Published online Dec 9, 2014. doi: 10.5497/wjp.v3.i4.209
Revised: October 15, 2014
Accepted: October 23, 2014
Published online: December 9, 2014
Processing time: 204 Days and 15.4 Hours
AIM: To the look at the current strength of evidence and the potential application of anti-oxidants in this setting.
METHODS: Two electronic databases (PubMed and Web of Knowledge) were searched to January 2013 to find studies addressing serum bilirubin levels in non-alcoholic fatty liver disease (NAFLD). The search used key word combinations in relation to NAFLD and serum bilirubin specific to human adults only. After screening selected studies were reviewed in depth by two independent reviewers. Data synthesis with further meta-analysis was planned but not possible due to the heterogeneity of the outcome measures in these studies.
RESULTS: Out of 416 studies screened only seven studies were considered suitable for inclusion. All seven studies consistently reported an inverse association of bilirubin with NAFLD despite the heterogeneous sample of studies. Only two studies were prospective. No negative studies were found.
CONCLUSION: Most studies suggest a correlation between high bilirubin levels of any type are inversely correlated with NAFLD. But to date most of these studies have been poorly designed to allow meaningful conclusions, except one cohort study. There is a need for a large prospective cohort study in multiple populations to test this hypothesis fully before mechanistic associations can be established and therapeutic options of the apparent anti-oxidant effect of bilirubin be explored in NAFLD. Furthermore these studies should include analysis of UGT1A1 gene to expound upon underlying cause of unconjugated hyperbilirubinaemia.
Core tip: This systematic review summarises and highlights the deficiencies in the current studies on the association of serum bilirubin with non-alcoholic fatty liver disease (NAFLD). It explores the potential underpinning of the mechanistic association of NAFLD with bilirubin. Potential novel therapeutic avenues of bilirubin are explored in NAFLD, a common condition with oxidative damage as a core pathogenetic factor. Although this area of study is still in its infancy, this review is a timely summary of current key studies in this subject area and provides an up to date thought perspective with focus on future direction and potential therapeutic application.
- Citation: Anwar MS, Dillon JF, Miller MH. Association of serum bilirubin and non-alcoholic fatty liver disease: A feasible therapeutic avenue? World J Pharmacol 2014; 3(4): 209-216
- URL: https://www.wjgnet.com/2220-3192/full/v3/i4/209.htm
- DOI: https://dx.doi.org/10.5497/wjp.v3.i4.209
Non-alcoholic fatty liver disease (NAFLD) has been recognised as the most prevalent liver disease, with current estimations that it affects around 20%-30% of the general population in the western world[1,2]. NAFLD is considered to be the hepatic manifestation of the metabolic syndrome as it is closely related to insulin resistance, obesity, hypercholesterolemia, type 2 diabetes, and coronary artery disease[3-5]. NAFLD is composed of a histological spectrum of hepatic dysfunctions ranging from simple steatosis (SS) to non-alcoholic steatohepatitis (NASH), cirrhosis and hepatocellular carcinoma[6]. The pathogenic processes underpinning NAFLD remain unclear, although oxidative stress, fat transportation and inflammation are implicated. Furthermore oxidative stress has been suggested as an aetiopathogenic mechanism in NAFLD[7,8]. Additionally, mounting evidence suggests a link between serum ferritin, insulin resistance, and NAFLD[9,10]. Excessive hepatic iron accumulation in NAFLD is likely one of the potential cofactors involved in the enhanced oxidative stress, which triggers liver cell necrosis and activation of hepatic stellate cells, both leading to fibrosis[11]. The significance of the association serum ferritin in NAFLD may relate to heme catabolism and the anti-oxidant state of play, which will be discussed later. Studies involving administration of a free radical-generating azo compound to mice or rats induced fat accumulation in the liver by increasing triacylglycerol and decreasing phospholipids. Likewise fat accumulation in the liver was suppressed through the simultaneous administration of free radical-scavenging antioxidants such as Vitamin E, Therefore antioxidant agents have been proposed as an effective treatment[12-15].
Bilirubin, the end product of heme catabolism, is known to be a potent physiological antioxidant cytoprotectant due to its inhibitory effect on the activity of NAD(P)H oxidase. In addition it scavenges peroxyl radicals, hydroxyl radicals, and reactive nitrogen species preventing oxidation of intracellular lipids[16-20]. Bilirubin has also been proposed as having an anti-inflammatory role and has major antifibrogenic properties via heme oxygenase-1 (HO-1)[21-23]. Previous studies have shown that unconjugated hyperbilirubinemia is inversely associated with ischaemic heart disease, carotid stenosis, insulin resistance, diabetes, vascular complication of diabetes, peripheral vascular disease and even cancer[24-29]. Furthermore there is strong clinical evidence for the beneficial cytoprotective effects of unconjugated bilirubin as observed in Gilbert’s syndrome[28-29].
