Revised: June 4, 2014
Accepted: June 5, 2014
Published online: June 9, 2014
Processing time: 150 Days and 18.7 Hours
Arsenic-contaminated drinking water is a public health problem in countries such as Taiwan, Bangladesh, United States, Mexico, Argentina, and Chile. The chronic ingestion of arsenic-contaminated drinking water increases the risk for ischemic heart disease, cerebrovascular disease, and prevalence of hypertension. Although toxic arsenic effects are controversial, there is evidence that a high concentration of arsenic may induce hypertension through increase in vascular tone and resistance. Vascular tone is regulated by the rhythmic contractions of the blood vessels, generated by calcium oscillations in the cytosol of vascular smooth muscle cells. To regulate the cytosolic calcium oscillations, the membrane oscillator model involves the participation of Ca2+ channels, calcium-activated K+ channels, Na+/Ca2+ exchange, plasma membrane Ca2+-ATPase, and the Na+/K+-ATPase. However, little is known about the role of K+ uptake by sodium transporters [Na+/K+-ATPase or Na+-K+-2Cl- (NKCC1)] on the rhythmic contractions. Vascular rhythmic contractions, or vasomotion are a local mechanism to regulate vascular resistance and blood flow. Since vascular rhythmic contractions of blood vessels are involved in modulating the vascular resistance, the blood flow, and the systemic pressure, we suggest a model explaining the participation of the sodium pump and NKCC1 co-transporter in low dose arsenic exposure effects on vasomotion and vascular dysfunction.
Core tip: Vascular tone is regulated in part by cytosolic calcium oscillations. Arsenic can induce an increase in vascular tone and resistance. We suggest a model explaining the participation of the sodium pump and Na+-K+-2Cl- co-transporter in low dose arsenic exposure effects on vasomotion and vascular dysfunction.
- Citation: Palacios J, Nwokocha CR, Cifuentes F. Arsenic exposure decreases rhythmic contractions of vascular tone through sodium transporters and K+ channels. World J Pharmacol 2014; 3(2): 18-23
- URL: https://www.wjgnet.com/2220-3192/full/v3/i2/18.htm
- DOI: https://dx.doi.org/10.5497/wjp.v3.i2.18
Arsenic toxicity is a global environmental health problem. The toxicity of this metalloid has been observed in various countries, including Taiwan[1], Bangladesh[2], Mexico[3], United States[4], Hungary[5], Argentina[6], and Chile[7]. Volcanic emission is one of the natural sources of arsenic, and individuals are majorly exposed through contaminated drinking water[8]. Smelting companies are also an important source of individual and population exposure to these kinds of heavy metals contamination. Contamination has been reported in Russia[9], United States[10], Mexico[11], Peru[12], and Chile[13]. There are few studies showing that Chinese workers in copper smelter, steel or iron have high levels of total arsenic in urine (50 g/g creatinine). These studies include those reported for Fushun city[14], Yunnan province[15], and Fuxin city[16].
There are epidemiologic studies that showed an association between chronic arsenic exposure and vascular diseases[17,18]. In fact, the ingestion of the arsenic-contaminated drinking water produced an increased risk for ischemic heart disease, cerebrovascular disease, and peripheral vascular resistance[19]. Other studies report positive associations between chronic arsenic exposure in drinking water, and the prevalence of hypertension[20-24].
Currently, arsenic effects on systemic blood pressure are controversial[25,26]. However, there is ample evidence that arsenic exposure mainly increases the vascular peripheral resistance[19,27], which defines the difficulty to blood flow through the blood vessels, particularly the small arteries.
Vascular rhythmic contractions, or vasomotion, are local mechanisms that regulate the vascular resistance and blood flow[28-30]. For instance, an increase in the amplitude of the rhythmic contractions cause an increased blood flow because the vascular resistance is reduced[31]. Since vascular rhythmic contractions of blood vessels are involved in modulating the vascular resistance, the blood flow, and the systemic pressure[28,29], the effects of chronic low dose exposures to arsenic on vascular rhythmic contractions becomes of great interest.
