Revised: December 13, 2011
Accepted: December 26, 2011
Published online: December 31, 2011
Unlike bone marrow (BM) mesenchymal stem cells (MSCs), whose in vivo identity has been actively explored in recent years, the biology of MSCs in the synovium remains poorly understood. Synovial MSCs may be of great importance to rheumatology and orthopedics because of the direct proximity and accessibility of the synovium to cartilage, ligament, and meniscus. Their excellent chondrogenic capabilities and suggested transit through the synovial fluid, giving unhindered access to the joint surface, further support a pivotal role for synovial MSCs in homeostatic joint repair. This review highlights several unresolved issues pertaining to synovial MSC isolation, topography, and their relationship with pericytes, synovial fibroblasts, and synovial fluid MSCs. Critically reviewing published data on synovial MSCs, we also draw from our experience of exploring the in vivo biology of MSCs in the BM to highlight key differences. Extending our knowledge of synovial MSCs in vivo could lead to novel therapeutic strategies for arthritic diseases.
-
Citation: Jones E, McGonagle D. Synovial mesenchymal stem cells
in vivo : Potential key players for joint regeneration. World J Rheumatol 2011; 1(1): 4-11 - URL: https://www.wjgnet.com/2220-3214/full/v1/i1/4.htm
- DOI: https://dx.doi.org/10.5499/wjr.v1.i1.4
Only a decade has passed since their original discovery[1], but synovial mesenchymal stem cells (MSCs) have already become primary candidates for joint regeneration strategies for osteoarthritis and traumatic joint injuries[2]. Their existence has been inferred from experiments where the synovium was digested and culture-expanded, with daughter cells being able to differentiate into bone, cartilage, fat, and muscle lineages[1]. Numerous original articles have demonstrated that culture-expanded synovial MSCs could represent an optimal MSC source for cartilage and meniscus regeneration[3-6]. In contrast, the nature of parental culture-initiating MSCs resident in vivo is much less understood. Given that the substantial regenerative capacity of synovial tissue following synovectomy[7], this points towards their immense in vivo potential for joint repair.
From this perspective, we first consider the biology of synovial MSCs in comparison to synovial fibroblasts (SFs). This is particularly relevant, because SFs are often considered as malevolent, destructive cells in arthritis[8,9], whereas synovial MSCs are believed to be beneficial, regenerative cells. Even in healthy individuals, the relationship between synovial MSCs and SFs remains unclear, and whether the regenerative and destructive potentials represent different faces of the same coin, remains to be determined. Secondly, we describe currently available data on the biology of synovial MSCs in vivo in the context of bone marrow (BM) MSCs, which we reviewed recently[10]. A comparative analysis of these two types of MSCs is likely to shed more light on tissue-specificity and heterogeneity of MSCs in vivo.
The normal synovium has a membrane and a sub-membrane fibro-fatty tissue, surrounded by a joint capsule. The synovial membrane is composed of type A (monocytic) and type B (fibroblastic), or synovial intimal, fibroblasts cells and is normally 1-2 cells thick. Normally, the synovial membrane plays a key role in joint lubrication, but can undergo substantial hyperplasia during chronic inflammatory processes[8]. The sub-synovium accumulates many myeloid and lymphoid lineage cells during chronic inflammation, which is associated with extensive tissue remodeling, new blood vessel formation, and related increase in proliferation of SFs. As arthritis develops, SFs change their gene expression, leading to further attraction and accumulation of inflammatory cells in the subsynovium[9,11]. In rheumatoid arthritis (RA) and other settings of chronic inflammation, increased proliferation of SFs may lead to the formation of an invasive stromal tissue, termed pannus. The combined hyperplasia of both components of the synovium leads to villus formation. Currently, the location of synovial MSCs, whether from one or both of these synovial compartments, and their contribution to pannus formation remain unclear.
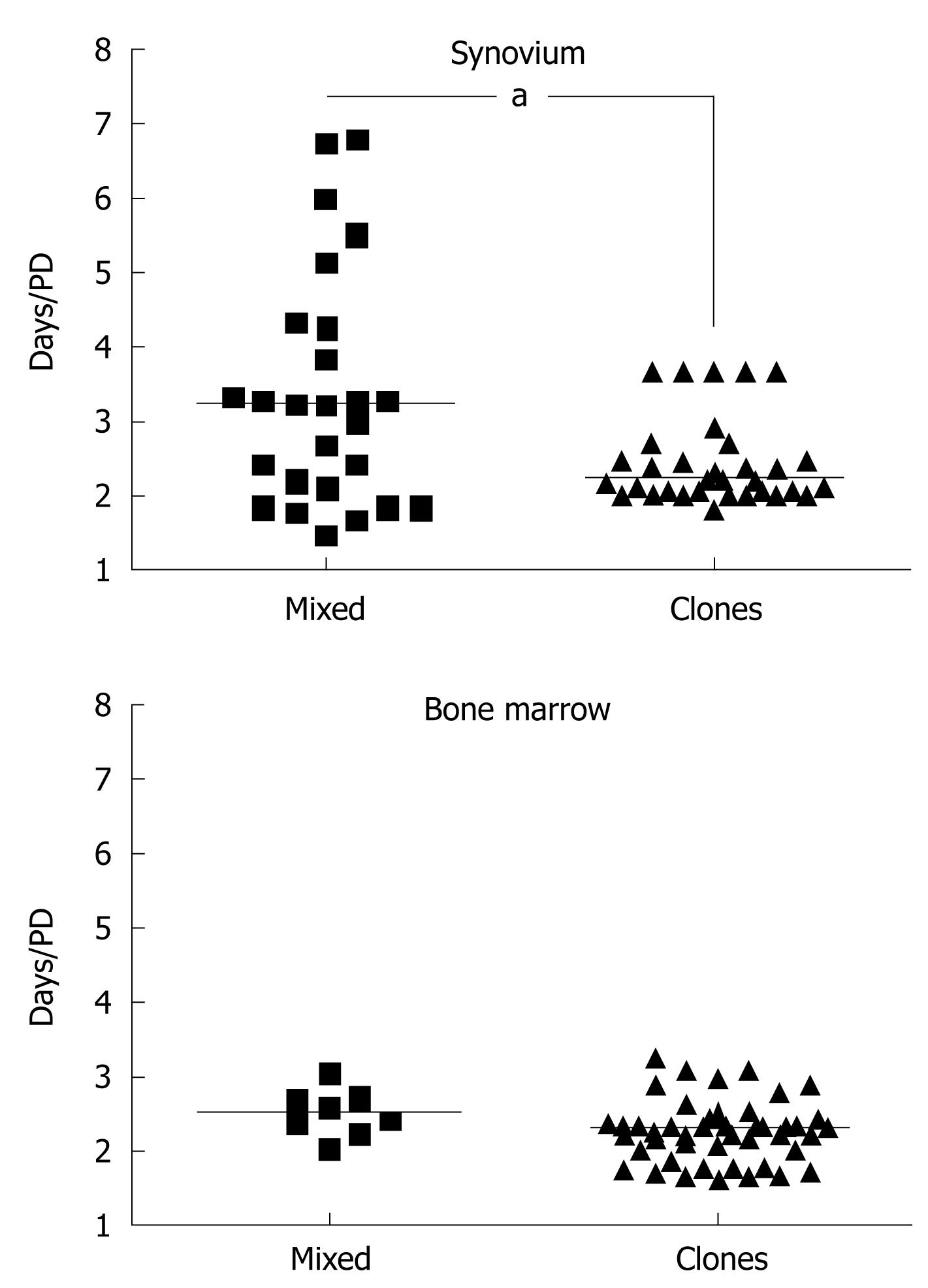
Similar to synovial MSCs, SFs are isolated by plastic adherence and cultured in serum-rich medium[1,12,13]. Surface markers, initially described to be specific for synovial MSCs[14,15], were later shown to cross-react with synovial and other types of fibroblasts[9,16-20]. The synovial MSCs, however, can be distinguished from SFs by their higher proliferative capacity and faster growth rates. MSCs are highly proliferative cells, capable of over 20 population doublings (PDs)[21]. According to Smith and Hayflick[22], the majority of fibroblasts have “a maximum doubling potential of about eight PDs”[22]. Based on these considerations, MSCs have been historically defined as cells initiating rapidly growing, highly proliferative clonal cultures[1,16,23-26]. MSC clones can be isolated either by plating synovial cells in limiting dilution conditions[1,16,23,24] or at a very low seeding density[3,5,27]. Under the latter conditions, MSCs are believed to “overgrow” SFs because of their faster proliferation rates. Indeed, clonal synovial cultures grown in our laboratory[18] demonstrate faster growth rates compared to mixed synovial cultures. The same trend was observed for BM-derived controls[28] (Figure 1).
Regardless of the cultivation conditions, senescent cells eventually accumulate in clonal synovial MSC cultures[26], suggesting that current in vitro cultivation conditions do not support indefinite “self-renewal” of synovial MSCs. In the BM, the fastest growing clonal MSCs are truly tripotential, whereas relatively slower-growing clones are bi- or unipotential[29-31]. Although fast- and slow-growing clonal MSCs can be also grown from the synovium, no link with their multipotentiality could be established[26]. Thus, it appears that in the BM, high and rapid proliferative capacity is linked with robust tripotentiality, whereas in the synovium, it might not be. From a practical perspective, this may explain why it has proving difficult to find a marker for synovial MSCs based solely on in vitro expansion/differentiation experiments.
In high-density expansion conditions, synovial MSCs may be “contaminated” with SFs. Several investigations attempted to purify synovial MSCs prospectively (Table 1). Unlike studies with BM MSCs[32-35], the expansion and differentiation capacities of unsorted control preparations are not commonly reported, making the evaluation of functional improvement following isolation somewhat difficult. Furthermore, sorting is sometimes performed from passage 0 cells, rather than from primary tissue digests, making cell adherence, and not sorting, the primary selection step.
| Candidate phenotype | Control standard culture:“without separation” | Control negative fraction:“opposite phenotype” | ||
| Expansion capacity | Differentiation capacity | Expansion capacity | Differentiation capacity | |
| CD9+CD90+ CD166+[14] | NR | Similar | NR | NR |
| SP (bovine)[98] | NR | NR | NR | Chondro: similar |
| SP (bovine)[99] | NR | NR | Significantly lower | Chondro: inferior Osteo: similar Myo: absent |
| CD105+[100] | NR | Chondro:present | NR | Chondro:present |
| CD45-CD31-[18] | NR | NR | Absent | NR |
In our recent studies, we optimized the cell sorting methodology for digested human synovium[18,36] and demonstrated an exclusive presence of clonogenic synovial MSCs in the CD45-CD31- (non-hematopoietic, non-endothelial) fraction[18]. Our most recent data show that highly-proliferative clonogenic MSCs (capable of over 20PDs) represent no more than 1% of synovial CD45-CD31- cells[18]. These data suggest that molecules with expression levels markedly above 1% are unlikely to be selective for synovial MSCs. These ineffectual markers include CD73, CD44, and CD90 (expressed on approximately 90% of freshly-isolated synovial cells)[18,37], and should be better categorized as markers of SFs and not MSCs. CD44 expression on SFs has been previously documented[17,38].
We, and others, have previously shown that CD271 is very selective for in vivo BM MSCs[32,33,39]. CD271 has been also proposed to be MSC-specific in adipose tissue[40-43]. The CD271-positive population represents approximately 10% of the CD45-CD31 fraction[36], making CD271 a fairly promising candidate for synovial MSC isolation. Indeed, CD271-positive cells isolated from mixed synovial cultures were found to be highly chondrogenic[44]. The CD73-positive subpopulation was less chondrogenic and the CD106-positive cells were most undifferentiated[44]. This agrees with studies from adipose tissue that have been unable to define a singular specific marker that is enriched in all cells with MSC activity[45,46]. In the latter study, adipose-derived MSCs were identified in both CD34-positive and CD271-positive fractions[46]. In the BM, all clonogenic MSCs reside in the CD34-CD271+ fraction[10]. In the synovium and adipose tissue, this fraction does not appear to be highly selective for MSCs, suggesting that a theory of the “common phenotype” of MSCs in different tissues is unlikely to hold true. The recent study by Kurth et al[47] also indicated that synovial MSCs may be more phenotypically heterogeneous than BM MSCs.
In addition to synovial MSCs, the CD45-CD31- synovial fraction is likely to contain more committed mature cells, myofibroblasts, and adipocytes. MSC differentiation towards these lineages is affected by inflammation[48]; therefore, studies on normal synovial tissue are needed to find markers for the isolation of these separate cell types alongside the MSCs.
