Published online May 25, 2015. doi: 10.5495/wjcid.v5.i2.44
Peer-review started: September 18, 2014
First decision: December 17, 2014
Revised: January 29, 2015
Accepted: April 27, 2015
Article in press: April 29, 2015
Published online: May 25, 2015
Processing time: 244 Days and 2.4 Hours
AIM: To corroborate the capacity of Phyto V7, a complex of phytochemicals, to improve the physical well-being of human immunodeficiency virus-1 (HIV-1) infected and acquired immune deficiency syndrome (AIDS) patients not undergoing antiretroviral treatment.
METHODS: Two hundred and thirty nine HIV-1 seropositive male and female voluntary inmates were recruited through the Uruguay National Program of AIDS. The study participants received for 90 consecutive days every eight hours two tablets (760 mg/each) of Phyto V7, containing a mix of the following phytochemicals: flavonols (Kaempferol, Quercetin), flavones (Apigenin, Luteolin), hydroxy-cinnamic acids (ferrulic acid), carotenoids (Lutein, Lycopene, Beta carotene) and organosulfur compounds, all from vegetal origin. The participants did not receive any antiretroviral treatment during the study. At days 0, 30, 60 and 90 (± 2 d) the participants were evaluated for body mass index (BMI), tolerance to Phyto V7 and Index of Quality of Life based on the Karfnosky scale. ANOVA, Tukey Post-test, χ2 test and Wilcoxon Signed Rank test were used to analyze the effect of treatment.
RESULTS: One hundred and nighty nine study participants finished the study. Already after 30 d of Phyto V7 consumption, the weight, BMI and Karnofsky score statistically significantly improved (P < 0.001), and continued to improve until the end of the study. The mean weight gain per participant during the 90 d was of 1.21 kg (approximately 2% of body weight). The overall increase in the mean Karnofsky score after 90 d was 14.08%. The lower the BMI and Karnofsky score of the participants were at the beginning of the study, the more notorious was the improvement over time. For example, the mean increment of Index of Quality of Life, among the participants with an initial Karnofsky score of 5 or below (n = 33) from day 0 to day 90, was of 35.67% (0.476 ± 0.044 vs 0.645 ± 0.09; P < 0.001). The tolerability to Phyto V7 was very good and no adverse reactions were recorded or reported.
CONCLUSION: Administration of the Phyto V7 can be an important tool to improve the well-being of HIV-1 seropositive individuals and AIDS patients, not undergoing antiretroviral treatment.
Core tip: Phyto V7 is a complex of phytochemicals and micronutrients. Phyto V7 has been found to stimulate the immune system and dramatically improve the physical well-being of terminal acquired immune deficiency syndrome (AIDS) patients. The current study demonstrates the capacity of Phyto V7 to improve the physical well-being of human immunodeficiency virus-1 (HIV-1) infected and AIDS patients not undergoing antiretroviral treatment, as demonstrated in 199 individuals. We conclude that administration of the food supplement Phyto V7 can be an important tool to improve the well-being of HIV-1 seropositive individuals and AIDS patients, not undergoing antiretroviral treatment.
- Citation: Wernik R, Priore JL, Goldman WF, Elias ADC, Borkow G. Improvement in human immunodeficiency virus-1/acquired immune deficiency syndrome patients’ well-being following administration of “Phyto V7”. World J Clin Infect Dis 2015; 5(2): 44-50
- URL: https://www.wjgnet.com/2220-3176/full/v5/i2/44.htm
- DOI: https://dx.doi.org/10.5495/wjcid.v5.i2.44
The energy needed for physical activity and for maintaining the body weight is higher in human immunodeficiency virus-1 (HIV-1) infected individuals than in non-HIV infected individuals[1,2]. Acquired immune deficiency syndrome (AIDS) patients spend approximately 20% to 30% more energy than healthy individuals in order to maintain their body weight, including when receiving highly active antiretroviral treatment (HAART)[3,4]. The World Health Organization has recommended including micronutrient supplementation as an integral part of all HIV treatment programs[5]. Micronutrient supplementation trials demonstrated a reduced mortality and improved clinical outcomes in HIV-1 infected individuals, regardless of their clinical stage and use of antiretrovirals[6-9].
Phytochemicals, chemical compounds that occur naturally in plants, in addition of serving as micronutrients, enhance nonspecific immunity[10], down regulate inflammatory diseases[11], possess radical scavenging activities[12], and inhibit disease progression[13-19]. For example, administration of phytochemicals reduced hepatotoxic, lithic, and hepatitis related adverse symptoms[19]. Some phytochemicals inhibit HIV-1 protease and integrase, and inhibit viral entry to target cells[12,20-24]. Phyto V7 is a complex of phytochemicals, which also contains micronutrients, registered as a nutritional supplement in several countries. Administration of Phyto V7 to chicks enhances their humoral immune responses against Newcastle Disease Virus following vaccination[25]. Furthermore, its administration to human papilloma virus (HPV) affected women undergoing electrosurgical excision of cervical lesions resulted in approximately two-fold higher elimination of HPV than in the control group of women. In the group of woman receiving Phyto V7 there was an increase in the local cellular immune responses, as exemplified by much higher elevated presence of NK cells and cytotoxic T-cells (CD8+) in the cervical smears 90 d after the electrosurgical excisional procedure[26]. We have also found an increase in CD4+ T-cells in HIV-1 infected individuals taking Phyto V7, without affecting their viral loads titers (manuscript in press). Taken together, the above findings indicate that Phyto V7 has immune-stimulatory properties. Remarkably, administration of Phyto V7 to 9 terminally ill AIDS patients resulted in a dramatic improvement in their physical status[27].
Antiretroviral treatment, which can effectively control viremia, requires high patient adherence for life. Low patient adherence results in the appearance of drug resistant viral isolates and necessitates different treatment protocols and salvage therapy options. Unfortunately, in many developing countries HIV-1 infected individuals are not treated at all. Many reasons account for that, such as inappropriate or non-existent centralized government treatment programs and elevated costs of antiretroviral treatments. One of the treatment neglected populations, in many developing countries, is prison inmates. The rates of HIV-1 infection are very high in this population[28,29]. Prison inmates are at higher risk of HIV-1 infection due to increased intravenous drug use, unprotected sexual activity, exposure to blood during fights, and tattooing.
In the current manuscript we report the very significant improvement in the well-being of 199 HIV-1 infected prison inmates, who did not receive any antiretroviral treatment while in prison, receiving only a daily administration of Phyto V7.
The methodological design of the study was analytical and longitudinal, conducted by mid-2010 in Uruguay through the patronage of Dr. Tabaré Vasquez, President of Uruguay, by the General Direction of Prisons and the Uruguay Association of Seropositives. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was reviewed and approved by the Ethical Medical Committee of the Ministry of Health of Uruguay. HIV-1 seropositive male and female inmates were recruited from the Libertad, La Tablada and Cabildo prisons. All study participants gave their informed consent prior to the commencement of the study.
Phyto V7 was donated by the Israel Project Life Foundation and Immune Nutrition Incorporated. Phyto V7 was registered as a food supplement (Registration Number 54221) at the Division of Health Products, Department of Food. Each Phyto V7 tablet contained 760 mg of the following phytochemicals: flavonols (Kaempferol, Quercetin), flavones (Apigenin, Luteolin), hydroxy-cinnamic acids (ferrulic acid), carotenoids (Lutein, Lycopene, Beta carotene) and organosulfur compounds, all from vegetal origin.
During the study, each participant was given every 8 h two Phyto V7 tablets. At days 0, 30, 60 and 90 (± 2 d) the participants were evaluated for body mass index (BMI), tolerance to Phyto V7 and well-being. The well-being was estimated according to the modified Karfnosky scale (Table 1). Each time the doctor in charge filled the questionnaire while examining and consulting each study participant, without seeing the previous already filled questionnaires. No data regarding the viral load or immune profile of the participants could be gathered. The participants did not receive any antiretroviral treatment during the study.
| 10 | No complaints, no signs of disease |
| 9 | Capable of normal activity, few symptoms or signs of disease |
| 8 | Normal activity with some difficulty, some symptoms or signs |
| 7 | Caring for self, not capable of normal activity or work |
| 6 | Requiring some help, can take care of most personal requirements |
| 5 | Requires help often, requires frequent medical care |
| 4 | Disabled, requires special care and help |
| 3 | Severely disabled, hospital admission indicated but no risk of death |
| 2 | Very ill, urgently requiring hospital admission, requires supportive measures or treatment |
| 1 | Moribund, rapidly progressive fatal disease processes |
The differences between weight and BMI were analyzed with Kruskal-Wallis One Way Analysis of Variance on Ranks (ANOVA) and Tukey Post test. The proportions of levels of quality of life were analyzed with the χ2 test. An Index of Quality of Life was defined by dividing the levels of the Karnofsky score by the maximal level (10) and applied the Wilcoxon Signed Rank Test to analyze the differences. The SigmaPlot 12 software was used to conduct the statistical analyses.
A total of 239 HIV-1 seropositive inmates were recruited. Forty participants did not finish the study due to various reasons, such as being transferred to other facilities and being released from prison. Thus, the data presented is of 199 participants.
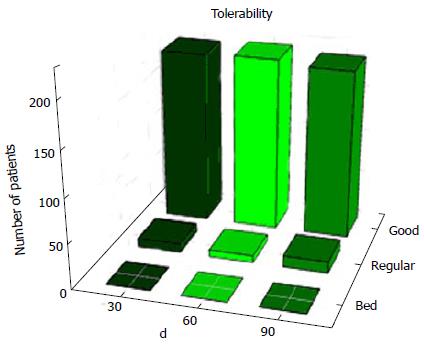
As reported by the study participants, after taking Phyto V7 for 30, 60 and 90 d, the tolerability to Phyto V7 was very good (Figure 1). No adverse reactions were recorded or reported.
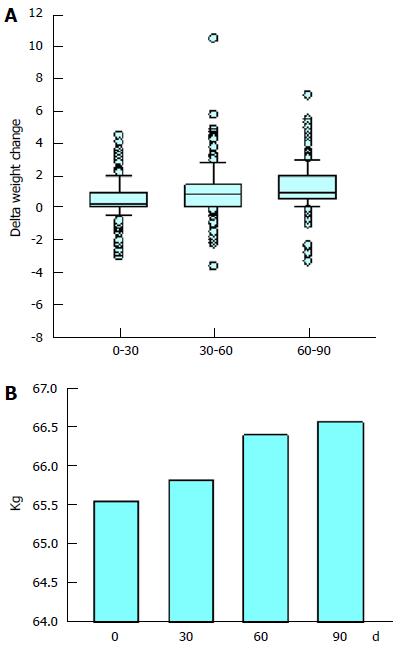
As can be seen in Table 2, the proportion of individuals that participated in the study in whom there was an increase in their weight was 53.8%, 72.4% and 81.9% after 30, 60 and 90 d, respectively. The increase in weight was statistically significant (P < 0.001). After 90 ds there was a decrease in weight in only 6% of the patients. The increase in the mean weight of the study participants can be appreciated in Figure 2B. The mean weight gain per participant during the 90 d was of 1.21 kg (approximately 2% of body weight).
| Weight | 30 d | 60 d | 90 d | |||
| n | % | n | % | n | % | |
| Decrease | 27 | 13.6 | 19 | 9.5 | 12 | 6 |
| Equal | 65 | 32.7 | 36 | 18.1 | 24 | 12.1 |
| Increase | 107 | 53.8 | 144 | 72.4 | 163 | 81.9 |
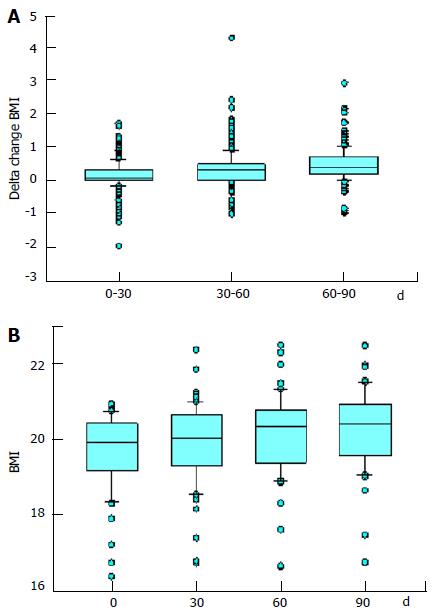
In accordance with the increase in the weight, also the BMI of the participants increased over time (Figure 3A). The mean of BMI increased from 23.18 on day 0 to 23.64 on day 90, a 1.98% increase. When analyzing the mean increase in the BMI of the group of participants that had a BMI of below 21 at the beginning of the study (n = 60), the increase in BMI is even more impressive (Figure 3B) - the mean in BMI among this group increased from 19.69 on day 0 to 20.24 on day 90, a 2.75% increase. Similarly, when looking into the 11 participants that had a BMI of below 19 at the beginning of the study (n = 11), the mean BMI increased from 18.02 on day 0 to 18.62 on day 90, a 3.04% increase.