Consequently, it can be hypothesised that elevated serum bilirubin levels reduce oxidative stress, decrease fibrosis and inflammation, and decrease the risk of NAFLD development and progression. If this hypothesis is confirmed then therapeutic options of inducing “iatrogenic” Gilbert’s syndrome would be a key area of research. This systematic review evaluates the studies carried out to date to assess the reported association between bilirubin and NAFLD.
This systematic review included studies published in electronic databases over the time period ranging from their inception to January 2013. We searched two main stream public-domain data bases, PubMed and Web of Knowledge. Three categories were devised (1) conditions (SS, NASH, NAFLD, FLD); (2) bilirubin (unconjugated hyperbilirubinemia, bilirubin, anti-oxidant, protective marker); and (3) subjects (human). Each possible combination was searched in the above two databases, also the bibliographies of relevant systematic reviews were manually searched.
We included all types of studies, which investigated the relationship between bilirubin and NAFLD. Paediatric studies were excluded due to the potential alternate pathophysiology in this category. Outcome measure for each study was bilirubin, but it should be noted there are three methods of reporting bilirubin. Total bilirubin which consists of direct (conjugated) and indirect (uncojugated) bilirubin. Each study focused on one of these during statistical analysis.
Each selected study was assessed independently by two reviewers for methodology, outcome measures, results, limitations, risks of bias. Data synthesis with further meta-analysis was not possible due to the heterogeneity of outcome measures, study designs and statistical analysis in each study. Therefore instead we have provided a summary of this for each study.
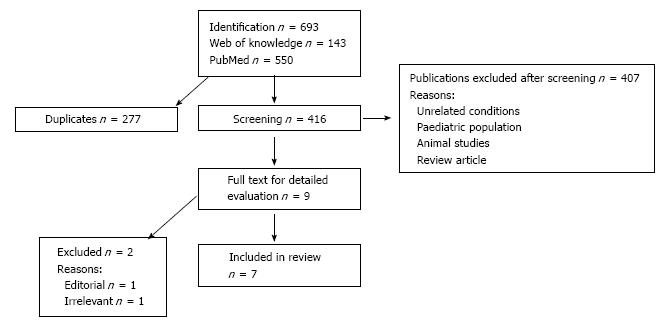
We considered 416 potentially relevant articles, after screening the abstracts and titles, 407 studies were excluded, (Figure 1). Nine articles were fully evaluated, with a further two excluded. First of these articles was an editorial rather than an original study[30] and the second article was not relevant, it discussed measures of oxidation stress in NASH[31]. Of the included studies, three were cross sectional, two retrospective and two prospective. Two of these studies had a large sample size and were conducted in South Korea[32,33]. The remaining five small sized studies used liver biopsy[34-38] to diagnose NAFLD but notably this does not include every patient in the study. Also only two studies[37,38] specifically states blinding of the pathologist to biochemical results and intention of study. All studies excluded patients using alcohol > 20 g/d, screened for viral hepatitis, alternative liver pathology and haemolysis, except the Tarantino[38] study which did not assess patient for possible haemolysis. The characteristics of each study are shown in Table 1. Due to the heterogeneity of these studies, further pooled analysis could not be carried out and therefore a summary of key results is provided instead in Table 2. Notably no study reported an insignificant association of bilirubin with NAFLD.