Surface markers may indeed be useful tools for stem cell isolation, but they rarely shed light on the stem cell nature of their target cells. CD34 is useful for hemopoietic stem cell isolation, but it is also expressed on endothelial cells and on adipose tissue MSCs[42,43], where its precise function remains unknown. Receptors and downstream intracellular molecules directly involved in specific stem cell maintenance and differentiation pathways may represent much more valuable tools. Molecules involved in BMP signaling (BMPR1A and pSMAD1/5) were the first to be used for the identification of MSCs in the synovium[49,50]. Similarly, lineage-specific transcription factors and downstream molecules activated by BMPs (Sox9, aggrecan and others) have been proposed to mark synovial chondroprogenitors[51]. Finally, Cadherin-11-expressing mesenchymal cells have been shown to orchestrate synovial tissue architecture[52]. Although these studies offer new opportunities for molecular analysis of marker-positive cells, the necessity to fix the cells for intracellular flow cytometry precludes downstream live cell experimentation. Furthermore, these mesenchymal lineage-related pathways may be equally active in SFs, in addition to MSCs, as shown earlier by their continuous activation in the inflamed synovium[53,54]. Therefore, their MSC-selectivity, even in the normal synovium, remains to be proven.
Most recently, the idea of a perivascular location of MSCs in diverse human tissues has become predominant[55-57]. This does make sense, because MSCs have been found in the majority of solid tissues where blood vessels may be the only common anatomical structure[56,58]. Furthermore, such a concept is very plausible, given that early embryonic limb development is characterized by epithelial-mesenchymal transition, where the mesenchyme acts as a “space filler” before the development of a vascular system[59]. Based on this “pericyte” concept, it has been suggested that MSC frequency directly correlates with blood vessel density in solid tissues[60], including the synovium[61].
Although many studies suggest that MSCs are perivascular and are possibly derived from pericytes, it should be noted that articular cartilage, an avascular tissue, has been reported to contain MSC-like cells[62-64], which argues against an exclusive perivascular location of MSCs. In the BM, MSC activity is associated with adventitial reticular cells, which are specialized pericytes of venous sinusoids[65], and also with cells lining bone surfaces[28,66]. Most recently, Feng et al[67] showed the existence of a non-pericyte stem cell population in a rodent incisor growth model. These non-perycitic MSCs were capable of migration toward areas of tissue damage and differentiation into odontoblasts. Similarly, lineage-tracing experiments in a mouse model of joint surface injury have proven the presence of slow-cycling MSCs in the synovium that were distinct from pericytes and differentiated to chondrocytes in response to injury[47].
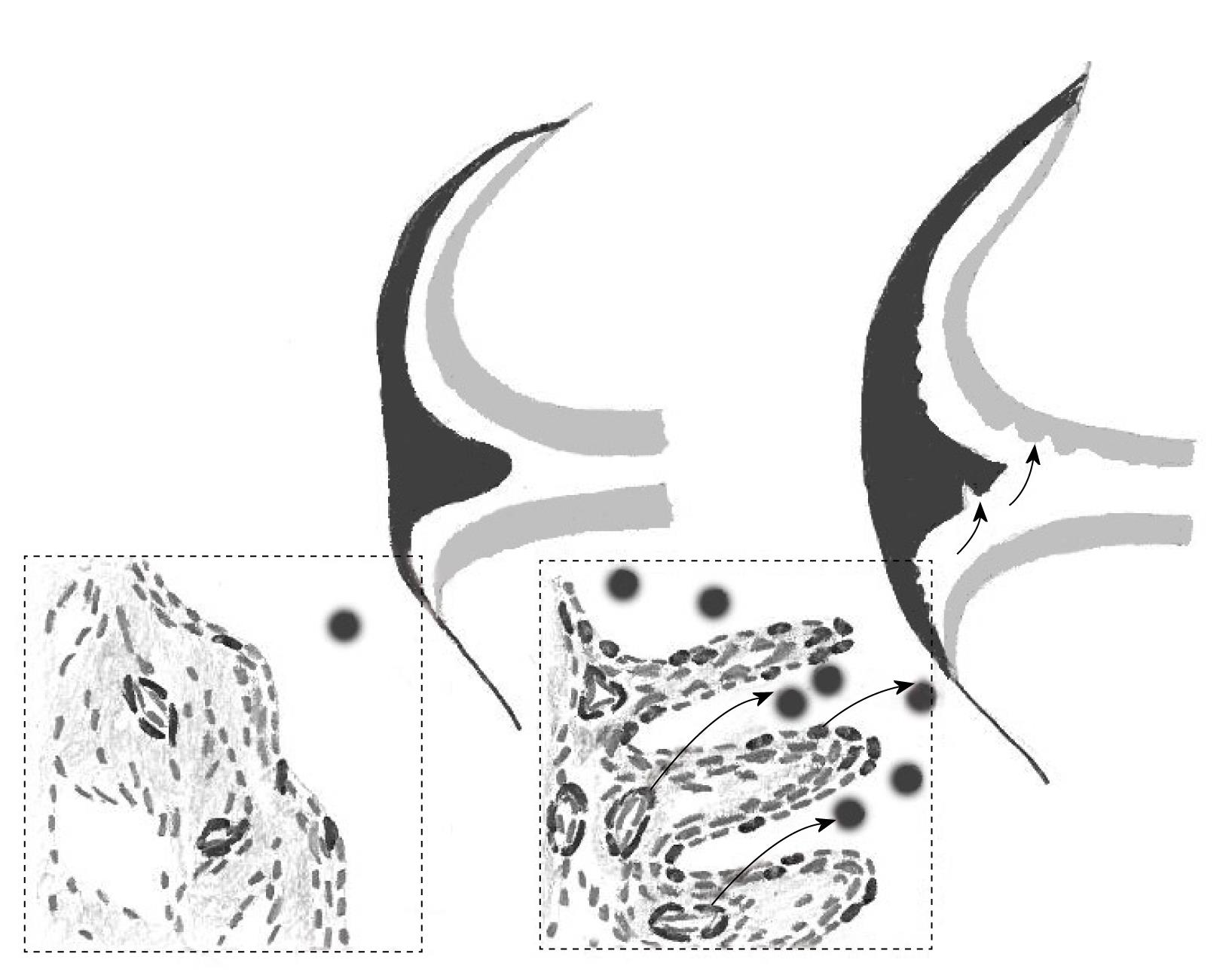
One study proposed the presence of MSCs in synovial tissue projections, i.e. the exterior areas of the tissue exposed to synovial fluid[68]. Although these observations need to be confirmed by direct isolation of candidate Stro-1+ cells, this correlates well with our findings relating to synovial fluid MSCs[16,23], which most likely originated in the synovium[23,69]. Both “synovial projection” and “pericyte” topographies allow easy egress of synovial MSCs into the fluid, without the need of extensive migration through several layers of cells and the extracellular matrix (Figure 2).
With the exception of joint cartilage, the synovium lines the entire joint surface, including intra-articular ligaments. As stated above, synovial fluid MSCs are most likely to originate from the synovium[23,69]; however, their superficial cartilage origin in healthy young individuals cannot be excluded[62]. Biophysical factors, trauma, and local injury-induced signaling centers[70] could potentially induce MSC egress into the fluid (Figure 2). This indicates a mechanism whereby synovial MSCs can gain access to cartilage and meniscal areas that are remote from the synovium, and explain how synovial MSCs can continuously effect homeostatic repair of microdamage in these tissues. Although synovial fluid MSCs are rare[16,23], their proliferative capacity is huge (normally a million-fold), which is likely to be sufficient for repairing small lesions in cartilage, considering its low cellularity[71]. Notably, physiological cartilage repair in humans[72] and animal models[73] has been documented, and the role of synovial fluid MSCs in these repair processes cannot be excluded. Most recently, a proof-of-concept study in rabbits demonstrated the regeneration of the entire articular surface of the synovial joint without cell transplantation, mediated by endogenous host cells, potentially derived from the synovium[74]. Further augmentation of MSC concentration in the fluid by injecting more MSCs facilitated good meniscal and cartilage repair in vivo[6,69,75]. The synovial fluid microenvironment can affect the migratory[76], proliferative[23], and differentiative potential of MSCs[77-80], which could further enhance their repair capabilities.
There is conflicting evidence for the presence of circulating BM MSCs[10,81,82]; however, in studies that suggest MSC circulation, very few colony-forming cells have been found[83]. RA SFs may be able to circulate in the SCID mouse model and contribute to the diffuse pattern of joint disease evident in RA[84]. Whether healthy SFs or synovial MSCs possess similar transmigration capacities, remains to be investigated. Even if rare MSC circulate, their tissue of origin remains to be determined.
It must be acknowledged that there are several unresolved controversies pertaining to the identification of synovial MSCs in vivo. The in vitro proliferative index of clonal synovial MSCs may be different from what actually happens in vivo. As mentioned above, the addition of synovial fluid to synovial MSCs can enhance their proliferation[23], indicating that in vivo factors may have a major effect on MSC divisions. SFs can be easily converted into pluripotent iPS cells in vitro[85], which involves the activation of a telomerase gene[86,87]. Synovial MSCs have low telomerase activity[26]. However, if a telomerase gene is activated in susceptible SFs in vivo, they may theoretically acquire an increased proliferative capacity, i.e. they may emerge as de novo MSCs. On the other hand, the in vivo inflammatory milieu can inhibit synovial MSC proliferation[18], as well as their differentiation and immunomodulatory capabilities[18,88]. This highlights the dynamic, rather than static, nature of the SF/MSC equilibrium in the synovium and may explain, at least in part, the massive pannus tissue formation in RA.
Finally, the in vitro conditions that are used to derive clonal synovial MSCs, by their very nature, artificially induce cellular senescence. Therefore, massively expanded clonal MSCs, when used for therapeutic implantation, may be near the end of their natural lifespans. This suggests that, for therapeutic applications, methodologies based on extensive synovial MSC proliferation should be avoided. In contrast, minimally expanded synovial MSCs may provide a better solution for joint tissue regeneration approaches.
In contrast to the BM MSC field, where there is a consensus on the MSC identity[10,89-91], data on synovial MSC topography and phenotype are scarce. Synovial MSCs and SF are intricately inter-related; in fact, one cannot exclude the possibility that mature SFs are direct descendants of ancestral MSCs and that SFs have a limited lifespan in vitro because of previous extensive proliferation of ancestral MSCs in vivo. An ideal marker for synovial MSCs would possibly be linked to their superior proliferative potential, showing notably lower levels of expression on SFs. Conversely, the majority SFs are likely to express higher levels of senescence-associated transcripts and shorter telomeres, which were initially proposed for BM MSCs and their progeny[31,92,93]. Furthermore, definitive markers may exist that identify SFs with increased reprogramming potential. Future studies analogous to those performed with BM MSCs[94-96] may discover such markers. Direct implantation of freshly isolated synovial MSCs based on these new markers, without culture-expansion and associated senescence, may ultimately be required to establish the in vivo phenotype of synovial MSCs.
A better understanding of the biology of synovial MSCs in vivo would not only lead to novel cell-based regenerative medicine approaches[2,97], but would also permit the development of cell-free interventions based on increased understanding of synovial MSC migration[74,76] and their metabolic responses to injury[47,70]. Therefore, the preliminary data on synovial MSCs, as outlined here, should serve as a platform for the pursuit of novel therapeutic strategies for joint degeneration. Novel methodologies, including lineage tracing, knockdown analysis, and laser-dissection microscopy of gene-marked cells in animal models, are likely to provide a much-needed breakthrough in this area.
The authors thank Anne English and Paul Emery for technical and organizational support.
Peer reviewer: Javier Alberto Cavallasca, MD, Staff Physician, Section of Rheumatology and Autoimmune Diseases, Hospital JB Iturraspe, Santa Fe, Argentina, Boulevard Pellegrini 3551, CP 3000, Santa Fe, Argentina
S- Editor Wang JL L- Editor Stewart G E- Editor Zheng XM
| 1. | De Bari C, Dell'Accio F, Tylzanowski P, Luyten FP. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001;44:1928-1942. [PubMed] |
| 2. | Fan J, Varshney RR, Ren L, Cai D, Wang DA. Synovium-derived mesenchymal stem cells: a new cell source for musculoskeletal regeneration. Tissue Eng Part B Rev. 2009;15:75-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 163] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 3. | Sakaguchi Y, Sekiya I, Yagishita K, Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. 2005;52:2521-2529. [PubMed] |
| 4. | Djouad F, Bony C, Häupl T, Uzé G, Lahlou N, Louis-Plence P, Apparailly F, Canovas F, Rème T, Sany J. Transcriptional profiles discriminate bone marrow-derived and synovium-derived mesenchymal stem cells. Arthritis Res Ther. 2005;7:R1304-R1315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 155] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 5. | Shirasawa S, Sekiya I, Sakaguchi Y, Yagishita K, Ichinose S, Muneta T. In vitro chondrogenesis of human synovium-derived mesenchymal stem cells: optimal condition and comparison with bone marrow-derived cells. J Cell Biochem. 2006;97:84-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 218] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 6. | Horie M, Sekiya I, Muneta T, Ichinose S, Matsumoto K, Saito H, Murakami T, Kobayashi E. Intra-articular Injected synovial stem cells differentiate into meniscal cells directly and promote meniscal regeneration without mobilization to distant organs in rat massive meniscal defect. Stem Cells. 2009;27:878-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 183] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 7. | Ostergaard M, Ejbjerg B, Stoltenberg M, Gideon P, Volck B, Skov K, Jensen CH, Lorenzen I. Quantitative magnetic resonance imaging as marker of synovial membrane regeneration and recurrence of synovitis after arthroscopic knee joint synovectomy: a one year follow up study. Ann Rheum Dis. 2001;60:233-236. [PubMed] |
| 8. | Firestein GS. Invasive fibroblast-like synoviocytes in rheumatoid arthritis. Passive responders or transformed aggressors? Arthritis Rheum. 1996;39:1781-1790. [PubMed] |
| 9. | Aidinis V, Plows D, Haralambous S, Armaka M, Papadopoulos P, Kanaki MZ, Koczan D, Thiesen HJ, Kollias G. Functional analysis of an arthritogenic synovial fibroblast. Arthritis Res Ther. 2003;5:R140-R157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Jones E, McGonagle D. Human bone marrow mesenchymal stem cells in vivo. Rheumatology. 2008;47:126-131. [RCA] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 199] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 11. | Parsonage G, Falciani F, Burman A, Filer A, Ross E, Bofill M, Martin S, Salmon M, Buckley CD. Global gene expression profiles in fibroblasts from synovial, skin and lymphoid tissue reveals distinct cytokine and chemokine expression patterns. Thromb Haemost. 2003;90:688-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 82] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 12. | Zimmermann T, Kunisch E, Pfeiffer R, Hirth A, Stahl HD, Sack U, Laube A, Liesaus E, Roth A, Palombo-Kinne E. Isolation and characterization of rheumatoid arthritis synovial fibroblasts from primary culture--primary culture cells markedly differ from fourth-passage cells. Arthritis Res. 2001;3:72-76. [PubMed] |
| 13. | Bilgen B, Ren Y, Pei M, Aaron RK, Ciombor DM. CD14-negative isolation enhances chondrogenesis in synovial fibroblasts. Tissue Eng Part A. 2009;15:3261-3270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Fickert S, Fiedler J, Brenner RE. Identification, quantification and isolation of mesenchymal progenitor cells from osteoarthritic synovium by fluorescence automated cell sorting. Osteoarthritis Cartilage. 2003;11:790-800. [PubMed] |
| 15. | Jo CH, Ahn HJ, Kim HJ, Seong SC, Lee MC. Surface characterization and chondrogenic differentiation of mesenchymal stromal cells derived from synovium. Cytotherapy. 2007;9:316-327. [PubMed] |
| 16. | Jones EA, English A, Henshaw K, Kinsey SE, Markham AF, Emery P, McGonagle D. Enumeration and phenotypic characterization of synovial fluid multipotential mesenchymal progenitor cells in inflammatory and degenerative arthritis. Arthritis Rheum. 2004;50:817-827. [PubMed] |
| 17. | Fiorito S, Magrini L, Adrey J, Mailhé D, Brouty-Boyé D. Inflammatory status and cartilage regenerative potential of synovial fibroblasts from patients with osteoarthritis and chondropathy. Rheumatology (Oxford). 2005;44:164-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Jones E, Churchman SM, English A, Buch MH, Horner EA, Burgoyne CH, Reece R, Kinsey S, Emery P, McGonagle D. Mesenchymal stem cells in rheumatoid synovium: enumeration and functional assessment in relation to synovial inflammation level. Ann Rheum Dis. 2010;69:450-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 19. | Ishii M, Koike C, Igarashi A, Yamanaka K, Pan H, Higashi Y, Kawaguchi H, Sugiyama M, Kamata N, Iwata T. Molecular markers distinguish bone marrow mesenchymal stem cells from fibroblasts. Biochem Biophys Res Commun. 2005;332:297-303. [PubMed] |
| 20. | Huang HI, Chen SK, Ling QD, Chien CC, Liu HT, Chan SH. Multilineage differentiation potential of fibroblast-like stromal cells derived from human skin. Tissue Eng Part A. 2010;16:1491-1501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 21. | Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143-147. [PubMed] |
| 22. | Smith JR, Hayflick L. Variation in the life-span of clones derived from human diploid cell strains. J Cell Biol. 1974;62:48-53. [PubMed] |
| 23. | Jones EA, Crawford A, English A, Henshaw K, Mundy J, Corscadden D, Chapman T, Emery P, Hatton P, McGonagle D. Synovial fluid mesenchymal stem cells in health and early osteoarthritis: detection and functional evaluation at the single-cell level. Arthritis Rheum. 2008;58:1731-1740. [PubMed] |
| 24. | De Bari C, Dell'Accio F, Karystinou A, Guillot PV, Fisk NM, Jones EA, McGonagle D, Khan IM, Archer CW, Mitsiadis TA. A biomarker-based mathematical model to predict bone-forming potency of human synovial and periosteal mesenchymal stem cells. Arthritis Rheum. 2008;58:240-250. [PubMed] |
| 25. | English A, Jones EA, Corscadden D, Henshaw K, Chapman T, Emery P, McGonagle D. A comparative assessment of cartilage and joint fat pad as a potential source of cells for autologous therapy development in knee osteoarthritis. Rheumatology (Oxford). 2007;46:1676-1683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 113] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 26. | Karystinou A, Dell'Accio F, Kurth TB, Wackerhage H, Khan IM, Archer CW, Jones EA, Mitsiadis TA, De Bari C. Distinct mesenchymal progenitor cell subsets in the adult human synovium. Rheumatology (Oxford). 2009;48:1057-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 27. | Mochizuki T, Muneta T, Sakaguchi Y, Nimura A, Yokoyama A, Koga H, Sekiya I. Higher chondrogenic potential of fibrous synovium- and adipose synovium-derived cells compared with subcutaneous fat-derived cells: distinguishing properties of mesenchymal stem cells in humans. Arthritis Rheum. 2006;54:843-853. [PubMed] |
| 28. | Jones E, English A, Churchman SM, Kouroupis D, Boxall SA, Kinsey S, Giannoudis PG, Emery P, McGonagle D. Large-scale extraction and characterization of CD271+ multipotential stromal cells from trabecular bone in health and osteoarthritis: implications for bone regeneration strategies based on uncultured or minimally cultured multipotential stromal cells. Arthritis Rheum. 2010;62:1944-1954. [PubMed] |
| 29. | Mareddy S, Crawford R, Brooke G, Xiao Y. Clonal isolation and characterization of bone marrow stromal cells from patients with osteoarthritis. Tissue Eng. 2007;13:819-829. [PubMed] |
| 30. | Mareddy S, Broadbent J, Crawford R, Xiao Y. Proteomic profiling of distinct clonal populations of bone marrow mesenchymal stem cells. J Cell Biochem. 2009;106:776-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | Mareddy S, Dhaliwal N, Crawford R, Xiao Y. Stem cell-related gene expression in clonal populations of mesenchymal stromal cells from bone marrow. Tissue Eng Part A. 2010;16:749-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Quirici N, Soligo D, Bossolasco P, Servida F, Lumini C, Deliliers GL. Isolation of bone marrow mesenchymal stem cells by anti-nerve growth factor receptor antibodies. Exp Hematol. 2002;30:783-791. [PubMed] |
| 33. | Jones EA, Kinsey SE, English A, Jones RA, Straszynski L, Meredith DM, Markham AF, Jack A, Emery P, McGonagle D. Isolation and characterization of bone marrow multipotential mesenchymal progenitor cells. Arthritis Rheum. 2002;46:3349-3360. [PubMed] |
| 34. | Poloni A, Maurizi G, Rosini V, Mondini E, Mancini S, Discepoli G, Biasio S, Battaglini G, Felicetti S, Berardinelli E. Selection of CD271(+) cells and human AB serum allows a large expansion of mesenchymal stromal cells from human bone marrow. Cytotherapy. 2009;11:153-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 35. | Jarocha D, Lukasiewicz E, Majka M. Adventage of mesenchymal stem cells (MSC) expansion directly from purified bone marrow CD105+ and CD271+ cells. Folia Histochem Cytobiol. 2008;46:307-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 36. | van Landuyt KB, Jones EA, McGonagle D, Luyten FP, Lories RJ. Flow cytometric characterization of freshly isolated and culture expanded human synovial cell populations in patients with chronic arthritis. Arthritis Res Ther. 2010;12:R15. [PubMed] |
| 37. | Hermida-Gómez T, Fuentes-Boquete I, Gimeno-Longas MJ, Muiños-López E, Díaz-Prado S, de Toro FJ, Blanco FJ. Quantification of cells expressing mesenchymal stem cell markers in healthy and osteoarthritic synovial membranes. J Rheumatol. 2011;38:339-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 38. | Henderson KJ, Edwards JC, Worrall JG. Expression of CD44 in normal and rheumatoid synovium and cultured synovial fibroblasts. Ann Rheum Dis. 1994;53:729-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 39. | Bühring HJ, Battula VL, Treml S, Schewe B, Kanz L, Vogel W. Novel markers for the prospective isolation of human MSC. Ann N Y Acad Sci. 2007;1106:262-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 326] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 40. | Quirici N, Brini AT, Scavullo C, de Girolamo L, Sartori MF, Onida , Lambertenghi DG. Isolation and characterization of l-NGFR( ) mesenchymal stem cells from adipose tissue and bone marrow: a comparison between the two sources. Haematologica-the Hematology Journal. 2008;93:S44-S44. |
| 41. | Yamada T, Akamatsu H, Hasegawa S, Yamamoto N, Yoshimura T, Hasebe Y, Inoue Y, Mizutani H, Uzawa T, Matsunaga K. Age-related changes of p75 neurotrophin receptor-positive adipose-derived stem cells. J Dermatol Sci. 2010;58:36-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 42. | Gronthos S, Franklin DM, Leddy HA, Robey PG, Storms RW, Gimble JM. Surface protein characterization of human adipose tissue-derived stromal cells. J Cell Physiol. 2001;189:54-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 780] [Cited by in RCA: 999] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 43. | Lin CS, Xin ZC, Deng CH, Ning H, Lin G, Lue TF. Defining adipose tissue-derived stem cells in tissue and in culture. Histol Histopathol. 2010;25:807-815. [PubMed] |
| 44. | Arufe MC, De la Fuente A, Fuentes I, de Toro FJ, Blanco FJ. Chondrogenic potential of subpopulations of cells expressing mesenchymal stem cell markers derived from human synovial membranes. J Cell Biochem. 2010;111:834-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 45. | Zimmerlin L, Donnenberg VS, Pfeifer ME, Meyer EM, Péault B, Rubin JP, Donnenberg AD. Stromal vascular progenitors in adult human adipose tissue. Cytometry A. 2010;77:22-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 340] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 46. | Quirici N, Scavullo C, de Girolamo L, Lopa S, Arrigoni E, Deliliers GL, Brini AT. Anti-L-NGFR and -CD34 monoclonal antibodies identify multipotent mesenchymal stem cells in human adipose tissue. Stem Cells Dev. 2010;19:915-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 47. | Kurth TB, Dell'accio F, Crouch V, Augello A, Sharpe PT, De Bari C. Functional mesenchymal stem cell niches in adult mouse knee joint synovium in vivo. Arthritis Rheum. 2011;63:1289-1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 150] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 48. | Yamasaki S, Nakashima T, Kawakami A, Miyashita T, Tanaka F, Ida H, Migita K, Origuchi T, Eguchi K. Cytokines regulate fibroblast-like synovial cell differentiation to adipocyte-like cells. Rheumatology (Oxford). 2004;43:448-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 49. | Marinova-Mutafchieva L, Taylor P, Funa K, Maini RN, Zvaifler NJ. Mesenchymal cells expressing bone morphogenetic protein receptors are present in the rheumatoid arthritis joint. Arthritis Rheum. 2000;43:2046-2055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 50. | Luyten FP, Lories RJ, Westhovens R, Verschueren PC. Detection, identification and evaluation of in vivo anti-TNF responsiveness of a BMP activated stromal precursor cell population in the human RA synovium. Arthritis Rheum. 2005;52:S474-S475. |
| 51. | Shintani N, Kurth T, Hunziker EB. Expression of cartilage-related genes in bovine synovial tissue. J Orthop Res. 2007;25:813-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 52. | Lee DM, Kiener HP, Agarwal SK, Noss EH, Watts GF, Chisaka O, Takeichi M, Brenner MB. Cadherin-11 in synovial lining formation and pathology in arthritis. Science. 2007;315:1006-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 316] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 53. | Lories RJ, Derese I, Ceuppens JL, Luyten FP. Bone morphogenetic proteins 2 and 6, expressed in arthritic synovium, are regulated by proinflammatory cytokines and differentially modulate fibroblast-like synoviocyte apoptosis. Arthritis Rheum. 2003;48:2807-2818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 134] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 54. | Seto H, Kamekura S, Miura T, Yamamoto A, Chikuda H, Ogata T, Hiraoka H, Oda H, Nakamura K, Kurosawa H. Distinct roles of Smad pathways and p38 pathways in cartilage-specific gene expression in synovial fibroblasts. J Clin Invest. 2004;113:718-726. [PubMed] |