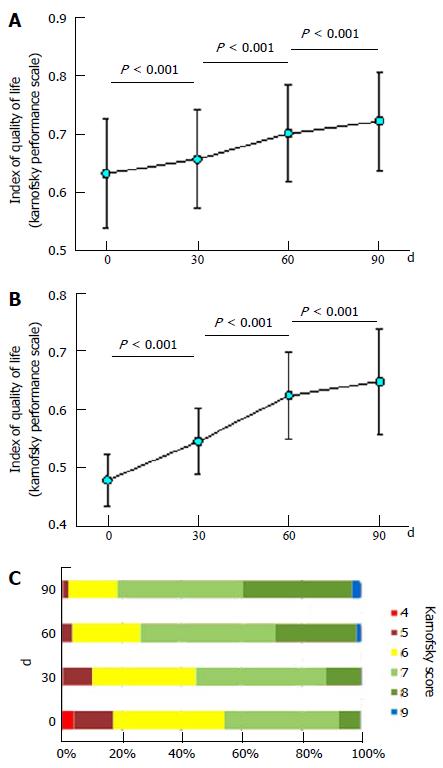
The overall quality of life of the participants increased over time, as determined by the Karnofsky scale determinations, and as exemplified for the Index of Quality of Life in Figure 4A. The Index of Quality of Life was already statistically significantly higher at day 30 compared to day 0 (mean of 0.657 vs 0.632; P < 0.001). The Index of Quality of Life continued to increase with Phyto V7 consumption, from a mean of 0.657 to 0.7 and 0.721 at days 30, 60 and 90, respectively (P < 0.001 between each data point). The overall increase in the mean Karnofsky score after 90 d was 14.08%.
When analyzing the changes in the Index of Quality of Life among the participants that at day 0 had a Karnofsky score of 5 or below (n = 33), the changes in the score from day 0 to day 90 are even more impressive, i.e., 35.67%, from 0.476 ± 0.044 to 0.645 ± 0.09 (P < 0.001; Figure 4B). The clear increase in the proportions of the Karnofsky score over time is depicted in Figure 4C. For example, the level score 8 increased from day 0 to day 90 by approximately 5 fold, from 7.5% to 36.7% for all study participants. In contrast, the level score 5 decreased from day 0 to day 90 from 13.1% to 1.5%.
At day 90, approximately 73% of the study participant’s felt that consumption of Phyto V7 was beneficial to them, while approximately 25% felt the same. This is in accordance with an increase in weight in 81.9% of the study participants. Two percent of the patients felt that their situation worsened during the 90 d study.
Since the institution of HAART, the number of individuals becoming ill with AIDS has declined significantly and the prognosis of AIDS patients has improved notably. However, low compliance, viral cross-resistance, and significant side effects caused by HAART, serve as reason to postpone HAART. In developing countries, wide implementation of HAART may be even more problematic due to high costs, infrastructure problems and high prevalence of other ailments such as anemia and co-infections[30,31]. Thus, new, non-expensive, safe, easy to take alternative or complementary remedies, that can improve the patient’s well-being, are very attractive for the treatment of individuals that fail HAART or antiretroviral naïve patients that can not get antiretroviral therapy.
Recently we published the results of a study that was conducted with 9 terminally ill AIDS patients living in a hospice[27]. All patients had very high HIV-1 viral loads and 8 out of the 9 patients were scored as C3 according to the United States Centers for Disease Control status index. Seven out of the 9 patients were antiretroviral naïve patients. During the study they did not receive antiretroviral treatment but only received the food supplement Phyto V7. While most of the patients at the commencement of the study could not eat, stand, dress or shower by themselves, after 3 mo of Phyto V7 supplementation all patients could eat, sit down, shower, stand up and dress without help. The well-being of the patients improved dramatically, both physically and mentally. The success of this trial was the incentive to conduct the current study.
As with the terminally ill AIDS patients, the administration of Phyto V7 to HIV-1 infected, asymptomatic and symptomatic individuals in the current study, resulted in a very significant improvement in the individuals’ well-being. The weight, BMI and Karnofsky score of the study participants increased notably, especially in those who had a low BMI and low Karnofsky score at the onset of the study. Increase in appetite, weight, and individuals mood, has a positive outcome in the individual well-being. Notably 83% of the participants adhered until the end of the trial and took Phyto V7, indicating the high likelihood that they will continue using Phyto V7 also finalizing the study. Part of the positive effect of Phyto V7 can also be explained as phytochemicals having radical scavenging activities[12], stimulating nonspecific immunity[10], and down regulating inflammatory responses[11]. Indeed, Phyto V7 has been shown to enhance humoral and cellular immune responses[25,26]. It is not clear from this study if crucial parameters relevant to the progression to AIDS were affected, such as the CD4+ T-cell counts and viremia. However, in a another study (manuscript in press) the administration of Phyto V7 resulted in the upregulation of CD4+ T-cell counts without affecting viral loads, indicating that Phyto V7 has an immuno-stimulating effect and no direct antiviral effect.
Administration of a food supplement, such as the Phyto V7, is extremely inexpensive as compared to HAART. Phyto V7 is from a natural source and as opposed to antiretrovirals, does not affect directly HIV-1. Thus its uptake with low adherence would not result in appearance of drug resistant viruses. Obviously, in order to increase its efficacy, high compliance is desired. Administration of Phyto V7 may potentially postpone the need to treat HIV-1 infected individuals with HAART, postponing the potential complications associated with this treatment. It may well be that Phyto V7 can be given in conjunction with HAART resulting in better prognosis. These assumptions need to be examined in placebo controlled studies.
We would like to thank Dr. Tabaré Vazquez, President of Uruguay during the study, who approved the Phyto V7 donation and encouraged all involved to conduct the study. We also thank Laboratorios Haymann, especially Prof. Nelson Lago, Technical Director of Laboratorios Haymann, who registered Phyto V7 under the name of GT+ in Uruguay. The study was funded by the Uruguay National Program of AIDS. This study is dedicated to Dr. Simon Raul Goldman who recently passed away. Dr. Goldman was the CEO of Immune Nutrition Incorporated and the driving force behind the development, testing and introduction of Phyto V7.
The immune system of human immunodeficiency virus-1 (HIV-1) infected individuals decays with the progression of time until they develop immunodeficiency. HIV-1 infected individuals also have increased energy needs than non-HIV infected individuals and many suffer from significant weight loss and wasting. Micronutrient supplementation is thus recommended as an integral part of all HIV treatment programs.
Micronutrient supplementation improves the physical condition of HIV-1 infected individuals and acquired immune deficiency syndrome (AIDS) patients regardless of their clinical status and antiretroviral treatment, as was demonstrated in several studies. The administration of micronutrients that also enhance the immune system may be significantly advantageous to the HIV-1 infected individuals.
The food supplement Phyto V7 is a complex of phytochemicals and micronutrients. Phyto V7 has been found to stimulate cellular and antibody immune responses against viruses both in humans and in chicks. Importantly, administration of Phyto V7 to 9 terminal AIDS patients resulted in dramatic improvement in their physical well-being. The current study corroborated the significant positive effect on Phyto V7 on the physical well-being of HIV-1 infected individuals. This was demonstrated by the significant increase in the body weight and physical well-being a very large group of HIV-1 infected individuals not undergoing antiviral treatment that only received a daily dose of Phyto V7 for a period of 90 consecutive days.
Administration of the Phyto V7 can be an important tool to improve the well-being of HIV-1 seropositive individuals and AIDS patients, not undergoing antiretroviral treatment. It may well be that administration of Phyto V7 together with antiviral treatment is highly advantageous. Further studies should test this hypothesis.
Phytochemicals are chemical compounds that occur naturally in plants. These chemicals, in addition of serving as micronutrients, have been found to enhance nonspecific immunity, down regulate inflammatory diseases, and inhibit disease progression. The Karfnosky score is a well-accepted scale used to assess the quality of life of patients. It was used by the examining physicians to address the well-being of the study participants during the study.
This is an interesting work with promising results in which Phyto V7, a phytocomponent mix, quickly and effectively improves the weight and makes most of the HIV patients treated to feel better. For these kinds of patients it is good to be able to help them and this treatment might prepare them for a future more aggressive antiviral therapy.
P- Reviewer: Blanco LP, Louwen R S- Editor: Tian YL L- Editor: A E- Editor: Wu HL
| 1. | Barron MA, Makhija M, Hagen LE, Pencharz P, Grunebaum E, Roifman CM. Increased resting energy expenditure is associated with failure to thrive in infants with severe combined immunodeficiency. J Pediatr. 2011;159:628-632.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | Batterham MJ. Investigating heterogeneity in studies of resting energy expenditure in persons with HIV/AIDS: a meta-analysis. Am J Clin Nutr. 2005;81:702-713. [PubMed] |
| 3. | Sutinen J, Yki-Järvinen H. Increased resting energy expenditure, fat oxidation, and food intake in patients with highly active antiretroviral therapy-associated lipodystrophy. Am J Physiol Endocrinol Metab. 2007;292:E687-E692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Shevitz AH, Knox TA, Spiegelman D, Roubenoff R, Gorbach SL, Skolnik PR. Elevated resting energy expenditure among HIV-seropositive persons receiving highly active antiretroviral therapy. AIDS. 1999;13:1351-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 78] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | World Health Organization. Nutrient requirements for people living with HIV/AIDS: Report of a technical consultation. 2003; Available from: http://www.who.int/nutrition/publications/Content_nutrient_requirements.pdf. |
| 6. | Fawzi WW, Msamanga GI, Spiegelman D, Wei R, Kapiga S, Villamor E, Mwakagile D, Mugusi F, Hertzmark E, Essex M. A randomized trial of multivitamin supplements and HIV disease progression and mortality. N Engl J Med. 2004;351:23-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 250] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 7. | Forrester JE, Sztam KA. Micronutrients in HIV/AIDS: is there evidence to change the WHO 2003 recommendations. Am J Clin Nutr. 2011;94:1683S-1689S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Kaiser JD, Campa AM, Ondercin JP, Leoung GS, Pless RF, Baum MK. Micronutrient supplementation increases CD4 count in HIV-infected individuals on highly active antiretroviral therapy: a prospective, double-blinded, placebo-controlled trial. J Acquir Immune Defic Syndr. 2006;42:523-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 94] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 9. | Siegfried N, Irlam JH, Visser ME, Rollins NN. Micronutrient supplementation in pregnant women with HIV infection. Cochrane Database Syst Rev. 2012;3:CD009755. [PubMed] |
| 10. | Sun LZ, Currier NL, Miller SC. The American coneflower: a prophylactic role involving nonspecific immunity. J Altern Complement Med. 1999;5:437-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Aggarwal BB, Shishodia S. Suppression of the nuclear factor-kappaB activation pathway by spice-derived phytochemicals: reasoning for seasoning. Ann N Y Acad Sci. 2004;1030:434-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 201] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 12. | Wang X, Liu Z, Qiao W, Cheng R, Liu B, She G. Phytochemicals and biological studies of plants from the genus Balanophora. Chem Cent J. 2012;6:79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | de Mejía EG, Ramírez-Mares MV. Ardisia: health-promoting properties and toxicity of phytochemicals and extracts. Toxicol Mech Methods. 2011;21:667-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Rao BN. Bioactive phytochemicals in Indian foods and their potential in health promotion and disease prevention. Asia Pac J Clin Nutr. 2003;12:9-22. [PubMed] |
| 15. | Kennedy DO, Wightman EL. Herbal extracts and phytochemicals: plant secondary metabolites and the enhancement of human brain function. Adv Nutr. 2011;2:32-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 309] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 16. | Kumar GP, Khanum F. Neuroprotective potential of phytochemicals. Pharmacogn Rev. 2012;6:81-90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 217] [Cited by in RCA: 265] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 17. | Traka MH, Mithen RF. Plant science and human nutrition: challenges in assessing health-promoting properties of phytochemicals. Plant Cell. 2011;23:2483-2497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 18. | Rajaram S. The effect of vegetarian diet, plant foods, and phytochemicals on hemostasis and thrombosis. Am J Clin Nutr. 2003;78:552S-558S. [PubMed] |
| 19. | Bagalkotkar G, Sagineedu SR, Saad MS, Stanslas J. Phytochemicals from Phyllanthus niruri Linn. and their pharmacological properties: a review. J Pharm Pharmacol. 2006;58:1559-1570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 113] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 20. | Berginc K, Trdan T, Trontelj J, Kristl A. HIV protease inhibitors: garlic supplements and first-pass intestinal metabolism impact on the therapeutic efficacy. Biopharm Drug Dispos. 2010;31:495-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Bunluepuech K, Sudsai T, Wattanapiromsakul C, Tewtrakul S. Inhibition on HIV-1 integrase activity and nitric oxide production of compounds from Ficus glomerata. Nat Prod Commun. 2011;6:1095-1098. [PubMed] |
| 22. | Mushi NF, Mbwambo ZH, Innocent E, Tewtrakul S. Antibacterial, anti-HIV-1 protease and cytotoxic activities of aqueous ethanolic extracts from Combretum adenogonium Steud. Ex A. Rich (Combretaceae). BMC Complement Altern Med. 2012;12:163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Tewtrakul S, Subhadhirasakul S, Cheenpracha S, Karalai C. HIV-1 protease and HIV-1 integrase inhibitory substances from Eclipta prostrata. Phytother Res. 2007;21:1092-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 24. | Xia CL, Mao QC, Li RM, Chen ZP, Jiang SB, Jiang ZH, Liu SW. Study of the mechanism of caffeoyl glucopyranoses in inhibiting HIV-1 entry using pseudotyped virus system. Nanfang Yike Daxue Xuebao. 2010;30:720-723. [PubMed] |
| 25. | Perelman D, Goldman WF, Wernik JR, Borkow G. Enhancement of Antibody Titers against Newcastle Disease Virus in Vaccinated Chicks by Administration of Phyto V7. Journal of Vaccines and Vaccination. 2013;4:7-8. |
| 26. | Goldman WF, Wernik R, Carmen-Elias A, Borkow G. Immunomodulating effect of Phyto V7 in preneoplastic cervical lesions. Med J Obstet Gynecol. 2014;2:1038-1042. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 27. | Lavandera DMM, Jiminian FAC, Wernik R, Goldman WF, Borkow G. Dramatic improvement in physical well-being of terminal AIDS patients following administration of phytochemicals. World J AIDS. 2013;3:287-293. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 28. | Prellwitz IM, Alves BM, Ikeda ML, Kuhleis D, Picon PD, Jarczewski CA, Osório MR, Sánchez A, Seuánez HN, Larouzé B. HIV behind bars: human immunodeficiency virus cluster analysis and drug resistance in a reference correctional unit from southern Brazil. PLoS One. 2013;8:e69033. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 29. | Kebede Y, Pickering J, McDonald JC, Wotton K, Zewde D. HIV infection in an Ethiopian prison. Am J Public Health. 1991;81:625-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | Subbaraman R, Chaguturu SK, Mayer KH, Flanigan TP, Kumarasamy N. Adverse effects of highly active antiretroviral therapy in developing countries. Clin Infect Dis. 2007;45:1093-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 83] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 31. | Obiako OR, Muktar HM. Challenges of HIV treatment in resource-poor countries: a review. Niger J Med. 2010;19:361-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |