Peer-review started: November 14, 2018
First decision: December 5, 2018
Revised: January 11, 2019
Accepted: January 21, 2019
Article in press: January 22, 2019
Published online: March 12, 2019
Processing time: 117 Days and 4.2 Hours
Resistant hypertension (RH) has a prevalence of around 12% and is associated with an increased risk of cardiovascular disease, progression to end-stage renal disease, and even mortality. In 2017, the American College of Cardiology and American Heart Association released updated guidelines that detail steps to ensure proper diagnosis of RH, including the exclusion of pseudoresistance. Lifestyle modifications, such as low salt diet and physical exercise, remain at the forefront of optimizing blood pressure control. Secondary causes of RH also need to be investigated, including screening for obstructive sleep apnea. Notably, the guidelines demonstrate a major change in medication management recommendations to include mineralocorticoid receptor antagonists. In addition to advances in medication optimization, there are several device-based therapies that have been showing efficacy in the treatment of RH. Renal denervation therapy has struggled to show efficacy for blood pressure control, but with a re-designed catheter device, it is once again being tested in clinical trials. Carotid baroreceptor activation therapy (BAT) via an implantable pulse generator has been shown to be effective in lowering blood pressure both acutely and in long-term follow up data, but there is some concern about the safety profile. Both a second-generation pulse generator and an endovascular implant are being tested in new clinical trials with hopes for improved safety profiles while maintaining therapeutic efficacy. Both renal denervation and carotid BAT need continued study before widespread clinical implementation. Central arteriovenous anastomosis has emerged as another possible therapy and is being actively explored. The ongoing pursuit of blood pressure control is a vital part of minimizing adverse patient outcomes. The future landscape appears hopeful for helping patients achieve blood pressure goals not only through the optimization of antihypertensive medications but also through device-based therapies in select individuals.
Core tip: Resistant hypertension (RH) is associated with an increased risk of cardiovascular events as well as mortality. Updated American College of Cardiology and American Heart Association guidelines in 2017 promote the use of mineralocorticoid receptor antagonists, which is a major change from prior guidelines. Device-based therapies such as renal denervation and carotid baroreceptor activation have emerged as innovative treatment modalities. They are continuing to be refined to improve their safety as well as efficacy profiles. Overall more validation is needed for device-based therapies, especially in the RH population.
- Citation: Schmidt K, Kelley W, Tringali S, Huang J. Achieving control of resistant hypertension: Not just the number of blood pressure medications. World J Hypertens 2019; 9(1): 1-16
- URL: https://www.wjgnet.com/2220-3168/full/v9/i1/1.htm
- DOI: https://dx.doi.org/10.5494/wjh.v9.i1.1
Resistant hypertension (RH) is defined as blood pressure (BP) elevation above goal despite the use of three or more anti-hypertensive medications of different classes with at least one being a diuretic, all at the maximum tolerated doses, after excluding pseudoresistance[1].
RH is achieving more recognition as a distinct category of hypertension. It has its own pathophysiology, patient characteristics, and consequences. This subgroup of patients carries higher risk for cardiovascular morbidity and mortality and may benefit from special diagnostic and therapeutic approaches to control their BP. Therefore, RH needs its own set of solutions, which may eventually include treatment modalities outside of lifestyle modification and medication-based therapies.
Following an accumulation of results from more recent clinical trials, major medical organizations across the globe have published specific RH guidelines with updated recommendations on proper diagnosis and multi-step treatment algorithms. The American College of Cardiology (ACC) and American Heart Association (AHA) were two societies to release joint updated guidelines in 2017[2]. AHA has also released an updated scientific statement that comprehensively reviews the current body of evidence pertaining to RH[1]. In 2018, the European Society of Cardiology/European Society of Hypertension (ESC/ESH) published updated guidelines for management of arterial hypertension, and included a section specifically for RH[3].
The pathogenesis of RH is not fully understood, but is thought to be a combination of fluid retention, sympathetic system activation, and arterial stiffness[4]. Aldosterone has emerged as a key player in these mechanisms, making mineralocorticoid receptor antagonists (MRA) that much more important in the treatment for RH[4].
Population-based studies estimate the prevalence of RH to be 10%-15%[1]. The negative health impact on the lives of this group of patients is far-reaching. Patients with RH have an increased risk of cardiovascular disease such as non-fatal myocardial infarction, congestive heart failure, coronary heart disease, and stroke[5-8]. In addition, these patients also are at risk of developing chronic kidney disease and end-stage renal disease, as well as increased all-cause mortality[7-11].
In addition to lifestyle modifications, medication-based treatment has been the mainstay of BP control since the 1930s, and continues to be the only widespread method currently used to treat RH. Standard treatment regimens consist of selecting not only from a variety of drug classes, but also mechanistically complementary ones. Angiotensin-converting enzyme inhibitors (ACEi), angiotensin receptor blockers (ARB), calcium channel blockers, diuretics, and beta-blockers continue to be the commonly used pharmacologic agents. The validation of MRAs in the treatment of RH from recent randomized clinical trials has led to adoption of evidence-based standards for their use[2].
Although medications are the basis of hypertension management, even as early as 1938 there was experimentation with a non-medication approach for BP reduction with the thoracolumbar sympathetic chain removal[12-14]. Despite the disastrous consequences of that particular modality, the concept of a non-medication approach endured. Today there are several device-based treatments being actively studied in clinical trials, including renal denervation (RDN), carotid baroreceptor activation therapy (BAT), and central arteriovenous (cAV) anastomosis.
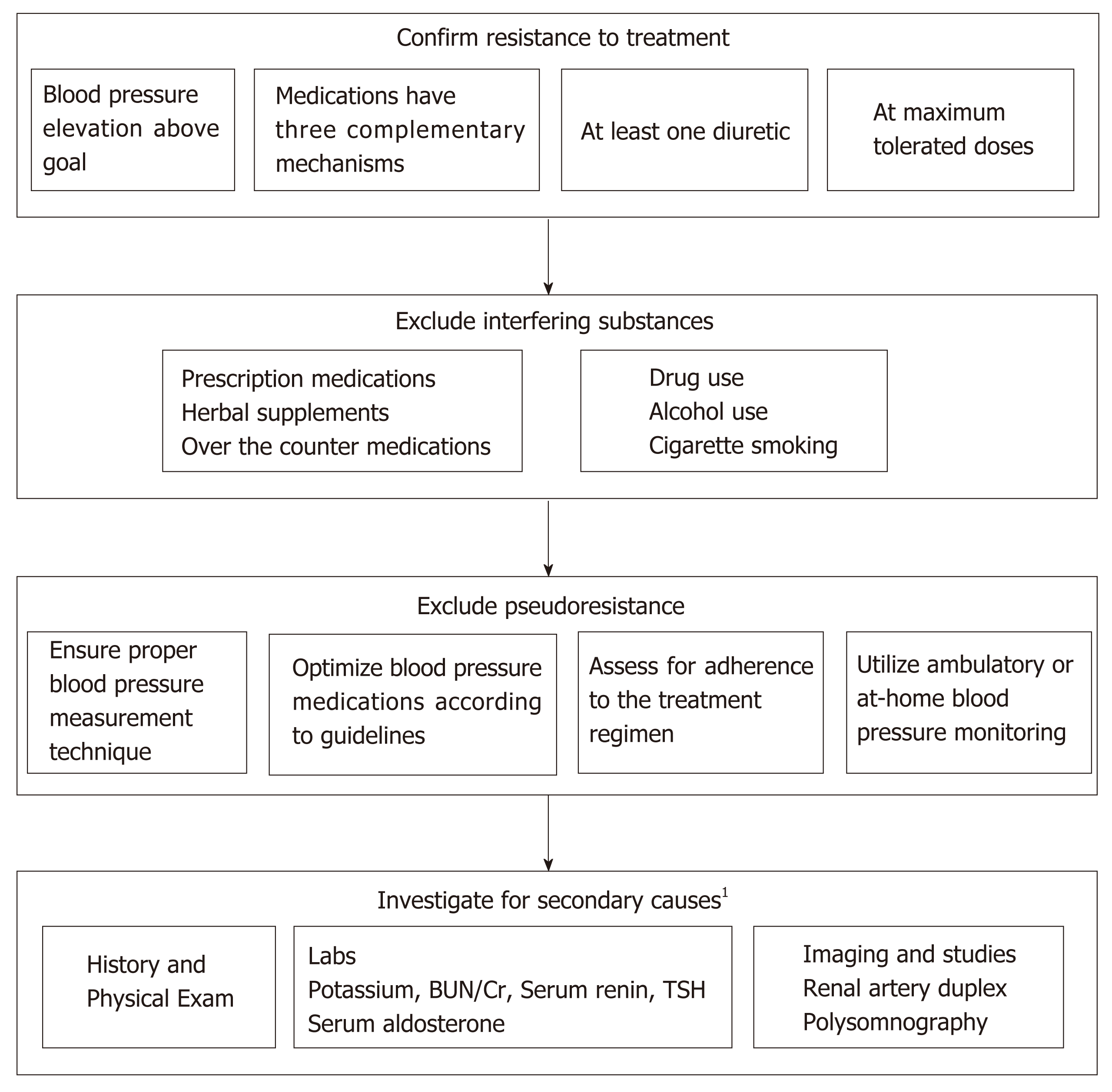
As previously stated, the definition of RH is BP elevation above goal despite the use of three or more anti-hypertensive medications of different classes, with at least one being a diuretic, all at the maximum tolerated doses after excluding pseudoresistance[1-3]. If BP is controlled with the use of four or more anti-hypertensive medications, this is called controlled RH[1]. A key part of this definition is that pseudoresistance must be ruled out first.
Pseudoresistance has four common etiologies including inappropriate measurement, sub-optimal prescribing (clinician inertia), white coat hypertension, and medication nonadherence. Medication nonadherence makes a sizeable contribution to pseudoresistance. One large meta-analysis concluded that between 31%-50% of those labeled as having RH are simply nonadherent to their medication regimens[15]. In all of the major clinical trials involving BP monitoring, ensuring adherence is always a notable concern. Otherwise, adherence can strongly influence the results. This was pointedly displayed in the SYMPLICITY-HTN 3 trial, where a post hoc analysis concluded that variable patterns of medication adherence played a significant part in producing results that were different from prior trials in the series, SYMPLICITY HTN 1 and 2[16,17]. Adherence to medication can be measured both indirectly through self-reported questionnaires (SRQs), and also via direct means. The imperfect gold standard currently used is the Medication Event Monitoring System (MEMS), where a sensor is placed in the medication vial cap to record every time the bottle is opened[18]. Trials have used a variety of methods to assess adherence, such as liquid chromatography and mass spectroscopy, patient self-reporting or pill count[17]. The inherent difficulty of properly measuring adherence is obvious, but not easily solved. The cost of implementing such direct measures is a significant barrier. As pharmacology literature suggests that there is at least moderate correlation between SRQs and MEMS, patient self-reporting will likely continue to be in widespread use[18].
A second major contributing factor to pseudoresistance is white coat hypertension, which is defined as in-office BP measurements above the threshold to be considered as hypertensive with out-of-office BP readings being lower and within goal range. The prevalence of white coat hypertension ranges from 15%-40% in the outpatient setting and should be ruled out in all cases of in-office hypertension[19]. Both ACC/AHA and ESC/ESH guidelines conclude that it is reasonable to screen for the presence of white coat hypertension using either ambulatory BP monitoring or home BP monitoring prior to the diagnosis of hypertension[2,3].
Similarly, inappropriate measurement of BP in the clinic setting should also be considered in cases of labeled RH. Bhatt et al[20] reported that in 130 patients noted to have uncontrolled hypertension by triage vitals, 33% of them were actually mislabeled and had controlled BP when checked with an automated BP machine during the same visit after sitting quietly in a room for 5 min. According to both European and United States major guidelines, patients should be seated with their backs supported and feet on the floor for at least 5 min, the cuff properly selected based on arm circumference, and arm supported at the level of the heart[2,3,21-25]. Although the gold standard is the use of mercury sphygmomanometer, many automated BP cuffs have been validated and are acceptable for use in clinical practice[21]. Whether using the manual or automated approach, the principles listed above still apply.
Although nonadherence, inappropriate measurement, and white coat hypertension are all major components of pseudoresistance, unfortunately there is also a contribution from the side of the healthcare providers. Clinician inertia is described as failure by the provider to intensify treatment as indicated[24]. An oft-cited but apropos study of 800 male patients receiving care at five outpatient Department of Veterans Affairs centers revealed that despite 40% of the patients having elevated BP readings at their clinic visits, only at 6.7% of the visits were the BP regimens intensified as indicated[25]. Another analysis from a large ambulatory care survey with over 19000 patients showed that providers were not prescribing anti-hypertensives according to the current ACC/AHA guidelines, even though they had been published two years earlier[26]. Some of the reasons behind clinician inertia include over-estimation of the care being given already, unsupported decision making that ends up delaying care, as well as lack of knowledge of how to achieve BP goals[24,27,28].
All cases of RH should be explored for secondary causes of hypertension as listed in Table 1[1,29,30]. Parts of the history may lead the provider to strongly consider one cause over another, but the appropriate lab and imaging workup should be done in all patients with confirmed RH. Of note, one of the most common causes of RH is obstructive sleep apnea (OSA), with estimates ranging from 60%-80%, making this an important patient population to identify early[31,32].
| Hyperaldosteronism | Serum renin-aldosterone ratio |
| Renal parenchymal disease | Serum creatinine |
| Obstructive sleep apnea | Polysomnography |
| Pheochromocytoma | Serum metanephrine and 24-h urine catecholamines |
| Renal artery stenosis | Renal artery duplex |
| Coarctation of the aorta | CT angiogram |
| Cushing’s disease | 24-h free urinary cortisol and late-night salivary cortiso1 |
| Thyroid disease | TSH and freeT4 |
| Acromegaly | Serum growth hormone |
As summarized in Table 2, there are many medications ranging from chemotherapy all the way to common over-the-counter remedies, herbal supplements, food items, and illicit substances that are known to raise BP[29,33,34]. Therefore any of these substances should be considered during the initial evaluation of a patient.
Diagnostic approach to RH is summarized in Figure 1.
| NSAIDs |
| Estrogens/Progestins |
| Anabolic Steroids |
| Corticosteroids |
| COX-2 Inhibitors |
| SSRI/SNRI |
| Tricyclic antidepressants |
| Lithium |
| Buspirone |
| Carbamazepine |
| Calcineurin inhibitors |
| Pseudoephedrine |
| Amphetamine derivatives |
| 1Chemotherapy agents |
| Caffeine |
| Methamphetamines |
| Cocaine |
| Ginseng, St John’s Wort, Ephedra, Yohimbine |
| Alcohol |
Our best estimate of the prevalence of RH based on the range of data accumulated is between seven and eighteen percent. The true prevalence is uncertain and varies depending on study populations and study settings. This is in part due to how past studies defined RH by BP readings and medication lists without being able to assess medication adherence and optimization. Undoubtedly, this ambiguity results in a percentage of patients being labeled as treatment resistant when in fact they are only nonadherent, or the doses of their medications are suboptimal. Apparent treatment RH is a new term that has been created recently to better characterize this known issue among studies defining the prevalence of RH. It is used when data on patient adherence, medication dose, or out of office BP data is missing, causing an inability to exclude pseudoresistance[1].
The proportion of patients with RH among treated hypertensive patients is reported to be around 10%-15% in population based studies, and slightly higher at 15%-18% in clinic-based studies[1]. Roughly 9% of hypertensive adults in the United States met criteria for RH in the National Health and Nutrition Examination Survey (NHANES) from 2003 through 2008[35,36]. This represented 12.8% of patients on antihypertensive drug therapy[36]. For any population data analysis, the influence of pseudoresistance on the data cannot be ignored. For example, in a cohort of 68045 patients in Spain, the prevalence of RH dropped from 12.2% to 7.6% after excluding white coat hypertension, one of the factors in pseudoresistance[37]. In addition, a recent meta-analysis of 91 worldwide population-based studies including 3.2 million patients on treatment for hypertension found the prevalence of true-RH to be 10.3% after excluding pseudoresistance [38].
The pathogenesis of RH is not fully understood, but is thought to be a combination of several mechanisms. Part of the difficulty of elucidating pathogenesis is the overlap between RH and other well-defined pathological states, such as obesity and chronic kidney disease (CKD), so that it is unclear if the mechanisms are unique to RH or merely stemming from the other concomitant conditions. The three most significant underlying processes that have been linked to RH are fluid retention, sympathetic system activation, and arterial stiffness[4]. Excess fluid retention can be caused by excess salt ingestion, impaired renal natriuresis, as well as increased aldosterone production[4]. Aldosterone is of particular interest in RH not only because it induces salt retention and thereby fluid retention, but also because of other non-genomic pro-inflammatory effects that can promote a pharmacologically resistant state[39,40]. Thus, mineralocorticoid receptor blockade becomes much more important in RH, a fact that has been reflected in the 2017 ACC/AHA guidelines and the 2018 AHA scientific statement, which now include MRAs as one of the front-runner drug classes for RH[1,2]. In addition to fluid and salt retention, sympathetic system activation is a cornerstone mechanism for RH[12]. Using norepinephrine regional spillover techniques, researchers have found statistically significant activation of renal sympathetic system in patients with RH when compared to both healthy subjects and those with non-RH[41]. Lastly, arterial stiffness is thought to be another major mechanism. There are numerous underlying processes in the pathogenesis of arterial stiffness, some of which include endothelial dysfunction, salt consumption, and sympathetic signaling[42]. Several studies have shown that RH patients have higher arterial stiffness than those with controlled hypertension[43-45]. Increasing arterial stiffness leads to increasing BP, which in turn increases arterial stiffness, and on and on the vicious cycle goes[44,45].
Before pharmacologic or other intervention for RH can commence, it is recommended to exclude secondary forms of hypertension and white coat hypertension. Guidelines from the ACC/AHA have been consistent on these evaluations for some time, though recently more emphasis has been placed on ambulatory BP monitoring to exclude white coat hypertension and detect masked hypertension.
According to the 2018 AHA Scientific Statement on the detection, evaluation, and management of RH, as well as ESC/ESH guidelines, patients should be on maximally tolerated doses of mechanistically complementary antihypertensive agents[1,3]. This would normally include an ACEi or ARB, a long acting calcium channel blocker, and a diuretic that is appropriate for the patient’s underlying kidney function[1,3]. One consideration to make before adding another medication to the patient’s therapeutic regimen would be whether the patient would benefit from switching from hydrochlorothiazide to either chlorthalidone or indapamide. Chlorthalidone or indapamide produce a more predictable natriuresis than hydrochlorothiazide below an eGFR of 45 mL/min/1.73 m2[1]. This produces a greater reduction in plasma volume which is of importance in RH linked to fluid overload. Switching from hydrochlorothiazide to the same daily dose of chlorthalidone has been shown to produce reductions in BP of 7-8 mmHg in studies comparing the two medications[46,47].
Recent changes to the treatment algorithm for RH now include emphasis on MRA. This new addition to the evidence-based algorithm for RH is based on multiple randomized controlled trials published over the last 10 years[47-50]. The addition of a MRA to a patient’s BP regimen is now recommended if his or her BP remains above goal despite maximally tolerated doses of three antihypertensive agents including a diuretic appropriately administered for the patient’s renal function. MRAs can be more effective than the addition of alpha- or beta-blockers in patients with low renin status or salt sensitivity[51]. The once daily administration of spironolactone, combined with its effectiveness at doses as low as 12.5 to 25 mg, make it an ideal fourth agent in most patients. Ideal patients for initiation of spironolactone have an eGFR > 45 mL/min·1.73 m2 and a baseline serum potassium of < 4.5 mmol/L due to the increased risk of hyperkalemia. Adverse effects at higher doses include gynecomastia and erectile dysfunction in men, and irregular menses in women.
Since the 1980s, it has been recognized that reducing dietary sodium intake can modestly reduce systolic blood pressure (SBP) 2-10 mmHg and diastolic blood pressure (DBP) 1-6 mmHg, and can improve the likelihood of successfully withdrawing anti-hypertensive medications[52-56]. It was during this era that the now ubiquitous Dietary Approaches to Stop Hypertension (DASH) diet, which limits salt intake to less than 2300 mg daily among other dietary restrictions, was shown to be effective not only for weight loss but also for BP reduction[52]. Multiple analyses since that time continue to show that lower salt intake can help in reducing BP[57,58]. All major guidelines for management of essential hypertension include a low salt diet, with recommended consumption limited to less than 2300 mg per day[2,3,59].
The RH population has also been studied in regard to low salt diet, and they had dramatic reductions of BP, on average 22.7/9.1 mmHg, which is much more than in patients with essential hypertension[60]. Since there is over-whelming evidence for BP reduction in the rest of the hypertensive population including CKD patients, it is recommended that patients with RH subscribe to a low salt diet of less than 2300 mg per day. However, there are more studies needed to identify the ideal level of salt restriction in the RH population.
The worldwide epidemic of obesity has been well described, with the prevalence of obesity tripling between 1975 and 2016[61]. According to the NCD Risk Factor Collaboration data, between 1975 and 2014, the obesity prevalence in men increased from 3.2% to 10.8, and in women 6.4% to 14.9%[62]. The highest rate of obesity in the world is within the United States with most recent CDC data reporting a prevalence of 39.8% of the adult population[63]. Various studies have shown that obesity correlates with having hypertension. The NHANES population data supports this, reporting that 36% of obese patients also have hypertension[64-66]. New evidence is emerging that obesity also increases the risk of RH. The underlying mechanism is multifaceted and involves signaling from adipokines, the renin-angiotensin-aldosterone pathway, and adipose-induced endothelial dysfunction[67]. There is a high level of evidence, including evidence from many randomized controlled trials, that even a 5 kg weight loss can lead to a reduction in BP in a population without RH[52,68-70]. A 5%-10% reduction of body weight continues to be a front-line recommendation in both older and newer hypertension management guidelines in both the United States and Europe[2,3]. However, it is worth noting that weight loss in patients with RH is understudied to date[1]. In addition, the long-term BP lowering effects appear to be somewhat attenuated, with the biggest gains happening in the initial stages of weight loss followed by a reduction in gains[71]. It is also possible that the long term effects are simply not being observed by the current studies due to limited duration of follow up[71,72]. Although weight loss has tremendous health benefits, it is very difficult for patients to sustain a lower weight over time[67,73]. Considering this, along with the lack of data in RH patients, weight loss may have limited potential as a long-term tool for BP reduction in this group.
Physical exercise has been extensively studied and has consistently been shown to reduce both SBP and DBP[74]. There are different forms of exercise including aerobic activity, isometric exercises, resistance training, and a combination of any of the above, and each category has been studied for BP lowering effect. Based on results from a large meta-analysis, aerobic activity had a mean BP reduction of 10.8/4.7 mmHg, which was the largest reduction of BP of all the exercise types[75]. Isometric exercises also show significant BP reduction[74,76-78]. Dynamic resistance seems to have a modest but significant BP lowering effect in hypertensive individuals when compared to normotensive individuals, with slightly greater effect when using larger muscle groups[78-80]. Exercise has been recommended in major guidelines for hypertension for many years, and continues to be a core lifestyle modification in both the recent ACC/AHA guidelines for RH as well as ESC/ESH guidelines for hypertension[2,3]. Although the RH population has not been studied extensively in this regard, there have been a few small trials that have already shown BP reduction with moderate level activity[81,82].
Pharmacologic therapy for the treatment of RH includes combinations of three or more drugs of different classes, including a diuretic[1]. The choice of agents should be tailored to the individual patient. Factors to consider include prior effect of medication, history of adverse events, intolerances, allergies, financial limitations, and the presence of diabetes, chronic kidney disease, and other comorbidities. NHANES data between 2005 and 2008 showed that in patients with uncontrolled BP on at least 3 medications, the most commonly used classes of medications other than diuretics were ACEi (50.8%), ARB (39.1%), beta-blockers (62.1%), dihydropyridine calcium channel blockers (40.0%), and non-dihydropyridine calcium channel blockers (18.9%)[83].
Over the last 10 years, observational studies and randomized controlled trials have accumulated evidence supporting MRAs, including eplerenone and spironolactone[48,49]. In 2011, the ASPIRANT trial included 111 patients who were all on diuretics, with a majority on a beta-blocker, calcium channel blocker, and either an ACEi or ARB[84]. In this study, patients randomized to 25 mg of spironolactone saw a significant reduction in SBP in the office (8.6 mmHg) and ambulatory setting (6.5 mmHg)[84].
A Denmark-based, multicenter, double blind, randomized, placebo-controlled study in 2013 added 25 mg of Spironolactone to 119 patients who were on triple therapy for RH[49]. Average daytime placebo-corrected SBP was reduced by 8.9 mmHg. Also, office, night-time and 24 h BP as well as pulse pressures were reduced significantly[49]. Urinary albumin to creatinine ratio was also significantly reduced in the spironolactone group.
A comparison between Spironolactone, Doxazosin, Bisoprolol, and a placebo was the focus of the PATHWAY-2 trial conducted at 12 different centers in the United Kingdom[48]. The mean reduction of baseline BP observed in the Spironolactone group was 14.4 mmHg. This was found to be statistically significant when compared to placebo, Doxazosin, or Bisoprolol, which reduced BP by 4.2 mmHg, 9.1 mmHg, and 8.4 mmHg respectively. No statistically significant difference was observed between agents with regards to serious adverse events or patient withdrawals due to adverse events.
A meta-analysis that included the three aforementioned randomized controlled trials in addition to a Brazilian open-label, parallel randomized trial (ReHOT), showed that adding on Spironolactone to the treatment regimen of patients with RH had a mean reduction of SBP and DBP of 16.67 mmHg and 6.11 mmHg, respectively[51,85]. It is important to note that the rates of serious adverse effects, including hyperkalemia, or patient withdrawals from the trials tended to be higher in patients treated with spironolactone than placebo[85]. This meta-analysis, in conjunction with these randomized controlled trials, has influenced the evidence-based treatment algorithm now published by the ACC/AHA in their latest guidelines.
Expert opinion also offers specific methods to guide treatment. The pharmacologic management of RH is summarized in the AHA 2018 scientific statement and is based on the currently available clinical evidence[1].
Briefly, if, after the addition of a MRA to the patient’s existing 3 BP medication regimen, the patient’s BP remains uncontrolled, this should trigger an assessment of the patient’s resting sympathetic tone and its contribution to the patient’s RH. A simple way to complete this assessment is to assess the patient’s resting heart rate. If his or her heart rate is above 70 beats per minute, an addition of a beta-blocker is a reasonable addition. If there exists a contraindication to beta-blockade, a central acting alpha-2 agonist, such as clonidine, can be considered. The patch form of clonidine has less risk of rebound hypertension after discontinuation or nonadherence.
If BP is not controlled after addition of either beta-blockade or clonidine, hydralazine is an additional option. Doses less than 150 mg per day decrease the chance of developing drug-induced lupus. The addition of nitrates to hydralazine in cases of heart failure is generally recommended.
Minoxidil should be withheld until all other options are exhausted due to its high rate of discontinuation from frequent dosing and adverse effects, including hirsutism. In addition, minoxidil must be given with both a loop diuretic and a beta-blocker due to a predictable increase in sympathetic tone and sodium retention. It is generally recommended that such individualized therapy must occur with close follow up for medication titration and monitoring for adverse effects.
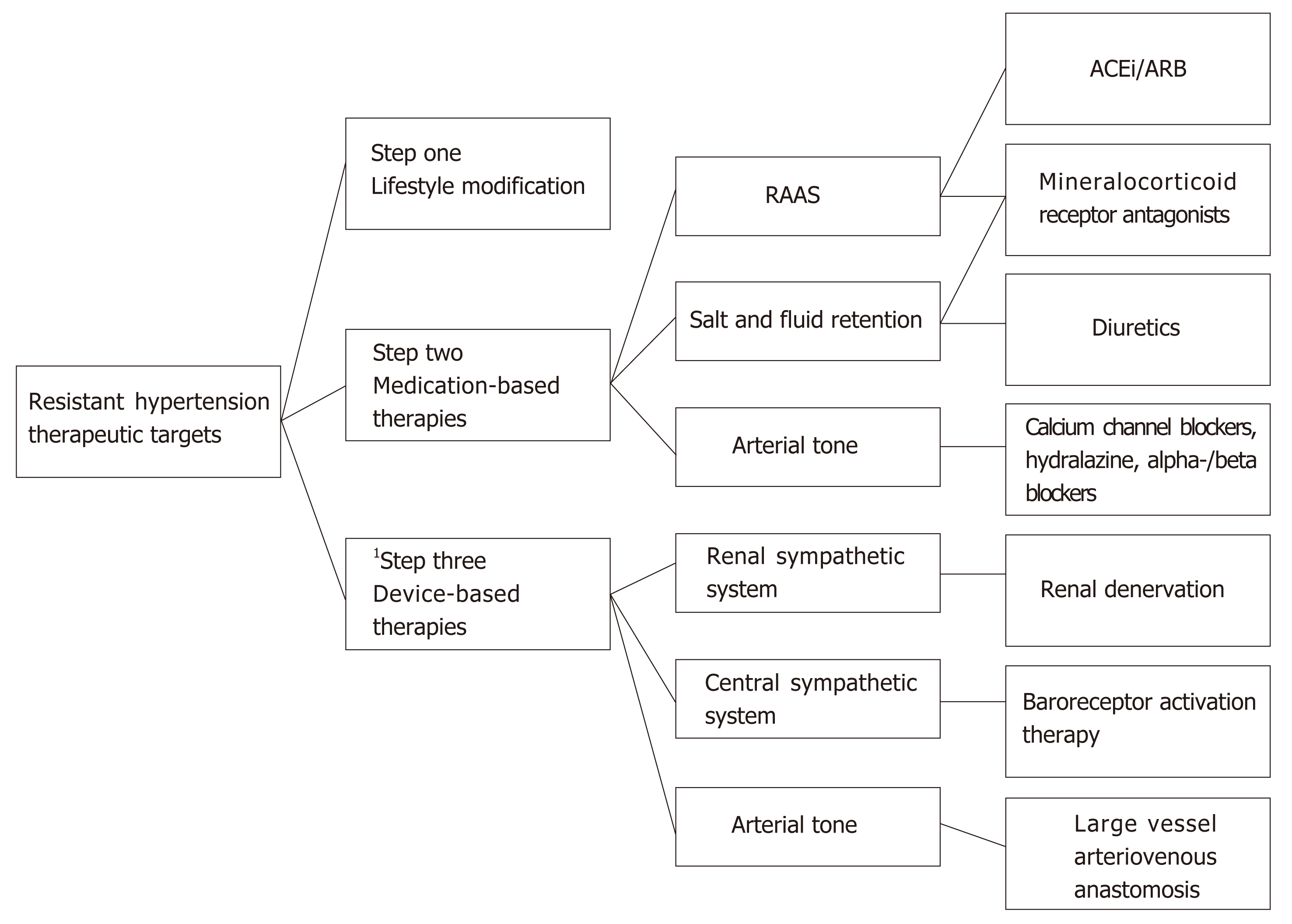
Evidence from numerous animal studies and physiological studies demonstrate that sympathetic activation is a key mechanism for not only essential hypertension, but also RH[12,41,86,87]. Most device-based therapies attempt to control BP by interfering with sympathetic signaling and thereby disrupting this crucial mechanism of hypertension.
The renal sympathetic supply has been a promising target given the physiological link between sympathetic input and hypertension[41]. Renal denervation therapy (RDN) is a device-based therapy to ablate the renal sympathetic nerves by radiofrequency or ultrasound waves emitted by an endovascular catheter. RDN showed early promise after both its human feasibility trial (SYMPLICITY HTN-1), and a larger randomized prospective study (SYMPLICITY HTN-2), demonstrated not only a reduction of in-office BP, but also an acceptable safety profile for the device[88,89]. The subsequent trial, SYMPLICITY HTN-3, halted the enthusiasm. SYMPLICITY HTN-3 was a single blind, randomized, sham-controlled trial, arguably the most robust design out of the studies. The results were surprising and also sobering to the field of RDN with no significant difference in in-office BP measurements between the sham procedure group and the RDN group[90]. Extensive analysis has been done to explain the contradictory findings. Prominent explanations include incomplete nerve ablation due to poorly designed catheter electrodes, proceduralists’ unfamiliarity with the technique, and variable BP medication adherence in the study participants[41,91,92].
Since SYMPLICITY HTN-3, the catheter ablation device has been re-designed to allow for circumferential and thus more complete ablation (Symplicity SpyralTM Multi-electrode Renal Denervation Catheter, MedTronic). With the newly designed device and specially trained proceduralists, new trials have already started. SPYRAL HTN-OFF MED is a proof of concept trial. Early results have shown significant blood pressure reduction; however, this study population of hypertensive patients are treatment naïve and therefore do not have RH by definition[93]. Similarly, the SPYRAL HTN-ON MED trial, which has selected a hypertensive population being treated with one to three BP medications, has shown significant reduction of BP in the RDN group compared to the sham-control[16].
RDN can also be accomplished via ultrasound (Paradise Renal Denervation System, ReCor Medical) instead of radiofrequency. This technique has also shown successful reduction of BP in its own proof of concept trial, RADIANCE HTN SOLO[94].
The results of SYMPLICITY HTN-3 have prompted persistent strides in the field of RDN to improve the technology and study designs, and to continue researching this methodology as a means of BP control. Although some of the preliminary results are promising, there is still more testing that is needed. As RH patients have the most to gain from such therapies, it will be imperative that future studies include this population.
The carotid baroreceptor reflex is triggered by wall stretch or a rapid rise of pressure within the carotid body leading to decreased sympathetic signaling to the vasculature, heart, and kidneys, with a combined effect of lowering systemic blood pressure[95,96]. Today there are human clinical trials underway to assess BAT as a means of hypertension treatment[97].
Two BAT devices are being actively studied in humans, an implantable pulse generator (RheosTM and BAROSTIM NEOTM) by CVRx, and an endovascular device MobiusHDTM by Vascular Dynamics. In the RheosTM system, an implantable pulse generator is surgically inserted into the subcutaneous tissue of the infraclavicular region, and bilateral electrode leads are then connected to the outside of the carotid body and tunneled subcutaneously[96,97]. The generator sends electrical impulses to the carotid sinus and activates the baroreceptor mechanism[96]. After two feasibility studies, The Rheos Feasibility Trial and the DEBuT-HT study[97,98], the Rheos Pivotal Trial was started with a sham control design in which the control group had the device implanted but not activated until 6 mo into the study. Although it failed to show a superiority of BAT to reduce BP compared to medical therapy after 6 mo (its short term efficacy end point), more than 50% of those with BAT were able to achieve a SBP < 140 mmHg[99]. In 2017, a 6-year follow up report of patients from all three aforementioned trials was released. The results showed a sustained reduction in both SBP and DBP[100].
There have been safety concerns related to the activation of the baroreceptor reflex, such as hypotension and bradycardia, but these effects were short in duration[97-100]. In addition, there are concerns related to the procedure itself, including cranial nerve injury, which occurred in 9.2% of The Rheos Pivotal Trial, albeit only 4.8% of patients had residual effects[99]. This event rate is similar to the range of 4%-12% reported in the cardiovascular surgery literature for patients undergoing carotid endarterec-tomies[101-103]. The investigators of the Rheos Pivotal Trial did note that about 76% of the patients with procedure-related events did not have long lasting sequelae.
The concern about the safety profile is being answered in several ways. Firstly, CVRx has designed a second generation pulse generator that has only a unilateral electrode, and requires a smaller procedure for insertion[104]. This has already completed a feasibility study, and is on schedule for a randomized, double blind, parallel study for further study (The Nordic BAT)[105,106]. A second answer to the safety concerns is the MobiusHDTM endovascular device by Vascular Dynamics. This device is a self-expanding nitinol implant that is deployed endovascularly[106]. Two large, open label, multicenter trials, CALM-FIM-EUR and CALM-FIM-US, recently published data which demonstrated safety of this approach as well as efficacy in lowering BP[106,107]. Currently, a randomized, double blind, sham controlled pivotal trial, CALM-2, is underway[108].
In addition to the well-studied RDN and BAT, there is also a surgical procedure utilizing the ROX Coupler (by ROX Medical) device, in which a small stent is placed through an endovascularly created anastomosis between the femoral artery and vein. This self-expanding stent is left in place, and will divert arterial blood flow in to the venous system. The Rox Coupler has been investigated in an open label randomized trial, and has shown efficacy in lowering BP[109]. There is ongoing study of this device, and the results and safety profile will be something to follow in the years ahead. Therapeutic targets in RH are summarized in Figure 2.
The progression of medication therapy for RH in the last 10 years is based on increasing evidence for current antihypertensive medications. The use of clonidine, minoxidil, and hydralazine for RH is now currently supported by expert opinion. In the future, we would expect the addition of one or more of these agents to the evidence-based algorithms supported by professional organizations once new trials have been conducted to establish their efficacy beyond currently recommended medical therapy.
The ongoing efforts of device and procedural refinement make both RDN and BAT potential options for BP control in the future. Over the next few years there will be more data coming out of RDN clinical trials, including the use of an ultrasound emission as opposed to radiofrequency. Although the early trial (SPYRAL HTN OFF MED) with the newly designed catheter assembly was conducted in patients without RH, the BP lowering effects are still notable, and likely there will be an expanded study to include patients with RH. BAT remains promising, with six-year follow-up data confirming ongoing BP control and device safety profile. This device-based therapy will be at the forefront of research in the years to come. cAV anastomosis is a unique approach and may be of particular benefit to those with increased arterial stiffness as a cause of RH, but the long-term safety of this approach is a major question that needs to be answered.
Patients with RH comprise a notable and formidable subset of the hypertensive population, who have increased cardiovascular risk and mortality. Providers must be able to promptly identify these patients, accurately assess the degree of treatment resistance by excluding pseudoresistance, then not only intensify but also optimize medication regimens to align with updated guidelines, including the routine use of MRA as indicated. Device based therapies such as renal denervation and BAT are on the horizon but need continued study, especially in the RH population.
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Bełtowski J, Chello M, Cheng TH, Iyalomhe GBS, Omboni S S- Editor: Cui LJ L- Editor: A E- Editor: Zhang YL
| 1. | Carey RM, Calhoun DA, Bakris GL, Brook RD, Daugherty SL, Dennison-Himmelfarb CR, Egan BM, Flack JM, Gidding SS, Judd E, Lackland DT, Laffer CL, Newton-Cheh C, Smith SM, Taler SJ, Textor SC, Turan TN, White WB; American Heart Association Professional/Public Education and Publications Committee of the Council on Hypertension; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Genomic and Precision Medicine; Council on Peripheral Vascular Disease; Council on Quality of Care and Outcomes Research; and Stroke Council. Resistant Hypertension: Detection, Evaluation, and Management: A Scientific Statement From the American Heart Association. Hypertension. 2018;72:e53-e90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 675] [Article Influence: 112.5] [Reference Citation Analysis (0)] |
| 2. | Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127-e248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3577] [Cited by in RCA: 3456] [Article Influence: 493.7] [Reference Citation Analysis (0)] |
| 3. | Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen SE, Kreutz R, Laurent S, Lip GYH, McManus R, Narkiewicz K, Ruschitzka F, Schmieder RE, Shlyakhto E, Tsioufis C, Aboyans V, Desormais I; ESC Scientific Document Group. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021-3104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7431] [Cited by in RCA: 6360] [Article Influence: 908.6] [Reference Citation Analysis (0)] |
| 4. | Hwang AY, Dietrich E, Pepine CJ, Smith SM. Resistant Hypertension: Mechanisms and Treatment. Curr Hypertens Rep. 2017;19:56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 5. | Daugherty SL, Powers JD, Magid DJ, Tavel HM, Masoudi FA, Margolis KL, O'Connor PJ, Selby JV, Ho PM. Incidence and prognosis of resistant hypertension in hypertensive patients. Circulation. 2012;125:1635-1642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 591] [Cited by in RCA: 664] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 6. | Kasiakogias A, Tsioufis C, Dimitriadis K, Konstantinidis D, Koumelli A, Leontsinis I, Andrikou E, Vogiatzakis N, Marinaki S, Petras D, Fragoulis C, Konstantinou K, Papademetriou V, Tousoulis D. Cardiovascular morbidity of severe resistant hypertension among treated uncontrolled hypertensives: a 4-year follow-up study. J Hum Hypertens. 2018;32:487-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Irvin MR, Booth JN, Shimbo D, Lackland DT, Oparil S, Howard G, Safford MM, Muntner P, Calhoun DA. Apparent treatment-resistant hypertension and risk for stroke, coronary heart disease, and all-cause mortality. J Am Soc Hypertens. 2014;8:405-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 111] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 8. | Muntner P, Davis BR, Cushman WC, Bangalore S, Calhoun DA, Pressel SL, Black HR, Kostis JB, Probstfield JL, Whelton PK, Rahman M; ALLHAT Collaborative Research Group. Treatment-resistant hypertension and the incidence of cardiovascular disease and end-stage renal disease: results from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Hypertension. 2014;64:1012-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 172] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 9. | De Nicola L, Gabbai FB, Agarwal R, Chiodini P, Borrelli S, Bellizzi V, Nappi F, Conte G, Minutolo R. Prevalence and Prognostic Role of Resistant Hypertension in Chronic Kidney Disease Patients. J Am Coll Cardiol. 2013;61:2461-2467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 116] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 10. | Tanner RM, Calhoun DA, Bell EK, Bowling CB, Gutiérrez OM, Irvin MR, Lackland DT, Oparil S, McClellan W, Warnock DG, Muntner P. Incident ESRD and treatment-resistant hypertension: the reasons for geographic and racial differences in stroke (REGARDS) study. Am J Kidney Dis. 2014;63:781-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Smith SM, Huo T, Gong Y, Handberg E, Gulati M, Merz CN, Pepine CJ, Cooper-DeHoff RM. Mortality Risk Associated With Resistant Hypertension Among Women: Analysis from Three Prospective Cohorts Encompassing the Spectrum of Women's Heart Disease. J Womens Health (Larchmt). 2016;25:996-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Voora R, Hinderliter AL. Modulation of Sympathetic Overactivity to Treat Resistant Hypertension. Curr Hypertens Rep. 2018;20:92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Smithwick RH, Thompson JE. Splanchnicectomy for essential hypertension; results in 1,266 cases. J Am Med Assoc. 1953;152:1501-1504. [PubMed] |
| 14. | Zintel HA, MACKIE JA, SELLERS AM, JEFFERS WA, HAFKENSCHIEL JH, LINDAUER MA. Results of thoracolumbar sympathectomy for essential hypertension; three-to-seven-year follow-up of one hundred patients. AMA Arch Surg. 1955;71:215-222. [PubMed] |
| 15. | Durand H, Hayes P, Morrissey EC, Newell J, Casey M, Murphy AW, Molloy GJ. Medication adherence among patients with apparent treatment-resistant hypertension: systematic review and meta-analysis. J Hypertens. 2017;35:2346-2357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 130] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 16. | Kandzari DE, Böhm M, Mahfoud F, Townsend RR, Weber MA, Pocock S, Tsioufis K, Tousoulis D, Choi JW, East C, Brar S, Cohen SA, Fahy M, Pilcher G, Kario K; SPYRAL HTN-ON MED Trial Investigators. Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomised trial. Lancet. 2018;391:2346-2355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 562] [Article Influence: 80.3] [Reference Citation Analysis (0)] |
| 17. | Berra E, Azizi M, Capron A, Høieggen A, Rabbia F, Kjeldsen SE, Staessen JA, Wallemacq P, Persu A. Evaluation of Adherence Should Become an Integral Part of Assessment of Patients With Apparently Treatment-Resistant Hypertension. Hypertension. 2016;68:297-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 148] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 18. | Shi L, Liu J, Fonseca V, Walker P, Kalsekar A, Pawaskar M. Correlation between adherence rates measured by MEMS and self-reported questionnaires: a meta-analysis. Health Qual Life Outcomes. 2010;8:99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 131] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 19. | Cuspidi C, Tadic M, Mancia G, Grassi G. White-Coat Hypertension: the Neglected Subgroup in Hypertension. Korean Circ J. 2018;48:552-564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Bhatt H, Siddiqui M, Judd E, Oparil S, Calhoun D. Prevalence of pseudoresistant hypertension due to inaccurate blood pressure measurement. J Am Soc Hypertens. 2016;10:493-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 21. | Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG, Roccella EJ. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation. 2005;111:697-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1494] [Cited by in RCA: 1619] [Article Influence: 81.0] [Reference Citation Analysis (0)] |
| 22. | Vischer AS, Burkard T. Principles of Blood Pressure Measurement – Current Techniques, Office vs Ambulatory Blood Pressure Measurement. Adv Exp Med Biol. 2017;956:85-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 23. | Krause T, Lovibond K, Caulfield M, McCormack T, Williams B; Guideline Development Group. Management of hypertension: summary of NICE guidance. BMJ. 2011;343:d4891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 340] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 24. | Phillips LS, Branch WT, Cook CB, Doyle JP, El-Kebbi IM, Gallina DL, Miller CD, Ziemer DC, Barnes CS. Clinical inertia. Ann Intern Med. 2001;135:825-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 999] [Cited by in RCA: 1036] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 25. | Berlowitz DR, Ash AS, Hickey EC, Friedman RH, Glickman M, Kader B, Moskowitz MA. Inadequate management of blood pressure in a hypertensive population. N Engl J Med. 1998;339:1957-1963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 631] [Cited by in RCA: 609] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 26. | Fontil V, Pletcher MJ, Khanna R, Guzman D, Victor R, Bibbins-Domingo K. Physician underutilization of effective medications for resistant hypertension at office visits in the United States: NAMCS 2006-2010. J Gen Intern Med. 2014;29:468-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Ogedegbe G. Barriers to optimal hypertension control. J Clin Hypertens. 10:644-646. [PubMed] |
| 28. | Borzecki AM, Oliveria SA, Berlowitz DR. Barriers to hypertension control. Am Heart J. 2005;149:785-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 85] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 29. | Charles L, Triscott J, Dobbs B. Secondary Hypertension: Discovering the Underlying Cause. Am Fam Physician. 2017;96:453-461. [PubMed] |
| 30. | Rimoldi SF, Scherrer U, Messerli FH. Secondary arterial hypertension: when, who, and how to screen? Eur Heart J. 2014;35:1245-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 210] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 31. | Pedrosa RP, Drager LF, Gonzaga CC, Sousa MG, de Paula LK, Amaro AC, Amodeo C, Bortolotto LA, Krieger EM, Bradley TD, Lorenzi-Filho G. Obstructive sleep apnea: the most common secondary cause of hypertension associated with resistant hypertension. Hypertension. 2011;58:811-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 420] [Cited by in RCA: 458] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 32. | Torres G, Sánchez-de-la-Torre M, Barbé F. Relationship Between OSA and Hypertension. Chest. 2015;148:824-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 115] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 33. | Grossman A, Messerli FH, Grossman E. Drug induced hypertension--An unappreciated cause of secondary hypertension. Eur J Pharmacol. 2015;763:15-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 34. | Clyburn EB, DiPette DJ. Hypertension induced by drugs and other substances. Semin Nephrol. 1995;15:72-86. [PubMed] |
| 35. | Roberie DR, Elliott WJ. What is the prevalence of resistant hypertension in the United States? Curr Opin Cardiol. 2012;27:386-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 36. | Persell SD. Prevalence of resistant hypertension in the United States, 2003-2008. Hypertension. 2011;57:1076-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 542] [Cited by in RCA: 577] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 37. | de la Sierra A, Segura J, Banegas JR, Gorostidi M, de la Cruz JJ, Armario P, Oliveras A, Ruilope LM. Clinical features of 8295 patients with resistant hypertension classified on the basis of ambulatory blood pressure monitoring. Hypertension. 2011;57:898-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 569] [Cited by in RCA: 558] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 38. | Noubiap JJ, Nansseu JR, Nyaga UF, Sime PS, Francis I, Bigna JJ. Global prevalence of resistant hypertension: a meta-analysis of data from 3.2 million patients. Heart. 2019;105:98-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 202] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 39. | Sowers JR, Whaley-Connell A, Epstein M. Narrative review: the emerging clinical implications of the role of aldosterone in the metabolic syndrome and resistant hypertension. Ann Intern Med. 2009;150:776-783. [PubMed] |
| 40. | Whaley-Connell A, Johnson MS, Sowers JR. Aldosterone: role in the cardiometabolic syndrome and resistant hypertension. Prog Cardiovasc Dis. 2010;52:401-409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 41. | Grassi G, Mark A, Esler M. The sympathetic nervous system alterations in human hypertension. Circ Res. 2015;116:976-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 435] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 42. | Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25:932-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1125] [Cited by in RCA: 1277] [Article Influence: 63.9] [Reference Citation Analysis (0)] |
| 43. | Chung CM, Cheng HW, Chang JJ, Lin YS, Hsiao JF, Chang ST, Hsu JT. Relationship between resistant hypertension and arterial stiffness assessed by brachial-ankle pulse wave velocity in the older patient. Clin Interv Aging. 2014;9:1495-1502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 44. | Pabuccu T, Baris N, Ozpelit E, Akdeniz B, Guneri S. The relationship between resistant hypertension and arterial stiffness. Clin Exp Hypertens. 2012;34:57-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 45. | Martins LC, Figueiredo VN, Quinaglia T, Boer-Martins L, Yugar-Toledo JC, Martin JFV, Demacq C, Pimenta E, Calhoun DA, Moreno H. Characteristics of resistant hypertension: ageing, body mass index, hyperaldosteronism, cardiac hypertrophy and vascular stiffness. J Hum Hypertens. 2011;25:532-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 46. | Ernst ME, Carter BL, Goerdt CJ, Steffensmeier JJ, Phillips BB, Zimmerman MB, Bergus GR. Comparative antihypertensive effects of hydrochlorothiazide and chlorthalidone on ambulatory and office blood pressure. Hypertension. 2006;47:352-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 285] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 47. | Khosla N, Chua DY, Elliott WJ, Bakris GL. Are chlorthalidone and hydrochlorothiazide equivalent blood-pressure-lowering medications? J Clin Hypertens (Greenwich). 2005;7:354-356. [PubMed] |
| 48. | Williams B, MacDonald TM, Morant S, Webb DJ, Sever P, McInnes G, Ford I, Cruickshank JK, Caulfield MJ, Salsbury J, Mackenzie I, Padmanabhan S, Brown MJ; British Hypertension Society's PATHWAY Studies Group. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet. 2015;386:2059-2068. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 723] [Cited by in RCA: 809] [Article Influence: 80.9] [Reference Citation Analysis (0)] |
| 49. | Oxlund CS, Henriksen JE, Tarnow L, Schousboe K, Gram J, Jacobsen IA. Low dose spironolactone reduces blood pressure in patients with resistant hypertension and type 2 diabetes mellitus: a double blind randomized clinical trial. J Hypertens. 2013;31:2094-2102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 50. | Widimský J. PATHWAY-2 Study: spironolactone vs placebo, bisoprolol and doxazosin to determine optimal treatment of resistant hypertension. Spironolactone high effective in lowering blood pressure in drug resistant hypertension. Vnitr Lek. 2015;61:1067-1071. [PubMed] |
| 51. | Krieger EM, Drager LF, Giorgi DMA, Pereira AC, Barreto-Filho JAS, Nogueira AR, Mill JG, Lotufo PA, Amodeo C, Batista MC, Bodanese LC, Carvalho ACC, Castro I, Chaves H, Costa EAS, Feitosa GS, Franco RJS, Fuchs FD, Guimarães AC, Jardim PC, Machado CA, Magalhães ME, Mion D, Nascimento RM, Nobre F, Nóbrega AC, Ribeiro ALP, Rodrigues-Sobrinho CR, Sanjuliani AF, Teixeira MDCB, Krieger JE; ReHOT Investigators. Spironolactone Versus Clonidine as a Fourth-Drug Therapy for Resistant Hypertension: The ReHOT Randomized Study (Resistant Hypertension Optimal Treatment). Hypertension. 2018;71:681-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 108] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 52. | Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM, Lin PH, Karanja N. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336:1117-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3896] [Cited by in RCA: 3728] [Article Influence: 133.1] [Reference Citation Analysis (0)] |
| 53. | Whelton PK, Appel LJ, Espeland MA, Applegate WB, Ettinger WH, Kostis JB, Kumanyika S, Lacy CR, Johnson KC, Folmar S, Cutler JA. Sodium reduction and weight loss in the treatment of hypertension in older persons: a randomized controlled trial of nonpharmacologic interventions in the elderly (TONE). TONE Collaborative Research Group. JAMA. 1998;279:839-846. [PubMed] |
| 54. | The Hypertension Prevention Trial: three-year effects of dietary changes on blood pressure. Hypertension Prevention Trial Research Group. Arch Intern Med. 1990;150:153-162. [PubMed] |
| 55. | Stamler R, Stamler J, Grimm R, Gosch FC, Elmer P, Dyer A, Berman R, Fishman J, Van Heel N, Civinelli J. Nutritional therapy for high blood pressure. Final report of a four-year randomized controlled trial--the Hypertension Control Program. JAMA. 1987;257:1484-1491. [PubMed] |
| 56. | Applegate WB, Miller ST, Elam JT, Cushman WC, el Derwi D, Brewer A, Graney MJ. Nonpharmacologic intervention to reduce blood pressure in older patients with mild hypertension. Arch Intern Med. 1992;152:1162-1166. [PubMed] |
| 57. | Midgley JP, Matthew AG, Greenwood CM, Logan AG. Effect of reduced dietary sodium on blood pressure: a meta-analysis of randomized controlled trials. JAMA. 1996;275:1590-1597. [PubMed] |
| 58. | He FJ, Li J, Macgregor GA. Effect of longer term modest salt reduction on blood pressure: Cochrane systematic review and meta-analysis of randomised trials. BMJ. 2013;346:f1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 806] [Cited by in RCA: 902] [Article Influence: 75.2] [Reference Citation Analysis (0)] |
| 59. | Nerenberg KA, Zarnke KB, Leung AA, Dasgupta K, Butalia S, McBrien K, Harris KC, Nakhla M, Cloutier L, Gelfer M, Lamarre-Cliche M, Milot A, Bolli P, Tremblay G, McLean D, Padwal RS, Tran KC, Grover S, Rabkin SW, Moe GW, Howlett JG, Lindsay P, Hill MD, Sharma M, Field T, Wein TH, Shoamanesh A, Dresser GK, Hamet P, Herman RJ, Burgess E, Gryn SE, Gregoire JC, Lewanczuk R, Poirier L, Campbell TS, Feldman RD, Lavoie KL, Tsuyuki RT, Honos G, Prebtani APH, Kline G, Schiffrin EL, Don-Wauchope A, Tobe SW, Gilbert RE, Leiter LA, Jones C, Woo V, Hegele RA, Selby P, Pipe A, McFarlane PA, Oh P, Gupta M, Bacon SL, Kaczorowski J, Trudeau L, Campbell NRC, Hiremath S, Roerecke M, Arcand J, Ruzicka M, Prasad GVR, Vallee M, Edwards C, Sivapalan P, Penner SB, Fournier A, Benoit G, Feber J, Dionne J, Magee LA, Logan AG, Cote AM, Rey E, Firoz T, Kuyper LM, Gabor JY, Townsend RR, Rabi DM, Daskalopoulou SS; Hypertension Canada. Hypertension Canada's 2018 Guidelines for Diagnosis, Risk Assessment, Prevention, and Treatment of Hypertension in Adults and Children. Can J Cardiol. 2018;34:506-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 434] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 60. | Pimenta E, Gaddam KK, Oparil S, Aban I, Husain S, Dell'Italia LJ, Calhoun DA. Effects of dietary sodium reduction on blood pressure in subjects with resistant hypertension: results from a randomized trial. Hypertension. 2009;54:475-481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 426] [Cited by in RCA: 378] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 61. | World Health Organization. Fact Sheets: Obesity and Overweight. [Accessed November 3, 2018]. Available from: http://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. |
| 62. | NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet. 2016;387:1377-1396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3952] [Cited by in RCA: 3513] [Article Influence: 390.3] [Reference Citation Analysis (0)] |
| 63. | Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of Obesity Among Adults and Youth: United States, 2015-2016. NCHS Data Brief. 2017;288:1-8. [PubMed] |
| 64. | Shihab HM, Meoni LA, Chu AY, Wang NY, Ford DE, Liang KY, Gallo JJ, Klag MJ. Body mass index and risk of incident hypertension over the life course: the Johns Hopkins Precursors Study. Circulation. 2012;126:2983-2989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 202] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 65. | Matsuo T, Sairenchi T, Suzuki K, Tanaka K, Muto T. Long-term stable obesity increases risk of hypertension. Int J Obes (Lond). 2011;35:1056-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 66. | Saydah S, Bullard KM, Cheng Y, Ali MK, Gregg EW, Geiss L, Imperatore G. Trends in cardiovascular disease risk factors by obesity level in adults in the United States, NHANES 1999-2010. Obesity (Silver Spring). 2014;22:1888-1895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 130] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 67. | Cohen JB. Hypertension in Obesity and the Impact of Weight Loss. Curr Cardiol Rep. 2017;19:98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 84] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 68. | Dengel DR, Galecki AT, Hagberg JM, Pratley RE. The independent and combined effects of weight loss and aerobic exercise on blood pressure and oral glucose tolerance in older men. Am J Hypertens. 1998;11:1405-1412. [PubMed] |
| 69. | Neter JE, Stam BE, Kok FJ, Grobbee DE, Geleijnse JM. Influence of weight reduction on blood pressure: a meta-analysis of randomized controlled trials. Hypertension. 2003;42:878-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 846] [Cited by in RCA: 893] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 70. | Stevens VJ, Obarzanek E, Cook NR, Lee IM, Appel LJ, Smith West D, Milas NC, Mattfeldt-Beman M, Belden L, Bragg C, Millstone M, Raczynski J, Brewer A, Singh B, Cohen J; Trials for the Hypertension Prevention Research Group. Long-term weight loss and changes in blood pressure: results of the Trials of Hypertension Prevention, phase II. Ann Intern Med. 2001;134:1-11. [PubMed] |
| 71. | Aucott L, Poobalan A, Smith WC, Avenell A, Jung R, Broom J. Effects of weight loss in overweight/obese individuals and long-term hypertension outcomes: a systematic review. Hypertension. 2005;45:1035-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 167] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 72. | Aucott L, Rothnie H, McIntyre L, Thapa M, Waweru C, Gray D. Long-term weight loss from lifestyle intervention benefits blood pressure?: a systematic review. Hypertension. 2009;54:756-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 73. | Look AHEAD Research Group; Wing RR, Bolin P, Brancati FL, Bray GA, Clark JM, Coday M, Crow RS, Curtis JM, Egan CM, Espeland MA, Evans M, Foreyt JP, Ghazarian S, Gregg EW, Harrison B, Hazuda HP, Hill JO, Horton ES, Hubbard VS, Jakicic JM, Jeffery RW, Johnson KC, Kahn SE, Kitabchi AE, Knowler WC, Lewis CE, Maschak-Carey BJ, Montez MG, Murillo A, Nathan DM, Patricio J, Peters A, Pi-Sunyer X, Pownall H, Reboussin D, Regensteiner JG, Rickman AD, Ryan DH, Safford M, Wadden TA, Wagenknecht LE, West DS, Williamson DF, Yanovski SZ. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369:145-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2142] [Cited by in RCA: 1961] [Article Influence: 163.4] [Reference Citation Analysis (0)] |
| 74. | Lopes S, Mesquita-Bastos J, Alves AJ, Ribeiro F. Exercise as a tool for hypertension and resistant hypertension management: current insights. Integr Blood Press Control. 2018;11:65-71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 75. | Börjesson M, Onerup A, Lundqvist S, Dahlöf B. Physical activity and exercise lower blood pressure in individuals with hypertension: narrative review of 27 RCTs. Br J Sports Med. 2016;50:356-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 184] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 76. | Inder JD, Carlson DJ, Dieberg G, McFarlane JR, Hess NC, Smart NA. Isometric exercise training for blood pressure management: a systematic review and meta-analysis to optimize benefit. Hypertens Res. 2016;39:88-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 163] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 77. | Carlson DJ, Dieberg G, Hess NC, Millar PJ, Smart NA. Isometric exercise training for blood pressure management: a systematic review and meta-analysis. Mayo Clin Proc. 2014;89:327-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 197] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 78. | Cornelissen VA, Smart NA. Exercise training for blood pressure: a systematic review and meta-analysis. J Am Heart Assoc. 2013;2:e004473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 761] [Cited by in RCA: 962] [Article Influence: 80.2] [Reference Citation Analysis (0)] |
| 79. | Casonatto J, Goessler KF, Cornelissen VA, Cardoso JR, Polito MD. The blood pressure-lowering effect of a single bout of resistance exercise: A systematic review and meta-analysis of randomised controlled trials. Eur J Prev Cardiol. 2016;23:1700-1714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 108] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 80. | MacDonald HV, Johnson BT, Huedo-Medina TB, Livingston J, Forsyth KC, Kraemer WJ, Farinatti PT, Pescatello LS. Dynamic Resistance Training as Stand-Alone Antihypertensive Lifestyle Therapy: A Meta-Analysis. J Am Heart Assoc. 2016;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 182] [Cited by in RCA: 162] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 81. | Dimeo F, Pagonas N, Seibert F, Arndt R, Zidek W, Westhoff TH. Aerobic exercise reduces blood pressure in resistant hypertension. Hypertension. 2012;60:653-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 235] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 82. | Guimaraes GV, de Barros Cruz LG, Fernandes-Silva MM, Dorea EL, Bocchi EA. Heated water-based exercise training reduces 24-hour ambulatory blood pressure levels in resistant hypertensive patients: a randomized controlled trial (HEx trial). Int J Cardiol. 2014;172:434-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 83. | Egan BM, Zhao Y, Axon RN, Brzezinski WA, Ferdinand KC. Uncontrolled and apparent treatment resistant hypertension in the United States, 1988 to 2008. Circulation. 2011;124:1046-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 439] [Cited by in RCA: 454] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 84. | Václavík J, Sedlák R, Plachy M, Navrátil K, Plásek J, Jarkovsky J, Václavík T, Husár R, Kociánová E, Táborsky M. Addition of spironolactone in patients with resistant arterial hypertension (ASPIRANT): a randomized, double-blind, placebo-controlled trial. Hypertension. 2011;57:1069-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 216] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 85. | Zhao D, Liu H, Dong P, Zhao J. A meta-analysis of add-on use of spironolactone in patients with resistant hypertension. Int J Cardiol. 2017;233:113-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 86. | Esler M, Lambert G, Brunner-La Rocca HP, Vaddadi G, Kaye D. Sympathetic nerve activity and neurotransmitter release in humans: translation from pathophysiology into clinical practice. Acta Physiol Scand. 2003;177:275-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 113] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 87. | Sata Y, Head GA, Denton K, May CN, Schlaich MP. Role of the Sympathetic Nervous System and Its Modulation in Renal Hypertension. Front Med (Lausanne). 2018;5:82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 118] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 88. | Krum H, Schlaich M, Whitbourn R, Sobotka PA, Sadowski J, Bartus K, Kapelak B, Walton A, Sievert H, Thambar S, Abraham WT, Esler M. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet. 2009;373:1275-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1591] [Cited by in RCA: 1545] [Article Influence: 96.6] [Reference Citation Analysis (0)] |
| 89. | Symplicity HTN-2 Investigators; Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Böhm M. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet. 2010;376:1903-1909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1656] [Cited by in RCA: 1614] [Article Influence: 107.6] [Reference Citation Analysis (0)] |
| 90. | Bhatt DL, Kandzari DE, O'Neill WW, D'Agostino R, Flack JM, Katzen BT, Leon MB, Liu M, Mauri L, Negoita M, Cohen SA, Oparil S, Rocha-Singh K, Townsend RR, Bakris GL; SYMPLICITY HTN-3 Investigators. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370:1393-1401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1571] [Cited by in RCA: 1594] [Article Influence: 144.9] [Reference Citation Analysis (0)] |
| 91. | Kandzari DE, Bhatt DL, Brar S, Devireddy CM, Esler M, Fahy M, Flack JM, Katzen BT, Lea J, Lee DP, Leon MB, Ma A, Massaro J, Mauri L, Oparil S, O'Neill WW, Patel MR, Rocha-Singh K, Sobotka PA, Svetkey L, Townsend RR, Bakris GL. Predictors of blood pressure response in the SYMPLICITY HTN-3 trial. Eur Heart J. 2015;36:219-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 428] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 92. | White WB, Galis ZS, Henegar J, Kandzari DE, Victor R, Sica D, Townsend RR, Turner JR, Virmani R, Mauri L. Renal denervation therapy for hypertension: pathways for moving development forward. J Am Soc Hypertens. 2015;9:341-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 93. | Townsend RR, Mahfoud F, Kandzari DE, Kario K, Pocock S, Weber MA, Ewen S, Tsioufis K, Tousoulis D, Sharp ASP, Watkinson AF, Schmieder RE, Schmid A, Choi JW, East C, Walton A, Hopper I, Cohen DL, Wilensky R, Lee DP, Ma A, Devireddy CM, Lea JP, Lurz PC, Fengler K, Davies J, Chapman N, Cohen SA, DeBruin V, Fahy M, Jones DE, Rothman M, Böhm M; SPYRAL HTN-OFF MED trial investigators*. Catheter-based renal denervation in patients with uncontrolled hypertension in the absence of antihypertensive medications (SPYRAL HTN-OFF MED): a randomised, sham-controlled, proof-of-concept trial. Lancet. 2017;390:2160-2170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 594] [Cited by in RCA: 543] [Article Influence: 67.9] [Reference Citation Analysis (0)] |
| 94. | Azizi M, Schmieder RE, Mahfoud F, Weber MA, Daemen J, Davies J, Basile J, Kirtane AJ, Wang Y, Lobo MD, Saxena M, Feyz L, Rader F, Lurz P, Sayer J, Sapoval M, Levy T, Sanghvi K, Abraham J, Sharp ASP, Fisher NDL, Bloch MJ, Reeve-Stoffer H, Coleman L, Mullin C, Mauri L; RADIANCE-HTN Investigators. Endovascular ultrasound renal denervation to treat hypertension (RADIANCE-HTN SOLO): a multicentre, international, single-blind, randomised, sham-controlled trial. Lancet. 2018;391:2335-2345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 571] [Cited by in RCA: 526] [Article Influence: 75.1] [Reference Citation Analysis (0)] |
| 95. | Lohmeier TE, Iliescu R. The baroreflex as a long-term controller of arterial pressure. Physiology (Bethesda). 2015;30:148-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 96. | Heusser K, Tank J, Engeli S, Diedrich A, Menne J, Eckert S, Peters T, Sweep FC, Haller H, Pichlmaier AM, Luft FC, Jordan J. Carotid baroreceptor stimulation, sympathetic activity, baroreflex function, and blood pressure in hypertensive patients. Hypertension. 2010;55:619-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 312] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 97. | Illig KA, Levy M, Sanchez L, Trachiotis GD, Shanley C, Irwin E, Pertile T, Kieval R, Cody R. An implantable carotid sinus stimulator for drug-resistant hypertension: surgical technique and short-term outcome from the multicenter phase II Rheos feasibility trial. J Vasc Surg. 2006;44:1213-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 84] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 98. | Scheffers IJ, Kroon AA, Schmidli J, Jordan J, Tordoir JJ, Mohaupt MG, Luft FC, Haller H, Menne J, Engeli S, Ceral J, Eckert S, Erglis A, Narkiewicz K, Philipp T, de Leeuw PW. Novel baroreflex activation therapy in resistant hypertension: results of a European multi-center feasibility study. J Am Coll Cardiol. 2010;56:1254-1258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 249] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 99. | Bisognano JD, Bakris G, Nadim MK, Sanchez L, Kroon AA, Schafer J, de Leeuw PW, Sica DA. Baroreflex activation therapy lowers blood pressure in patients with resistant hypertension: results from the double-blind, randomized, placebo-controlled rheos pivotal trial. J Am Coll Cardiol. 2011;58:765-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 429] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 100. | de Leeuw PW, Bisognano JD, Bakris GL, Nadim MK, Haller H, Kroon AA; DEBuT-HT and Rheos Trial Investigators. Sustained Reduction of Blood Pressure With Baroreceptor Activation Therapy: Results of the 6-Year Open Follow-Up. Hypertension. 2017;69:836-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 101. | Kakisis JD, Antonopoulos CN, Mantas G, Moulakakis KG, Sfyroeras G, Geroulakos G. Cranial Nerve Injury After Carotid Endarterectomy: Incidence, Risk Factors, and Time Trends. Eur J Vasc Endovasc Surg. 2017;53:320-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 102. | Ferguson GG, Eliasziw M, Barr HW, Clagett GP, Barnes RW, Wallace MC, Taylor DW, Haynes RB, Finan JW, Hachinski VC, Barnett HJ. The North American Symptomatic Carotid Endarterectomy Trial: surgical results in 1415 patients. Stroke. 1999;30:1751-1758. [PubMed] |
| 103. | Chisci E, Rehring TF, Pigozzi C, Colon S, Borgheresi A, Tramacere L, Ercolini L, Michelagnoli S. Cranial nerve injury is associated with dual antiplatelet therapy use and cervical hematoma after carotid endarterectomy. J Vasc Surg. 2016;64:985-989.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 104. | Iliescu R, Tudorancea I, Lohmeier TE. Baroreflex activation: from mechanisms to therapy for cardiovascular disease. Curr Hypertens Rep. 2014;16:453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 105. | Gordin D, Fadl Elmula FEM, Andersson B, Gottsäter A, Elf J, Kahan T, Christensen KL, Vikatmaa P, Vikatmaa L, Bastholm Olesen T, Groop PH, Olsen MH, Tikkanen I; Nordic BAT Study Group. The effects of baroreflex activation therapy on blood pressure and sympathetic function in patients with refractory hypertension: the rationale and design of the Nordic BAT study. Blood Press. 2017;26:294-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 106. | Spiering W, Williams B, Van der Heyden J, van Kleef M, Lo R, Versmissen J, Moelker A, Kroon A, Reuter H, Ansel G, Stone GW, Bates M; CALM-FIM_EUR investigators. Endovascular baroreflex amplification for resistant hypertension: a safety and proof-of-principle clinical study. Lancet. 2017;390:2655-2661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 107. | Van der Heyden J. Controlling and lowering blood pressure with the MobiusHD device: first-in-man results (CALM-FIM study). J Am Soc Hypertens. 2016;10:e12. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 108. | Williams B, Stone G. Introduction of the CALM II study: a multi-center, blinded, sham-controlled pivotal trial of an endovascular carotid barostimulation implant for the treatment of resistant hypertension. J Am Coll Cardiol. 2017;69:1726. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 109. | Lobo MD, Ott C, Sobotka PA, Saxena M, Stanton A, Cockcroft JR, Sulke N, Dolan E, van der Giet M, Hoyer J, Furniss SS, Foran JP, Witkowski A, Januszewicz A, Schoors D, Tsioufis K, Rensing BJ, Scott B, Ng GA, Schmieder RE. Central Iliac Arteriovenous Anastomosis for Uncontrolled Hypertension: One-Year Results From the ROX CONTROL HTN Trial. Hypertension. 2017;70:1099-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |