Published online Nov 23, 2015. doi: 10.5494/wjh.v5.i4.119
Peer-review started: June 1, 2015
First decision: July 3, 2015
Revised: September 17, 2015
Accepted: October 20, 2015
Article in press: October 27, 2015
Published online: November 23, 2015
Processing time: 175 Days and 9.7 Hours
The dietary approaches to stop hypertension (DASH) diet has been developed and popularized as a non-pharmaceutical intervention for high blood pressure reduction since 1995. However, to date, a comprehensive description of the biochemical rationale behind the diet’s principal guidelines has yet to be compiled. With rising interest for healthy and reliable life-style modifications to combat cardiovascular disease, this review aims to compile the most recent and relevant studies on this topic and make an informed assessment as to the efficacy of and underlying mechanisms operant in the DASH diet. Specifically, the merits of lowering dietary intake of sodium and saturated fat, as well as increasing the intake of fruits, vegetables, fiber, and dairy, have been shown to attenuate hypertension individually. Upon review of this evidence, we conclude that the combination of dietary patterns proposed in the DASH diet is effective in attenuating high blood pressure. We also suggest that efforts to more widely implement adoption of the DASH diet would be beneficial to public health.
Core tip: As a non-pharmaceutical intervention for hypertension, the dietary approaches to stop hypertension (DASH) diet have emerged as the most prevalent choice. Based on the principles of salt restrictions, lowering fat and sugar intake, increasing fruit, vegetable, and fiber intake, this program serves great promise for patients diagnosed with high blood pressure. This review aimed to assess the biochemical rational behind the diet’s principle guidelines to evaluate the efficacy of the DASH diet in the treatment of hypertension. We conclude that the combined tenets of the DASH diet are effective in lowering blood pressure.
- Citation: Shah PT, Maxwell KD, Shapiro JI. Dashing away hypertension: Evaluating the efficacy of the dietary approaches to stop hypertension diet in controlling high blood pressure. World J Hypertens 2015; 5(4): 119-128
- URL: https://www.wjgnet.com/2220-3168/full/v5/i4/119.htm
- DOI: https://dx.doi.org/10.5494/wjh.v5.i4.119
Currently suspected as an underlying cause of approximately 7.1 million (13% of) deaths worldwide, hypertension remains a prevalent affliction[1]. To complicate the matter, the etiology of primary (essential) hypertension which accounts for 90%-95% of adult cases is still unclear[2]. Uncontrolled hypertension serves to be a major risk factor for the development of a multitude of neural[3], cardiac[4], and renal[5] disorders. Similar to the “All roads lead to Rome” adage, several factors have been implicated in the pathogenesis of hypertension including, but not limited to: Oxidative stress, genetic factors, renal injury, inadequate nutrient intake, overproduction of sodium retaining hormones, disruption of the renin-angiotensin or kallikrein-kinin systems, deficiencies in vasodilators[6]. This review utilized studies dating back to the early 1900’s in order to establish a background for the research being conducted today, however, the majority of data compiled for review in this paper was conducted from the INTERSALT study[7] in 1988 through present day.
Until recently, hypertension has been classified into three stages that increase in severity: Pre-hypertension, [systolic blood pressure (BP) of 120-139 mmHg and diastolic BP of 80-89 mmHg], stage 1 (systolic BP of 140-159 mmHg and diastolic BP of 90-99 mmHg), and stage 2 (systolic BP of 160-179 mmHg and diastolic BP of 90-99 mmHg). These classifications arose in 2003 with the 7th JNC report on hypertension[8]. More recently in 2014, the 8th JNC report was released to the public. The 8th edition of the report modified the above specifications, and took an alternate approach regarding the BP threshold of intervention, which treatment should be administered, and also the target BP to be achieved. JNC 8 proposes to begin treatment for those over the age of 60 at an increased BP threshold of > 150/90 mmHg, while the goal BP for those below the age of 60 is still < 140/90[9]. The change originated from multidisciplinary analysis of many randomly controlled trial studies. The idea behind this decision was based on the reevaluation of the risks of side effects vs the benefits of treatment of hypertension[9]. A great deal of controversy has arisen surrounding the 8th JNC report, some dissent arising from the JNC panel itself. In a paper published shortly after the JNC 8, Wright et al[10] cited insufficient evidence for increasing the benchmark of treatment in patients over 60, proposing that the goal BP eligible for treatment should be lowered. Wright et al[10] argued that increasing the benchmark BP of this age group would also increase the group’s risk of cardiovascular disease (CVD), especially in high risk populations, and would undo the progress of steadily decreasing levels of cardiovascular mortality. The team proposed that it would be more appropriate to raise the BP threshold for treatment in individuals aged 80 and above, as the benefits of therapy would be far more likely to exceed the risks[10]. For the purpose of this review, we refer to JNC 7 stages of hypertension, due to the vast majority of research published before the presentation of the new guidelines.
To help manage and attenuate disease pathogenesis, intervention by way of lifestyle modification and/or pharmaceutical therapies are strongly recommended. However, due to the economic burden and potential adverse side effects associated with pharmaceutical therapies, a growing demand for alternate means of treatment quickly arose. Consequently, before the turn of the century, a dietary plan known as dietary approaches to stop hypertension (DASH) was publicized after successful results following two multicenter, randomized outpatient feeding studies[11-13]. The principal guidelines of the DASH diet[14] (depicted in Table 1) focus on increased consumption of fruits, vegetables, whole grains, fish, poultry, beans, and seeds; in addition to consuming low- and non-fat dairy products, the diet calls for limited intake of: Sodium, saturated and trans fats, sugar, and red meat. This diet further encourages consumption of mineral-rich foods containing potassium, calcium, and magnesium, and foods rich in fiber.
| Food group | Daily servings | Nutritional value |
| Grains/dietary fiber | 7-8 | Rich in dietary fiber |
| Vegetables | 4-5 | Rich in nitrate, fiber, potassium, and magnesium |
| Fruits | 4-5 | Rich in nitrate, fiber, potassium, and magnesium |
| Protein (poultry, fish, etc.) | 2-5 | High in bioactive proteins and magnesium |
| Low and non-fat dairy | 2-3 | High in calcium, vitamin D, and bioactive proteins |
| Nuts, seeds, and dry beans | 4-5 | High in potassium, magnesium, proteins, and fiber |
| Fats and oil | 2-3 | Accounts for 27% of caloric intake and essential fats and oils |
| Sugar | 5 per week | Sweets should be low in fat |
This review aims to breakdown and examine the biochemical rationales behind specific tenets of the DASH diet in an effort to evaluate its efficacy.
Sodium plays an integral role in the biomechanical function of muscle and nerve fibers, and is largely responsible for the auto regulation of fluid balance at the cellular level. Yet, similar to other essential nutrients, excessive sodium levels can yield damaging effects physiologically. Research has delved into the relationship between sodium intake and hypertension for the past 50 years, and it is widely regarded as a major component to the development of high BP[15]. Investigators Ambard et al[16] first evidenced this relationship in 1904, in which six hypertensive patients were placed on three separate diets with modified salt and protein content. Outcomes of the study revealed that sodium content, irrespective of protein content, was inversely related to BP within and across each diet. Specifically, when sodium intake was reduced, corresponding decreases in BP were observed.
Similar beneficial effects of dietary sodium restriction were reported in 1948, when Walter Kempner’s Rice Diet became publicized. The initial study involved 500 hypertensive patients placed on a diet predominantly consisting of rice, supplemented with fruits. Dietary salt content was maintained below 500 mg, and the subsequent effects of the diet were tremendous; along with attenuation of high BP, the patients’ demonstrated reduced cardiac hypertrophy and amelioration of hypertensive retinopathy[17]. Although the practicality of maintaining this fairly tasteless diet proved bleak, the implications of the study were inescapable.
More recently, in 1980 Srinivasan et al[18] tested the effects of a high salt vs high salt/sucrose diets on spider monkeys. Findings from the study indicated that both high salt and high salt/sucrose diets resulted in elevated BP, and most importantly, in a dose-dependent manner[18]. In 1988, the monumental Intersalt study was compiled. Examining approximately 10000 patients from 52 examination centers worldwide, Intersalt remains the largest study of its kind to date. The major findings extended over international population and individual levels, and supported the direct correlation between sodium intake and hypertension. Regional differences in salt intake were also found to correlate with regional levels of hypertension. Perhaps of even greater relevance, the increases in systolic BP noted with age appeared to correlate on a population level even better with sodium intake than absolute levels of BP[7].
Although the relationship between salt and BP is readily accepted, the exact biochemical mechanism behind salt’s role in the development of hypertension remains unclear. The renal renin-angiotensin-aldosterone system (RAAS) appears to be largely implicated in the development of salt driven hypertension and is the site of many pharmacological treatments for stage 2 hypertension, including angiotensin-converting enzyme (ACE) inhibitors and angiotensin II (Ang II) receptor blockers[19].
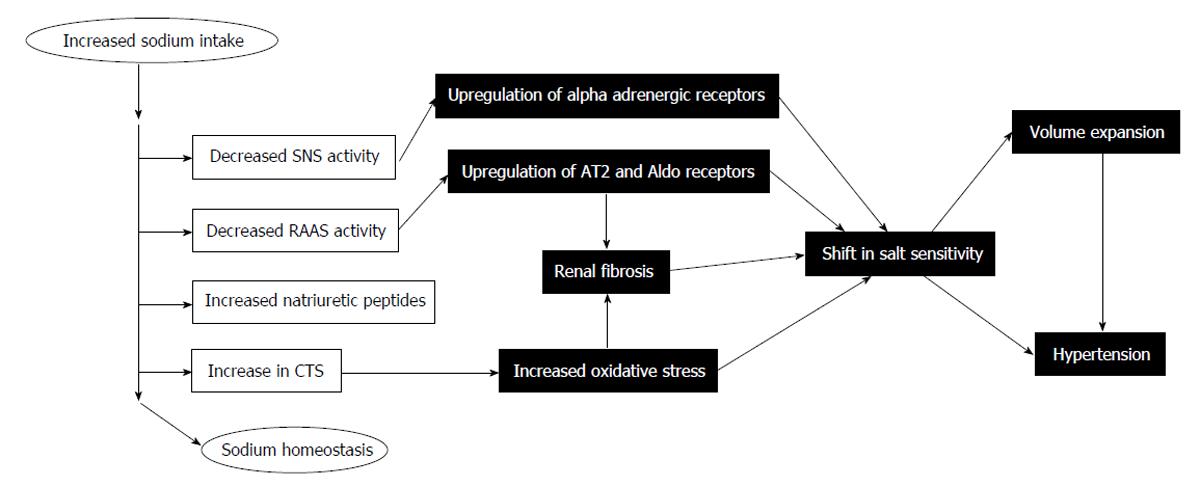
Under physiological conditions, high salt diets typically suppress Ang II levels through BP control mechanisms[20]. Despite this, 40%-50% of patients with essential hypertension do not engage this expected renal response to Ang II and thus, do not react appropriately to changes in dietary sodium intake (depicted in Figure 1)[20,21]. This is one avenue of salt sensitivity, and while it has been documented clinically, its mechanisms have yet to be resolved[22,23]. Salt sensitivity has also been attributed to genetic mutations of the renin and ACE genes[24]. These individuals appear to exhibit high BP and low plasma renin levels in response to salt intake[25].
Additionally, RAAS, nitric oxide (NO), and superoxide anion (O2-) in the kidney, work together in a regulatory fashion. Activation of RAAS leads to the production of O2-, a vasoconstrictor, and NO, a vasodilator, and both molecules readily react with one another. Dysfunction within the RAAS can lead to an imbalance of NO and O2-, which has been linked to salt sensitivity and hypertension[26,27]. In short, high levels of dietary sodium appear to induce inappropriate RAAS activity, leading to vascular maladaptation[28]. While certainly not the only avenue of hypertension, these studies highlight the importance of the RAAS in management of hypertension.
Sustained levels of high dietary sodium intake have been implicated not only in the pathogenesis of hypertension, but also albuminuria, altered gene expression, and even renal structural damage[29]. Further experiments have been crafted to test the efficacy of lowering sodium intake on hypertensive patients. He et al[30] demonstrated that a diet modified to lower only sodium content, with no other dietary restrictions, caused a significant reduction in BP in both normotensive and hypertensive patients. Moreover, as recently as 2015, a study conducted by Barros et al[31] highlighted the use of “light salt”, a salt developed with lower sodium content and higher potassium levels as a significantly effective agent in reducing BP.
The first major tenet of the DASH diet is a substantial reduction in sodium intake[11]. It is estimated that the average adult American consumes roughly 3700 mg of sodium per day; by contrast, the DASH diet recommends that dietary sodium should be limited to < 2300 mg/d (< 1500 mg/d for high-risk individuals)[32]. In a comprehensive analysis of the effect of sodium consumption on BP, Bray et al[33] evaluated variations of sodium intake within the context of the standard American diet vs the DASH diet. In total, the study consisted of six groups: A control diet similar to the standard American diet and a DASH diet group were each divided into low, moderate, and average (high) sodium groups[33]. The researchers demonstrated a dose-dependent relationship between sodium consumption and BP; the findings corresponded with the results of previous experiments (discussed above): The lower the sodium intake, the greater the drop in BP. Furthermore, while both control and DASH groups experienced a drop in BP, limiting dietary sodium had an additive relationship when coupled with the DASH diet; these results demonstrate that patients consuming lower dietary sodium, in accompaniment with the rest of the DASH dietary patterns, experience an even greater drop in BP[30,33]. Biochemical analysis of the DASH diet yield similar optimistic results. Using the same six-group approach as Bray et al[33], analysis was performed by examining their corresponding pressure-natriuresis curves. The DASH diet appeared to decrease tubular sodium reabsorption without increasing glomerular filtration rate[34]; thus, the DASH diet is natriuretic in a sustainable way. Compiling the evidence-based research together, as a whole, we find overwhelming support of sodium restriction aiding the efficacy of the DASH diet in controlling hypertension.
As discussed above, the origin and development of hypertension is complex, yet substantial literature also suggests that a sustained increase in peripheral vascular resistance due to arterial structural remodeling plays a prominent role in the pathogenesis of this disease[6,35,36]. Research suggests that the high inorganic nitrate (NO3-) content present in many vegetables may play vasoprotective[37-39] and cardioprotective[40] roles, via endogenous conversion to NO - a potent vasodilator. Consequently, endothelial dysfunction often characterized by a reduction in NO bioavailability has been largely implicated in patients with essential hypertension[41-43]. Discussion considering the physiological biosynthesis and function of NO can be found in several references[40,44-48].
It is estimated that the largest source of dietary nitrates (roughly 80%) comes directly from vegetable consumption; alternate dietary sources of nitrate and nitrite (a reduced form of nitrate) can also be found in fruits, vegetables, and processed meats[38]. In 2008, Webb et al[37] provided the first clinical evidence supporting the vasoprotective role of dietary nitrate harbored by a vegetable-rich diet in normotensive volunteers. The team of researchers utilized beetroot juice to display that consumption of an acute nitrate load corresponds with a significant reduction of both systolic and diastolic BP, as well as a reduction of platelet activation; these effects appear to be associated with the simultaneous rise in circulating nitrite levels[37] via entero-salivary conversion of the original dietary nitrate load, and further reduction of nitrite to NO[37,49,50].
The DASH diet calls for 4-5 servings of fruits and 4-5 servings of vegetables per day (based on a 2000 calorie diet); the range of daily servings per food group may fluctuate depending on an individual’s daily caloric needs, which take one’s age and activity level into account[14]. In response to the substantial literature present supporting the physiological benefits of dietary nitrate and nitrite, Hord et al[38] extended these findings to assess the high fruit and vegetable recommendations emphasized by the DASH diet. The researchers utilized High-performance liquid chromatography (HPLC) on a convenience sample of foods in order to quantify and compare various nitrate and nitrite concentrations. Results from HPLC indicated a wide range of nitrate and nitrite content amongst various fruits and vegetables. From this data, the researchers generated two hypothetical high- and low-nitrate vegetable and fruit DASH diet patterns (i.e., 1222 mg nitrate vs 174 mg); the analysis revealed that the general dietary pattern of fruits and vegetables outlined in the DASH guidelines have the potential to vary drastically in terms of nitrate intake, based on specific vegetable and fruit selection[38]. The results further suggest that simply increasing fruit and vegetable intake does not directly translate to higher dietary nitrate and nitrite consumption, this may alter the extent of vasoprotective and cardioprotective implications mediated by these molecules (as discussed above).
In the pathologic state, increasing evidence implicates oxidative stress to largely influence the induction and progression of hypertension[51]. The unregulated production of reactive oxygen species (ROS) can largely disrupt function of essential cellular lipids and proteins[52,53]. Accordingly, several natural antioxidant components of fruits and vegetables (i.e., vitamins, minerals, polyphenols) are shown to assist the bodies’ ROS scavenging system via multiple mechanisms, and are greatly implicated in controlling high BP and endothelial dysfunction[54-56]. The rich vitamin and mineral content found within fruits and vegetables contribute to both the enzymatic and non-enzymatic (direct ROS scavengers) antioxidant defense systems[57]. In regards to enzymatic ROS quenching, many of these enzymes exist as metalloenzymes. Three isoforms of superoxide dismutase help confer vascular protection as potent defenders of superoxide via dismutation[58], and utilize metals such as zinc, copper, and manganese. Additionally, glutathione peroxidases readily quench reactive hydrogen peroxide (by product of previous reaction), and are selenium-dependent enzymes[57]. Fruits and vegetables are often great sources of these essential minerals, and thus can contribute to enzymatic ROS defense.
In a similar vein, other micronutrients abundant in fruits and vegetables further bolster antioxidant defense by non-enzymatic means via direct scavenging of ROS. Lipid-soluble vitamin E and water-soluble vitamin C are both capable of reacting with peroxyl radicals[59]. It was later reported that vitamin E might synergistically interact with vitamin C in order to enhance its role as a peroxyl radical scavenger[60]. Additionally, Pierdomenico et al[61] observed drastically reduced plasma levels of vitamin C in hypertensive patients compared to their normotensive counterparts. Subsequently, Block et al[62] supported this observation by demonstrating that vitamin C depletion in normotensive subjects resulted in increased diastolic and systolic BP[62]. However, it should be noted that the relative antioxidant capacity[63] and content[64] of various constituents in fruits and vegetables is variable. This disparity is seen with plant-derived polyphenolic flavonoids, which are found to possess nearly four times the antioxidant activity than vitamin E analogue[63].
As of recent, fructose and its relation to BP has been a popular topic of contention. In the United States, this monosaccharide - naturally available in fruits - is most abundantly obtained from added fructose-glucose sweeteners such as, table sugar (sucrose) and high-fructose corn syrup[65]. Although, high fructose consumption is evidenced to be associated with insulin resistance[66], obesity[67,68], and renal[69] complications, epidemiological studies are controversial[70-72]; moreover, the source of dietary fructose may be of importance. The rich antioxidant properties associated with fruits (discussed above) may counter the potential harmful effects suggested by fructose. While this relationship or mechanism has yet to be established, Forman et al[70] found no association with fructose consumption and hypertension, in which individuals consumed a high intake of fruits. Excluding fructose content from natural fruit, a 2010 cross sectional analysis of United States adults with no prior history of hypertension reported an independent association between high fructose intake (from added sugar) and elevated BP[71]. Cumulatively, the multitude of micronutrients found in fruits and vegetables (as evidenced above) make them a beneficial and necessary inclusion in the major tenets of the DASH diet program.
Dairy products and fatty acids are closely intertwined biochemically, and comprise one of the major tenets of the DASH diet program. The effect of saturated fats on BP has been a popular research topic during the last twenty years. It has been observed that populations consuming diets low in both total and saturated fats, are often the ones exhibiting low to moderate BPs[73]. Analysis of three vegetarian diets: the first consisting of high carbohydrates and low fat, the second high in polyunsaturated fat, and the third low in saturated fat content, revealed that all conditions were shown to reduce BP in their respective populations[73-75]. Further support for the relationship between dietary saturated fat content and BP was demonstrated by a study compiled in Finland; the experimental group that consumed high levels of saturated fats subsequently had higher BP than their control counterparts[76,77].
Unlike saturated fatty acids, diets high in polyunsaturated fats[78] and monounsaturated fats[79] have been shown to have an inverse relationship with BP. Fish oil, high in polyunsaturated fat, has been shown to have protective effects against the risk factors for CVD, including hypertension[80]. The fatty acids found in fish oil are precursors for 3-series prostaglandins, known for their antiaggregatory and vasodilator properties[81]; furthermore, polyunsaturated fats (such as linoleic acid and those found in fish oil) have been shown to lower low-density lipoprotein (LDL) cholesterol levels. This is vitally important in protection against CVD because the oxidized form of LDLs are absorbed by macrophages, creating foam cells that latch onto the walls of arteries, leading to atherosclerosis[82]. Frenoux et al[81] study went on to show that polyunsaturated fatty acids had antihypertensive effects, and also boosted resistance to free radical aggregation and lipid peroxidation.
Dairy, in contrast, consists of a more complex makeup of constituents: 89% water, 3.5% protein, 4.6% carbohydrates, and 3.3% lipid[83]. The protein constituents in dairy products are made up of bioactive peptides, known as lactotripeptides[84], and are similar to those found in lean red meat; both varieties of bioactive peptides have been shown to block ACE[85]. The lipid components of dairy products generally consist of about 60% saturated fatty acids (FA) and about 30% unsaturated FA[86]. The other about 10% consist of short to medium length FA chains that are readily absorbed into the bloodstream, where they preferentially become oxidized rather than stored as triglycerides; and, in short, is associated with weight loss[86,87]. An inverse relationship was found to exist between dairy intake and BP; and, this relationship was strengthened if saturated fat intake was below about 11%[88].
Additionally, dairy intake, while not associated with overall mortality, appears to be inversely related to the multitude of risk factors for CVD including hypertension[89]. More recently, Drouin-Chartier et al[84] reported dairy intake to improve endothelial function and attenuate mild to moderate hypertension. Subsequent studies have shown that vitamin D alone also displays a curative effect on hypertension and other CVD risk factors[90]. The body of evidence here supports the DASH diet’s recommendations in emphasizing the consumption of fat-free and low-fat dairy products, as well as reducing the intake of saturated and trans fats.
Dietary fiber is the indigestible cell wall component found in plants[91]. It is divided into two categories, soluble fiber and insoluble fiber. Soluble fibers include pectins, gums, mucilages, and some hemicelluloses; alternatively, insoluble fibers include lignins, cellulose, and the remainder of the hemicelluloses[92]. Early studies, conducted by Burkitt[93] in 1975, demonstrated that low dietary fiber intake is linked to many diseases, such as cardiovascular disease. This lent credence to the idea that dietary fiber may also be related to hypertension, a major risk factor in CVD. The recommended daily intake value of dietary fiber is between 20-35 g/d[94]. This is twice the amount of fiber that the average American typically consumes[95]. Initially, studies sought to analyze high-fiber vegetarian diets, which were previously shown to attenuate hypertension[91]. Subsequently, there was a rise in controversy; critics suggested that the decrease in BP seen in these studies could have easily arisen from other factors present in a vegetarian diet. Another setback arose with the discovery that increases in dietary fiber alone had little to no effect on normotensive patients[96].
Despite these setbacks, new research emerged showing the benefits of dietary fiber in regards to cardiac distress. In 1997, Stamler et al[97] demonstrated an inverse relationship between increased dietary fiber intake and BP. Additionally, whole grains were shown to be associated with lowering BP[98]. Whole grains themselves offer much nutritional value, providing complex carbohydrates, resistant starch, dietary fiber, minerals, vitamins, phytochemicals (which serve as antioxidants) and other nutrients[99,100]. A 4-fold decrease was seen in the cardiovascular death rate between men who ingested a high fiber diet (> 37 g/d) vs those who ingested a low fiber diet (< 20 g/d)[101]. Similar results were demonstrated, during a 6-year prospective study, in which an inverse relationship was found between dietary fiber intake and CVD rates[102]. Additionally, cereal fiber was revealed to be strongly associated with a reduction in CVD death rates; this result was further supported by the documented protective nature of whole grains[103,104].
Adding support to the inverse relationship between dietary fiber and cardiovascular risk factors, Lairon et al[105] further demonstrated that this correlation existed for all forms of fiber (Soluble, insoluble, fruits, grains, vegetables, cereal, etc.), specifically in regards to the prevalence of hypertension. While mechanistic data is yet to be confirmed, it is thought to be associated with a reduction of abdominal obesity and increased vascular reactivity[105]. Fiber has also been shown to attenuate endothelial dysfunction associated with hypercholesterolemia[106]; this finding suggests that fiber may indirectly play a protective role against hypertension, as the endothelium serves as an important regulator of vascular tone in response to altering needs for blood amongst different organs and tissues[107,108]. Taken together, a substantial body of evidence suggests that increasing dietary fiber plays a beneficial role in the battle against hypertension.
The DASH diet has provided the general public with a non-pharmaceutical option to combat hypertension. The last twenty years have been filled with studies breaking down each aspect of the DASH program. Many of these studies showed great success in highlighting the benefits of lowering sodium and saturated fat intake, while increasing intake of fruits, vegetables, dairy, and dietary fiber. However, the biochemical mechanisms behind these dietary guidelines have only been vaguely illuminated. Dietary biochemistry itself is a very complex story to tell, consisting of a diverse array of mechanistic pathways and byproducts. Although a complete understanding of each of these mechanisms and their implications has not yet been established, substantial effort has been made to fill in the gaps. This review attempted to compile the most recent available research in order to paint as vivid a picture as possible of how individual aspects of the DASH diet effect BP, and ultimately draw a conclusion as to the efficacy of such a diet for the treatment of hypertension. Despite the limitations of the total scope of our analysis, the abundant evidence in support of these dietary modifications is compelling, and we recommend the DASH diet to be an effective non-pharmaceutical treatment of hypertension. Further publicity and implementation could dramatically reduce the number of hypertensives both nationally and internationally. In American culture, focus should be placed on the maintainability of the diet. Stigmata has been placed on healthy diets for being overly expensive, and out of reach for the average family. According to Sacks et al[109], the DASH program should cost approximately $130.00 per week for a family of 4. Adjusting for inflation, this translates to less than $200.00 per week today, still a very low total for a family of 4. Internationally, focus should be shifted to modification of diets depending on the cultural tendencies of the various groups of people who are regionally predisposed to high salt diets. These actions taken together could have a great impact on the health of individuals worldwide. Future research will need to elucidate the biochemical mechanisms inherent in the DASH diet. This could lead to further, more tailored and effective, dietary modifications for the masses; we predict that as further comprehensive understanding of this anti-hypertensive diet grows, we will see an overall attenuation of this disease.
P- Reviewer: de Frutos P, Salles GF, Xiao DL S- Editor: Tian YL L- Editor: A E- Editor: Liu SQ
| 1. | Guilbert JJ. The world health report 2002 - reducing risks, promoting healthy life. Educ Health (Abingdon). 2003;16:230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 1106] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 2. | Bolívar JJ. Essential hypertension: an approach to its etiology and neurogenic pathophysiology. Int J Hypertens. 2013;2013:547809. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 3. | Ariesen MJ, Claus SP, Rinkel GJ, Algra A. Risk factors for intracerebral hemorrhage in the general population: a systematic review. Stroke. 2003;34:2060-2065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 482] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 4. | Sowers JR, Epstein M, Frohlich ED. Diabetes, hypertension, and cardiovascular disease: an update. Hypertension. 2001;37:1053-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 801] [Cited by in RCA: 808] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 5. | Tylicki L, Rutkowski B. [Hypertensive nephropathy: pathogenesis, diagnosis and treatment]. Pol Merkur Lekarski. 2003;14:168-173. [PubMed] |
| 6. | Oparil S, Zaman MA, Calhoun DA. Pathogenesis of hypertension. Ann Intern Med. 2003;139:761-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 393] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 7. | Intersalt: an international study of electrolyte excretion and blood pressure. Results for 24 hour urinary sodium and potassium excretion. Intersalt Cooperative Research Group. BMJ. 1988;297:319-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1741] [Cited by in RCA: 1552] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 8. | Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jones DW, Materson BJ, Oparil S, Wright JT. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560-2572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13416] [Cited by in RCA: 13292] [Article Influence: 604.2] [Reference Citation Analysis (0)] |
| 9. | James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5429] [Cited by in RCA: 5503] [Article Influence: 500.3] [Reference Citation Analysis (0)] |
| 10. | Wright JT, Fine LJ, Lackland DT, Ogedegbe G, Dennison Himmelfarb CR. Evidence supporting a systolic blood pressure goal of less than 150 mm Hg in patients aged 60 years or older: the minority view. Ann Intern Med. 2014;160:499-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 233] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 11. | Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336:1117-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3896] [Cited by in RCA: 3732] [Article Influence: 133.3] [Reference Citation Analysis (0)] |
| 12. | Svetkey LP, Simons-Morton D, Vollmer WM, Appel LJ, Conlin PR, Ryan DH, Ard J, Kennedy BM. Effects of dietary patterns on blood pressure: subgroup analysis of the Dietary Approaches to Stop Hypertension (DASH) randomized clinical trial. Arch Intern Med. 1999;159:285-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 327] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 13. | Svetkey LP, Sacks FM, Obarzanek E, Vollmer WM, Appel LJ, Lin PH, Karanja NM, Harsha DW, Bray GA, Aickin M. The DASH Diet, Sodium Intake and Blood Pressure Trial (DASH-sodium): rationale and design. DASH-Sodium Collaborative Research Group. J Am Diet Assoc. 1999;99:S96-S104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 143] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 14. | NIH National Heart, Lung, and Blood Institutes. Your Guide to Lowering Your Blood Pressure With DASH. [Accessed 2006]. Available from: https://www.nhlbi.nih.gov/health/resources/heart/hbp-dash-index. |
| 15. | Obarzanek E, Proschan MA, Vollmer WM, Moore TJ, Sacks FM, Appel LJ, Svetkey LP, Most-Windhauser MM, Cutler JA. Individual blood pressure responses to changes in salt intake: results from the DASH-Sodium trial. Hypertension. 2003;42:459-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 127] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 16. | Ambard L, Beaujard E. Causes of arterial hypertension. Arch Gen Med. 1904;1:520-533. |
| 17. | Kempner W. Treatment of hypertensive vascular disease with rice diet. Am J Med. 1948;4:545-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Srinivasan SR, Berenson GS, Radhakrishnamurthy B, Dalferes ER, Underwood D, Foster TA. Effects of dietary sodium and sucrose on the induction of hypertension in spider monkeys. Am J Clin Nutr. 1980;33:561-569. [PubMed] |
| 19. | Pichler RH, de Boer IH. Dual renin-angiotensin-aldosterone system blockade for diabetic kidney disease. Curr Diab Rep. 2010;10:297-305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Williams GH, Hollenberg NK. Sodium-sensitive essential hypertension: emerging insights into an old entity. J Am Coll Nutr. 1989;8:490-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Franco V, Oparil S. Salt sensitivity, a determinant of blood pressure, cardiovascular disease and survival. J Am Coll Nutr. 2006;25:247S-255S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 120] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 22. | Campese VM. Salt sensitivity in hypertension. Renal and cardiovascular implications. Hypertension. 1994;23:531-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 248] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 23. | Weinberger MH. Salt sensitivity of blood pressure in humans. Hypertension. 1996;27:481-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 554] [Cited by in RCA: 572] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 24. | Hasimu B, Nakayama T, Mizutani Y, Izumi Y, Asai S, Soma M, Kokubun S, Ozawa Y. Haplotype analysis of the human renin gene and essential hypertension. Hypertension. 2003;41:308-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Poch E, González D, Giner V, Bragulat E, Coca A, de La Sierra A. Molecular basis of salt sensitivity in human hypertension. Evaluation of renin-angiotensin-aldosterone system gene polymorphisms. Hypertension. 2001;38:1204-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 142] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 26. | Kopkan L, Husková Z, Vanourková Z, Thumová M, Skaroupková P, Cervenka L, Majid DS. Superoxide and its interaction with nitric oxide modulates renal function in prehypertensive Ren-2 transgenic rats. J Hypertens. 2007;25:2257-2265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Kopkan L, Husková Z, Vanourková Z, Thumová M, Skaroupková P, Malý J, Kramer HJ, Dvorák P, Cervenka L. Reduction of oxidative stress does not attenuate the development of angiotensin II-dependent hypertension in Ren-2 transgenic rats. Vascul Pharmacol. 2009;51:175-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Bayorh MA, Ganafa AA, Emmett N, Socci RR, Eatman D, Fridie IL. Alterations in aldosterone and angiotensin II levels in salt-induced hypertension. Clin Exp Hypertens. 2005;27:355-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Gu JW, Young E, Pan ZJ, Tucker KB, Shparago M, Huang M, Bailey AP. Long-term high salt diet causes hypertension and alters renal cytokine gene expression profiles in Sprague-Dawley rats. Beijing Daxue Xuebao. 2009;41:505-515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 30. | He FJ, MacGregor GA. How far should salt intake be reduced? Hypertension. 2003;42:1093-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 239] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 31. | Barros CL, Sousa AL, Chinem BM, Rodrigues RB, Jardim TS, Carneiro SB, Souza WK, Jardim PC. Impact of light salt substitution for regular salt on blood pressure of hypertensive patients. Arq Bras Cardiol. 2015;104:128-135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 32. | Cogswell ME, Zhang Z, Carriquiry AL, Gunn JP, Kuklina EV, Saydah SH, Yang Q, Moshfegh AJ. Sodium and potassium intakes among US adults: NHANES 2003-2008. Am J Clin Nutr. 2012;96:647-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 200] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 33. | Bray GA, Vollmer WM, Sacks FM, Obarzanek E, Svetkey LP, Appel LJ. A further subgroup analysis of the effects of the DASH diet and three dietary sodium levels on blood pressure: results of the DASH-Sodium Trial. Am J Cardiol. 2004;94:222-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 134] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 34. | Akita S, Sacks FM, Svetkey LP, Conlin PR, Kimura G. Effects of the Dietary Approaches to Stop Hypertension (DASH) diet on the pressure-natriuresis relationship. Hypertension. 2003;42:8-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 91] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 35. | Folkow B, Grimby G, Thulesius O. Adaptive structural changes of the vascular walls in hypertension and their relation to the control of the peripheral resistance. Acta Physiol Scand. 1958;44:255-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 370] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 36. | Folkow B. Physiological aspects of primary hypertension. Physiol Rev. 1982;62:347-504. [PubMed] |
| 37. | Webb AJ, Patel N, Loukogeorgakis S, Okorie M, Aboud Z, Misra S, Rashid R, Miall P, Deanfield J, Benjamin N. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension. 2008;51:784-790. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 856] [Cited by in RCA: 774] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 38. | Hord NG, Tang Y, Bryan NS. Food sources of nitrates and nitrites: the physiologic context for potential health benefits. Am J Clin Nutr. 2009;90:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 633] [Cited by in RCA: 607] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 39. | Lundberg JO, Carlström M, Larsen FJ, Weitzberg E. Roles of dietary inorganic nitrate in cardiovascular health and disease. Cardiovasc Res. 2011;89:525-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 223] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 40. | Moore C, Tymvios C, Emerson M. Functional regulation of vascular and platelet activity during thrombosis by nitric oxide and endothelial nitric oxide synthase. Thromb Haemost. 2010;104:342-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 41. | Panza JA, Quyyumi AA, Brush JE, Epstein SE. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N Engl J Med. 1990;323:22-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1763] [Cited by in RCA: 1697] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 42. | Panza JA, Casino PR, Kilcoyne CM, Quyyumi AA. Role of endothelium-derived nitric oxide in the abnormal endothelium-dependent vascular relaxation of patients with essential hypertension. Circulation. 1993;87:1468-1474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 402] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 43. | Taddei S, Virdis A, Mattei P, Ghiadoni L, Sudano I, Salvetti A. Defective L-arginine-nitric oxide pathway in offspring of essential hypertensive patients. Circulation. 1996;94:1298-1303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 184] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 44. | Radomski MW, Palmer RM, Moncada S. Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet. 1987;2:1057-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1034] [Cited by in RCA: 1018] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 45. | Palmer RM, Ashton DS, Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988;333:664-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3437] [Cited by in RCA: 3374] [Article Influence: 91.2] [Reference Citation Analysis (0)] |
| 46. | Förstermann U, Closs EI, Pollock JS, Nakane M, Schwarz P, Gath I, Kleinert H. Nitric oxide synthase isozymes. Characterization, purification, molecular cloning, and functions. Hypertension. 1994;23:1121-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 756] [Cited by in RCA: 760] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 47. | Hermann M, Flammer A, Lüscher TF. Nitric oxide in hypertension. J Clin Hypertens (Greenwich). 2006;8:17-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 252] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 48. | Hirst DG, Robson T. Nitric oxide physiology and pathology. Methods Mol Biol. 2011;704:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 79] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 49. | Duncan C, Dougall H, Johnston P, Green S, Brogan R, Leifert C, Smith L, Golden M, Benjamin N. Chemical generation of nitric oxide in the mouth from the enterosalivary circulation of dietary nitrate. Nat Med. 1995;1:546-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 474] [Cited by in RCA: 467] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 50. | McKnight GM, Smith LM, Drummond RS, Duncan CW, Golden M, Benjamin N. Chemical synthesis of nitric oxide in the stomach from dietary nitrate in humans. Gut. 1997;40:211-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 223] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 51. | Kizhakekuttu TJ, Widlansky ME. Natural antioxidants and hypertension: promise and challenges. Cardiovasc Ther. 2010;28:e20-e32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 114] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 52. | Davies KJ. Protein damage and degradation by oxygen radicals. I. general aspects. J Biol Chem. 1987;262:9895-9901. [PubMed] |
| 53. | Rochette L, Zeller M, Cottin Y, Vergely C. Diabetes, oxidative stress and therapeutic strategies. Biochim Biophys Acta. 2014;1840:2709-2729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 349] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 54. | Moore TJ, Vollmer WM, Appel LJ, Sacks FM, Svetkey LP, Vogt TM, Conlin PR, Simons-Morton DG, Carter-Edwards L, Harsha DW. Effect of dietary patterns on ambulatory blood pressure : results from the Dietary Approaches to Stop Hypertension (DASH) Trial. DASH Collaborative Research Group. Hypertension. 1999;34:472-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 87] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 55. | John JH, Ziebland S, Yudkin P, Roe LS, Neil HA. Effects of fruit and vegetable consumption on plasma antioxidant concentrations and blood pressure: a randomised controlled trial. Lancet. 2002;359:1969-1974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 322] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 56. | Ulker S, McKeown PP, Bayraktutan U. Vitamins reverse endothelial dysfunction through regulation of eNOS and NAD(P)H oxidase activities. Hypertension. 2003;41:534-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 169] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 57. | Lampe JW. Health effects of vegetables and fruit: assessing mechanisms of action in human experimental studies. Am J Clin Nutr. 1999;70:475S-490S. [PubMed] |
| 58. | Faraci FM, Didion SP. Vascular protection: superoxide dismutase isoforms in the vessel wall. Arterioscler Thromb Vasc Biol. 2004;24:1367-1373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 362] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 59. | Sies H, Stahl W, Sundquist AR. Antioxidant functions of vitamins. Vitamins E and C, beta-carotene, and other carotenoids. Ann N Y Acad Sci. 1992;669:7-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 493] [Cited by in RCA: 446] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 60. | Niki E. Role of vitamin E as a lipid-soluble peroxyl radical scavenger: in vitro and in vivo evidence. Free Radic Biol Med. 2014;66:3-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 403] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 61. | Pierdomenico SD, Costantini F, Bucci A, De Cesare D, Cuccurullo F, Mezzetti A. Low-density lipoprotein oxidation and vitamins E and C in sustained and white-coat hypertension. Hypertension. 1998;31:621-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 62. | Block G, Mangels AR, Norkus EP, Patterson BH, Levander OA, Taylor PR. Ascorbic acid status and subsequent diastolic and systolic blood pressure. Hypertension. 2001;37:261-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 53] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 63. | Rice-Evans CA, Miller NJ, Bolwell PG, Bramley PM, Pridham JB. The relative antioxidant activities of plant-derived polyphenolic flavonoids. Free Radic Res. 1995;22:375-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1325] [Cited by in RCA: 1116] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 64. | Carlsen MH, Halvorsen BL, Holte K, Bøhn SK, Dragland S, Sampson L, Willey C, Senoo H, Umezono Y, Sanada C. The total antioxidant content of more than 3100 foods, beverages, spices, herbs and supplements used worldwide. Nutr J. 2010;9:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 589] [Cited by in RCA: 500] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 65. | White JS. Straight talk about high-fructose corn syrup: what it is and what it ain’t. Am J Clin Nutr. 2008;88:1716S-1721S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 133] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 66. | Stanhope KL, Schwarz JM, Keim NL, Griffen SC, Bremer AA, Graham JL, Hatcher B, Cox CL, Dyachenko A, Zhang W. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest. 2009;119:1322-1334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1178] [Cited by in RCA: 1205] [Article Influence: 75.3] [Reference Citation Analysis (0)] |
| 67. | Bray GA, Nielsen SJ, Popkin BM. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr. 2004;79:537-543. [PubMed] |
| 68. | Malik VS, Popkin BM, Bray GA, Després JP, Hu FB. Sugar-sweetened beverages, obesity, type 2 diabetes mellitus, and cardiovascular disease risk. Circulation. 2010;121:1356-1364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1269] [Cited by in RCA: 1182] [Article Influence: 78.8] [Reference Citation Analysis (0)] |
| 69. | Shoham DA, Durazo-Arvizu R, Kramer H, Luke A, Vupputuri S, Kshirsagar A, Cooper RS. Sugary soda consumption and albuminuria: results from the National Health and Nutrition Examination Survey, 1999-2004. PLoS One. 2008;3:e3431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 82] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 70. | Forman JP, Choi H, Curhan GC. Fructose and vitamin C intake do not influence risk for developing hypertension. J Am Soc Nephrol. 2009;20:863-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 71. | Jalal DI, Smits G, Johnson RJ, Chonchol M. Increased fructose associates with elevated blood pressure. J Am Soc Nephrol. 2010;21:1543-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 143] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 72. | Nguyen S, Choi HK, Lustig RH, Hsu CY. Sugar-sweetened beverages, serum uric acid, and blood pressure in adolescents. J Pediatr. 2009;154:807-813. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 283] [Cited by in RCA: 240] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 73. | Sacks FM, Rosner B, Kass EH. Blood pressure in vegetarians. Am J Epidemiol. 1974;100:390-398. [PubMed] |
| 74. | Sacks FM, Kass EH. Low blood pressure in vegetarians: effects of specific foods and nutrients. Am J Clin Nutr. 1988;48:795-800. [PubMed] |
| 75. | Rouse IL, Beilin LJ, Mahoney DP, Margetts BM, Armstrong BK, Vandongen R. Vegetarian diet and blood pressure. Lancet. 1983;2:742-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 76. | Salonen JT, Tuomilehto J, Tanskanen A. Relation of blood pressure to reported intake of salt, saturated fats, and alcohol in healthy middle-aged population. J Epidemiol Community Health. 1983;37:32-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 77. | Salonen JT, Salonen R, Ihanainen M, Parviainen M, Seppänen R, Kantola M, Seppänen K, Rauramaa R. Blood pressure, dietary fats, and antioxidants. Am J Clin Nutr. 1988;48:1226-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 78. | Berry EM, Hirsch J. Does dietary linolenic acid influence blood pressure? Am J Clin Nutr. 1986;44:336-340. [PubMed] |
| 79. | Rasmussen BM, Vessby B, Uusitupa M, Berglund L, Pedersen E, Riccardi G, Rivellese AA, Tapsell L, Hermansen K. Effects of dietary saturated, monounsaturated, and n-3 fatty acids on blood pressure in healthy subjects. Am J Clin Nutr. 2006;83:221-226. [PubMed] |
| 80. | Chen HW, Lii CK, Chen WT, Wang ML, Ou CC. Blood pressure-lowering effect of fish oil is independent of thromboxane A2 level in spontaneously hypertensive rats. Prostaglandins Leukot Essent Fatty Acids. 1996;54:147-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 81. | Frenoux JM, Prost ED, Belleville JL, Prost JL. A polyunsaturated fatty acid diet lowers blood pressure and improves antioxidant status in spontaneously hypertensive rats. J Nutr. 2001;131:39-45. [PubMed] |
| 82. | Suzukawa M, Abbey M, Howe PR, Nestel PJ. Effects of fish oil fatty acids on low density lipoprotein size, oxidizability, and uptake by macrophages. J Lipid Res. 1995;36:473-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 83. | German JB, Dillard CJ. Composition, structure and absorption of milk lipids: a source of energy, fat-soluble nutrients and bioactive molecules. Crit Rev Food Sci Nutr. 2006;46:57-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 193] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 84. | Drouin-Chartier JP, Gigleux I, Tremblay AJ, Poirier L, Lamarche B, Couture P. Impact of dairy consumption on essential hypertension: a clinical study. Nutr J. 2014;13:83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 85. | McGregor RA, Poppitt SD. Milk protein for improved metabolic health: a review of the evidence. Nutr Metab (Lond). 2013;10:46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 125] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 86. | Molkentin J. Occurrence and biochemical characteristics of natural bioactive substances in bovine milk lipids. Br J Nutr. 2000;84 Suppl 1:S47-S53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 46] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 87. | Dougkas A, Reynolds CK, Givens ID, Elwood PC, Minihane AM. Associations between dairy consumption and body weight: a review of the evidence and underlying mechanisms. Nutr Res Rev. 2011;24:72-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 88. | Djoussé L, Pankow JS, Hunt SC, Heiss G, Province MA, Kabagambe EK, Ellison RC. Influence of saturated fat and linolenic acid on the association between intake of dairy products and blood pressure. Hypertension. 2006;48:335-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 89. | Soedamah-Muthu SS, Ding EL, Al-Delaimy WK, Hu FB, Engberink MF, Willett WC, Geleijnse JM. Milk and dairy consumption and incidence of cardiovascular diseases and all-cause mortality: dose-response meta-analysis of prospective cohort studies. Am J Clin Nutr. 2011;93:158-171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 316] [Cited by in RCA: 294] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 90. | Zhang Y, Hao X, Sun W, Ma H, Yang Y, Zhu Y, Zhu H. The Study Advance on The Role of Vitamin D in Hypertension and Cardiovascular Disease (CVD). J Am SCI. 2015;11:120-125. |
| 91. | Anderson JW, Smith BM, Gustafson NJ. Health benefits and practical aspects of high-fiber diets. Am J Clin Nutr. 1994;59:1242S-1247S. [PubMed] |
| 92. | Brown L, Rosner B, Willett WW, Sacks FM. Cholesterol-lowering effects of dietary fiber: a meta-analysis. Am J Clin Nutr. 1999;69:30-42. [PubMed] |
| 93. | Burkitt D. Food fiber and disease prevention. Compr Ther. 1975;1:19-22. [PubMed] |
| 94. | Pilch SM. Physiological Effects and Health Consequences of Dietary Fiber. Bethesda, MD: Life Sciences Research Office 1987; 55-67. |
| 95. | Anderson JW, Bridges SR, Tietyen J, Gustafson NJ. Dietary fiber content of a simulated American diet and selected research diets. Am J Clin Nutr. 1989;49:352-357. [PubMed] |
| 96. | Swain JF, Rouse IL, Curley CB, Sacks FM. Comparison of the effects of oat bran and low-fiber wheat on serum lipoprotein levels and blood pressure. N Engl J Med. 1990;322:147-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 145] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 97. | Stamler J, Caggiula AW, Grandits GA. Relation of body mass and alcohol, nutrient, fiber, and caffeine intakes to blood pressure in the special intervention and usual care groups in the Multiple Risk Factor Intervention Trial. Am J Clin Nutr. 1997;65:338S-365S. [PubMed] |
| 98. | Anderson JW, Hanna TJ. Whole grains and protection against coronary heart disease: what are the active components and mechanisms? Am J Clin Nutr. 1999;70:307-308. [PubMed] |
| 99. | Anderson JW, Bridges SR. Dietary fiber content of selected foods. Am J Clin Nutr. 1988;47:440-447. [PubMed] |
| 100. | Anderson JW, Deakins DA, Floore TL, Smith BM, Whitis SE. Dietary fiber and coronary heart disease. Crit Rev Food Sci Nutr. 1990;29:95-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 130] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 101. | Kromhout D, Bosschieter EB, de Lezenne Coulander C. Dietary fibre and 10-year mortality from coronary heart disease, cancer, and all causes. The Zutphen study. Lancet. 1982;2:518-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 97] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 102. | Rimm EB, Ascherio A, Giovannucci E, Spiegelman D, Stampfer MJ, Willett WC. Vegetable, fruit, and cereal fiber intake and risk of coronary heart disease among men. JAMA. 1996;275:447-451. [PubMed] [DOI] [Full Text] |
| 103. | Jacobs DR, Meyer KA, Kushi LH, Folsom AR. Whole-grain intake may reduce the risk of ischemic heart disease death in postmenopausal women: the Iowa Women’s Health Study. Am J Clin Nutr. 1998;68:248-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 135] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 104. | Liu S, Stampfer MJ, Hu FB, Giovannucci E, Rimm E, Manson JE, Hennekens CH, Willett WC. Whole-grain consumption and risk of coronary heart disease: results from the Nurses’ Health Study. Am J Clin Nutr. 1999;70:412-419. [PubMed] |
| 105. | Lairon D, Arnault N, Bertrais S, Planells R, Clero E, Hercberg S, Boutron-Ruault MC. Dietary fiber intake and risk factors for cardiovascular disease in French adults. Am J Clin Nutr. 2005;82:1185-1194. [PubMed] |
| 106. | Momenizadeh A, Heidari R, Sadeghi M, Tabesh F, Ekramzadeh M, Haghighatian Z, Golshahi J, Baseri M. Effects of oat and wheat bread consumption on lipid profile, blood sugar, and endothelial function in hypercholesterolemic patients: A randomized controlled clinical trial. ARYA Atheroscler. 2014;10:259-265. [PubMed] |
| 107. | Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003;23:168-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1535] [Cited by in RCA: 1621] [Article Influence: 73.7] [Reference Citation Analysis (0)] |
| 108. | Weissberg P. Mechanisms modifying atherosclerotic disease - from lipids to vascular biology. Atherosclerosis. 1999;147 Suppl 1:S3-S10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 109. | Sacks FM, Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Bray GA, Vogt TM, Cutler JA, Windhauser MM. A dietary approach to prevent hypertension: a review of the Dietary Approaches to Stop Hypertension (DASH) Study. Clin Cardiol. 1999;22:III6-II10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 138] [Article Influence: 5.3] [Reference Citation Analysis (0)] |