Copyright
©The Author(s) 2016.
World J Psychiatr. Mar 22, 2016; 6(1): 18-30
Published online Mar 22, 2016. doi: 10.5498/wjp.v6.i1.18
Published online Mar 22, 2016. doi: 10.5498/wjp.v6.i1.18
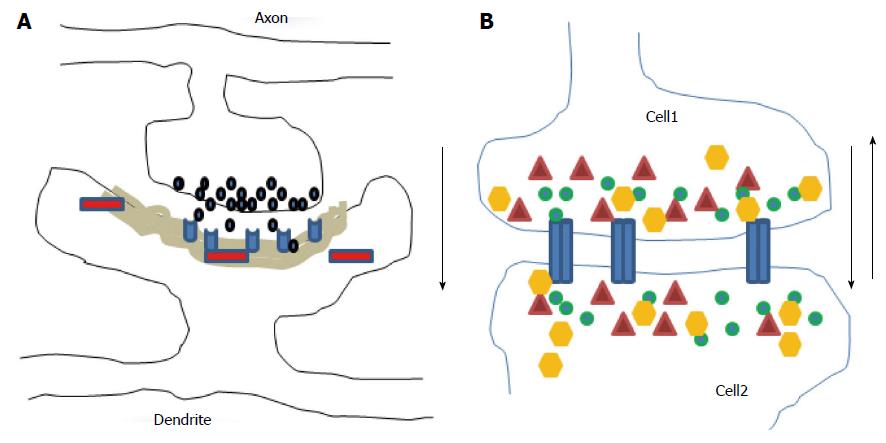
Figure 1 Diagrams illustrate chemical (A) and electrical (B) synapses.
At chemical synapses, neurotransmitters (black) released from axonal boutons bind to postsynaptic receptors (light blue) and trigger specific signaling pathways via activation of proteins (red) in postsynaptic cells with prominent postsynaptic densities component (grey area). The information transmission is unidirectional (black arrow). At electrical synapses, gap junction channels (blue) directly connect the two adjacent cells, thus enable the bidirectional passage of electrical currents (black arrows) carried by ions (green), and of small peptides (yellow) or second messengers (dark red).
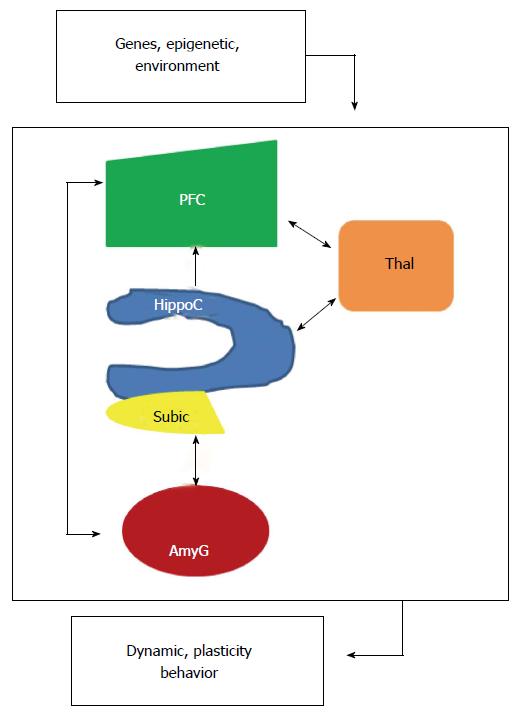
Figure 2 Localized interregional connectivity.
Diagram shows pathway connections of hippocampus (blue), amygdala (dark red), thalamus (orange) and prefrontal cortex (green). HippoC-PFC pathway originating from the subiculum and the CA1 of the hippocampus to the PFC is unidirectional, direct in a monosynaptic manner in rodents and primates. HippoC-AmyG pathway shows bidirectional connection between ventral HippoC with AmyG and the pathway AmyG-PFC has also bidirectional connections. Together, both the HippoC and PFC are reciprocally connected with the AmyG and disruption of these pathways, anatomically or functionally may be a common origin of mental diseases. Moreover, there are bidirectional connections between PFC/HippoC with Thalamus. PFC: Prefrontal cortex.
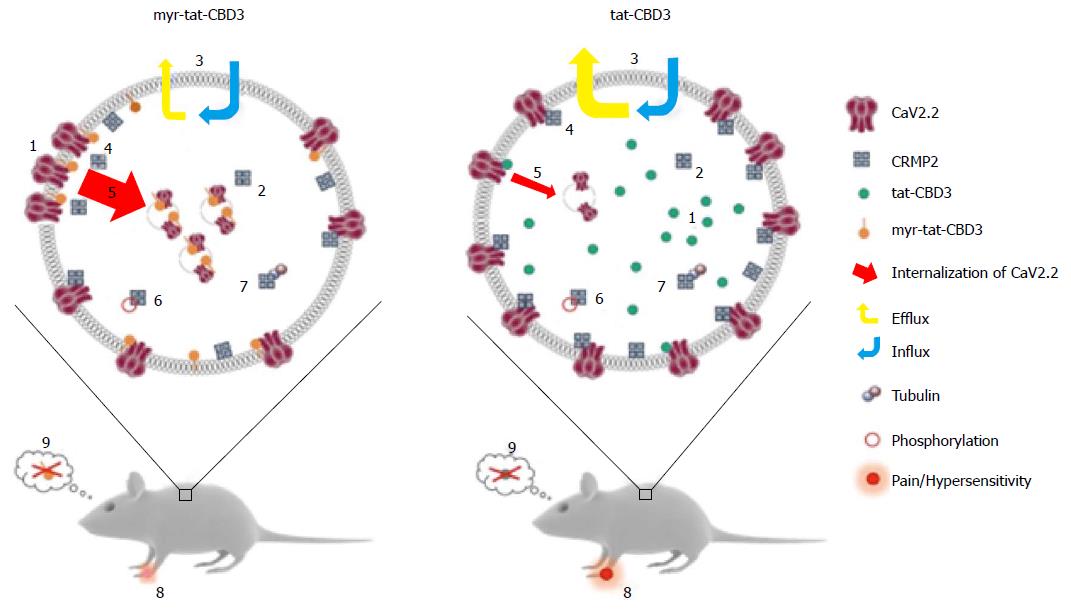
Figure 3 A possible model for N-myristoylated collapsin response mediator protein 2 peptide’s actions on CaV2.
2 trafficking and efficacy in neuropathic pain model. Application of an N-myristoylated tat-conjugated CRMP2 peptide (myr-tat-CBD3) results in membrane-delimited “rimming” of the peptide whereas the non-myristoylated version (tat-CBD3) appears to be spatially diffusely distributed in the cell cytoplasm. Analysis of penetration of peptides into GPMVs, which are “blebs” of membrane devoid of organelles and actin cytoskeleton, reveals an unrestricted distribution of the membrane sensitive dye (di-4-ANNEPDHQ) with tat-CBD3, whereas the myristoylated peptide induces a lateral heterogeneity of the fluorescent signal resulting in dye aggregation into micro domains within these model membranes[122]. While CRMP2 has been demonstrated to exist as a tetramer, the oligomeric state of membrane proximal CRMP2 is as yet unknown; however, neither peptide appears to affect CRMP2 oligomerization[123]. Whereas the cells take up both forms of the peptide with similar efficiency, the myristoylated peptide demonstrates a lesser degree of efflux[124]. The apparent increase in retention of myr-tat-CBD3 translates into a superior potency and efficacy in inhibition of evoked calcium influx in sensory neurons presumably via greater uncoupling of CRMP2-CaV2.2 interactions at or juxta-membrane[125]. Increased inhibition of CaV2.2 surface trafficking induced by myr-tat-CBD3 compared with tat-CBD3 may account[126] for the more pronounced restriction of calcium influx imposed by the myristoylated peptide. Cdk5-phosphorylated CRMP2 has been demonstrated to have an enhanced interaction with CaV2.2[129]; however myr-tat-CBD3 does not affect the levels of Cdk5-phosphorylated CRMP2[127], thereby ruling out a role of phosphorylated CRMP2 in regulating calcium influx. CRMP2 binding to tubulin is strengthened by the peptides[130]; the consequences of this are currently unknown. Importantly, where tat-CBD3 is completely ineffective in reversing mechanical hypersensitivity in a rat neuropathic pain model (tibial nerve injury), the myristoylated peptide reverses this hypersensitivity when administered in vivo[122]. Neither peptide elicits any reward-like addictive behaviors. GPMVs: Giant plasma membrane vesicles; CRMP: Collapsin response mediator proteins.
- Citation: Quach TT, Lerch JK, Honnorat J, Khanna R, Duchemin AM. Neuronal networks in mental diseases and neuropathic pain: Beyond brain derived neurotrophic factor and collapsin response mediator proteins. World J Psychiatr 2016; 6(1): 18-30
- URL: https://www.wjgnet.com/2220-3206/full/v6/i1/18.htm
- DOI: https://dx.doi.org/10.5498/wjp.v6.i1.18