Published online May 23, 2015. doi: 10.5494/wjh.v5.i2.98
Peer-review started: October 5, 2014
First decision: December 12, 2014
Revised: January 31, 2015
Accepted: February 10, 2015
Article in press: February 12, 2015
Published online: May 23, 2015
Processing time: 232 Days and 15.2 Hours
AIM: To study patients with atrial fibrillation and hypertension who had successful catheter ablation for changes in blood pressure 1 year later.
METHODS: A retrospective study was performed on patients who had catheter ablation for atrial fibrillation (AF) and hypertension (HTN) which included local autonomic ganglionated plexi denervation and pulmonary veins isolation. Of the records of 119 patients, follow-up data was found in order to determine the presence of sinus rhythm and data on systolic (SBP) and diastolic blood pressure at 2 wk, 3 mo, 6 mo and 1 year after the ablation procedure. Transthoracic echocardiograms were taken at the time of the catheter procedure to determine left atrial dimensions (LADs) and left ventricular size.
RESULTS: There was no significant difference in the pre-ablation mean blood pressures between the two groups (P = 0.08). After 1 year 33 of the 60 with AF and HTN were in sinus rhythm, of whom 12 had normal LADs, ≤ 4 cm Group 1, and 21 had enlarged left atria (LADs > 4 cm, Group 2). For Group 1, at 1 year of follow up, there was a significant difference in the SBP (119.2 ± 13 mmHg) compared to pre-ablation (142.6 ± 13.7 mmHg, P = 0.001). For Group 2, there was no significant difference in the SBP, pre-ablation (130.3 ± 17.5 mmHg) and at 1 year of follow up (130.4 ± 13.4 mmHg, P = 0.75). All patients were on similar anti-hypertensive medications. There was a trend for a greater left ventricular size in Group 2 compared to Group 1.
CONCLUSION: We suggest that Group 1 had HTN due to sympathetic hyperactivity, neurogenic HTN; whereas HTN in Group 2 was based on arterial vasoconstriction.
Core tip: A retrospective study of 119 patient with atrial fibrillation (AF) and hypertension (HTN) underwent catheter ablation consisting of pulmonary vein isolation and local cardiac autonomic denervation. After 1 year 33 were in sinus rhythm and fell into 2 categories based on significant differences in left atrial dimensions (LADs). Although similarly medicated, Group I (LADs ≤ 4 cm) had a significant decrease in blood pressure compared to Group 2, LAD > 4 cm. We conclude that HTN in Group 1 was neurogenic and ameliorated by neural ablation; whereas HTN in Group 2, manifested arterial vasoconstriction as the mechanism for HTN.
- Citation: Sharma T, Scherlag BJ, Nakagawa H, Jackman WM, Lazzara R, Po SS. Catheter ablation for atrial fibrillation in a subset of patients with concomitant hypertension. World J Hypertens 2015; 5(2): 98-103
- URL: https://www.wjgnet.com/2220-3168/full/v5/i2/98.htm
- DOI: https://dx.doi.org/10.5494/wjh.v5.i2.98
Early studies seeking the mechanism for clinical hypertension (HTN) focused on the sympathetic nervous system as the underlying cause. It was well known that sympathetic stimulation of the heart led to increased contractility leading to high blood pressure. Indeed, a concept was put forth by several investigators suggesting that HTN was neurogenic in origin[1-5]. The seminal studies of Goldblatt et al[6], induced chronic HTN in animals who had a clip (stenosis) on the renal arteries which provided the basis for the discovery of the role of the renin/angiotensin/aldosterone syndrome as a cause of HTN[7]. This concept became the prevalent view and has become the mainstay of therapeutic strategies to control HTN, i.e., use of angiotensin blocking agents and diuretics. The acceptance of a sympathetic contribution is evidenced by the addition of beta-blockers to the antihypertensive regimen. The Framingham study has provided evidence linking HTN and atrial fibrillation (AF). In a multivariate analysis it was found that HTN was an independent predictor for developing AF, with an odds ratio of 1.5 for men and 1.4 for women[1]. The sequence of pathological events in this association might start with increased vascular resistance followed by ventricular hypertrophy and atrial dilatation, the last providing the substrate for AF[8].
The recent dramatic effects of renal sympathetic denervation[9] has revived the neurogenic concept[10] particularly in regard to the treatment for resistant forms of hypertension. In this regard, Schlaich et al[11] cited experimental[12] and clinical[13] evidence that afferent nerve denervation may play a more significant role in “the sustained blood pressure-lowering (by) renal denervation …via the removal of renal afferent activity and the subsequent effects on central sympathetic outflow”. It is interesting to note that renal sympathetic denervation has also been applied to patients with concomitant hypertension and atrial fibrillation. Scherlag et al[14] reported that renal artery denervation reduces systolic and diastolic blood pressure in patients with drug-resistant hypertension and reduces AF recurrences when combined with pulmonary vein isolation (PVI).
Since 2004 the procedure for catheter ablation in patients with AF in our clinical electrophysiological practice has consisted of PVI plus ablation of hyperactive autonomic nerve clusters called ganglionated plexi (GP) at the PV-atrial junctions. This combined procedure has been shown to increase the success rates for maintaining sinus rhythm compared to PVI alone[15,16]. It was in this context that we hypothesized that a subset of our patients presenting with HTN and AF would manifest the neurogenic form of HTN based on hyperactivity of the intrinsic cardiac autonomic nervous system. Furthermore, based on the previous report[15] we surmised that the patients with the neurogenic and drug resistant form of HTN would respond with a significant blood pressure reduction due to the decrease of autonomic hyperactivity caused by PVI plus GP ablation.
We performed a retrospective study of 119 patients who had undergone catheter ablation using an irrigated tip ablation catheter (Biosense/Webster, Navi-Star, Thermocool catheter, Diamond Bar, CA, United States) for mapping and ablation.
The procedure for catheter ablation has been previously described in detail[15]. Briefly, General anesthesia was administered in all patients. Localization of GP was obtained by application of high-frequency stimulation to each GP (HFS; 20 Hz, 10-150 V and pulse width 1-10 ms; S-88 stimulator, Grass Instruments Division, Astro Med Inc., Warwick, RI, United States). Within 5 s of HFS, a marked parasympathetic response is elicited, which is arbitrarily defined as a ≥ 50% increase in mean R-R interval during AF. Each parasympathetic response is verified by both hypotension and high grade AV block. For GP ablation, radiofrequency (RF) current is delivered at 25-35 W for 40-60 s during saline irrigation at each site of positive parasympathetic response to HFS. RF applications are repeated until the parasympathetic response to HFS is eliminated.
After the 4 left atrial GP are ablated, pulmonary vein antrum isolation is performed. The endpoint of PV antrum isolation is elimination of potentials within the isolated antral area. As antrum isolation typically transects the ARGP and SLGP areas, we use the ARGP and SLGP ablation sites as the starting points for right and left antrum isolation, respectively. Echocardiographic studies were accomplished transthoracically which provided an anterior-posterior measurement of the left atrial dimensions.
Inclusion criteria were: (1) Successful catheter ablation for AF involving both GP ablation and PV isolation (patient should have been in sinus rhythm after one year); (2) Co-existence of AF and HTN; (3) Knowledge of left atrial dimension (LAD) by echocardiographic measurement; and (4) All patients were on anti-hypertensive drug regimens.
The exclusion criteria included: (1) Recurrence of AF at the end of one year; (2) No GP ablation; and (3) No follow up blood pressure data.
Of the 119 patients reviewed, there were 60 patients with co-existing AF and HTN. Of these 60 patients, only 33 patients who had been contacted were in sinus rhythm at the end of one year of follow up. The purpose of the study was to determine the differences in the blood pressure levels before ablation and at different periods of follow-up. The pre-ablation systolic (SBP) and diastolic (DBP) blood pressures of the patients in the two groups were compared with the post-ablation SBP and DBP at two weeks, three months, six months and one year of follow-up.
Statistical analyses were done using the SAS software (V 9.1). All statistical tests were carried out at an alpha of 0.05. Data is expressed as mean ± SD. Repeated measures analysis of variance (ANOVA) was used to determine if the mean SBP and DBP changed over the follow up periods for the two groups. Post-hoc analysis was done to compare the mean blood pressures across the individual follow up periods.
All of the 33 patients included in the chart review were on single or multiple antihypertensive medications prior to the ablation procedure (Table 1). Although 8 patients were on a single medication all the others were taking multiple anti-hypertensive agents and all continued these same regimens during the follow-up period.
| No. of patients | Anti-hypertensive agents |
| 7 | ACE inhibitor |
| 7 | Calcium channel blockers |
| 2 | Beta blockers |
| 1 | Angiotensin 2 receptor blocker |
| 6 | ACE inhibitor, beta blocker |
| 3 | Calcium channel blocker, angiotensin 2 receptor blocker |
| 2 | ACE inhibitor blocker. Calcium channel blocker |
| 2 | ACE inhibitor, diuretic |
| 1 | Calcium channel blocker, beta blocker, diuretic |
| 1 | Calcium channel blocker, beta blocker |
| 1 | ACE inhibitor, calcium channel blocker, diuretic |
There were 12 patients with normal sized left atria (LAD ≤ 4.0 cm) and 21 patients with enlarged left atria (LAD > 4.0 cm). On the basis of the blood pressure responses during progressive periods of follow-up, the 33 patients could be divided into two groups based on their LAD, viz., < 4.0 or > 4.0. Table 2 compares the descriptive statistics for all 33 patients that met the inclusion criteria divided into the 2 groups. Although there was a greater absolute mean value in group 1 (142/83 mmHg) vs group 2 (130/80 mmHg) the difference was not statistically significant prior to ablation (P = 0.08) For patients with LA dimensions ≤ 4.0 cm (Group 1), there was a significant difference in the mean SBP levels, at 2 wk after the ablation procedure (126.8 ± 19.4 mmHg) compared to SBP, pre-ablation (142 ± 13.7 mmHg, P = 0.008). This change persisted at three and six months of follow up. The mean SBP levels at one year of follow up (119.2 ± 13 mmHg) were significantly lower than the pre-ablation mean SBP levels (142 ± 13.7 mmHg), for patients with LADs ≤ 4.0 cm, P = 0.001.
