INTRODUCTION
According to World Health Organization estimates, 16.7 million people around the globe die of cardiovascular disease (CVD) each year[1]. Of the total annual CVD deaths, about 8.6 million occur in women. Heart attack and stroke deaths are responsible for twice as many deaths in women as all cancers combined[2]. In 2001, CVD contributed to nearly one-third of global deaths. Low and middle income countries contributed to 85 percent of CVD deaths[3].
On the other hand, antiretroviral therapy has dramatically improved the life expectancy of patients with human immunodeficiency virus (HIV)[4]. In the present highly active antiretroviral therapy (HAART) era, many HIV-infected patients are experiencing health problems that accompany the aging process, mainly the risk of CVD. There is renewed interest in a link between atherosclerotic CVD and as yet poorly defined environmental exposures, including infectious agents. If epidemiologic and laboratory evidence eventually supports this association, atherosclerosis could emerge as another non-communicable chronic condition related to infection. Chlamydophila pneumoniae (C. pneumoniae) has been one of the most important agents associated with atherosclerosis. Two types of observational studies are used to study an association between C. pneumoniae and atherosclerosis: seroepidemiology and the demonstration of the organism in atherosclerotic tissue. The first suggestion that C. pneumoniae might be associated with atherosclerosis was in 1988 when Saikku et al[5] showed that persons with coronary artery disease (CAD) more frequently had C. pneumoniae antibody than population controls. These seroepidemiologic findings have now been confirmed by many investigators around the world, and several studies with similar findings have been reported. Mainly, there are three types of studies on the etiologic role for C. pneumoniae in atherosclerosis: animal models, possible mechanisms and clinical treatment trials. The animal model studies focus on determining if C. pneumoniae can initiate or accelerate the atherosclerotic process. Clinical trials will provide evidence for the role of C. pneumoniae in complications of atherosclerosis, usually myocardial infarction (MI) and related syndromes.
C. PNEUMONIAE AND ATHEROSCLEROSIS
C. pneumoniae is an important cause of pneumonia. Its prevalence was determined in community-acquired pneumonia during a period of 7 years in Italy. Serum samples from patients with pneumonia were evaluated using microimmunofluorescence (MIF) assays to detect C. pneumoniae-specific IgG and IgM antibodies. 12.5% patients complied with the diagnostic criteria of acute C. pneumoniae infection[6].
Implicating C. pneumoniae in the etiology of atherosclerosis is quite a complicated process because the Koch postulates should be fulfilled before concluding that this pathogen is causally involved in human atherosclerosis.
Lines of evidence associating C. pneumoniae with atherosclerosis include seroepidemiologic studies, direct detection of bacterial components in atherosclerotic lesions, occasional isolation of viable organisms from coronary and carotid atheromatous tissue, and in vitro and animal experiments (previewed in[7-9]). Most cross-sectional and prospective studies have correlated seroprevalence with MI, chronic coronary heart disease (CHD), or stroke[7]. My colleagues and I evaluated the association between CVD and antibodies against Chlamydophila in a Mexican population. Study subjects included 70 CVD hospitalized patients. Serum IgG and IgM antibodies against C. pneumoniae were determined by MIF and compared with those from 140 healthy individuals, matched by age and sex. IgG antibodies against C. pneumoniae were found in 94.3% patients, as compared to only 37% of healthy individuals (P < 0.001); therefore, an association between IgG antibodies against C. pneumoniae and CVD was found[10]. Likewise, we determined antibodies against C. pneumoniae in patients with acute MI and coronary risk factors. We studied 100 patients hospitalized in the Coronary Unit of La Raza Medical Center, Mexico. The patients had increased seropositivity for C. pneumoniae since 70% presented antibodies[11].
The strongest evidence associating C. pneumoniae with atherosclerotic CVD has been detection of bacterial components in atherosclerotic lesions. C. pneumoniae appears to display tropism for atheromas[9].
Detection of C. pneumoniae antigens or DNA in intimal thickened tissue and fatty streaks of young adults and Alaskan Natives (the latter group at low risk for coronary atherosclerosis) supports an early microbial role in its pathogenesis[12]. Postmortem, the Alaskan retrospective study also positively correlated with prior systemic infection due to the presence of C. pneumoniae in atherosclerotic lesions. Studies from Seattle reported a slightly higher detection rate in late stage lesions[9].