| 55. | Shi S, Gronthos S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res. 2003;18:696-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1091] [Cited by in RCA: 1031] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 56. | da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119:2204-2213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1715] [Cited by in RCA: 1707] [Article Influence: 89.8] [Reference Citation Analysis (0)] |
| 57. | Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2900] [Cited by in RCA: 2866] [Article Influence: 168.6] [Reference Citation Analysis (0)] |
| 58. | da Silva Meirelles L, Caplan AI, Nardi NB. In search of the in vivo identity of mesenchymal stem cells. Stem Cells. 2008;26:2287-2299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 778] [Cited by in RCA: 719] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 59. | Risau W, Flamme I. Vasculogenesis. Annu Rev Cell Dev Biol. 1995;11:73-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1190] [Cited by in RCA: 1111] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 60. | da Silva Meirelles L, Sand TT, Harman RJ, Lennon DP, Caplan AI. MSC frequency correlates with blood vessel density in equine adipose tissue. Tissue Eng Part A. 2009;15:221-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 61. | Nagase T, Muneta T, Ju YJ, Hara K, Morito T, Koga H, Nimura A, Mochizuki T, Sekiya I. Analysis of the chondrogenic potential of human synovial stem cells according to harvest site and culture parameters in knees with medial compartment osteoarthritis. Arthritis Rheum. 2008;58:1389-1398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 62. | Dowthwaite GP, Bishop JC, Redman SN, Khan IM, Rooney P, Evans DJ, Haughton L, Bayram Z, Boyer S, Thomson B. The surface of articular cartilage contains a progenitor cell population. J Cell Sci. 2004;117:889-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 579] [Cited by in RCA: 601] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 63. | Dell'Accio F, De Bari C, Luyten FP. Microenvironment and phenotypic stability specify tissue formation by human articular cartilage-derived cells in vivo. Exp Cell Res. 2003;287:16-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 93] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 64. | Alsalameh S, Amin R, Gemba T, Lotz M. Identification of mesenchymal progenitor cells in normal and osteoarthritic human articular cartilage. Arthritis Rheum. 2004;50:1522-1532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 377] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 65. | Bianco P, Riminucci M, Gronthos S, Robey PG. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells. 2001;19:180-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1417] [Cited by in RCA: 1348] [Article Influence: 56.2] [Reference Citation Analysis (0)] |
| 66. | Tuli R, Tuli S, Nandi S, Wang ML, Alexander PG, Haleem-Smith H, Hozack WJ, Manner PA, Danielson KG, Tuan RS. Characterization of multipotential mesenchymal progenitor cells derived from human trabecular bone. Stem Cells. 2003;21:681-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 204] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 67. | Feng J, Mantesso A, De Bari C, Nishiyama A, Sharpe PT. Dual origin of mesenchymal stem cells contributing to organ growth and repair. Proc Natl Acad Sci U S A. 2011;108:6503-6508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 308] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 68. | Giurea A, Rüger BM, Hollemann D, Yanagida G, Kotz R, Fischer MB. STRO-1+ mesenchymal precursor cells located in synovial surface projections of patients with osteoarthritis. Osteoarthritis Cartilage. 2006;14:938-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 69. | Morito T, Muneta T, Hara K, Ju YJ, Mochizuki T, Makino H, Umezawa A, Sekiya I. Synovial fluid-derived mesenchymal stem cells increase after intra-articular ligament injury in humans. Rheumatology (Oxford). 2008;47:1137-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 160] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 70. | Dell'Accio F, De Bari C, El Tawil NM, Barone F, Mitsiadis TA, O'Dowd J, Pitzalis C. Activation of WNT and BMP signaling in adult human articular cartilage following mechanical injury. Arthritis Res Ther. 2006;8:R139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 120] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 71. | Hunziker EB, Quinn TM, Häuselmann HJ. Quantitative structural organization of normal adult human articular cartilage. Osteoarthritis Cartilage. 2002;10:564-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 350] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 72. | Koshino T, Wada S, Ara Y, Saito T. Regeneration of degenerated articular cartilage after high tibial valgus osteotomy for medial compartmental osteoarthritis of the knee. Knee. 2003;10:229-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 168] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 73. | Eltawil NM, De Bari C, Achan P, Pitzalis C, Dell'accio F. A novel in vivo murine model of cartilage regeneration. Age and strain-dependent outcome after joint surface injury. Osteoarthritis Cartilage. 2009;17:695-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 95] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 74. | Lee CH, Cook JL, Mendelson A, Moioli EK, Yao H, Mao JJ. Regeneration of the articular surface of the rabbit synovial joint by cell homing: a proof of concept study. Lancet. 2010;376:440-448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 517] [Cited by in RCA: 456] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 75. | Murphy JM, Fink DJ, Hunziker EB, Barry FP. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum. 2003;48:3464-3474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 765] [Cited by in RCA: 743] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 76. | Zhang S, Muneta T, Morito T, Mochizuki T, Sekiya I. Autologous synovial fluid enhances migration of mesenchymal stem cells from synovium of osteoarthritis patients in tissue culture system. J Orthop Res. 2008;26:1413-1418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 77. | Yang KG, Saris DB, Verbout AJ, Creemers LB, Dhert WJ. The effect of synovial fluid from injured knee joints on in vitro chondrogenesis. Tissue Eng. 2006;12:2957-2964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 78. | Hegewald AA, Ringe J, Bartel J, Krüger I, Notter M, Barnewitz D, Kaps C, Sittinger M. Hyaluronic acid and autologous synovial fluid induce chondrogenic differentiation of equine mesenchymal stem cells: a preliminary study. Tissue Cell. 2004;36:431-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 129] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 79. | Krüger JP, Endres M, Neumann K, Häupl T, Erggelet C, Kaps C. Chondrogenic differentiation of human subchondral progenitor cells is impaired by rheumatoid arthritis synovial fluid. J Orthop Res. 2010;28:819-827. [PubMed] |
| 80. | Zhu H, Jiang XX, Guo ZK, Li H, Su YF, Yao HY, Wang XY, Li XS, Wu Y, Liu YL. Tumor necrosis factor-alpha alters the modulatory effects of mesenchymal stem cells on osteoclast formation and function. Stem Cells Dev. 2009;18:1473-1484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 81. | Zvaifler NJ, Marinova-Mutafchieva L, Adams G, Edwards CJ, Moss J, Burger JA, Maini RN. Mesenchymal precursor cells in the blood of normal individuals. Arthritis Res. 2000;2:477-488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 499] [Cited by in RCA: 484] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 82. | Mansilla E, Marín GH, Drago H, Sturla F, Salas E, Gardiner C, Bossi S, Lamonega R, Guzmán A, Nuñez A. Bloodstream cells phenotypically identical to human mesenchymal bone marrow stem cells circulate in large amounts under the influence of acute large skin damage: new evidence for their use in regenerative medicine. Transplant Proc. 2006;38:967-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 138] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 83. | Kuznetsov SA, Mankani MH, Gronthos S, Satomura K, Bianco P, Robey PG. Circulating skeletal stem cells. J Cell Biol. 2001;153:1133-1140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 515] [Cited by in RCA: 486] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 84. | Lefèvre S, Knedla A, Tennie C, Kampmann A, Wunrau C, Dinser R, Korb A, Schnäker EM, Tarner IH, Robbins PD. Synovial fibroblasts spread rheumatoid arthritis to unaffected joints. Nat Med. 2009;15:1414-1420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 502] [Cited by in RCA: 496] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 85. | Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14327] [Cited by in RCA: 14323] [Article Influence: 842.5] [Reference Citation Analysis (0)] |
| 86. | Marion RM, Strati K, Li H, Tejera A, Schoeftner S, Ortega S, Serrano M, Blasco MA. Telomeres acquire embryonic stem cell characteristics in induced pluripotent stem cells. Cell Stem Cell. 2009;4:141-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 378] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 87. | Stadtfeld M, Maherali N, Breault DT, Hochedlinger K. Defining molecular cornerstones during fibroblast to iPS cell reprogramming in mouse. Cell Stem Cell. 2008;2:230-240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 685] [Cited by in RCA: 626] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 88. | Djouad F, Fritz V, Apparailly F, Louis-Plence P, Bony C, Sany J, Jorgensen C, Noël D. Reversal of the immunosuppressive properties of mesenchymal stem cells by tumor necrosis factor alpha in collagen-induced arthritis. Arthritis Rheum. 2005;52:1595-1603. [PubMed] |
| 89. | Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2:313-319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1248] [Cited by in RCA: 1126] [Article Influence: 66.2] [Reference Citation Analysis (0)] |
| 90. | Bianco P, Robey PG, Saggio I, Riminucci M. "Mesenchymal" stem cells in human bone marrow (skeletal stem cells): a critical discussion of their nature, identity, and significance in incurable skeletal disease. Hum Gene Ther. 2010;21:1057-1066. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 122] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 91. | Tormin A, Li O, Brune JC, Walsh S, Schütz B, Ehinger M, Ditzel N, Kassem M, Scheding S. CD146 expression on primary nonhematopoietic bone marrow stem cells is correlated with in situ localization. Blood. 2011;117:5067-5077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 329] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 92. | Tarte K, Gaillard J, Lataillade JJ, Fouillard L, Becker M, Mossafa H, Tchirkov A, Rouard H, Henry C, Splingard M. Clinical-grade production of human mesenchymal stromal cells: occurrence of aneuploidy without transformation. Blood. 2010;115:1549-1553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 342] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 93. | Baxter MA, Wynn RF, Jowitt SN, Wraith JE, Fairbairn LJ, Bellantuono I. Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion. Stem Cells. 2004;22:675-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 571] [Cited by in RCA: 590] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 94. | Yanada S, Ochi M, Kojima K, Sharman P, Yasunaga Y, Hiyama E. Possibility of selection of chondrogenic progenitor cells by telomere length in FGF-2-expanded mesenchymal stromal cells. Cell Prolif. 2006;39:575-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 95. | Wagner W, Horn P, Castoldi M, Diehlmann A, Bork S, Saffrich R, Benes V, Blake J, Pfister S, Eckstein V. Replicative senescence of mesenchymal stem cells: a continuous and organized process. PLoS One. 2008;3:e2213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 758] [Cited by in RCA: 854] [Article Influence: 50.2] [Reference Citation Analysis (0)] |
| 96. | Kubo H, Shimizu M, Taya Y, Kawamoto T, Michida M, Kaneko E, Igarashi A, Nishimura M, Segoshi K, Shimazu Y. Identification of mesenchymal stem cell (MSC)-transcription factors by microarray and knockdown analyses, and signature molecule-marked MSC in bone marrow by immunohistochemistry. Genes Cells. 2009;14:407-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 97. | Tuan RS. Stemming cartilage degeneration: adult mesenchymal stem cells as a cell source for articular cartilage tissue engineering. Arthritis Rheum. 2006;54:3075-3078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 69] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 98. | Yamane S, Reddi AH. Induction of chondrogenesis and superficial zone protein accumulation in synovial side population cells by BMP-7 and TGF-beta1. J Orthop Res. 2008;26:485-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 99. | Teramura T, Fukuda K, Kurashimo S, Hosoi Y, Miki Y, Asada S, Hamanishi C. Isolation and characterization of side population stem cells in articular synovial tissue. BMC Musculoskelet Disord. 2008;9:86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 100. | Arufe MC, De la Fuente A, Fuentes-Boquete I, De Toro FJ, Blanco FJ. Differentiation of synovial CD-105(+) human mesenchymal stem cells into chondrocyte-like cells through spheroid formation. J Cell Biochem. 2009;108:145-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 101. | Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797-1806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2213] [Cited by in RCA: 2120] [Article Influence: 73.1] [Reference Citation Analysis (0)] |
| 102. | Jackson KA, Majka SM, Wang H, Pocius J, Hartley CJ, Majesky MW, Entman ML, Michael LH, Hirschi KK, Goodell MA. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest. 2001;107:1395-1402. [PubMed] |