Vascular rhythmic contractions may be considered as a compensatory mechanism to preserve the perfusion of tissues[31], especially in patients with hypertension[32,33] or ischemia[34]. The mechanisms of the vascular rhythmic contractions may account for 3 states of contraction in blood vessels with different levels of calcium. These include small, medium, and tonic contraction, but only the medium concentrations produce rhythmic contractions[35]. The changes of vascular tone are generated by calcium oscillations in the cytosol of vascular smooth muscle cells[36]. To regulate the cytosolic calcium oscillations, the membrane oscillator model considers that activity of Ca2+ channels, calcium-activated K+ channels, Na+/Ca2+ exchange, plasma membrane Ca2+-ATPase, and the Na+/K+-ATPase, voltage-dependent calcium channel, and transient receptor potential channel are essential for maintaining calcium oscillations[37].
Little is known about the role of K+ uptake through Na+/K+-ATPase and Na+-K+-2Cl- (NKCC1) on the rhythmic contractions. Na+/K+-ATPase and NKCC1 cotransporter are responsible for the major K+ uptake in vascular smooth muscle cells[38-40]. Recent reports demonstrates that rhythmic contractions were associated with tonic and phasic responses, the tonic dependent on [Ca2+]i and the phasic on potassium efflux (through K+ channels) and potassium uptake[41,42].
Na+/K+-ATPase is responsible for the electrochemical gradient of sodium and potassium ions, it also plays a vital role in the regulations of ionic homeostasis in tissues and cells. In vascular smooth muscle cells, Na+/K+-ATPase plays a major role in the regulation of vascular tone[43,44], an increase in Na+/K+-ATPase activity leads to hyperpolarization and relaxation of smooth muscle[45], while its inhibition blunts rhythmic contractions in vascular smooth muscle cells[46].
It was postulated that the inhibition of KATP channels reduces extracellular K+ and Na+/K+-ATPase activity, increases intracellular calcium concentration via Na+/Ca2+ exchanger, uncouples vascular smooth muscle cells via gap junctions, and eliminates vascular rhythmic contractions[47,48]. Also, the inhibition of inward-rectifier K+ channels (Kir) decrease Na+/K+-ATPase activity in vascular smooth muscle cells[49]. It is important to remember that the Na+/K+-ATPase participates in relaxation of vascular smooth muscle cells through K+ channels. For instance, Na+/K+-ATPase is involved in K+-induced vasodilatation of hamster cremasteric arterioles[50], and vasodilation in the human forearm[51]. When K+ (1 to 15 mmol/L) accumulates in the extracellular space, Na+/K+-ATPase activity increases efflux of potassium through Kir. This leads to hyperpolarization and vasodilatation of the vascular smooth muscle cells[49,52]. In contrast, the opening of calcium-activated K+ channels inhibits the Na+/K+-ATPase function[53,54], and vascular rhythmic contractions[28].
NKCC1 is an obligatory symport system with an apparent stoichiometry of 1:1:2 sodium, potassium and chloride ratios respectively. Although the co-transporter is bidirectional in resting vascular smooth muscle cells, the sum of the electrochemical gradients for the three transported ion species determines net influx[55].
Evidence for the role of NKCC1 co-transporter on vascular rhythmic contractions is scanty, but it is worthy of note that the inward current of Cl- decreases rhythmic contractions by increasing vasoconstriction[47]. NKCC1 is responsible in part to keep intracellular Cl- concentration above the electrochemical equilibrium[56] as such helping to maintain the electrochemical gradient and cellular reactivity. Phenylephrine-induced stimulation of NKCC1 increases intracellular Cl- concentration, depolarize vascular smooth muscle cells[57], open L-type calcium channels[58] and produce vasoconstriction. In the vascular oscillator model[59], the release of intracellular Ca2+ from the reticulum stimulates the inward current of Cl-via the calcium-activated Cl- channel[60] and cyclic guanosine monophosphate (cGMP)-activated Ca2+-dependent Cl- channels[61]. This leads to membrane depolarization, opening L-type calcium channels and reduction in the oscillations of vascular tone. Therefore these findings suggest that the cotransporter NKCC1 would be responsible, in part, for vasoconstriction by chloride.
Vascular rhythmic contractions are dependent in part on endothelial nitric oxide (NO)[46], but there are few studies showing that the arsenic reduces vasomotion (vascular rhythmic contractions) by decreasing the NO bioavailability[62].
It is well established that heavy metals such as arsenic induce increases in vascular resistance by inducing vascular endothelial dysfunction (VED)[62,63]. VED consists of a reduction in endothelium-dependent vasorelaxation caused by a decrease in the release of endothelial NO[64]. Arsenic-induced VED is caused in part by oxidative stress.
Oxidative stress from pollutants like arsenic causes an increase in the reactive oxygen species, this leads to a modification of amino acids of proteins, mainly sulfur-containing amino acids methionine and cysteine[65]. Arsenic causes oxidative stress through peroxynitrite generation in aortic endothelial cells, producing loss of biological activity in enzymes and proteins[66,67]. In this context we had shown that chronic arsenic exposure in drinking water reduced acetylcholine-induced relaxation in female rat aorta[68], impairment of the endothelial nitric oxide synthase activity and decreasing of endothelial NO production[69,70].
NO is reported to activates Na+/K+-ATPase function[71], we observed that acetylcholine and sodium nitroprusside (SNP) induces activation of Na+/K+-ATPase activity, and SNP effect is abolished by inhibition of PKG (KT-5823)[72]. Cogolludo et al[73] (2001) showed that SNP activates Na+/K+-ATPase in mesenteric piglet’s arteries while Tamaoki et al[74] (1997) found that cGMP activates Na+/K+-ATPase in pulmonary artery smooth muscle cells.
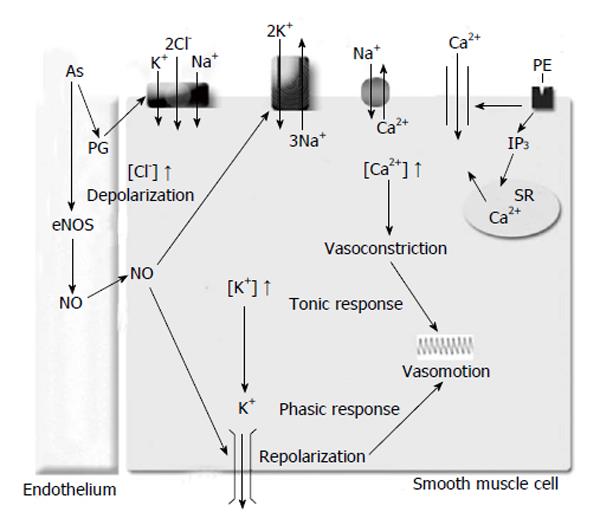
Since arsenic decreases the NO bioavailability[62], and the NO increases Na+/K+-ATPase function[71] which enhances the vascular rhythmic contractions, we may suggest that arsenic decreases the vascular rhythmic contractions by Na+/K+-ATPase function (Figure 1). Similar conclusions would be expected with the Kir channel, as Chen et al[75] (2010) demonstrated that arsenic trioxide produces down-regulation of Kir channel in cardiomyocytes of rats, and the Kir channel function increases Na+/K+-ATPase activity[49].
Although the endothelial NO does not affect NKCC1 co-transporter function[76], the endothelial prostaglandins increase NKCC1 activity thereby enhancing the contractile response to agonist in rat aorta[77-80]. Moreover, the endothelial prostaglandins increase agonist-induced rhythmic contractions in rat aorta[81], rat mesenteric artery[82], and arterioles of the cheek pouch of male hamsters[42]. Furthermore, arsenic increases the cyclooxygenase-2 (COX-2) protein in aortic endothelial cells[67], COX-2 in HUVEC[83], and enhances COX-1 and COX-2 activities in hind paw muscle of male rats[84]. Therefore, as a result of the prostaglandins effect on the vascular contractility through NKCC1 described above, arsenic might increase the vascular rhythmic contractions by NKCC1 co-transporter function.
The major toxic species of arsenic used in several studies are arsenite (trivalent inorganic arsenic, i.e., arsenic trioxide) or arsenate (pentavalent inorganic arsenic). Although the concentration of arsenate in drinking water is higher than those of arsenite, toxic effects of arsenate have not been properly documented. Arsenate is mainly metabolized by organisms as monomethylarsonic acid and dimethylarsinic acid, which significantly are not toxic[85]. However, this theory of the methylation of inorganic arsenic as a detoxification process has been revised[86] as other trivalent methylated species with higher toxicity have been reported[87]. Possibly, the biological effect of arsenate is mainly by reduction to arsenite[88].
P- Reviewer: Chen F, Sandow SL S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
| 1. | Guo HR, Chiang HS, Hu H, Lipsitz SR, Monson RR. Arsenic in drinking water and incidence of urinary cancers. Epidemiology. 1997;8:545-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 91] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 2. | Smith AH, Lingas EO, Rahman M. Contamination of drinking-water by arsenic in Bangladesh: a public health emergency. Bull World Health Organ. 2000;78:1093-1103. [PubMed] |
| 3. | Del Razo LM, Arellano MA, Cebrián ME. The oxidation states of arsenic in well-water from a chronic arsenicism area of northern Mexico. Environ Pollut. 1990;64:143-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 96] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | Lewis DR, Southwick JW, Ouellet-Hellstrom R, Rench J, Calderon RL. Drinking water arsenic in Utah: A cohort mortality study. Environ Health Perspect. 1999;107:359-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 216] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 5. | Börzsönyi M, Bereczky A, Rudnai P, Csanady M, Horvath A. Epidemiological studies on human subjects exposed to arsenic in drinking water in southeast Hungary. Arch Toxicol. 1992;66:77-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 68] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Hopenhayn-Rich C, Biggs ML, Fuchs A, Bergoglio R, Tello EE, Nicolli H, Smith AH. Bladder cancer mortality associated with arsenic in drinking water in Argentina. Epidemiology. 1996;7:117-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 212] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 7. | Borgoño JM, Vicent P, Venturino H, Infante A. Arsenic in the drinking water of the city of Antofagasta: epidemiological and clinical study before and after the installation of a treatment plant. Environ Health Perspect. 1977;19:103-105. [PubMed] [DOI] [Full Text] |
| 8. | Nordstrom DK. Public health. Worldwide occurrences of arsenic in ground water. Science. 2002;296:2143-2145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1176] [Cited by in RCA: 832] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 9. | Bustueva KA, Revich BA, Bezpalko LE. Cadmium in the environment of three Russian cities and in human hair and urine. Arch Environ Health. 1994;49:284-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Hwang YH, Bornschein RL, Grote J, Menrath W, Roda S. Environmental arsenic exposure of children around a former copper smelter site. Environ Res. 1997;72:72-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 49] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Díaz-Barriga F, Santos MA, Mejía JJ, Batres L, Yáñez L, Carrizales L, Vera E, del Razo LM, Cebrián ME. Arsenic and cadmium exposure in children living near a smelter complex in San Luis Potosí, Mexico. Environ Res. 1993;62:242-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 65] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Ramírez AV. [Environmental pollution by cadmium in a metallurgy plant]. Bol Oficina Sanit Panam. 1986;101:514-521. [PubMed] |
| 13. | Rivara MI, Cebrián M, Corey G, Hernández M, Romieu I. Cancer risk in an arsenic-contaminated area of Chile. Toxicol Ind Health. 1997;13:321-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Xi S, Zheng Q, Zhang Q, Sun G. Metabolic profile and assessment of occupational arsenic exposure in copper- and steel-smelting workers in China. Int Arch Occup Environ Health. 2011;84:347-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Wen J, Wen W, Li L, Liu H. Methylation capacity of arsenic and skin lesions in smelter plant workers. Environ Toxicol Pharmacol. 2012;34:624-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Xu Y, Wang Y, Zheng Q, Li B, Li X, Jin Y, Lv X, Qu G, Sun G. Clinical manifestations and arsenic methylation after a rare subacute arsenic poisoning accident. Toxicol Sci. 2008;103:278-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Chiou HY, Huang WI, Su CL, Chang SF, Hsu YH, Chen CJ. Dose-response relationship between prevalence of cerebrovascular disease and ingested inorganic arsenic. Stroke. 1997;28:1717-1723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 178] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 18. | Wang CH, Hsiao CK, Chen CL, Hsu LI, Chiou HY, Chen SY, Hsueh YM, Wu MM, Chen CJ. A review of the epidemiologic literature on the role of environmental arsenic exposure and cardiovascular diseases. Toxicol Appl Pharmacol. 2007;222:315-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 159] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 19. | Chen WY, Yen TS. Experimental studies on the drinking water of blackfoot endemic area. 2. studies on the effects of drinking water of blackfoot endemic area on peripheral vascular perfusion of the hind limbs of frogs. Tsa Chih Gaoxiong Yi Xue Yuan Tong Xue Hui. 1964;63:150-158. [PubMed] |