Detection of C. pneumoniae in plaques has not correlated well with serology[7,11], so investigators have attempted to predict endovascular infection through polymerase chain reaction (PCR) recognition of microbial DNA in peripheral blood monocytes. The prevalence of C. pneumoniae DNA in these mononuclear cells has varied between studies (perhaps due to differences in both assay sensitivity and extraction procedures), but was 59% in coronary angiography patients compared with 44% in blood donors in one series; the rate appears to increase with age[13,14]. However, some authors have not detected circulating C. pneumoniae in CAD[15].
In vitro studies support hypothesis that C. pneumoniae might directly promote atherosclerosis. Infection of human endothelial cells augments the production of inflammatory cytokines and modulates expression of adhesion molecules, enhancing recruitment of inflammatory leukocytes to the vessel wall[7]. Animal experiments have also explored the link between C. pneumoniae and atherosclerosis. Intranasal or intratracheal C. pneumoniae inoculation of New Zealand white rabbits fed a normal diet produced inflammatory changes of the aorta[16].
Inflammatory changes without foam cells and typical atheromas have also been observed in C. pneumoniae infected mice fed a normal chow diet[9,17]. However, inoculation of C. pneumoniae in mouse models led to aggravation of experimental atherosclerosis induced by a cholesterol-enriched diet[18].
C. PNEUMONIAE ANTIBODIES, ELEVATED BODY MASS INDEX AND CVD
The relationship between seropositivity for IgG antibodies of C. pneumoniae and lipid levels has been studied[19]. The presence of IgG antibodies to C. pneumoniae is evidence of exposure to the organism but a single measurement will not distinguish between a primary infection, a repeat infection or a chronic infection. Previous studies showing an association between C. pneumoniae infection and lipid levels[20,21] largely ignored the role that confounding factors may have played in their findings. Several factors may confound this association: for example, low socioeconomic status, has been related to both elevated total cholesterol[22] and C. pneumoniae infection[23], although these are not consistent findings[24,25]. The date at which blood was collected may also have confounded the association. Seasonal variation in total cholesterol and high-density lipoprotein (HDL) cholesterol is a recognized phenomenon, with a total cholesterol peak[26,27] and HDL cholesterol trough[28] usually occurring during the winter months. The findings of the study[19] are consistent with the observation by Laurila et al[20], in a cross sectional study of male reindeer herders in Northern Finland, of decreased HDL, and HDL/total cholesterol ratio in subjects with serological evidence of C. pneumoniae infection. However, as in other studies of C. pneumoniae infection in which total cholesterol was measured[24,25,29], Laurila et al[21] did not observe an association between seropositivity and cholesterol levels, though in a follow up study, total cholesterol was increased in subjects with evidence of chronic infection.
The most commonly reported infection-induced lipid abnormalities in man and experimental animals have been decreased HDL, cholesterol and elevated triglycerides and very low density lipoprotein[30,31]. These effects are part of the acute phase response, appear to be mediated by cytokines such as tumor necrosis factor (TNF), interleukin (IL)-1, and IL-6[31], and have been observed in persons with serological evidence of C. pneumoniae infection[20]. The acute phase response to infection may therefore explain the association observed in the study[19], with C. pneumoniae seropositivity and lowered HDL cholesterol but does not convincingly explain the elevated total cholesterol. The lipid response to chronic infection may be different from that which occurs in acute infection.
This was the first study[19] to examine the relation between infection with C. pneumoniae and lipid levels in both men and women. In both sexes, an effect on total cholesterol in the same direction was documented, but infected men had lower HDL cholesterol concentrations while infected women had higher concentrations. An epidemiological robust association[19] was demonstrated between an atherogenic lipid profile and evidence of infection with C. pneumoniae and although the possible mechanism of action may not be clear, this finding may have substantial public health implications. If treatment of C. pneumoniae infection resulted in a sustained reduction in total cholesterol similar in magnitude to the increase seen in infected subjects in this study, data from meta-analyses of cholesterol lowering trials[32] would suggest an expected decrease in the risk of ischemic heart disease in treated individuals (potentially 70% of the population) of at least 20%.
Manifestations of CVD have been also associated with chronic infection by Helicobacter pylori (H. pylori). This was investigated in subjects classified according to serology titers due to infection with C. pneumoniae and H. pylori, association between seropositivity and the degree of obesity and fasting insulin levels, as well as social factors. Frozen samples from serum of 310 middle-aged treated hypertensive and 288 age-matched and gender-matched normotensive controls from a defined population were analyzed. The body mass index (BMI) was calculated as kg/m2. Fasting blood samples were drawn for measurement of serum lipid levels, blood glucose, plasma insulin and serum lipids, including total cholesterol and triglycerides. The titers for C. pneumoniae were determined by MIF. Subjects with combined positive serology for H. pylori and C. pneumoniae are characterized by greater age, lower socio-economic class and higher BMI, as well as higher fasting levels of insulin compared to seronegative subjects. Obesity might be a marker not only for lower socioeconomic class but also for a greater than normal susceptibility to such infections[33].
C. pneumoniae infection has been linked to the development of CHD, but its relationship to CHD risk factors is less clear. The relation between past infection with C. pneumoniae and risk factors for CHD, including body weight amongst subjects with and without CHD was determined. Antibodies to C. pneumoniae and a range of CHD risk factors were measured in 170 subjects, of whom 43 had recent onset angina. The prevalence of seropositivity was similar for subjects with and without CHD and for those with or without hypertension. However on multivariate analysis, only BMI remained significant (P < 0.05). Although the study failed to find a greater prevalence of antibodies to C. pneumoniae amongst subjects with recent onset angina, there were associations with a number of cardiovascular risk factors. An increase in body weight appears to underscore these relationships[34,35].
Whether serum chlamydial lipopolysaccharide (cLPS), C. pneumoniae antibodies and high-sensitivity C-reactive protein (hsCRP) levels are associated with BMI was explored. The study population consisted of 174 patients with symptomatic carotid stenosis, abdominal aortic aneurysm or occlusive aortic disease. Information on BMI, diabetes, smoking, hypercholesterolemia, and statin therapy was available. Serum C. pneumoniae IgG and IgA antibodies, cLPS, hsCRP and total endotoxin acitivity (totLPS) were measured. BMI correlated with cLPS (r = 0.197, P < 0.01) and with hsCRP (rho = 0.195, P < 0.01); in addition, there was a positive correlation between cLPS and hsCRP (rho = 0.499, P < 0.01). A trend of an increasing proportion of C. pneumoniae IgG positivity (titer ≥ 64, P = 0.018) and higher serum cLPS (P = 0.01) concentrations was observed across the BMI range. Elevated serum cLPS levels were associated with an elevated BMI. This is a novel finding and it strengthens the link between chlamydial infection and obesity. A lack of association between totLPS and BMI suggests that the association between infection and an elevated BMI may be specific to certain pathogens[36].
C. PNEUMONIAE INFECTION AND HIV
In 1991, the case of an HIV infected adult with C. pneumoniae was reported. The patient presented with a clinical picture suggestive of Pneumocystis carinii pneumonia (PCP) but did not respond to standard anti-PCP therapy. The diagnosis was eventually confirmed by bronchoscopy and serology. C. pneumoniae pneumonia should be considered in the differential of pathogens that cause interstitial infiltrates in HIV infected individuals[37].
Seven hundred and sixty-four healthy subjects, 96 HIV infected and 50 children with vertically transmitted HIV infection were studied. In the HIV infected population, C. pneumoniae seroprevalence was higher than in immunocompetent controls (children, 26% vs 11%; adults, 60% vs 40%). HIV infected subjects seem to be at higher risk of developing C. pneumoniae infections. Further studies are needed to elucidate fully the pathogenic role of C. pneumoniae in HIV infected subjects, because this high antibody prevalence could be the result of either a greater rate of infection in immunocompromised subjects or a polyclonal immunoglobulin activation commonly found in HIV patients[38].
The significance of chlamydia serum IgG and IgA antibodies was studied by immunoperoxidase assay in 210 homosexual men at various stages of HIV infection. Cross-sectional analysis of chlamydia IgG antibodies indicated a significantly higher prevalence rate among acquired immune deficiency syndrome (AIDS) patients (27%) as compared to asymptomatic HIV seronegative subjects (6.0%) (P = 0.022). The geometric mean titer of IgG antibodies to chlamydia was also significantly higher in AIDS patients (106.4) as compared to HIV seronegative subjects (58.2) (P = 0.022)[39]. The prevalence of C. pneumoniae antibodies was evaluated in an Italian population of HIV infected and uninfected individuals in relation to the presence of HIV risk factors. A statistically significant higher C. pneumoniae seroprevalence was found to be related, by multivariate analysis, to sex, age, and presence of HIV risk factors but not to the presence of HIV infection itself. Likewise, HIV infected subjects seem to have progressively lost their C. pneumoniae IgG antibodies in mid and advanced stages of HIV infection. High C. pneumoniae IgG titers are rarely found in advanced stage HIV infected patients[40]. Later, the same authors described C. pneumoniae infection in patients seropositive for the HIV. They concluded that C. pneumoniae is a possible cause of severe respiratory infection in Italian HIV infected immunocompromised patients, and its presence must be suspected when patients do not respond to therapy with beta lactam agents or to anti-Pneumocystis carinii treatment[41].
The incidence of C. pneumoniae respiratory tract infection was determined in HIV positive or AIDS patients. Twenty of 82 patients were found to have IgG antibodies to C. pneumoniae at titers ranging between 1:16 and 1:1024. Seven of the patients had evidence of acute C. pneumoniae infection. Results of this study indicate that C. pneumoniae may play a role in the etiology of respiratory tract infections in HIV positive patients; this fact should affect empirical antibiotic therapy[42].
On the other hand, C. pneumoniae DNA was investigated by PCR, in cerebrospinal fluid (CSF) specimens from patients suffering from HIV-associated dementia complex (HADC). Four (17.3%) cases of C. pneumoniae infection were identified among 23 HADC individuals with DNA amplification of the major outer membrane protein gene and 16S rRNA gene sequences. Sequence analysis revealed significant homologies with C. pneumoniae compared to Chlamydia trachomatis (C. trachomatis) and Chlamydophila psittaci (C. psittaci). High mean levels of CSF specific anti-C. pneumoniae antibodies and C. pneumoniae antibody specific index values were significantly elevated by enzyme-linked immunosorbent assay in these patients. The results suggest a hypothetical role for C. pneumoniae in the pathogenesis or progression of HADC[43].
C. pneumoniae seropositivity is associated with CVD and HIV infection. Cell-mediated immune responses are important in the control of Chlamydia pneumoniae, and such responses may be impaired in HIV infected patients. An assay for detection of interferon (IFN)-γ in whole blood stimulated with Chlamydia pneumoniae antigen was developed and studied in HIV infected patients and uninfected controls. Among 34 HIV infected patients, none had an IFN-γ response to C. pneumoniae antigen, compared with five of 32 healthy controls (P < 0.001). Fewer HIV infected individuals elicited a serum IgG response when tested with a commercial enzyme immunoassay (P = 0.009), but this was not so for serum IgA (P = 0.12). Additionally, the IFN-γ and antibody assays showed a trend towards a bivariate response in normal controls. This indicates that cellular and antibody responses against C. pneumoniae may be mutually exclusive, with potential implications for the role of this organism in the genesis of CVD in both immunocompetent and HIV infected populations[44].
To better understand the possible role of C. pneumoniae infection in the pathogenesis of epi-aortic lesions in HIV positive patients, the presence of anti-C. pneumoniae antibodies was evaluated in a group of individuals subjected to ultrasonography of the epi-aortic vessels. The presence of specific antibodies in 129 subjects was determined; 59 patients were HIV positive, 30 had carotid plaques and 29 had no lesions. The control group was composed of 70 subjects. All were subjected to ultrasonography of the epi-aortic vessels. IgG, IgM and IgA anti-C. pneumoniae antibodies were measured with MIF and positive sera were tested for C. trachomatis and C. psittaci. No subjects were positive for IgM. Neither IgA nor IgG levels differed significantly in the three groups. The only highly significant variable was the use of protease inhibitors. Results suggest that the damage to the carotid wall in HIV patients was not due to C. pneumoniae[45]. In contrast, our team determined whether the infection by C. pneumoniae is a risk factor for atherosclerosis in patients with AIDS. A case-control study of 43 patients with AIDS under HAART (16 cases and 27 controls) was conducted. To document atherosclerosis, a carotid and transcranial Doppler ultrasound was performed. Anti-C. pneumoniae antibodies were determined using a MIF test for IgM and IgG levels. To study the atherosclerosis risk factors, odds ratios were calculated for each IgG anti-C. pneumoniae antibody titer. A titer of 1:64 significantly increased the risk of atherosclerosis. These results suggest that hypertriglyceridemia and C. pneumoniae infection coexistence significantly increases the risk of atherosclerosis. The inverse geometric average of the antibody titers against C. pneumoniae in individuals with atheromatous plaque fell to 64, (two titers above controls). This difference turned out to be statistically significant. Exposure to C. pneumoniae with positive antibody (IgG) titers should be considered in any HIV diagnosed patient as a risk factor for atherosclerosis, having found that the inverse geometric averages of antibody titers are significantly different when comparing cases and controls, especially in patients with dyslipidemia, hypertriglyceridemia or in patients whose treatments could cause these conditions. An assessment of the therapy against C. pneumoniae and HAART should always be conducted. When we studied patients with concomitant hypertriglyceridemia, we found that the association increased three-fold. It is advisable to serologically test HIV-AIDS patients to determine exposure to C. Pneumoniae and to assess treatment options[46].
ATHEROMAS IN HIV AND C. PNEUMONIA INFECTED INDIVIDUALS
The development of anti-HIV antiretroviral therapies has significantly prolonged the life span of infected individuals, leading to the need to further understand the virus’ impact on chronic disease processes such as atherosclerosis[47].
HIV infection is associated to accelerated atherosclerosis and vasculopathy, and although the involved mechanisms have yet to be elucidated, there are observations substantiating it: (1) An increase in the prevalence of cardiac risk factors in HIV-infected individuals; (2) The dyslipidemia reported after therapy with certain anti-HIV antiretrovirals; and (3) The pro-inflammatory effects due to monocyte/macrophage infiltration in HIV-infected individuals[47].
It has not been determined whether HIV per se can infect smooth muscle cells (SMCs) and hence hastens the development of vascular disease[47].
Atherosclerotic lesions are characterized by thickening of the intima and plaque formation, consisting of fibrous tissue encapsulating a lipid core. These lesions have SMCs some of which contain phagocytosed lipids. The presence of abundant macrophages is also recognized, particularly surrounding the lipid core: this is known as an atheroma. Macrophages play a crucial role in lipid phagocytosis and their function is referred to as “scavenging” since they attempt to remove the fat particles infiltrating the intima. These macrophages may “super-phagocytose” and lead to cell death and subsequent “fat spillover” thus leading to atheroma formation. In terms of pathogenesis, atherosclerosis has been mainly considered a lipid disorder whereby an excessive lipid influx within the arterial wall and macrophage activity eventually foment lesion development in an attempt to eliminate lipids. It is currently known that macrophages do not only act as scavenger cells but rather as veritable immunocompetent cells that interact with other inflammatory cells such as lymphocytes and mast cells[48].
Immunohistochemical analysis of atherosclerotic plaques reveal the presence of macrophages and T lymphocytes, frequently in close cell to cell contact[49,50]. Furthermore, a variable number of these T lymphocytes appear to be activated[51]. Aside from macrophages and T lymphocytes, the atherosclerotic plaque also appears to contain clusters of activated mast cells, particularly in the posterior area of the lesions[52]. These aforementioned observations have led to the understanding that an immune intraplaque mediating cellular function is present within the atherosclerotic plaque. Consequently, the release of inflammation mediators or cytokines such as growth factors and other proteins from these cells may regulate a vast variety of cellular processes affecting the components of the atherosclerotic plaque; this would lead to a fine balance promoting plaque instability and its complications or, its stabilization[53].
Cytokines tend to be Th2 cell dependent, such as IFN-γ that may inhibit smooth muscle proliferation and decrease its ability to synthesize extracellular matrix. IFN-γ also increases the recruitment of immunocompetent cells thus regulating the expression of adhesion molecules on endothelial cells and playing an important role in the perpetuation of the inflammatory process. Other cytokines such as IL-1 and TNF may induce SMC apoptosis[54].
Macrophages destabilize plaque by releasing matrix metalloproteinases (MMPs). The macrophage layer, particularly when foam-laden, produces several MMPs including interstitial collagenase (MMP-1), stromelysin (MMP-3) and gelatinases MMP-2 and MMP-9. The release of these proteinases is controlled by inflammatory cytokines such as TNF and IL-1. Mast cells may orchestrate this cytokine release since the severity of the coronary syndrome correlates directly with their activation and not with that of macrophages or T cells; mast cells may thus play a functional role since they can release neutral proteases such as tryptase and chymase, both capable of destroying components of the extracellular matrix[55].
Finally, we must mention another mechanism operating in the process of immune activation within the arterial wall and it relates to the influx of modified lipoproteins such as oxidized low-density lipoprotein (OxLDL) within macrophages; these can induce non-antigen specific immune activation, transforming monocytes into foam cells that in turn, lead to functional changes such as the release of growth factors, cytokines and enzymes. The specific activation of the adaptive immune response may play an important role in atherosclerotic disease; some studies have even shown increased levels of anti-OxLDL antibodies as well as elevated titers against infectious agents. Some implicated microorganisms include c, H. pylori and C. pneumoniae, the most suspect. A relationship between MI and CAD has been established with antibody titers against C. pneumoniae, as first described by Saikku et al[5] and subsequently confirmed by other groups[56]. The fact that chronic or past exposure to the above mentioned microorganisms exacerbates inflammation and contributes to atherosclerosis has also been reported[57].
Chlamydia pneumoniae has been detected in atherosclerotic lesions via several methods such as electron microscopy, immunohistochemistry, PCR and the microorganism has also been cultured from atherosclerotic plaques[8].
HIV infection has been associated with abnormal lipid profiles[58] and HAART may also cause lipid abnormalities and insulin resistance, both risk factors for atherosclerosis[59]. However, the role of HIV infection per se and its effects on the vascular wall in atherosclerosis pathogenesis are not well understood, although the effect of HIV infection on endothelial dysfunction and HIV infiltration of macrophages and monocytes has been studied[60]. It has been shown that arterial SMCs express three relevant HIV receptors: CD4, CCR5 and CXCR4. They play a role in leukocyte viral entry[61]. SMCs are the predominant cell type in arteries.
One possibility correlating HIV infection and atheroma formation suggests that HIV can directly infect SMCs and if so, hasten vascular disease. SMC infection by HIV has been demonstrated in vivo and in vitro. HIV p24 protein has been detected by confocal fluorescence microscopy in SMCs of atherosclerotic plaques from HIV-infected individuals. Also, SMCs can be infected in vitro via CD4-dependent mechanisms, CXCR4 or CCR5 chemokine receptors and endocytosis; this leads to a marked secretion of CCl2/MCP-1 by SMCs, a critical mediator of atherosclerosis. SMC infection by HIV may be part of a multifactorial mechanism that could explain the exacerbated atherosclerosis and vasculopathy seen in HIV-infected individuals[47].
DYSLIPIDEMIA, METABOLIC INFLAMMATORY CHANGES AND HIV
CVD risk associated with the fat redistribution seen among HIV infected individuals remains unexplained, but may be increased due to associated hyperlipidemia, hyperinsulinemia, increased visceral adiposity, and the prothrombotic state associated with these metabolic abnormalities[62]. Increased tissue type plasminogen activator (tPA) antigen, a marker of impaired fibrinolysis, predicts increased risk of CAD mortality among patients with a history of angina pectoris and CVD[63], as well as cerebrovascular events among individuals without a prior history of CVD[64]. Further, there is a strong association between hyperinsulinemia and impaired glucose tolerance and levels of plasminogen activator inhibitor-1 (PAI-1) and tPA in otherwise healthy adults[65], suggesting a mechanistic link between insulin resistant states and increased CVD. PAI-1 and tPA markers of fibrinolysis were characterized and correlated with an increased CVD risk in HIV positive lipodystrophic patients compared with controls. The effect of treatment with metformin on PAI-1 and tPA antigen levels in patients with HIV associated fat redistribution was also analyzed. The data demonstrated impaired fibrinolysis in association with hyperinsulinemia and an increased waist to hip ratio in patients with HIV infection and fat redistribution. This suggests that metformin improves the overall cardiovascular risk profile in HIV infected patients with fat redistribution[66].
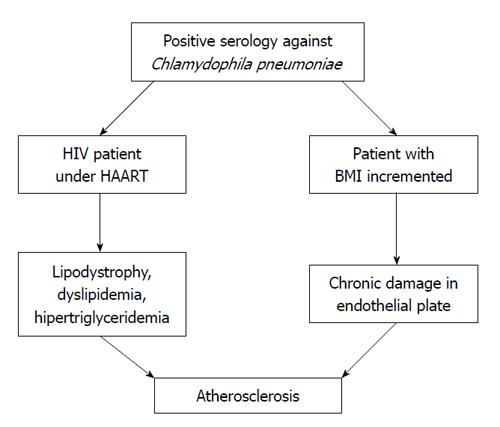
We would suggest that (Figure 1) on the one hand, the patient with HIV and lipodystrophy caused by HAART and exacerbated by C. pneumoniae infection, could be a risk factor for atherosclerosis. An assessment of the therapy against C. pneumoniae and HAART should always be carried out. It is advisable that HIV-AIDS patients undergo a serological test to determine exposure to C. pneumoniae, and to assess treatment options. On the other hand, in patients with a positive serology to C. pneumoniae, an increase in BMI has been found; therefore, it is probable that the recurrent infection may play an important role in creating adverse endothelial conditions that allow the infection by C. pneumoniae in its chronic form to damage the endothelial plate. Vascular studies would be necessary for corroboration. In obesity, adipose tissue is inflamed and many inflammatory molecules, such as IL-6 and TNF, are produced. This low-grade inflammation in adipose tissue induces insulin resistance and obesity, which are linked to metabolic syndrome, type 2 diabetes and CHD. Slightly elevated serum C-reactive protein (CRP) levels act as a marker of systemic inflammation, and have been shown to increase the risk of CHD. The synthesis of CRP is regulated by IL-6, which has been assumed to originate largely from adipose tissue.
Figure 1 In the patient co infected with human immunodeficiency virus /chlamydia, under highly active antiretroviral therapy, a factor of risk can occur to develop lipodystrophy and other disorders of the metabolism of lipids as dyslipidemia or hipertriglyceridemia, conducting finally to atherosclerosis.
Thus same, in patients with re infections and obesity, would be able to occur an activation in the mechanism of inflammation that has an important role in the pathogenesis of atherosclerosis resulting in a cycle of inflammation, activation and continuous cell recruitment.
CONCLUSION
C. pneumoniae is an obligate, intracellular bacterium associated with a wide variety of acute and chronic diseases. C. pneumoniae infection is characterized by persistence and immunopathological damage to host target tissues, including the lung. Over the past 20 years, a variety of studies have investigated a possible link between C. pneumoniae infection and atherosclerosis, because of its role in all stages of atherosclerosis, from initial inflammatory lesions to plaque rupture. In the present HAART era, many HIV-infected patients are experiencing the health problems that accompany the aging process, mainly the risk of CVD. There is renewed interest in a link between atherosclerotic CVD and as yet poorly defined environmental exposures, including infectious agents. In addition, there is an association between possible chronic C. pneumoniae infection and obesity.
Peer reviewer: Andrés Moya, Professor, Department of Genomics and Health, University of Valencia and Public Health Research Center of Valencia, Catedrático José Beltrán 2, Paterna 46980, Spain
S- Editor Wu X L- Editor A E- Editor Zheng XM