| Ref. | Study designTotal number of patients | Study population | Main outcome measure |
| Chang et al[32] | Cohort-longitudinal | Korean men from a single large semiconductor company (South Korea) | Conjugated bilirubin |
| n = 5900 | |||
| Kwak et al[33] | Cross-section | Health check-general | |
| n = 17348 | Population (South Korea) | Total bilirubin | |
| Duseja et al[34] | Prospective | Hospital setting unclear, probably Tertiary (India) | Unconjugated bilirubin |
| n = 67 | |||
| Hjelkrem et al[35] | Retrospective | Tertiary hospital patients undergoing liver biopsy (United States) | Unconjugated bilirubin |
| n = 641 | |||
| Kumar et al[36] | Cross-sectional | Tertiary hospital, outpatient NAFLD clinic (India) | Unconjugated bilirubin |
| n = 204 | |||
| Chisholm et al[37] | Cross-sectional | Liver biopsy prior to bariatric surgery (United States) | Total bilirubin |
| n = 370 | |||
| Tarantino et al[38] | Cross-sectional | Tertiary hospital, outpatient likely liver clinic (Italy) | Unconjugated bilirubin |
| n = 186 |
| Ref. | Study analysis | Results |
| Chang et al[32] | (+) Large sample (+) Prospective (-) NAFLD on US | Hazard ratio for NAFLD comparing the highest to the lower quartile of serum conjugated bilirubin = 0.61 (95%CI: 0.54-0.68), after adjusting metabolic parameters 0.86a (95%Cl : 0.76-0.98) Total bilirubin inversely associated with NAFLD OR = 0.88, 95%CI: 0.80-0.97. An inverse, dose-dependent association between NAFLD and serum total bilirubin levels OR = 0.80b, 95%CI: 0.71-0.90 in the fourth quartile vs lowest quartile |
| Kwak et al[33] | (+) Large sample (-) NAFLD on US | |
| Duseja et al[34] | (-) Small sample (+) Prospective (+) NASH on biopsy (selected) | Patient sample too small to make any meaningful statistical inference |
| Hjelkrem et al[35] | (-)Retrospective design (-) Small sample (+) NAFLD on biopsy | Unconjugated hyperbilirubinaemia inversely associated with NASH OR = 16.1b, 95%CI: 3.7-70.8 |
| Kumar et al[36] | (-) Small sample (+/-) NAFLD on biopsy but not all patients | Unconjugated hyperbilirubinaemia (UCHB) and NAS score-negative correlation: r: -0.48b. UCHB and stage of fibrosis: r: -0.28d |
| Chisholm et al[37] | (-) Small sample (+) NAFLD on biopsy | Binary logistic regression showed independent association of total bilirubin with NASH cP = 0.016 Unconjugated bilirubin elevated in all spectra of NAFLD, except healthy control fP = 0.008 High UCHB in advanced NASH group |
| Tarantino et al[38] | (-) Retrospective | |
| (-) Small sample | ||
| (+) NASH on biopsy (selected) |
Of these studies, two were prospective[32,33] and one[32] of these was a large cohort study of young Korean men (5900) and showed all types of raised bilirubin were inversely associated with developing NAFLD but focused on conjugated hyperbilirubinaemia. In this study multivariate model analysis showed only conjugated hyperbilirubinaemia as independently association with risk of developing NAFLD. The study adjusted for confounding factors such as age, body mass index, current smoking, alcohol intake, exercise, diabetes mellitus, history of cardiovascular disease and history of malignancy, high density lipoprotein cholesterol, triglycerides, glucose, insulin, and uric acid.
The second[33] large sample study was also carried out in South Korea and showed an inverse association between total bilirubin and NAFLD. But due to the cross-sectional design of this study a causal relationship cannot be confirmed. These large sample studies confirmed NAFLD on the basis of typical ultrasound findings instead of a liver biopsy. This limitation is essentially unavoidable in such a large sample size given the morbidity and mortality associated with this procedure.
The smaller studies which were based at tertiary centres did carry out liver biopsies albeit not for every patient. Duseja[34] carried out the first study looking into association of hyperbilirubinaemia and NASH. This was a prospective study consisting of only 67 subjects and therefore did not show a statistically significant association between hyperbilirubinaemia and NASH. Given the small sample size of this study, it cannot be considered sufficient to have shown negative or positive correlation between hyperbilirubinemia and NASH. Furthermore the setting and criterion for patient selection is not adequately defined.
Hjelkrem et al[35] and Kumar et al[36] suggested that degree of fibrosis also appears to be related to bilirubin levels alongside NAFLD development and progression, although neither study blinded the pathologist to study intent. Interestingly although 508 patients had liver biopsy in the Hjelkrem et al[35] study including only thirty five who had elevated unconjugated bilirubin, their statistical analysis did not suggest any association between NAFLD severity and unconjugated bilirubin but this is probably due to small sample of elevated unconjugated bilirubin patients in this group. Kumar et al[36] reported a negative correlation between serum unconjugated levels and histopathological NAS score, stages of fibrosis on the basis of biopsy results from 42 patients in total. Given the sample size and study design of these two studies, these results can only be regarded as speculative at this stage.
Chisholm et al[37] carried out a study in bariatric patients prior to surgery for evaluation of markers predictive of steatohepatitis, they devised a ROC curve for prediction curve of NASH which included 3 variables: total bilirubin, ALT and HOMA-IR (Homeostasis model of assessment-insulin resistance). Of 370 patients in this study 275 had a liver biopsy, with the pathologist blinded to the intent of the study but a cohort of patients were also given very low calorie diet to study the influence of this on histology findings. Unfortunately insufficient data is given to infer any association between degree of fibrosis and bilirubin level.
Tarantino et al[38] study analysed hepatocytes after exposing them to free fatty acids with expected mitochondrial damage for the presence of anti-oxidant substances such as cytochrome c, gamma-glutamyl transferase, triglycerides and unconjugated bilirubin in patients with NAFLD of differing severity (n = 186) and controls (n = 27). The study showed unconjugated bilirubin to be elevated in all spectra of NAFLD, except in healthy controls (P = 0.008). The study authors felt the elevated unconjugated bilirubin represented anti-oxidant effect in this setting and unconjugated bilirubin could be used to monitor response to disease modifying treatment. Interestingly the level of unconjugated bilirubin was the highest in NASH subgroup (n = 44) with the most severe histological findings, contradicting all previous studies. All previous studies had suggested unconjugated bilirubin was protective for NASH and less commonly elevated in the advanced cohort of NASH. It is worth noting that the study did not demonstrate cytochrome c to be elevated as expected which contradicts multiple previous studies and notably did not check for underlying haemolysis in patient cohort either therefore a cautious approach should be taken with all results of this study.
Despite the heterogeneous nature of studies addressing the association of bilirubin with NAFLD including large variation in sample size and specific bilirubin type (s) measured there was consistent inverse association of raised total bilirubin, conjugated and uncojugated hyperbilirubinemia with NAFLD, across populations in Asia and America. Although Tarantino et al[38] data contradicted these findings, the poor study design and failure to demonstrate other expected findings make result interpretation from this study highly speculative. But given the sample size and study design variation of the remaining studies, these results can only be regarded as showing a tentative association with disease reduction but combined with a plausible biological mechanism, they raise the intriguing possibility of a causative relationship.
This review is limited due to the small number of studies on this topic and the heterogeneity of these studies. Given this pooled analysis could not be carried out. Despite the shortcoming of the studies in this review, there is a consistent reported inverse association between high bilirubin and NAFLD, except in one study, which had very small sample size, and therefore no statistical inference can be attained from this study. Notably to date most studies have suggested undiagnosed Gilbert’s syndrome as the cause of unconjugated hyperbilirubinemia although none of the studies have validated this with analysis of UGT1A1 genetic mutation. Although Gilbert’s syndrome may account for some of these patients, another possibility is that unconjugated hyperbilirubinemia may be an initial acquired response to oxidative stress and thus represents the liver’s intrinsic anti-oxidative capacity, but is not sustainable due to repeated insults. Notably Gilbert’s syndrome is more commonly diagnosed in men and sex steroids may influence bilirubin metabolism with higher production in men[39]. Two studies included in this review[32,36] showed higher preponderance of men with unconjugated hyperbilirubinemia. Therefore future studies are required to verify the inverse correlation of bilirubin to NAFLD in large, prospective cohort study in multiple populations along with the analysis of UGT1A1 gene to expound upon underlying cause of unconjugated hyperbilirubinaemia in this cohort.
Although a direct effect of bilirubin maybe likely, other parts of the heme catabolism such as the effect on iron homeostasis may also be relevant. Heme catabolism represents a key function in mobilising macrophage iron derived from ingested erythrocytes. Importantly, the storage and processing of iron from erythrophagocytosis by macrophages within plaque appear to play a vital role in plaque progression[40]. Accordingly, it has been demonstrated that erythrocytes induce plaque vulnerability in a dose-dependent manner in a rabbit model of intra-plaque haemorrhage[41]. Furthermore, it has been found that the effect of HO-1 on iron homeostasis within macrophages may represent a new tool to prevent foam cell formation and atherosclerotic lesion progression. A protective effect of iron depletion that may have multiple beneficial consequences is decreased availability of redox-active iron in vivo. The amount of free iron available at sites of oxidative or inflammatory injury appears to be a function of the stored iron level. Indeed, a recent study found that the cytoprotective effect of HO-1 induction or forced expression (which usually leads to concomitant elevated serum bilirubin level) may derive from temporary elevated expression of ferritin, and consequent reduction of redox-active iron[42]. Alongside this, there is accumulating evidence to suggest that excessive hepatic iron accumulation in NAFLD is one of the potential cofactors involved in the enhanced oxidative stress, which triggers liver cell necrosis and eventually fibrosis[9-11]. There are potentially multiple mechanistic underpinnings for the beneficial association of bilirubin with NAFLD.
The development of antioxidant therapeutics is gaining prominence as the pathophysiological foundation of oxidative stress in cardiovascular disease, neoplasia and NAFLD is better understood[43]. Significant numbers of studies have focused on using vitamin based antioxidant therapy to assess prevention of cardiovascular or neoplastic disease. Despite replicable in vitro evidence to support antioxidant vitamin use, this has poor correlation with in vivo subjects showing conflicting results at best to date. Further ongoing large randomised controlled trials results are awaited[44,45]. This disparity is likely due to any exogenous vitamin antioxidants’ inability to meaningfully influence intracellular antioxidant levels[46].
Bilirubin is recognised to have antioxidant activity so inducing “iatrogenic Gilbert’s syndrome” is another strategy for advancing antioxidant therapeutics which involves using drugs that promote the unconjugated hyperbilirubinaemic state. This strategy may overcome the difficulties of achieving sufficient antioxidants at intracellular level as bilirubin’s main antioxidant action appears to act not as a direct radical scavenger given its low concentration in tissue. But instead as a potent and specific inhibitor of the membrane bound NADPH oxidase, a key source oxidants in both phagocytic and non-phagocytic cells[47]. Probenecid, a uricosuric agent is known to decrease hepatic glucuronidation activity, leading to hyperbilirubinaemia which has been observed only in multiple case reports but not formally evaluated in studies in dose-dependent manner[47,48]. It is known to be well tolerated but decreasing glucuronidation activity will increase half life of commonly used medication such as paracetamol and lorazepam and thus may hinder the application of this drug and strategy[49,50]. Rifampicin could be another possible candidate, it causes hyperbilirubinaemia by inhibiting the transporter which accelerates hepatocyte uptake of bilirubin[51-53]. But rifampicin can cause significant side effects which include liver failure although in a small proportion of patients, this would entail closer monitoring. Sodium valproate has a similar mechanism of action to rifampicin but due to many side effects and poor tolerability would be unsuitable for this application.
Oral administration of bilirubin or its precursor biliverdin which is more soluble is another possible avenue but this strategy is limited by the lack of evidence showing increased bilirubin levels after oral administration other than in mice and rat models[41,54]. Even if clear evidence was obtained the mass production of bilirubin is complex and costly thus would be a major obstacle to overcome[55]. Phycobilins that are structural analogues of biliverdin are produced readily by plants, algae and cynobacteia which may provide feasible alternatives to bilirubin[56]. Both in animal models and in vitro studies phycobilins have been shown to have comparable effect to bilirubin[57,58]. Further research in this area with human subjects is awaited.
To conclude once the protective association between uncojugated hyperbilirubinaemia and NAFLD is verified. The focus of preventing NAFLD progression and development should consider bilirubin induction therapy randomised double blind controlled trials to assess the clinical value of this treatment. Thereafter it remains to be shown how popular this treatment would be as patient will be icteric. Although equally with weight reduction as the current mainstay of NAFLD prevention, this strategy has its own limitations and therefore optimal treatment options which are acceptable to patients continues to be a key challenge in this area.
Obesity is a growing epidemic in the western world and if it is not controlled and reversed it is associated with type 2 diabetes, hypercholesterolaemia and coronary artery disease. The hepatic manifestation of this multi-organ disease is non-alcoholic fatty liver disease (NAFLD). This hepatic dysfunction can eventually lead to cirrhosis and hepatocellular carcinoma. At present there is no effective treatment available and the key current management is prevention by weight reduction. High levels of bilirubin (hyperbilirubinaemia), which is naturally occurring anti-oxidant in the human body has been shown to prevent progression of NAFLD in some studies. This paper evaluates all studies in relation to this, to the look at the current strength of evidence and the potential application of anti-oxidants in this setting.
Preventing NAFLD progression is a key area of research, inducing hyperbilirubinaemia represents a potential solution. Laboratory based research as well as on animal models has shown high levels of bilirubin to have protective and preventative effect on NAFLD and other diseases processes related to obesity. But this relationship needs to be substantiated in human subjects.
Although most studies evaluated in this review suggest there is likely a protective association between high bilirubin levels and NAFLD progression and prevention. The poor design of these studies is prohibitive to allow any meaningful conclusion. There is a need for good quality large prospective cohort study in multiple populations to test this hypothesis fully, which should also included analysis of UGT1A1 gene to expound upon underlying cause of unconjugated hyperbilirubinaemia.
This review allows readers to appreciate and evaluate current progress in this area of research. The case for protective association of hyperbilirubinaemia with NAFLD needs to be corroborated with good quality studies. They are multiple drug options available to induce hyperbilirubinaemia but these drugs need to be tested in randomised controlled trial setting.
Bilirubin is a naturally occuring substance in the blood which comes from break down of red blood cells. It is an anti-oxidant (protects cells again harmful substances) and appears to also have an anti-inflammatory effect. High level of bilirubin gives the jaundiced appearance.
It is a good review article, the authors provide updates of the fatty liver diseases with a Q and A format for easy reads.
P- Reviewer: Ahmed M, Mascitelli L, Oeda S, Shen WJ, Tamano M S- Editor: Song XX L- Editor: A E- Editor: Lu YJ
| 1. | Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2633] [Cited by in RCA: 2696] [Article Influence: 128.4] [Reference Citation Analysis (3)] |
| 2. | Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 2003;37:1202-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1488] [Cited by in RCA: 1478] [Article Influence: 67.2] [Reference Citation Analysis (0)] |
| 3. | Choi SY, Kim D, Kim HJ, Kang JH, Chung SJ, Park MJ, Kim YS, Kim CH, Choi SH, Kim W. The relation between non-alcoholic fatty liver disease and the risk of coronary heart disease in Koreans. Am J Gastroenterol. 2009;104:1953-1960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 100] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 4. | Speliotes EK, Massaro JM, Hoffmann U, Vasan RS, Meigs JB, Sahani DV, Hirschhorn JN, O’Donnell CJ, Fox CS. Fatty liver is associated with dyslipidemia and dysglycemia independent of visceral fat: the Framingham Heart Study. Hepatology. 2010;51:1979-1987. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 324] [Cited by in RCA: 318] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 5. | Chen SH, He F, Zhou HL, Wu HR, Xia C, Li YM. Relationship between nonalcoholic fatty liver disease and metabolic syndrome. J Dig Dis. 2011;12:125-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 6. | Salamone F, Bugianesi E. Nonalcoholic fatty liver disease: the hepatic trigger of the metabolic syndrome. J Hepatol. 2010;53:1146-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Oliveira CP, da Costa Gayotto LC, Tatai C, Della Bina BI, Janiszewski M, Lima ES, Abdalla DS, Lopasso FP, Laurindo FR, Laudanna AA. Oxidative stress in the pathogenesis of nonalcoholic fatty liver disease, in rats fed with a choline-deficient diet. J Cell Mol Med. 2002;6:399-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 93] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Sumida Y, Nakashima T, Yoh T, Furutani M, Hirohama A, Kakisaka Y, Nakajima Y, Ishikawa H, Mitsuyoshi H, Okanoue T. Serum thioredoxin levels as a predictor of steatohepatitis in patients with nonalcoholic fatty liver disease. J Hepatol. 2003;38:32-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 155] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 9. | Zelber-Sagi S, Nitzan-Kaluski D, Halpern Z, Oren R. NAFLD and hyperinsulinemia are major determinants of serum ferritin levels. J Hepatol. 2007;46:700-707. [PubMed] |
| 10. | Ryan MC, Itsiopoulos C, Thodis T, Ward G, Trost N, Hofferberth S, O’Dea K, Desmond PV, Johnson NA, Wilson AM. The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J Hepatol. 2013;59:138-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 578] [Cited by in RCA: 538] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 11. | Mascitelli L, Goldstein MR. Might some of the beneficial effects of the Mediterranean diet on non-alcoholic fatty liver disease be mediated by reduced iron stores? J Hepatol. 2013;59:639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Morita M, Ishida N, Uchiyama K, Yamaguchi K, Itoh Y, Shichiri M, Yoshida Y, Hagihara Y, Naito Y, Yoshikawa T. Fatty liver induced by free radicals and lipid peroxidation. Free Radic Res. 2012;46:758-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Terao K, Niki E. Damage to biological tissues induced by radical initiator 2,2’-azobis(2-amidinopropane) dihydrochloride and its inhibition by chain-breaking antioxidants. J Free Radic Biol Med. 1986;2:193-201. [PubMed] |
| 14. | Shimasaki H, Saypil WH, Ueta N. Free radical-induced liver injury. II. Effects of intraperitoneally administered 2,2’-azobis(2-amidinopropane) dihydrochloride on the fatty acid profiles of hepatic triacylglycerol and phospholipids. Free Radic Res Commun. 1991;14:247-252. [PubMed] |
| 15. | Sanyal AJ, Mofrad PS, Contos MJ, Sargeant C, Luketic VA, Sterling RK, Stravitz RT, Shiffman ML, Clore J, Mills AS. A pilot study of vitamin E versus vitamin E and pioglitazone for the treatment of nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2004;2:1107-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 298] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 16. | Baranano DE, Rao M, Ferris CD, Snyder SH. Biliverdin reductase: a major physiologic cytoprotectant. Proc Natl Acad Sci U S A. 2002;99:16093-16098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 800] [Cited by in RCA: 832] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 17. | Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2595] [Cited by in RCA: 2637] [Article Influence: 69.4] [Reference Citation Analysis (0)] |
| 18. | Stocker R. Antioxidant activities of bile pigments. Antioxid Redox Signal. 2004;6:841-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 215] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 19. | Inoguchi T, Li P, Umeda F, Yu HY, Kakimoto M, Imamura M, Aoki T, Etoh T, Hashimoto T, Naruse M. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C--dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes. 2000;49:1939-1945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1121] [Cited by in RCA: 1132] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 20. | Keshavan P, Deem TL, Schwemberger SJ, Babcock GF, Cook-Mills JM, Zucker SD. Unconjugated bilirubin inhibits VCAM-1-mediated transendothelial leukocyte migration. J Immunol. 2005;174:3709-3718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 120] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 21. | Willis D, Moore AR, Frederick R, Willoughby DA. Heme oxygenase: a novel target for the modulation of the inflammatory response. Nat Med. 1996;2:87-90. [PubMed] |
| 22. | Li L, Grenard P, Nhieu JT, Julien B, Mallat A, Habib A, Lotersztajn S. Heme oxygenase-1 is an antifibrogenic protein in human hepatic myofibroblasts. Gastroenterology. 2003;125:460-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Cheriyath P, Gorrepati VS, Peters I, Nookala V, Murphy ME, Srouji N, Fischman D. High Total Bilirubin as a Protective Factor for Diabetes Mellitus: An Analysis of NHANES Data From 1999 - 2006. J Clin Med Res. 2010;2:201-206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 24. | Lin JP, O’Donnell CJ, Schwaiger JP, Cupples LA, Lingenhel A, Hunt SC, Yang S, Kronenberg F. Association between the UGT1A1*28 allele, bilirubin levels, and coronary heart disease in the Framingham Heart Study. Circulation. 2006;114:1476-1481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 251] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 25. | Perlstein TS, Pande RL, Creager MA, Weuve J, Beckman JA. Serum total bilirubin level, prevalent stroke, and stroke outcomes: NHANES 1999-2004. Am J Med. 2008;121:781-788.e1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 150] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 26. | Lin LY, Kuo HK, Hwang JJ, Lai LP, Chiang FT, Tseng CD, Lin JL. Serum bilirubin is inversely associated with insulin resistance and metabolic syndrome among children and adolescents. Atherosclerosis. 2009;203:563-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 116] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 27. | Zucker SD, Horn PS, Sherman KE. Serum bilirubin levels in the U.S. population: gender effect and inverse correlation with colorectal cancer. Hepatology. 2004;40:827-835. [PubMed] |
| 28. | Vítek L, Jirsa M, Brodanová M, Kalab M, Marecek Z, Danzig V, Novotný L, Kotal P. Gilbert syndrome and ischemic heart disease: a protective effect of elevated bilirubin levels. Atherosclerosis. 2002;160:449-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 315] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 29. | Inoguchi T, Sasaki S, Kobayashi K, Takayanagi R, Yamada T. Relationship between Gilbert syndrome and prevalence of vascular complications in patients with diabetes. JAMA. 2007;298:1398-1400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 135] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 30. | Jang BK. Elevated serum bilirubin levels are inversely associated with nonalcoholic fatty liver disease. Clin Mol Hepatol. 2012;18:357–359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 31. | Madan K, Bhardwaj P, Thareja S, Gupta SD, Saraya A. Oxidant stress and antioxidant status among patients with nonalcoholic fatty liver disease (NAFLD). J Clin Gastroenterol. 2006;40:930-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 32. | Chang Y, Ryu S, Zhang Y, Son HJ, Kim JY, Cho J, Guallar E. A cohort study of serum bilirubin levels and incident non-alcoholic fatty liver disease in middle aged Korean workers. PLoS One. 2012;7:e37241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 33. | Kwak MS, Kim D, Chung GE, Kang SJ, Park MJ, Kim YJ, Yoon JH, Lee HS. Serum bilirubin levels are inversely associated with nonalcoholic fatty liver disease. Clin Mol Hepatol. 2012;18:383-390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 34. | Duseja A, Das A, Das R, Dhiman RK, Chawla Y, Bhansali A. Unconjugated hyperbilirubinemia in nonalcoholic steatohepatitis--is it Gilbert's syndrome? Trop Gastroenterol. 2005;26:123-125. [PubMed] |
| 35. | Hjelkrem M, Morales A, Williams CD, Harrison SA. Unconjugated hyperbilirubinemia is inversely associated with non-alcoholic steatohepatitis (NASH). Aliment Pharmacol Ther. 2012;35:1416-1423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 36. | Kumar R, Rastogi A, Maras JS, Sarin SK. Unconjugated hyperbilirubinemia in patients with non-alcoholic fatty liver disease: a favorable endogenous response. Clin Biochem. 2012;45:272-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 37. | Chisholm J, Seki Y, Toouli J, Stahl J, Collins J, Kow L. Serologic predictors of nonalcoholic steatohepatitis in a population undergoing bariatric surgery. Surg Obes Relat Dis. 2012;8:416-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 38. | Tarantino G, Colao A, Capone D, Conca P, Tarantino M, Grimaldi E, Chianese D, Finelli C, Contaldo F, Scopacasa F. Circulating levels of cytochrome C, gamma-glutamyl transferase, triglycerides and unconjugated bilirubin in overweight/obese patients with non-alcoholic fatty liver disease. J Biol Regul Homeost Agents. 2011;25:47-56. [PubMed] |
| 39. | Muraca M, Fevery J. Influence of sex and sex steroids on bilirubin uridine diphosphate-glucuronosyltransferase activity of rat liver. Gastroenterology. 1984;87:308-313. [PubMed] |
| 40. | Finn AV, Nakano M, Polavarapu R, Karmali V, Saeed O, Zhao X, Yazdani S, Otsuka F, Davis T, Habib A. Hemoglobin directs macrophage differentiation and prevents foam cell formation in human atherosclerotic plaques. J Am Coll Cardiol. 2012;59:166-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 263] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 41. | Lin HL, Xu XS, Lu HX, Zhang L, Li CJ, Tang MX, Sun HW, Liu Y, Zhang Y. Pathological mechanisms and dose dependency of erythrocyte-induced vulnerability of atherosclerotic plaques. J Mol Cell Cardiol. 2007;43:272-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 42. | Lanceta L, Li C, Choi AM, Eaton JW. Haem oxygenase-1 overexpression alters intracellular iron distribution. Biochem J. 2013;449:189-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 43. | Schwertner HA, Vítek L. Gilbert syndrome, UGT1A1*28 allele, and cardiovascular disease risk: possible protective effects and therapeutic applications of bilirubin. Atherosclerosis. 2008;198:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 175] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 44. | Christen WG, Gaziano JM, Hennekens CH. Design of Physicians’ Health Study II--a randomized trial of beta-carotene, vitamins E and C, and multivitamins, in prevention of cancer, cardiovascular disease, and eye disease, and review of results of completed trials. Ann Epidemiol. 2000;10:125-134. [PubMed] |
| 45. | Hennekens CH, Buring JE, Manson JE, Stampfer M, Rosner B, Cook NR, Belanger C, LaMotte F, Gaziano JM, Ridker PM. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N Engl J Med. 1996;334:1145-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1708] [Cited by in RCA: 1431] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 46. | Juránek I, Nikitovic D, Kouretas D, Hayes AW, Tsatsakis AM. Biological importance of reactive oxygen species in relation to difficulties of treating pathologies involving oxidative stress by exogenous antioxidants. Food Chem Toxicol. 2013;61:240-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 47. | McCarty MF. ‘’Iatrogenic Gilbert syndrome’’--a strategy for reducing vascular and cancer risk by increasing plasma unconjugated bilirubin. Med Hypotheses. 2007;69:974-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 101] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 48. | Bokoch GM, Knaus UG. NADPH oxidases: not just for leukocytes anymore! Trends Biochem Sci. 2003;28:502-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 280] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 49. | Abernethy DR, Greenblatt DJ, Ameer B, Shader RI. Probenecid impairment of acetaminophen and lorazepam clearance: direct inhibition of ether glucuronide formation. J Pharmacol Exp Ther. 1985;234:345-349. [PubMed] |
| 50. | Vree TB, van den Biggelaar-Martea M, Verwey-van Wissen CP, van Ewijk-Beneken Kolmer EW. Probenecid inhibits the glucuronidation of indomethacin and O-desmethylindomethacin in humans. A pilot experiment. Pharm World Sci. 1994;16:22-26. [PubMed] |
| 51. | Turner KC, Brouwer KL. In vitro mechanisms of probenecid-associated alterations in acetaminophen glucuronide hepatic disposition. Drug Metab Dispos. 1997;25:1017-1021. [PubMed] |
| 52. | Murthy GD, Byron D, Shoemaker D, Visweswaraiah H, Pasquale D. The utility of rifampin in diagnosing Gilbert’s syndrome. Am J Gastroenterol. 2001;96:1150-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 53. | Campbell SD, de Morais SM, Xu JJ. Inhibition of human organic anion transporting polypeptide OATP 1B1 as a mechanism of drug-induced hyperbilirubinemia. Chem Biol Interact. 2004;150:179-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 108] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 54. | Vítek L, Carey MC. Enterohepatic cycling of bilirubin as a cause of ‘black’ pigment gallstones in adult life. Eur J Clin Invest. 2003;33:799-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 67] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 55. | Meyer UA. [Heme biosynthesis]. Schweiz Med Wochenschr. 1975;105:1165-1168. [PubMed] |
| 56. | Brown SB, Houghton JD, Vernon DI. Biosynthesis of phycobilins. Formation of the chromophore of phytochrome, phycocyanin and phycoerythrin. J Photochem Photobiol B. 1990;5:3-23. [PubMed] |
| 57. | Romay C, González R. Phycocyanin is an antioxidant protector of human erythrocytes against lysis by peroxyl radicals. J Pharm Pharmacol. 2000;52:367-368. [PubMed] |
| 58. | Bhat VB, Madyastha KM. C-phycocyanin: a potent peroxyl radical scavenger in vivo and in vitro. Biochem Biophys Res Commun. 2000;275:20-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 256] [Article Influence: 10.2] [Reference Citation Analysis (0)] |