| 20. | Borgoño JM, Greiber R. [Epidemiologic study of arsenic poisoning in the city of Antofagasta]. Rev Med Chil. 1971;99:702-707. [PubMed] |
| 21. | Rosenberg HG. Systemic arterial disease and chronic arsenicism in infants. Arch Pathol. 1974;97:360-365. [PubMed] |
| 22. | Zaldívar R. Arsenic contamination of drinking water and foodstuffs causing endemic chronic poisoning. Beitr Pathol. 1974;151:384-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 65] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Zierold KM, Knobeloch L, Anderson H. Prevalence of chronic diseases in adults exposed to arsenic-contaminated drinking water. Am J Public Health. 2004;94:1936-1937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 138] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 24. | Chen Y, Factor-Litvak P, Howe GR, Graziano JH, Brandt-Rauf P, Parvez F, van Geen A, Ahsan H. Arsenic exposure from drinking water, dietary intakes of B vitamins and folate, and risk of high blood pressure in Bangladesh: a population-based, cross-sectional study. Am J Epidemiol. 2007;165:541-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 94] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 25. | Abir T, Rahman B, D’Este C, Farooq A, Milton AH. The Association between Chronic Arsenic Exposure and Hypertension: A Meta-Analysis. J Toxicol. 2012;2012:198793. [PubMed] |
| 26. | Islam MR, Khan I, Attia J, Hassan SM, McEvoy M, D’Este C, Azim S, Akhter A, Akter S, Shahidullah SM. Association between hypertension and chronic arsenic exposure in drinking water: a cross-sectional study in Bangladesh. Int J Environ Res Public Health. 2012;9:4522-4536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 27. | Lee MY, Lee YH, Lim KM, Chung SM, Bae ON, Kim H, Lee CR, Park JD, Chung JH. Inorganic arsenite potentiates vasoconstriction through calcium sensitization in vascular smooth muscle. Environ Health Perspect. 2005;113:1330-1335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Nilsson H, Aalkjaer C. Vasomotion: mechanisms and physiological importance. Mol Interv. 2003;3:79-89, 51. [PubMed] |
| 29. | Funk W, Endrich B, Messmer K, Intaglietta M. Spontaneous arteriolar vasomotion as a determinant of peripheral vascular resistance. Int J Microcirc Clin Exp. 1983;2:11-25. [PubMed] |
| 30. | Gratton RJ, Gandley RE, McCarthy JF, Michaluk WK, Slinker BK, McLaughlin MK. Contribution of vasomotion to vascular resistance: a comparison of arteries from virgin and pregnant rats. J Appl Physiol (1985). 1998;85:2255-2260. [PubMed] |
| 31. | Pradhan RK, Chakravarthy VS. Informational dynamics of vasomotion in microvascular networks: a review. Acta Physiol (Oxf). 2011;201:193-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 32. | Hollenberg NK, Sandor T. Vasomotion of renal blood flow in essential hypertension. Oscillations in xenon transit. Hypertension. 1984;6:579-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | Frielingsdorf J, Kaufmann P, Seiler C, Vassalli G, Suter T, Hess OM. Abnormal coronary vasomotion in hypertension: role of coronary artery disease. J Am Coll Cardiol. 1996;28:935-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 34. | Intaglietta M. Arteriolar vasomotion: implications for tissue ischemia. Blood Vessels. 1991;28 Suppl 1:1-7. [PubMed] |
| 35. | Koenigsberger M, Sauser R, Bény JL, Meister JJ. Role of the endothelium on arterial vasomotion. Biophys J. 2005;88:3845-3854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 36. | Peng H, Matchkov V, Ivarsen A, Aalkjaer C, Nilsson H. Hypothesis for the initiation of vasomotion. Circ Res. 2001;88:810-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 191] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 37. | Parthimos D, Edwards DH, Griffith TM. Minimal model of arterial chaos generated by coupled intracellular and membrane Ca2+ oscillators. Am J Physiol. 1999;277:H1119-H1144. [PubMed] |
| 38. | Garrahan PJ, Glynn IM. The sensitivity of the sodium pump to external sodium. J Physiol. 1967;192:175-188. [PubMed] |
| 39. | Sachs JR. Ouabain-insensitive sodium movements in the human red blood cell. J Gen Physiol. 1971;57:259-282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 102] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 40. | Russell JM. Sodium-potassium-chloride cotransport. Physiol Rev. 2000;80:211-276. [PubMed] |
| 41. | Palacios J, Vega JL, Paredes A, Cifuentes F. Effect of phenylephrine and endothelium on vasomotion in rat aorta involves potassium uptake. J Physiol Sci. 2013;63:103-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 42. | de Souza Md, Bouskela E. Arteriolar diameter and spontaneous vasomotion: importance of potassium channels and nitric oxide. Microvasc Res. 2013;90:121-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 43. | Blaustein MP. Sodium ions, calcium ions, blood pressure regulation, and hypertension: a reassessment and a hypothesis. Am J Physiol. 1977;232:C165-C173. [PubMed] |
| 44. | Clausen T, Nielsen OB. The Na+,K(+)-pump and muscle contractility. Acta Physiol Scand. 1994;152:365-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 45. | Rapoport RM, Schwartz K, Murad F. Effects of Na+,K+-pump inhibitors and membrane depolarizing agents on acetylcholine-induced endothelium-dependent relaxation and cyclic GMP accumulation in rat aorta. Eur J Pharmacol. 1985;110:203-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 63] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 46. | Gustafsson H, Nilsson H. Rhythmic contractions in isolated small arteries of rat: role of K+ channels and the Na+,K(+)-pump. Acta Physiol Scand. 1994;150:161-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 47. | Matchkov VV, Gustafsson H, Rahman A, Briggs Boedtkjer DM, Gorintin S, Hansen AK, Bouzinova EV, Praetorius HA, Aalkjaer C, Nilsson H. Interaction between Na+/K+-pump and Na+/Ca2+-exchanger modulates intercellular communication. Circ Res. 2007;100:1026-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 48. | Glavind-Kristensen M, Matchkov V, Hansen VB, Forman A, Nilsson H, Aalkjaer C. KATP-channel-induced vasodilation is modulated by the Na,K-pump activity in rabbit coronary small arteries. Br J Pharmacol. 2004;143:872-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 49. | Haddy FJ, Vanhoutte PM, Feletou M. Role of potassium in regulating blood flow and blood pressure. Am J Physiol Regul Integr Comp Physiol. 2006;290:R546-R552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 204] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 50. | Burns WR, Cohen KD, Jackson WF. K+-induced dilation of hamster cremasteric arterioles involves both the Na+/K+-ATPase and inward-rectifier K+ channels. Microcirculation. 2004;11:279-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 83] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 51. | Dawes M, Sieniawska C, Delves T, Dwivedi R, Chowienczyk PJ, Ritter JM. Barium reduces resting blood flow and inhibits potassium-induced vasodilation in the human forearm. Circulation. 2002;105:1323-1328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 52. | Ulusoy HB, Kaya MG. Potassium induced dilation in bovine coronary artery involves both inward rectifier potassium channels and Na+ /K+ ATPase. Acta Physiol Hung. 2009;96:427-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 53. | Dora KA, Ings NT, Garland CJ. K(Ca) channel blockers reveal hyperpolarization and relaxation to K+ in rat isolated mesenteric artery. Am J Physiol Heart Circ Physiol. 2002;283:H606-H614. [PubMed] |
| 54. | Weston AH, Richards GR, Burnham MP, Félétou M, Vanhoutte PM, Edwards G. K+-induced hyperpolarization in rat mesenteric artery: identification, localization and role of Na+/K+-ATPases. Br J Pharmacol. 2002;136:918-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 66] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 55. | O’Donnell ME, Owen NE. Regulation of ion pumps and carriers in vascular smooth muscle. Physiol Rev. 1994;74:683-721. [PubMed] |
| 56. | Chipperfield AR, Harper AA. Chloride in smooth muscle. Prog Biophys Mol Biol. 2000;74:175-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 133] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 57. | Davis JP, Chipperfield AR, Harper AA. Accumulation of intracellular chloride by (Na-K-Cl) co-transport in rat arterial smooth muscle is enhanced in deoxycorticosterone acetate (DOCA)/salt hypertension. J Mol Cell Cardiol. 1993;25:233-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 58. | Anfinogenova YJ, Baskakov MB, Kovalev IV, Kilin AA, Dulin NO, Orlov SN. Cell-volume-dependent vascular smooth muscle contraction: role of Na+, K+, 2Cl- cotransport, intracellular Cl- and L-type Ca2+ channels. Pflugers Arch. 2004;449:42-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 59. | Berridge MJ. Smooth muscle cell calcium activation mechanisms. J Physiol. 2008;586:5047-5061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 282] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 60. | Haddock RE, Hill CE. Differential activation of ion channels by inositol 1,4,5-trisphosphate (IP3)- and ryanodine-sensitive calcium stores in rat basilar artery vasomotion. J Physiol. 2002;545:615-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 61. | Piper AS, Large WA. Single cGMP-activated Ca(+)-dependent Cl(-) channels in rat mesenteric artery smooth muscle cells. J Physiol. 2004;555:397-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 62. | Lee MY, Jung BI, Chung SM, Bae ON, Lee JY, Park JD, Yang JS, Lee H, Chung JH. Arsenic-induced dysfunction in relaxation of blood vessels. Environ Health Perspect. 2003;111:513-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 91] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 63. | Jindal S, Singh M, Balakumar P. Effect of bis (maltolato) oxovanadium (BMOV) in uric acid and sodium arsenite-induced vascular endothelial dysfunction in rats. Int J Cardiol. 2008;128:383-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 64. | Tsou TC, Tsai FY, Hsieh YW, Li LA, Yeh SC, Chang LW. Arsenite induces endothelial cytotoxicity by down-regulation of vascular endothelial nitric oxide synthase. Toxicol Appl Pharmacol. 2005;208:277-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 65. | Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996;271:C1424-C1437. [PubMed] |
| 66. | Del Razo LM, Quintanilla-Vega B, Brambila-Colombres E, Calderón-Aranda ES, Manno M, Albores A. Stress proteins induced by arsenic. Toxicol Appl Pharmacol. 2001;177:132-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 188] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 67. | Bunderson M, Coffin JD, Beall HD. Arsenic induces peroxynitrite generation and cyclooxygenase-2 protein expression in aortic endothelial cells: possible role in atherosclerosis. Toxicol Appl Pharmacol. 2002;184:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 63] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 68. | Cifuentes F, Bravo J, Norambuena M, Stegen S, Ayavire A, Palacios J. Chronic exposure to arsenic in tap water reduces acetylcholine-induced relaxation in the aorta and increases oxidative stress in female rats. Int J Toxicol. 2009;28:534-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 69. | Pi J, Horiguchi S, Sun Y, Nikaido M, Shimojo N, Hayashi T, Yamauchi H, Itoh K, Yamamoto M, Sun G. A potential mechanism for the impairment of nitric oxide formation caused by prolonged oral exposure to arsenate in rabbits. Free Radic Biol Med. 2003;35:102-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 82] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 70. | Kumagai Y, Pi J. Molecular basis for arsenic-induced alteration in nitric oxide production and oxidative stress: implication of endothelial dysfunction. Toxicol Appl Pharmacol. 2004;198:450-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 78] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 71. | Pavlovic D, Hall AR, Kennington EJ, Aughton K, Boguslavskyi A, Fuller W, Despa S, Bers DM, Shattock MJ. Nitric oxide regulates cardiac intracellular Na+ and Ca²+ by modulating Na/K ATPase via PKCΕ and phospholemman-dependent mechanism. J Mol Cell Cardiol. 2013;61:164-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 72. | Palacios J, Marusic ET, Lopez NC, Gonzalez M, Michea L. Estradiol-induced expression of N(+)-K(+)-ATPase catalytic isoforms in rat arteries: gender differences in activity mediated by nitric oxide donors. Am J Physiol Heart Circ Physiol. 2004;286:H1793-H1800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 73. | Cogolludo AL, Pérez-Vizcaíno F, Zaragozá-Arnáez F, Ibarra M, López-López G, López-Miranda V, Tamargo J. Mechanisms involved in SNP-induced relaxation and [Ca+]i reduction in piglet pulmonary and systemic arteries. Br J Pharmacol. 2001;132:959-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 74. | Tamaoki J, Tagaya E, Nishimura K, Isono K, Nagai A. Role of Na(+)-K+ ATPase in cyclic GMP-mediated relaxation of canine pulmonary artery smooth muscle cells. Br J Pharmacol. 1997;122:112-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 75. | Chen X, Shan H, Zhao J, Hong Y, Bai Y, Sun I, Pan Z, Zhang Y, Yang B, Du Z. L-type calcium current (ICa,L) and inward rectifier potassium current (IK1) are involved in QT prolongation induced by arsenic trioxide in rat. Cell Physiol Biochem. 2010;26:967-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 76. | Koltsova SV, Kotelevtsev SV, Tremblay J, Hamet P, Orlov SN. Excitation-contraction coupling in resistance mesenteric arteries: evidence for NKCC1-mediated pathway. Biochem Biophys Res Commun. 2009;379:1080-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 77. | Palacios J, Espinoza F, Munita C, Cifuentes F, Michea L. Na+-K+-2Cl- cotransporter is implicated in gender differences in the response of the rat aorta to phenylephrine. Br J Pharmacol. 2006;148:964-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 78. | Oppermann M, Hansen PB, Castrop H, Schnermann J. Vasodilatation of afferent arterioles and paradoxical increase of renal vascular resistance by furosemide in mice. Am J Physiol Renal Physiol. 2007;293:F279-F287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 79. | Mtabaji JP, Manku MS, Horrobin DF. Vascular actions of furosemide and bumetanide on the rat superior mesenteric vascular bed: interactions with prolactin and prostaglandins. Can J Physiol Pharmacol. 1976;54:357-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 80. | Pickkers P, Dormans TP, Russel FG, Hughes AD, Thien T, Schaper N, Smits P. Direct vascular effects of furosemide in humans. Circulation. 1997;96:1847-1852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 74] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 81. | Mauban JR, Wier WG. Essential role of EDHF in the initiation and maintenance of adrenergic vasomotion in rat mesenteric arteries. Am J Physiol Heart Circ Physiol. 2004;287:H608-H616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 82. | Okazaki K, Seki S, Kanaya N, Hattori J, Tohse N, Namiki A. Role of endothelium-derived hyperpolarizing factor in phenylephrine-induced oscillatory vasomotion in rat small mesenteric artery. Anesthesiology. 2003;98:1164-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 83. | Tsai SH, Liang YC, Chen L, Ho FM, Hsieh MS, Lin JK. Arsenite stimulates cyclooxygenase-2 expression through activating IkappaB kinase and nuclear factor kappaB in primary and ECV304 endothelial cells. J Cell Biochem. 2002;84:750-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 84. | Ahmad W, Prawez S, Chanderashekara HH, Tandan SK, Sankar P, Sarkar SN. Subacute arsenic exposure through drinking water reduces the pharmacodynamic effects of ketoprofen in male rats. Environ Toxicol Pharmacol. 2012;33:267-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 85. | Vahter M, Concha G. Role of metabolism in arsenic toxicity. Pharmacol Toxicol. 2001;89:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 173] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 86. | Kitchin KT. Recent advances in arsenic carcinogenesis: modes of action, animal model systems, and methylated arsenic metabolites. Toxicol Appl Pharmacol. 2001;172:249-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 548] [Cited by in RCA: 492] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 87. | Dopp E, von Recklinghausen U, Diaz-Bone R, Hirner AV, Rettenmeier AW. Cellular uptake, subcellular distribution and toxicity of arsenic compounds in methylating and non-methylating cells. Environ Res. 2010;110:435-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 88. | Huang RN, Lee TC. Cellular uptake of trivalent arsenite and pentavalent arsenate in KB cells cultured in phosphate-free medium. Toxicol Appl Pharmacol. 1996;136:243-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 55] [Article Influence: 1.9] [Reference Citation Analysis (0)] |