Published online Apr 26, 2022. doi: 10.5495/wjcid.v12.i1.20
Peer-review started: September 2, 2021
First decision: November 22, 2021
Revised: December 3, 2021
Accepted: February 12, 2022
Article in press: February 12, 2022
Published online: April 26, 2022
Processing time: 234 Days and 19.6 Hours
In the United States, Staphylococcus aureus (S. aureus) kills tens of thousands of individuals each year and the formation of a biofilm contributes to lethality. Biofilm-associated infections are hard to treat once the biofilm has formed. A new stilbene drug, labeled SK-03-92, was shown to kill S. aureus and affected transcription of two genes tied to a putative two-component system (TCS) we have named brpR (biofilm regulating protein regulator) and brpS (biofilm regulating protein sensor).
To determine if BrpR and BrpS regulate biofilm formation, brpR and brpS mutants were assessed using biofilm assays compared to wild-type S. aureus.
A combination of biofilm and quantitative real-time-polymerase chain reaction assays were used. In addition, bioinformatic software tools were also utilized.
Significantly more biofilm was created in the brpR and brpS mutants vs wild-type cells. Quantitative real-time polymerase chain reactions showed the brpS mutant had differences in transcription of biofilm associated genes that were eight-fold higher for srtA, two-fold lower for lrgA, and 1.6-fold higher for cidA compared to wild-type. Bioinformatic analysis demonstrated that the S. aureus brpR/brpS TCS had homology to streptococcal late-stage competence proteins involved in cell-death, increased biofilm production, and the development of persister cells.
Our study suggests that brpR/brpS is a TCS that may repress S. aureus biofilm production and be linked to late-stage competence in S. aureus.
Core Tip:
- Citation: Zank A, Schulte L, Brandon X, Carstensen L, Wescott A, Schwan WR. Mutations of the brpR and brpS genes affect biofilm formation in Staphylococcus aureus. World J Clin Infect Dis 2022; 12(1): 20-32
- URL: https://www.wjgnet.com/2220-3176/full/v12/i1/20.htm
- DOI: https://dx.doi.org/10.5495/wjcid.v12.i1.20
Staphylococcus aureus (S. aureus) is a significant pathogen of humans, causing more than 700000 skin/soft tissue infections, nearly 120000 bloodstream infections, and close to 20000 deaths per year in the United States[1-3]. Because drug resistance within this species continues to increase, new drugs are needed to treat human infections. Our research group has developed a new stilbene drug labeled SK-03-92 with efficacy against all Gram-positive bacteria that were tested, including methicillin-resistant S. aureus[4]. An mRNA microarray was performed on SK-03-92 treated vs untreated S. aureus cells to try to elucidate the mechanism of action of the drug[5]. From this microarray, the genes for a putative two-component system (TCS) (annotated as MW2284/MW2285) were the most downregulated at the transcriptional level. Moreover, transcription of the srtA gene (encoding sortase A) was upregulated and the lrgA gene encoding an anti-holin was downregulated following SK-03-92 treatment. Additionally, SK-03-92 treatment led to a high degree of persister cells and greater biofilm formation. Because of the effect on biofilm formation, the MW22284 gene was labeled brpR (biofilm regulating protein regulator) and the MW2285 gene was labeled brpS (biofilm regulating protein sensor).
Transcriptional changes of the srtA and lrgA genes as well as high numbers of persister cells suggested that SK-03-92 treatment may induce late-stage competence in S. aureus. Although competence allows DNA uptake to occur in heavily stressed cells, transformation is only one effect of bacterial competence. During early competence, which occurs prior to transformation, a large proportion of the stressed bacterial population die via holin-induced autolysis[6]. It is this phenomenon that supplies environmental DNA (eDNA) to the remaining cells for DNA uptake. Additionally, the surplus eDNA provides scaffolding for the rapid formation of a biofilm[7]. The final stage of natural competence is metabolic dormancy[8]. Current estimates show that only the youngest 1% of the original population survive to become a dormant cell. Thus, when faced with resource competition, a thriving bacterial colony has the ability to rapidly transform itself into a small group of latent (i.e. persister) cells living within a biofilm. These surviving cells re-emerge once environmental resources again become plentiful. This is one strategy used by bacterial cells to survive antibiotic challenge and re-infect the host[9].
The initiation of competence has been shown to rely on a symphony of genetic switches that begin to harmonize when short-sequence amino acids, known as competence stimulating pheromones (CSPs) bind to certain specific membrane proteins. The initiation of the CSP alarmone response has been well characterized in streptococcal species[10]. These membrane proteins are autoinducers that comprise one half of a specific TCS[11]. In Streptococcus pneumoniae (S. pneumoniae) and Streptococcus mutans (S. mutans), this response is initiated following the interaction with self-produced autoinducing pheromones known as CSPs, which are short, 14 residue peptides. The CSP is then received by the membrane bound sensor kinase ComD (S. pneumoniae)[12] or BrsM (S. mutans)[13]. Next, ComD or BrsM phosphorylate the cytoplasmic response regulator ComE (S. pneumoniae)[12] or BrsR (S. mutans)[13], which ultimately controls programmed cell death and persistence. In S. aureus, neither the CSP nor the TCS by which CSPs are received have yet been identified.
In this study, we have shown that mutations of the brpR and brpS genes in S. aureus strain Newman showed greater biofilm formation and transcriptional changes of the srtA and lrgA genes than wild-type S. aureus. Furthermore, we have used bioinformatic tools to show that the brpR/brpS TCS has homology to the BrsR/BrsM[13] and ComE/ComD[12] late-stage competence TCSs in S. mutans and S. pneumoniae, respectively. These findings suggest that the brpR/brpS TCS may be specific for the reception and resultant signal cascade of a molecule that induces late-stage competence in S. aureus.
All of the bacterial strains and plasmids used in this study are shown in Table 1. The S. aureus parent strain Newman was isolated from a human infection[14]. The JE2 strain, created by the University of Nebraska Medical Center, is the S. aureus parent strain USA300 LAC CA-MRSA cured of its plasmids[15]. Strains NE272 (brpS mutant), NE671 (brpR mutant), and NE1787 (srtA mutant) are erythromycin-resistant (EmR) mutants representing part of the Nebraska Transposon Mutant Library created by the University of Nebraska Medical Center by mariner transposon mutagenesis[15] and obtained from the Network on Antimicrobial Resistance in S. aureus (NARSA) strain repository (Table 1). The E. coli strain DH5α is a cloning strain with mutations that enable high-efficiency transformation[16]. S. aureus strain RN4220 is a transformation efficient strain of S. aureus[17].
| Bacterial strain | Description | Ref. |
| E. coli | ||
| DH5 | Transformation efficient E. coli strain | [16] |
| S. aureus | ||
| Newman | S. aureus clinical isolate | [14] |
| JE2 | S. aureus USA300 MRSA strain | [15] |
| NE272 | S. aureus JE2 brpS::mariner mutant | [15] |
| NE671 | S. aureus JE2 brpR::mariner mutant | [15] |
| NE1787 | S. aureus JE2 srtA::mariner mutant | [15] |
| RN4220 | Transformation-efficient S. aureus strain | [17] |
| Newman brpR | S. aureus Newman brpR::mariner mutant | This study |
| Newman brpS | S. aureus Newman brpS::mariner mutant | This study |
| Newman srtA | S. aureus Newman srtA::mariner mutant | This study |
| Plasmids | ||
| pXB3-1 | pALC2073 plasmid with the brpR gene inserted | This study |
| pALC2073 | Cloning vector with Apr, Cmr, and Tcr genes, and a Tc-inducible promoter | [18] |
| pAMZ1-3 | pALC2073 plasmid with the brpS gene inserted | This study |
To clone the brpR and brpS genes for complementation studies, plasmid pALC2073 was used. This plasmid carries ampicillin and chloramphenicol resistance genes, E. coli and Gram-positive origins of replication, and a xyl/tetO tetracycline inducible promoter[18].
All media was purchased from Thermo Fisher Scientific (Thermo Fisher Scientific, Pittsburgh, PA, United States). All antibiotics were purchased from Sigma-Aldrich (Sigma-Aldrich, St. Louis, MO, United States). E. coli strains were grown in Luria (LB) broth shaken at 250 rpm at 37 °C or on Luria agar (LA) incubated at 37 °C. The E. coli strains carrying the pALC2073 plasmid were selected for in media containing 100 μg/mL ampicillin.
All S. aureus strains were grown in brain heart infusion (BHI) broth with 1% (wt/vol) glucose (BHI-G) or trypticase soy broth shaken at 250 rpm at 37 °C. Agar grown S. aureus cultures were passaged on BHI agar at 37 °C. The mariner transposon mutant strains were grown in media with 5 μg/mL of erythromycin (Em5). S. aureus strains carrying the pALC2073, pXB3-1, or pAMZ1-3 plasmid were selected for in media containing 10 μg/mL of chloramphenicol (Cm10). To induce the xyl/tetO promoter on the pXB3-1 and pAMZ1-3 plasmids, tetracycline at 0.25 μg/mL was added to the growth medium.
The brpR::mariner, brpS::mariner, and srtA::mariner mutations were transduced into strain Newman using the a ϕ80a bacteriophage[19]. The transductants were then selected for on BHI agar containing Em5. All mutations were verified by polymerase chain reaction (PCR) and biofilm assays.
The brpR complementing plasmid was constructed using the pALC2073 backbone[18]. Isolation of pALC2073 plasmid DNA followed the manufacturer’s instructions for the Qiagen QiaPrep plasmid isolation kit (Qiagen, Germantown, MD, United States). The full-length coding region of the S. aureus strain MW2 brpR gene was PCR amplified using the MW2284I/MW2284M primers (Integrated DNA Technologies, Coralville, IA, United States; Table 2) and the following PCR conditions: 35 cycles, 94 °C 1 min, 57 °C 1 min, 72 °C 1 min. S. aureus strain MW2 chromosomal DNA served as a template. The brpR DNA was amplified to have a KpnI site on the 5’ end and an EcoRI site on the 3’ end. PCR amplified brpR gene product was digested with KpnI and EcoRI (New England Biolabs, Ipswich, MA, United States), and then ligated with KpnI/EcoRI cut pALC2073 plasmid DNA using T4 DNA ligase (New England Biolabs). Ligated DNA was transformed into E. coli strain DH5α cells[16]. Transformants were selected on LA containing 100 μg/mL ampicillin, and one resulting plasmid, plasmid pXB3-1, was used for further experiments.
| Primer | Sequence |
| GEX-5XC | 5’- CCTAGGAGATCTCTTTCTGTC -3’ |
| GEX-5XB | 5’- GTTAATTTTACTAAACTTAAG -3’ |
| MW2284I | 5’- GAGCAGGTACCATGATGAAACTCAATTTATTTATCAATGCAAAAG-3’ |
| MW2284M | 5’- CAGCGAATTCTCAATGGTGATGGTGATGTATTGATAATCGCTCCTTTATAGATTTTAAAA -3’ |
| CidA1 | 5’- TGCAACGATACATGTTCCTATG -3’ |
| CidA2 | 5’- CTACAACTAGGAATCATCATTGTG -3’ |
| LrgA1 | 5’- GCATCAAAACCAGCACACTTT -3’ |
| LrgA2 | 5’- GACTTCGCCTAACTTAACAGC -3’ |
| SaFtsZ1 | 5’- GGTGTAGGTGGTGGCGGTAA -3’ |
| SaFtsZ2 | 5’- TCATTGGCGTAGATTTGTC -3’ |
| SrtA1 | 5’- TCGCTGGTGTGGTACTTATC -3’ |
| SrtA2 | 5’- CAGGTGTTGCTGGTCCTGGA -3’ |
A recombinant brpS complementing plasmid was also constructed. Isolation of pALC2073 plasmid DNA used the same plasmid isolation kit described above. The full-length coding region of the S. aureus strain MW2 brpS gene was PCR amplified using the GEX-5XB/GEX-5XC primers (Table 2) and the following PCR conditions: 35 cycles, 94 °C 1 min, 57 °C 1 min, 72 °C 1 min. S. aureus strain MW2 chromosomal DNA was used as the template. The brpS DNA was amplified to have a KpnI site on the 5’ end and an EcoRI site on the 3’ end. PCR amplified brpS gene product was digested with KpnI and EcoRI (New England Biolabs), and then ligated with KpnI/EcoRI cut pALC2073 plasmid DNA using T4 DNA ligase (New England Biolabs) immediately downstream from the tetracycline-inducible xyl/tetO promoter on pALC2073. Ligated DNA was transformed into E. coli strain DH5α cells[16]. Transformants were selected on LA containing 100 mg/mL ampicillin, and one resulting plasmid, plasmid pAMZ1-3, was used for further experiments.
Plasmid DNA from E. coli was purified with a Qiagen Plasmid Miniprep Kit (Qiagen) and electroporated into the S. aureus strain RN4220[20] using a GenePulser, (Bio-Rad, Hercules, CA, United States) under the following conditions: 100 W capacitance, 25 mF resistance, 2.5 kV charge voltage, 4 s. Transformants were selected for on BHI agar containing Cm10 after one hour of expression in BHI broth. Finally, plasmid DNA was re-isolated from one S. aureus strain RN4200 transformant carrying either the pXB3-1 or pAMZ1-3 plasmid using the method noted above with one alteration. The S. aureus cells were incubated with 50 μL of lysostaphin (10 mg/mL; Remel, San Diego, CA, United States) for 60 min at 37 °C prior to the first step to facilitate lysis of the staphylococcal cells. Each isolated plasmid DNA sample was then cut with the KpnI and EcoRI restriction endonucleases to verify the insertion. The S. aureus strain Newman was then transformed with 10 mL of pXB3-1 or pAMZ1-3 plasmid DNA using electroporation as outlined above and transformants selected for on BHI agar containing Cm10.
To determine the effect of the brpR and brpS mutations on the ability of S. aureus Newman to form a biofilm, biofilm assays were performed[21]. Briefly, cultures of the S. aureus were grown at 37 °C with shaking (250 rpm) overnight in BHIG broth with the appropriate antibiotic(s). Each strain was then diluted 1:100 in BHI-G and 220 μL of the solution was placed in microtiter wells in triplicate in a 96-well microtiter plate. The microtiter plates were statically incubated for 24 h at 37 °C to allow a biofilm to form. Each well was then rinsed three times with sterile water. The biofilms were then allowed to settle (10 min), stained with crystal violet dye (0.1% wt/vol) for 10 min, and then washed with sterile water. After allowing the well contents to dry fully in a sterile hood, the dried contents were incubated in 33% acetic acid at room temperature for 30 min. The contents of the well were vigorously curettaged. The optical densities were measured on a SpectraMax M3 96-well microtiter plate reader (Molecular Devices, San Jose, CA, United States) at an optical density of 570 nm. In addition to wild-type Newman cells, Newman brpR and Newman brpS mutant strains as well as brpR and brpS mutants containing the pXB3-1 or pAMZ1-3 plasmids were tested and compared with a Newman srtA transposon mutant strain that served as a negative control. To achieve statistical significance, the biofilm assays were performed a minimum of five times in triplicate for each strain.
Total RNA was isolated from S. aureus strains grown to early logarithmic phase in BHI broth with shaking (250 rpm) incubated at 37 °C using a High Pure RNA Isolation kit ∆∆(Roche Diagnostics, Indianapolis, IN, United States) with an additional lysostaphin treatment step to help lyse the S. aureus cell walls and a DNase I digestion to digest contaminating DNA. To confirm RNA concentration and ensure the integrity of each RNA sample, an aliquot of each RNA sample was analyzed on a Nanodrop machine (Thermo Scientific, Waltham, MA, United States) and electrophoresed through 0.8% agarose gels. The cDNA for each strain was then synthesized from 2 μg of total RNA according to manufacturer’s instruction using a First-Strand Synthesis kit (Life Technologies, Carlsbad, CA, United States). All quantitative real-time PCR (qRT-PCR) trials were performed according to manufacturer’s instruction using the iTaq Universal SYBR Supermix kit (BioRad, Hercules, CA, United States). Oligonucleotide primers that targeted the ftsZ, srtA, lrgA, and cidA genes were synthesized (Table 2) by Integrated DNA Technologies. To perform qRT-PCR, the minimum information for publication of quantitative real-time PCR experiments guidelines were followed and the ftsZ housekeeping gene was used as a standardization control[22]. All replicates were performed at least three times on a CFX96 qPCR instrument (BioRad, Hercules, CA, United States) under the following conditions: 94 °C, 20 s; 55 °C, 30 s; and 72 °C, 1 min for 35 cycles. The level of target gene transcript from each strain was estimated against the ftsZ gene standard curve. Additionally, crossover points for all genes were standardized to the crossover points for ftsZ in each sample using the 2-ΔΔCT formula[23].
The sequenced genomes of S. aureus strains MW2 and Newman used in this study are publicly available on GenBank (NCBI, genome assembly ASM1126v1)[24-26]. The protein annotations for all of the bacterial strains included in this study were found on BioCyc or GenBank[26,27]. BioCyc was also used to search for brpRS homologs downstream of the mqo2 gene. The UniProt Consortium was used to obtain amino acid FASTA sequences[28]. Domain motifs were sought using NetPHOS, ExPASy, Prosite, and GenomeNet[28-30]. Protter was used to two-dimensionally visualize brpS and brsM[31]. I-TASSER and PyMOL were used together to three dimensionally visualize BrpR and BrpS[32,33]. I-TASSER and PyMol were also used to visually verify DNA binding in residues predicted by DP-Bind[34]. Finally, protein sequence homology analyses were performed by BLASTp (NCBI) with the following parameters: Max target sequences = 100, automatically adjusted parameters for short input sequences, expect threshold = 10, word size = 3, max matches in a query range = 0, matrix = BLOSUM62, gap costs = 11 existence and 1 extension, and a conditional compositional score matrix adjustment[35].
Calculation of the means, standard deviations, and paired Student’s t-tests were performed using Microsoft Excel. P < 0.05 were considered significant.
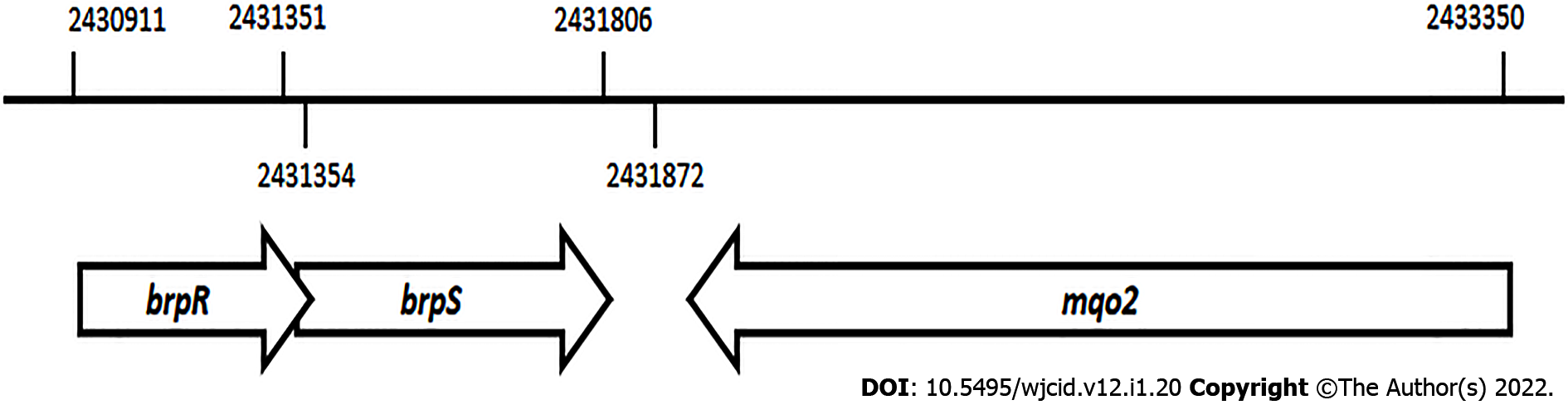
An alignment of the brpR and brpS genes that encode the BrpR and BrpS proteins is seen on the S. aureus MW2 genome sequence (Figure 1)[24]. These genes overlap in a unidirectional in-tandem sequence. The overlapping brpRS genes lie just 66 base pairs upstream from the mqo2 gene, encoding one of the two malate: Quinone oxidoreductases (MQO2) produced by S. aureus. The bi-functional MQO2 protein is able to generate oxaloacetate through an oxidation of malate as well as donate electrons to the electron transport chain[36].
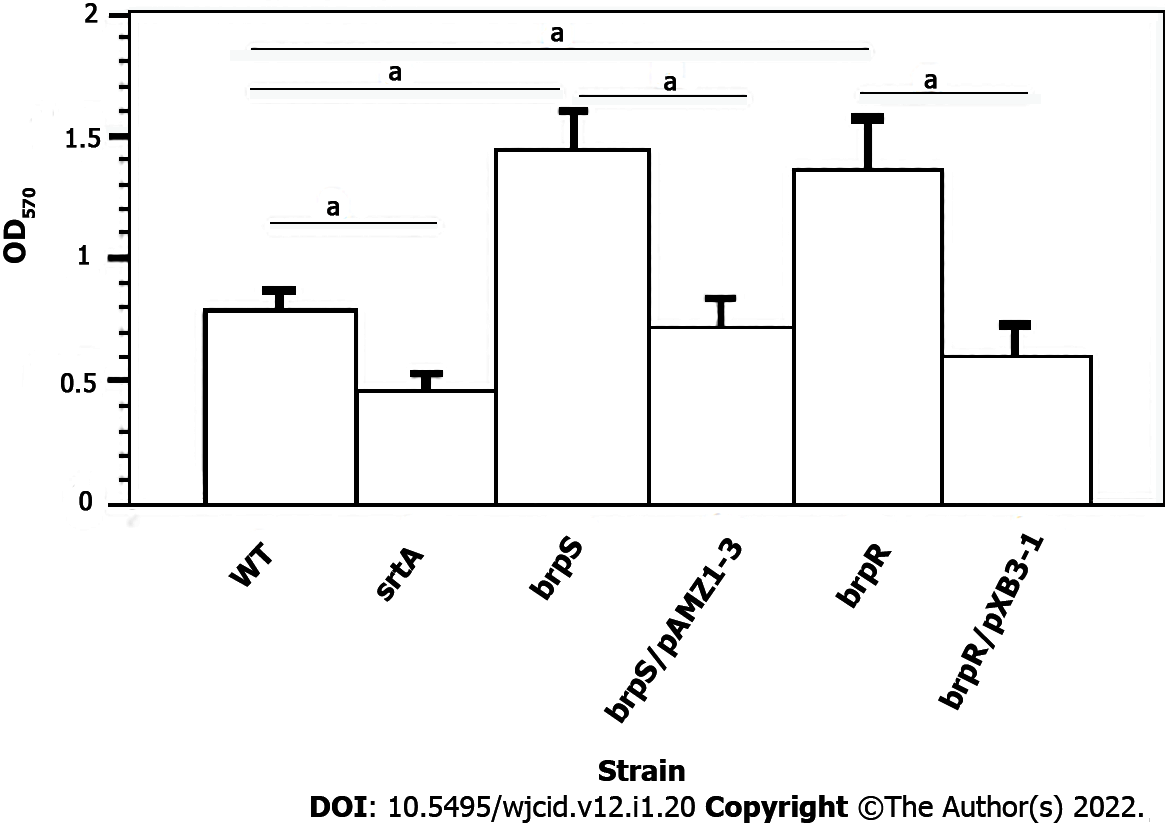
To confirm biofilm formation was linked to BrpR and BrpS, individual brpR and brpS mutations were moved to the S. aureus strain JE2 background[15] to the S. aureus strain Newman via transduction. Biofilm production of both mutants was compared to the unmutated wild-type S. aureus Newman strain. Significantly more biofilm material was produced by the brpS and brpR mutants (1.8-fold and 1.73-fold higher, respectively, P < 0.001) compared to wild-type. Complementation of the brpR and brpS mutants caused biofilm expression to either return to wild-type levels or there was less biofilm material formed (Figure 2). The srtA mutant displayed a 1.73-fold decline in the biofilm forming ability compared to the wild-type strain (P < 0.001). This suggested that the putative BrpRS TCS may repress S. aureus biofilm production.
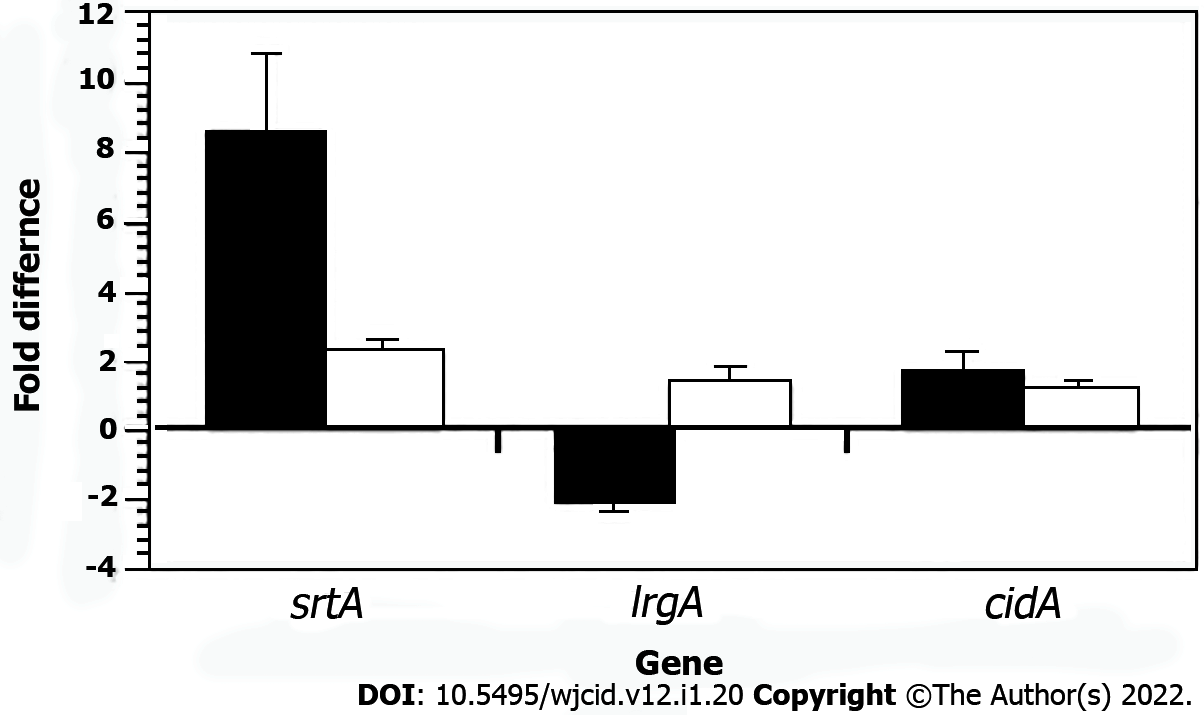
Previously, we showed srtA transcription was elevated and lrgA transcription was lower after SK-03-92 treatment of S. aureus cells compared to untreated cells[5]. The srtA gene encodes sortase A[37] and the lrgA gene encodes an anti-holin[38] that are important for the formation of biofilms[39,40]. Total RNA was collected during the mid-exponential growth phase from the S. aureus Newman brpS mutant, S. aureus Newman brpS mutant containing the pAMZ1-3 plasmid, and wild-type S. aureus Newman cells Each RNA sample was converted to cDNA for qRT-PCRs analysis. The brpS mutant displayed 8.5-fold higher srtA transcription (P < 0.008), 2-fold lower lrgA transcription (P < 0.016), and 1.6-fold higher cidA transcription (P < 0.43) vs the wild-type strain (Figure 3). Complementation of the brpS mutation caused srtA transcription to drop to a 2.2-fold increase, a 1.3-fold increase in lrgA transcription, and a 1.2-fold increase in cidA transcription. These results demonstrated that transcription of some biofilm-associated genes was regulated by a mutation in the brpS gene.
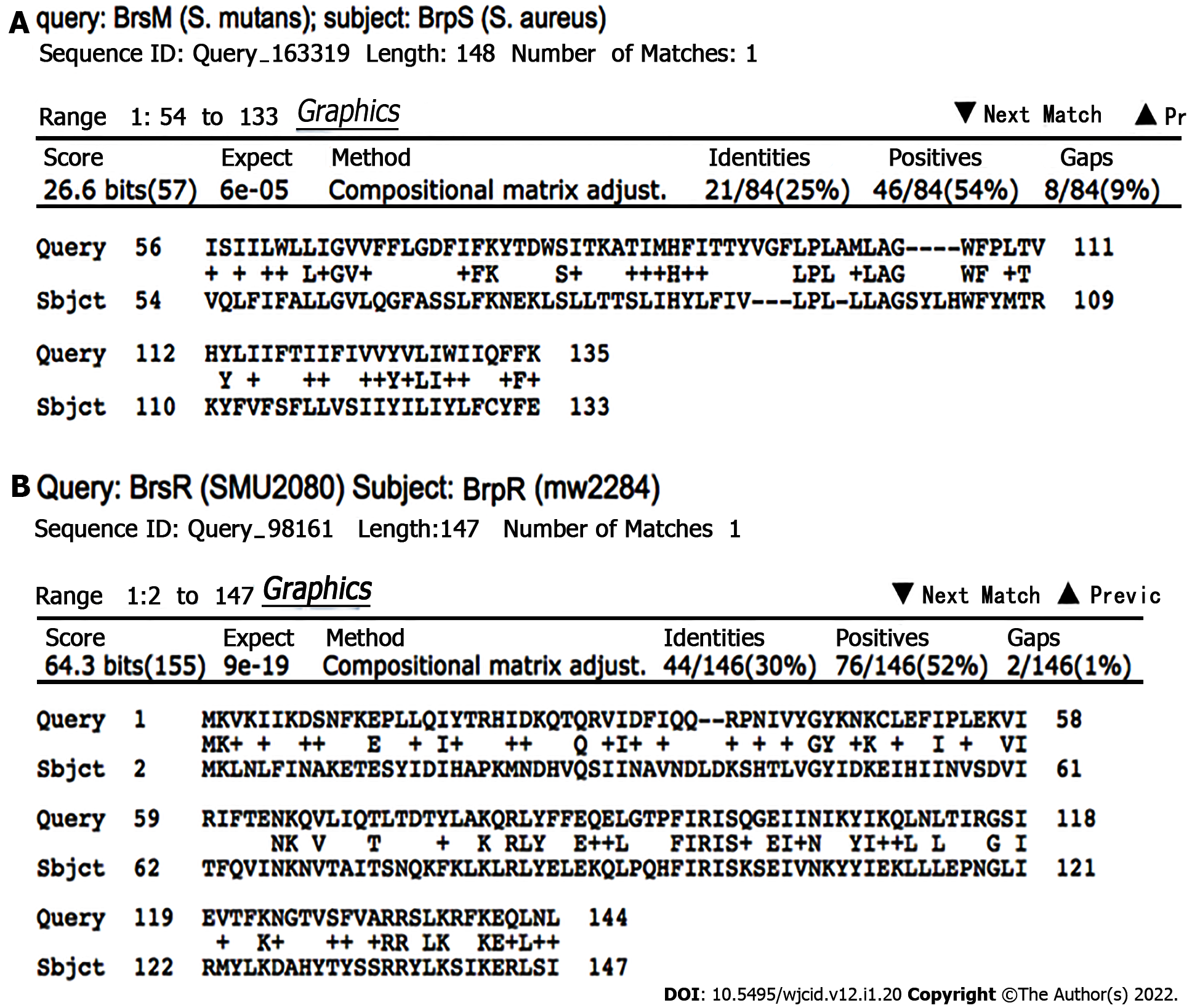
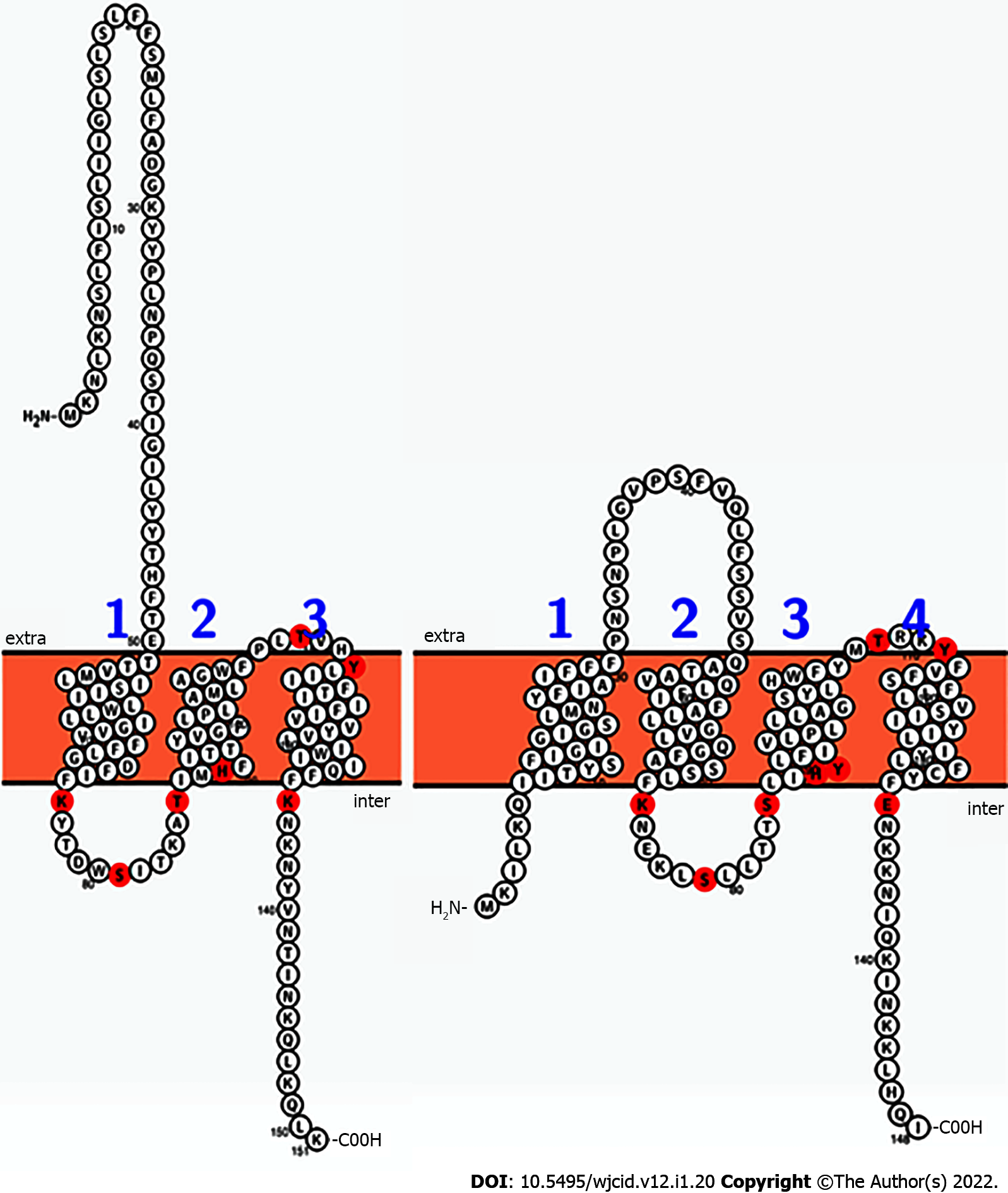
BrpR and BrpS homologs were identified by BLAST analyses in multiple Gram-positive bacterial pathogens, including Bacillus cereus, Clostridioides difficile, Enterococcus faecalis, Lactobacillus species, Staphylococcus haemolyticus, Streptococcus pneumoniae ComD/ComE, and S. mutans BrsR/BrsM as well as three other bacterial species (Escherichia coli YehT/YehU, Mycobacterium tuberculosis YehT/YehU, and Chlamydia trachomatis)[26,35]. Of these, the S. mutans BrsR/BrsM TCS that senses CSP and then induces late-stage competence showed the highest homology (Figure 4)[12].
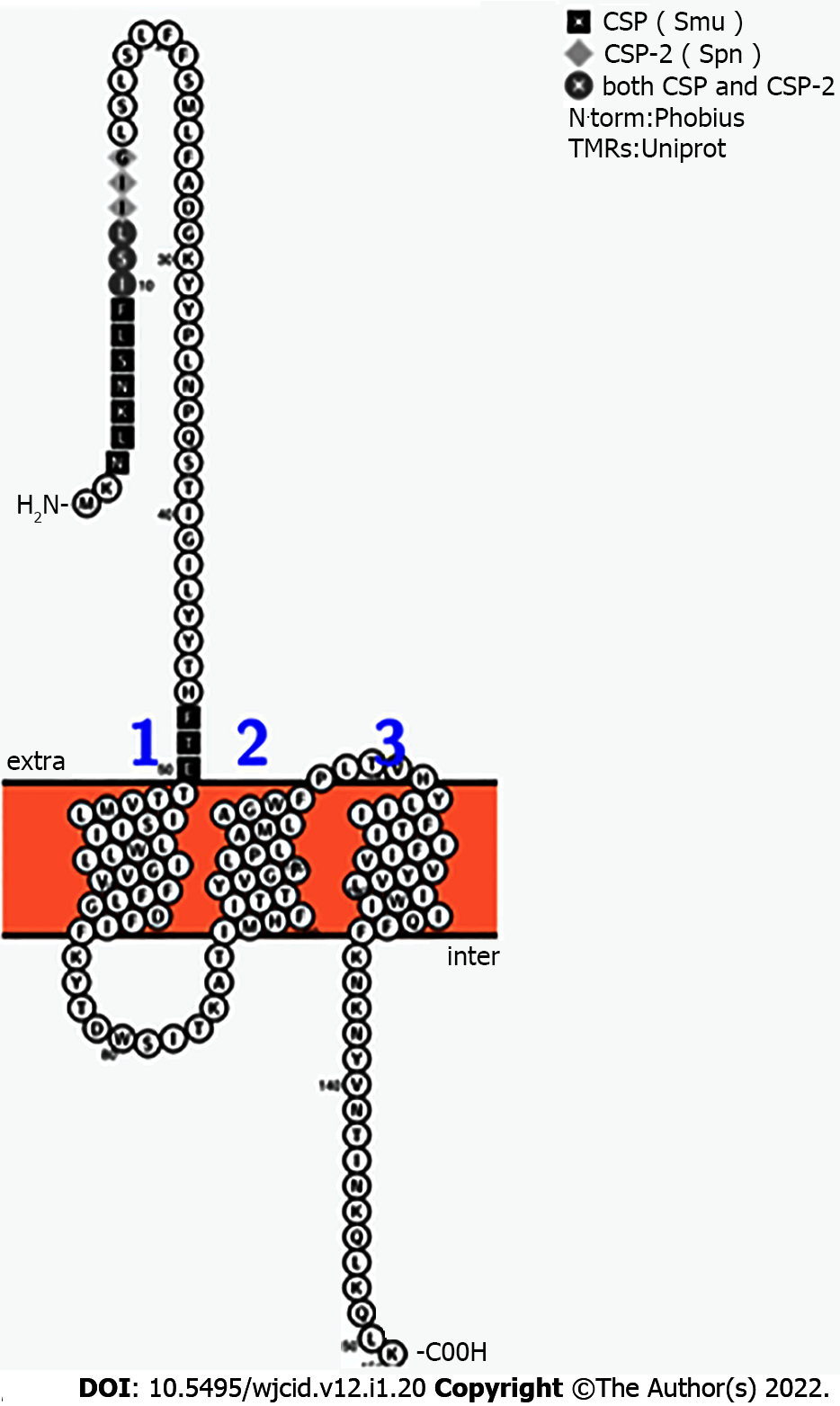
S. aureus BrpS and S. mutans BrsM have a similar arrangement of reactive residues. Lysine, serine, threonine, histidine, tyrosine, and glutamic acid residues were illuminated on a 2-dimensional Protter-generated image of each protein (Figure 5)[29]. This mapping suggested that BrpS and BrsM are partitioned into distinct functional domains separated by the membrane. Functionality appears to occur at the intercellular loop (staphylococcal N’-76-KYTDWSITKAT-86-C’), at the extracellular loop (staphylococcal N’-108-PLTVHY-113-C’), and within a single reactive residue near the membrane at K135 (staphylococcal) within the C’-terminal tail region.
Additionally, the region at the N’-terminus of brpS displays sequence homology with the secreted S. mutans and S. pneumoniae CSPs (Figure 6). The segment of brpS spanning the regions from N’-1-MKNLKNSLFISLIIGLSLSLFFSMLFADGKYYPLNPQSTIGILYYTHFT-50-C’ showed 56% similarity with CSP (S. mutans, 1-SGSLSTFFRLFNRSFTQ A-18) and 30% similarity to CSP-2 (S. pneumoniae, 1-EMRISRIILDFLFLRKK-17).
S. aureus causes 65% of biofilm-associated infections per year[40,41]. Biofilms provide a defense against host immune defenses as well as most antibiotics. An understanding of what regulates S. aureus biofilm formation could lead to treatment options that target this process in S. aureus.
Both sortase A (SrtA) and antiholin (LrgA) are important S. aureus proteins needed for creation and maintenance of biofilms. Sortase A promotes the covalent anchoring of surface proteins to the cell wall of S. aureus[42] that are important in the first stage of biofilm formation. Cell death releases eDNA that is tied to holin/antiholin action. An integral part of mature S. aureus biofilms is eDNA[7]. The function of the antiholin LrgA is to prevent cell autolysis by complexing with CidA holins[38,43].
In this study, we have shown that mutations in either the brpR or brpS gene cause an increase in biofilm formation as well as transcriptional changes of the srtA and lrgA genes, which are linked events. Previous studies with transposon mutants of what was an uncharacterized gene, that we have named brpS, displayed better biofilm formation than the wild-type strain[44,45]. Strains with a mutated lrgA gene have also been shown to produce increased levels of biofilms[46]. Another study has shown that cell lysis caused eDNA to be rapidly produced that could act as a scaffolding for newly forming biofilms[10].
The in silico data; biofilm results with the brpR and brpS mutants; and the data from the transcript abundance changes of the lrgA, srtA, brpR, and brpS genes suggest that BrpR/BrpS comprise a TCS that may be involved in late-stage competence. From the in silico analysis, we speculate that the BrpR protein (that possesses an apparent LytTR DNA binding-motif[47]) may repress srtA transcription. Other proteins that have LytTR motifs, such as BrsR and ComE, have been shown to have multifunctional activities tied to activation and repression[47-49]. Further analysis is required to show that BrpR is capable of binding to this region.
From the data presented, we speculate that BrpS is a receptor for a CSP-like pheromone secreted by S. aureus as a response to competition for resources. The leader peptide of BrpS may function to competitively antagonize the extracellular receptor portion of BrpS from the CSPs of competitive species, such as S. mutans and S. pneumoniae, that inhabit the human upper respiratory tract.
A number of previously completed studies focused on biofilm production and bacterial cell viability due to interactions with CSP-like pheromones. A study by Zhang et al[49] demonstrated that within S. mutans there was a 76.3% decline in cell viability and biofilm mass increased by 89.3% following the addition of CSP to bacterial growth media[49]. In addition, supernatant collected from S. mutans that was co-cultured with Aggregatibacter actinomycetemcomitans caused a 1.3-fold rise in biofilm production within S. mutans[50]. Ample evidence of cell death after CSP exposure has been documented by several studies, however, biofilm production has not been normalized to the viable bacterial cells that remain[51-53]. Nevertheless, a number of Gram-positive bacterial species show cell viability and subsequent biofilm production correlate with the level of CSP added to the media. Further studies should be done to assess the actual increase in biofilm formation by taking into account the findings that competence is accompanied by massive cellular death.
We also believe that there may be a connection between metabolic dormancy and the BrpR/BrpS TCS. If malate production is interrupted after BrpR binds to the sigma factor binding sites, malate conversion to oxalacetate would be halted. As a consequence of this interruption, any acetyl groups generated by acetyl-CoA would not interact with citrate within the citric-acid cycle. Thus, too much acetyl-CoA would arise within the cell. Because these functional groups would be liberated, it is possible that there would be an epigenetic modification and BrpR would be rapidly released from the srtA gene enhancer region. By freeing BrpR from the srtA gene enhancer region, additional BrpR molecules would be available to interact with sigma factors, blocking transcription of brpRS that would lead to even less transcription of the mqo2 gene. The work by Zhang et al[54] used profiling of lysine acetylomes in S. aureus and E. coli to identify a sequence motif, which supports our idea that BrpR may epigenetically block DNA-binding[54]. As part of that study, 412 proteins and 1361 lysine sites were cross-referenced against each other, which led to a conserved motif, N’-RLYELExQLxxxFIRISKxxEIVN-C’, being identified. BrpR has this conserved motif, which is very well conserved among a number of bacterial species. By shutting down malate expression, persister cells could form suddenly as a response to late-stage competence or treatment with the SK-03-92 drug. Thus, BrpR repression of malate production could be connected to formation of persister cells that is a feature of late-stage competence.
Our study suggests that BrpR/BrpS is a TCS that may repress S. aureus biofilm production and be linked to late-stage competence in S. aureus.
Staphylococcus aureus (S. aureus) is a primary cause of skin/soft tissue infections. Biofilm formation is a key component of S. aureus pathogenesis. Thus, an understanding of what regulates biofilm formation in S. aureus is important.
We were interested in characterizing two open reading frames that we thought were tied to biofilm formation in S. aureus.
Determine if mutations in the brpR and brpS genes affected biofilm formation and what the respective proteins had homologies with.
We used biofilm assays and quantitative real-time-polymerase chain reaction (qRT-PCR) analysis to test brpR and brpS mutants compared to the parent strain of S. aureus. Bioinformatic tools were used to determine what roles the BrpR and BrpS proteins may play in S. aureus cells.
The biofilm and qRT-PCR analyses demonstrated that mutations in the brpR and brpS genes affected biofilm formation in S. aureus and led to transcriptional differences in key biofilm-related genes as compared to the parent strain. Further, the BrpR and BrpS proteins share homologies with proteins involved in late-stage competence in streptococcal species.
BrpR/BrpS are likely a new two-component system which regulates biofilm formation in S. aureus.
A better understanding of a new regulator of S. aureus biofilm formation has been identified.
We wish to thank Jean Lee, Ambrose Cheung, Jo Handelsman, and NARSA for bacterial strains and plasmids used in this study as well as Jennifer Klein and the Molecular Biology Laboratory students who ran some of the qRT-PCRs.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Infectious diseases
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Barik S, Sun Y S-Editor: Fan JR L-Editor: A P-Editor: Cai YX
| 1. | Suaya JA, Mera RM, Cassidy A, O'Hara P, Amrine-Madsen H, Burstin S, Miller LG. Incidence and cost of hospitalizations associated with Staphylococcus aureus skin and soft tissue infections in the United States from 2001 through 2009. BMC Infect Dis. 2014;14:296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 123] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 2. | Klein EY, Sun L, Smith DL, Laxminarayan R. The changing epidemiology of methicillin-resistant Staphylococcus aureus in the United States: a national observational study. Am J Epidemiol. 2013;177:666-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 121] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 3. | Klein EY, Mojica N, Jiang W, Cosgrove SE, Septimus E, Morgan DJ, Laxminarayan R. Trends in methicillin-resistant Staphylococcus aureus hospitalizations in the United States, 2010-2014. Clin Infect Dis. 2017;65:1921-1923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 85] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 4. | Schwan WR, Kabir MS, Kallaus M, Krueger S, Monte A, Cook JM. Synthesis and minimum inhibitory concentrations of SK-03-92 against Staphylococcus aureus and other gram-positive bacteria. J Infect Chemother. 2012;18:124-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Schwan WR, Polanowski R, Dunman PM, Medina-Bielski S, Lane M, Rott M, Lipker L, Wescott A, Monte A, Cook JM, Baumann DD, Tiruveedhula VVNPB, Witzigmann CM, Mikel C, Rahman MT. Identification of Staphylococcus aureus cellular pathways affected by the stilbenoid lead drug SK-03-92 using a microarray. Antibiotics (Basel). 2017;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (1)] |
| 6. | Rice KC, Bayles KW. Molecular control of bacterial death and lysis. Microbiol Mol Biol Rev. 2008;72:85-109, table of contents. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 275] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 7. | Okshevsky M, Meyer RL. The role of extracellular DNA in the establishment, maintenance and perpetuation of bacterial biofilms. Crit Rev Microbiol. 2015;41:341-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 348] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 8. | Wood TK, Knabel SJ, Kwan BW. Bacterial persister cell formation and dormancy. Appl Environ Microbiol. 2013;79:7116-7121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 471] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 9. | Zhang Y. Persisters, persistent infections and the Yin-Yang model. Emerg Microbes Infect. 2014;3:e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 153] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 10. | Dufour D, Lévesque CM. Bacterial behaviors associated with the quorum-sensing peptide pheromone ('alarmone') in streptococci. Future Microbiol. 2013;8:593-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Cozzone AJ. ATP-dependent protein kinases in bacteria. J Cell Biochem. 1993;51:7-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Pestova EV, Håvarstein LS, Morrison DA. Regulation of competence for genetic transformation in Streptococcus pneumoniae by an auto-induced peptide pheromone and a two-component regulatory system. Mol Microbiol. 1996;21:853-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 330] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 13. | Xie Z, Okinaga T, Niu G, Qi F, Merritt J. Identification of a novel bacteriocin regulatory system in Streptococcus mutans. Mol Microbiol. 2010;78:1431-1447. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Duthie ES, Lorenz LL. Staphylococcal coagulase; mode of action and antigenicity. J Gen Microbiol. 1952;6:95-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 438] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 15. | Fey PD, Endres JL, Yajjala VK, Widhelm TJ, Boissy RJ, Bose JL, Bayles KW. A genetic resource for rapid and comprehensive phenotype screening of nonessential Staphylococcus aureus genes. mBio. 2013;4:e00537-e00512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 499] [Cited by in RCA: 690] [Article Influence: 57.5] [Reference Citation Analysis (0)] |
| 16. | Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8089] [Cited by in RCA: 8663] [Article Influence: 206.3] [Reference Citation Analysis (0)] |
| 17. |
Novick RP.
The |
| 18. | Bateman BT, Donegan NP, Jarry TM, Palma M, Cheung AL. Evaluation of a tetracycline-inducible promoter in Staphylococcus aureus in vitro and in vivo and its application in demonstrating the role of sigB in microcolony formation. Infect Immun. 2001;69:7851-7857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 157] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 19. | Kloss WE, Pattee PA. Transduction analysis of the histidine region in Staphylococcus aureus. J Gen Microbiol. 1965;39:195-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Iandolo JJ, Kraemer GR. High frequency transformation of Staphylococcus aureus by electroporation. Curr Microbiol. 1990;21:373-376. [RCA] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 137] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 21. | Stepanović S, Vuković D, Jezek P, Pavlović M, Svabic-Vlahović M. Influence of dynamic conditions on biofilm formation by staphylococci. Eur J Clin Microbiol Infect Dis. 2001;20:502-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55:611-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9915] [Cited by in RCA: 11144] [Article Influence: 696.5] [Reference Citation Analysis (0)] |
| 23. | Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149116] [Cited by in RCA: 133922] [Article Influence: 5580.1] [Reference Citation Analysis (1)] |
| 24. | Baba T, Takeuchi F, Kuroda M, Yuzawa H, Aoki K, Oguchi A, Nagai Y, Iwama N, Asano K, Naimi T, Kuroda H, Cui L, Yamamoto K, Hiramatsu K. Genome and virulence determinants of high virulence community-acquired MRSA. Lancet. 2002;359:1819-1827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1028] [Cited by in RCA: 987] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 25. | Baba T, Bae T, Schneewind O, Takeuchi F, Hiramatsu K. Genome sequence of Staphylococcus aureus strain Newman and comparative analysis of staphylococcal genomes: polymorphism and evolution of two major pathogenicity islands. J Bacteriol. 2008;190:300-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 416] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 26. | Clark K, Karsch-Mizrachi I, Lipman DJ, Ostell J, Sayers EW. GenBank. Nucleic Acids Res. 2016;44:D67-D72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 811] [Cited by in RCA: 914] [Article Influence: 91.4] [Reference Citation Analysis (0)] |
| 27. | Karp PD, Billington R, Caspi R, Fulcher CA, Latendresse M, Kothari A, Keseler IM, Krummenacker M, Midford PE, Ong Q, Ong WK, Paley SM, Subhraveti P. The BioCyc collection of microbial genomes and metabolic pathways. Brief Bioinform. 2019;20:1085-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 592] [Cited by in RCA: 558] [Article Influence: 93.0] [Reference Citation Analysis (0)] |
| 28. | UniProt Consortium T. UniProt: the universal protein knowledgebase. Nucleic Acids Res. 2018;46:2699. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1090] [Cited by in RCA: 1257] [Article Influence: 179.6] [Reference Citation Analysis (0)] |
| 29. | Omasits U, Ahrens CH, Müller S, Wollscheid B. Protter: interactive protein feature visualization and integration with experimental proteomic data. Bioinformatics. 2014;30:884-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 762] [Cited by in RCA: 974] [Article Influence: 81.2] [Reference Citation Analysis (0)] |
| 30. | Blom N, Sicheritz-Pontén T, Gupta R, Gammeltoft S, Brunak S. Prediction of post-translational glycosylation and phosphorylation of proteins from the amino acid sequence. Proteomics. 2004;4:1633-1649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1425] [Cited by in RCA: 1558] [Article Influence: 74.2] [Reference Citation Analysis (0)] |
| 31. | de Castro E, Sigrist CJ, Gattiker A, Bulliard V, Langendijk-Genevaux PS, Gasteiger E, Bairoch A, Hulo N. ScanProsite: detection of PROSITE signature matches and ProRule-associated functional and structural residues in proteins. Nucleic Acids Res. 2006;34:W362-W365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1081] [Cited by in RCA: 1204] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 32. | Yang J, Yan R, Roy A, Xu D, Poisson J, Zhang Y. The I-TASSER Suite: protein structure and function prediction. Nat Methods. 2015;12:7-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3815] [Cited by in RCA: 4236] [Article Influence: 423.6] [Reference Citation Analysis (0)] |
| 33. | Delano WL. The PyMOL Molecular Graphics System, Version 1.2r3pre, Schrödinger, LLC.. |
| 34. | Hwang S, Gou Z, Kuznetsov IB. DP-Bind: a web server for sequence-based prediction of DNA-binding residues in DNA-binding proteins. Bioinformatics. 2007;23:634-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 175] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 35. | Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57523] [Cited by in RCA: 61987] [Article Influence: 1771.1] [Reference Citation Analysis (0)] |
| 36. | Molenaar D, van der Rest ME, Petrović S. Biochemical and genetic characterization of the membrane-associated malate dehydrogenase (acceptor) from Corynebacterium glutamicum. Eur J Biochem. 1998;254:395-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 79] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 37. | Mazmanian SK, Liu G, Ton-That H, Schneewind O. Staphylococcus aureus sortase, an enzyme that anchors surface proteins to the cell wall. Science. 1999;285:760-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 780] [Cited by in RCA: 804] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 38. | Ranjit DK, Endres JL, Bayles KW. Staphylococcus aureus CidA and LrgA proteins exhibit holin-like properties. J Bacteriol. 2011;193:2468-2476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 119] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 39. | Kavanaugh JS, Flack CE, Lister J, Ricker EB, Ibberson CB, Jenul C, Moormeier DE, Delmain EA, Bayles KW, Horswill AR. Identification of extracellular DNA-binding proteins in the biofilm matrix. mBio. 2019;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 111] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 40. | Archer NK, Mazaitis MJ, Costerton JW, Leid JG, Powers ME, Shirtliff ME. Staphylococcus aureus biofilms: properties, regulation, and roles in human disease. Virulence. 2011;2:445-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 553] [Cited by in RCA: 667] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 41. | Otto M. Staphylococcal biofilms. Curr Top Microbiol Immunol. 2008;322:207-228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 339] [Cited by in RCA: 525] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 42. | Marraffini LA, Dedent AC, Schneewind O. Sortases and the art of anchoring proteins to the envelopes of gram-positive bacteria. Microbiol Mol Biol Rev. 2006;70:192-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 535] [Cited by in RCA: 526] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 43. | Sadykov MR, Bayles KW. The control of death and lysis in staphylococcal biofilms: a coordination of physiological signals. Curr Opin Microbiol. 2012;15:211-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 44. | Kadurugamuwa JL, Sin L, Albert E, Yu J, Francis K, DeBoer M, Rubin M, Bellinger-Kawahara C, Parr TR Jr, Contag PR. Direct continuous method for monitoring biofilm infection in a mouse model. Infect Immun. 2003;71:882-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 186] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 45. | Xiong YQ, Willard J, Kadurugamuwa JL, Yu J, Francis KP, Bayer AS. Real-time in vivo bioluminescent imaging for evaluating the efficacy of antibiotics in a rat Staphylococcus aureus endocarditis model. Antimicrob Agents Chemother. 2005;49:380-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 81] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 46. | Brunskill EW, Bayles KW. Identification of LytSR-regulated genes from Staphylococcus aureus. J Bacteriol. 1996;178:5810-5812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 116] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 47. | Okinaga T, Xie Z, Niu G, Qi F, Merritt J. Examination of the hdrRM regulon yields insight into the competence system of Streptococcus mutans. Mol Oral Microbiol. 2010;25:165-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 48. | Kreth J, Hung DCI, Merritt J, Perry J, Zhu L, Goodman SD, Cvitkovitch DG, Shi W, Qi F. The response regulator ComE in Streptococcus mutans functions both as a transcription activator of mutacin production and repressor of CSP biosynthesis. Microbiology (Reading). 2007;153:1799-1807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 49. | Zhang K, Ou M, Wang W, Ling J. Effects of quorum sensing on cell viability in Streptococcus mutans biofilm formation. Biochem Biophys Res Commun. 2009;379:933-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 50. | Szafrański SP, Deng ZL, Tomasch J, Jarek M, Bhuju S, Rohde M, Sztajer H, Wagner-Döbler I. Quorum sensing of Streptococcus mutans is activated by Aggregatibacter actinomycetemcomitans and by the periodontal microbiome. BMC Genomics. 2017;18:238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 51. | Qi F, Kreth J, Lévesque CM, Kay O, Mair RW, Shi W, Cvitkovitch DG, Goodman SD. Peptide pheromone induced cell death of Streptococcus mutans. FEMS Microbiol Lett. 2005;251:321-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 52. | Ween O, Gaustad P, Håvarstein LS. Identification of DNA binding sites for ComE, a key regulator of natural competence in Streptococcus pneumoniae. Mol Microbiol. 1999;33:817-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 117] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 53. | Perry JA, Cvitkovitch DG, Lévesque CM. Cell death in Streptococcus mutans biofilms: a link between CSP and extracellular DNA. FEMS Microbiol Lett. 2009;299:261-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 104] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 54. | Zhang Y, Wu ZX, Wan XL, Liu P, Zhang JB, Ye Y, Zhao YM, Tan MJ. Comprehensive profiling of lysine acetylome in Staphylococcus aureus. Sci China Chem. 2014;57:732-738. [RCA] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |