. Short-term and long-term epoprostenol (prostacyclin) therapy in pulmonary hypertension secondary to connective tissue diseases: results of a pilot study. Eur Respir J. 1999;13:1351-1356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 65] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 48. | Sitbon O, Humbert M, Nunes H, Parent F, Garcia G, Hervé P, Rainisio M, Simonneau G. Long-term intravenous epoprostenol infusion in primary pulmonary hypertension: prognostic factors and survival. J Am Coll Cardiol. 2002;40:780-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 983] [Cited by in RCA: 888] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 49. | McLaughlin VV, Shillington A, Rich S. Survival in primary pulmonary hypertension: the impact of epoprostenol therapy. Circulation. 2002;106:1477-1482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 805] [Cited by in RCA: 733] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 50. | Kowal-Bielecka O, Landewé R, Avouac J, Chwiesko S, Miniati I, Czirjak L, Clements P, Denton C, Farge D, Fligelstone K. EULAR recommendations for the treatment of systemic sclerosis: a report from the EULAR Scleroderma Trials and Research group (EUSTAR). Ann Rheum Dis. 2009;68:620-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 378] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 51. | Simonneau G, Barst RJ, Galie N, Naeije R, Rich S, Bourge RC, Keogh A, Oudiz R, Frost A, Blackburn SD. Continuous subcutaneous infusion of treprostinil, a prostacyclin analogue, in patients with pulmonary arterial hypertension: a double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med. 2002;165:800-804. [PubMed] |
| 52. | Oudiz RJ, Schilz RJ, Barst RJ, Galié N, Rich S, Rubin LJ, Simonneau G. Treprostinil, a prostacyclin analogue, in pulmonary arterial hypertension associated with connective tissue disease. Chest. 2004;126:420-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 165] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 53. | Olschewski H, Simonneau G, Galiè N, Higenbottam T, Naeije R, Rubin LJ, Nikkho S, Speich R, Hoeper MM, Behr J. Inhaled iloprost for severe pulmonary hypertension. N Engl J Med. 2002;347:322-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1191] [Cited by in RCA: 1104] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 54. | Launay D, Hachulla E, Hatron PY, Goullard L, Onimus T, Robin S, Fauchais AL, Queyrel V, Michon-Pasturel U, Hebbar M. Aerosolized iloprost in CREST syndrome related pulmonary hypertension. J Rheumatol. 2001;28:2252-2256. [PubMed] |
| 55. | Dupuis J, Hoeper MM. Endothelin receptor antagonists in pulmonary arterial hypertension. Eur Respir J. 2008;31:407-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 122] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 56. | Channick RN, Simonneau G, Sitbon O, Robbins IM, Frost A, Tapson VF, Badesch DB, Roux S, Rainisio M, Bodin F. Effects of the dual endothelin-receptor antagonist bosentan in patients with pulmonary hypertension: a randomised placebo-controlled study. Lancet. 2001;358:1119-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1079] [Cited by in RCA: 990] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 57. | Rubin LJ, Badesch DB, Barst RJ, Galie N, Black CM, Keogh A, Pulido T, Frost A, Roux S, Leconte I. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med. 2002;346:896-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1937] [Cited by in RCA: 1767] [Article Influence: 76.8] [Reference Citation Analysis (0)] |
| 58. | Denton CP, Humbert M, Rubin L, Black CM. Bosentan treatment for pulmonary arterial hypertension related to connective tissue disease: a subgroup analysis of the pivotal clinical trials and their open-label extensions. Ann Rheum Dis. 2006;65:1336-1340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 167] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 59. | Denton CP, Pope JE, Peter HH, Gabrielli A, Boonstra A, van den Hoogen FH, Riemekasten G, De Vita S, Morganti A, Dölberg M. Long-term effects of bosentan on quality of life, survival, safety and tolerability in pulmonary arterial hypertension related to connective tissue diseases. Ann Rheum Dis. 2008;67:1222-1228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 99] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 60. | Girgis RE, Mathai SC, Krishnan JA, Wigley FM, Hassoun PM. Long-term outcome of bosentan treatment in idiopathic pulmonary arterial hypertension and pulmonary arterial hypertension associated with the scleroderma spectrum of diseases. J Heart Lung Transplant. 2005;24:1626-1631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 70] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 61. | McLaughlin VV. Survival in patients with pulmonary arterial hypertension treated with first-line bosentan. Eur J Clin Invest. 2006;36 Suppl 3:10-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 67] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 62. | Williams MH, Das C, Handler CE, Akram MR, Davar J, Denton CP, Smith CJ, Black CM, Coghlan JG. Systemic sclerosis associated pulmonary hypertension: improved survival in the current era. Heart. 2006;92:926-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 156] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 63. | Barst RJ. Sitaxsentan: a selective endothelin-A receptor antagonist, for the treatment of pulmonary arterial hypertension. Expert Opin Pharmacother. 2007;8:95-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 64. | Barst RJ, Langleben D, Badesch D, Frost A, Lawrence EC, Shapiro S, Naeije R, Galie N. Treatment of pulmonary arterial hypertension with the selective endothelin-A receptor antagonist sitaxsentan. J Am Coll Cardiol. 2006;47:2049-2056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 301] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 65. | Langleben D, Brock T, Dixon R, Barst R. STRIDE 1: effects of the selective ET(A) receptor antagonist, sitaxsentan sodium, in a patient population with pulmonary arterial hypertension that meets traditional inclusion criteria of previous pulmonary arterial hypertension trials. J Cardiovasc Pharmacol. 2004;44 Suppl 1:S80-S84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 66. | Galié N, Badesch D, Oudiz R, Simonneau G, McGoon MD, Keogh AM, Frost AE, Zwicke D, Naeije R, Shapiro S. Ambrisentan therapy for pulmonary arterial hypertension. J Am Coll Cardiol. 2005;46:529-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 306] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 67. | Galiè N, Ghofrani HA, Torbicki A, Barst RJ, Rubin LJ, Badesch D, Fleming T, Parpia T, Burgess G, Branzi A. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2005;353:2148-2157. [PubMed] |
| 68. | Badesch DB, Hill NS, Burgess G, Rubin LJ, Barst RJ, Galiè N, Simonneau G. Sildenafil for pulmonary arterial hypertension associated with connective tissue disease. J Rheumatol. 2007;34:2417-2422. [PubMed] |
| 69. | Galiè N, Brundage BH, Ghofrani HA, Oudiz RJ, Simonneau G, Safdar Z, Shapiro S, White RJ, Chan M, Beardsworth A. Tadalafil therapy for pulmonary arterial hypertension. Circulation. 2009;119:2894-2903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 699] [Cited by in RCA: 728] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 70. | O'Callaghan DS, Gaine SP. Combination therapy and new types of agents for pulmonary arterial hypertension. Clin Chest Med. 2007;28:169-85, ix. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 71. | McLaughlin VV, Oudiz RJ, Frost A, Tapson VF, Murali S, Channick RN, Badesch DB, Barst RJ, Hsu HH, Rubin LJ. Randomized study of adding inhaled iloprost to existing bosentan in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2006;174:1257-1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 387] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 72. | Simonneau G, Rubin LJ, Galiè N, Barst RJ, Fleming TR, Frost AE, Engel PJ, Kramer MR, Burgess G, Collings L. Addition of sildenafil to long-term intravenous epoprostenol therapy in patients with pulmonary arterial hypertension: a randomized trial. Ann Intern Med. 2008;149:521-530. [PubMed] |
| 73. | Mathai SC, Girgis RE, Fisher MR, Champion HC, Housten-Harris T, Zaiman A, Hassoun PM. Addition of sildenafil to bosentan monotherapy in pulmonary arterial hypertension. Eur Respir J. 2007;29:469-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 107] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 74. | Opitz C, Klein-Weigel PF, Riemekasten G. Systemic sclerosis - a systematic overview: part 2 - immunosuppression, treatment of SSc-associated vasculopathy, and treatment of pulmonary arterial hypertension. Vasa. 2011;40:20-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 75. | Mathai SC, Hassoun PM. Pulmonary arterial hypertension associated with systemic sclerosis. Expert Rev Respir Med. 2011;5:267-279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 76. | Fukumoto Y, Shimokawa H. Recent progress in the management of pulmonary hypertension. Circ J. 2011;75:1801-1810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 77. | Ghofrani HA, Seeger W, Grimminger F. Imatinib for the treatment of pulmonary arterial hypertension. N Engl J Med. 2005;353:1412-1413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 310] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 78. | Souza R, Sitbon O, Parent F, Simonneau G, Humbert M. Long term imatinib treatment in pulmonary arterial hypertension. Thorax. 2006;61:736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 101] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 79. | ten Freyhaus H, Dumitrescu D, Bovenschulte H, Erdmann E, Rosenkranz S. Significant improvement of right ventricular function by imatinib mesylate in scleroderma-associated pulmonary arterial hypertension. Clin Res Cardiol. 2009;98:265-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 80. | Ghofrani HA, Morrell NW, Hoeper MM, Olschewski H, Peacock AJ, Barst RJ, Shapiro S, Golpon H, Toshner M, Grimminger F. Imatinib in pulmonary arterial hypertension patients with inadequate response to established therapy. Am J Respir Crit Care Med. 2010;182:1171-1177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 314] [Cited by in RCA: 274] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 81. | Maurer B, Busch N, Jüngel A, Pileckyte M, Gay RE, Michel BA, Schett G, Gay S, Distler J, Distler O. Transcription factor fos-related antigen-2 induces progressive peripheral vasculopathy in mice closely resembling human systemic sclerosis. Circulation. 2009;120:2367-2376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 90] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 82. | Bull TM. Screening and therapy of pulmonary hypertension in systemic sclerosis. Curr Opin Rheumatol. 2007;19:598-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 83. | Schachna L, Medsger TA, Dauber JH, Wigley FM, Braunstein NA, White B, Steen VD, Conte JV, Yang SC, McCurry KR. Lung transplantation in scleroderma compared with idiopathic pulmonary fibrosis and idiopathic pulmonary arterial hypertension. Arthritis Rheum. 2006;54:3954-3961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 105] [Article Influence: 5.8] [Reference Citation Analysis (0)] |