| Variable | Group 1 (LAD ≤4 cm), mean ± SD | Group 2 (LAD > 4 cm), mean ± SD |
| AGE (yr) | 58.3 ± 9.2 | 60.4 ± 6.8 |
| LAD (cm) | 3.63 ± 0.34 | 4.54 ± 0.4 |
| Pre-ablation SBP (mmHg) | 142.6 ± 13.7 | 130.3 ± 17.5 |
| Pre-ablation DBP (mmHg) | 83.8 ± 11.6 | 80.6 ± 15.6 |
| SBP - 2 wk (mmHg) | 126.8 ± 19.4a | 129.6 ± 16.9 |
| DBP - 2 wk (mmHg) | 76.2 ± 13.5 | 78.9 ± 11.5 |
| SBP - 3 mo (mmHg) | 129.1 ± 15.4a | 132.1 ± 13.6 |
| DBP - 3 mo (mmHg) | 77.6 ± 12.8 | 79.1 ± 12.2 |
| SBP - 6 mo (mmHg) | 123.7 ± 16.8ab | 134.3 ± 14.4 |
| DBP - 6 mo (mmHg) | 76 ± 10.1 | 77.7 ± 9.5 |
| SBP - 1 yr (mmHg) | 119.2 ± 13ab | 130.4 ± 13.4 |
| DBP - 1 yr (mmHg) | 70.4 ± 12.2 | 78.7 ± 9.1 |
For patients with LADs > 4.0 cm (Group 2), there was no significant difference in the mean SBP levels, pre-ablation (130 ± 17.5 mmHg) and at 2 wk of follow-up (129 ± 16.9 mmHg) (P = 0.92). For patients with LADs > 4.0 cm, there was no significant difference in the mean SBP levels, pre-ablation and at 1 year of follow up (130 ± 13.4 mmHg, P = 0.75). There was no significant difference in the mean DBP throughout the follow up periods for both groups.
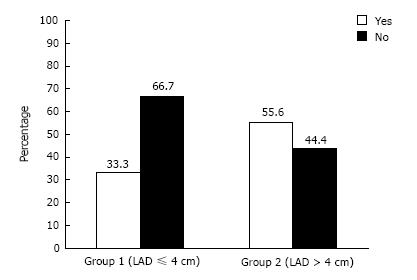
Figure 1 compares the proportion of patients with left ventricular hypertrophy (LVH) in both groups. Although the number of patients with or without LVH were not significantly different, the trend showed a lower incidence of LVH in Group 1 (33%) vs Group 2 (56%) and a corresponding higher number lacking LVH in Group 1 (67%) vs Group 2 (44%). The small sample size and wide range of standard deviation may have precluded obtaining statistical significance (P = 0.08).
Although there was no significant difference between the initial blood pressures of the two groups, the response to GP ablation and PVI were dramatically different during the follow-up periods. Specifically, in Group 1 patients, there was a marked decrease in the mean SBP over the short term (from 142 ± 13.7 mmHg to 126.8 ± 19.4 mmHg within 2 wk) which was maintained after 3 and 6 mo. At 1 year the mean SBP was even more significantly decreased compared to the initial values (119 ± 13 mmHg, P = 0.001). In contrast, in Group 2, there was no change in SBP over the same time periods, even though the response to AF ablation was exactly the same as in Group 1. Since the anti-hypertensive drug history was heterogeneously distributed for both groups, these findings suggest that Group 1 patients with the putative “neurogenic” form of HTN, i.e., due to increased ventricular contractility, represented a sub-population of HTN resistant to drugs prior to catheter ablation. It is of interest that recent studies of another group of patients with a resistant form of HTN have been shown to respond to renal artery denervation with an endovascular method for applying radiofrequency ablation to adventitial sympathetic nerves[10,11]. Experimental evidence has shown that the neurogenic form of HTN derives from an increase of sympathetic activity which increases the BP through enhanced ventricular contractility, so-called “cardiogenic hypertension”. Sustained HTN has been shown to occur experimentally by chronic electrical stimulation of the left stellate ganglion[17].
In a recent study from our laboratory, we developed an acute model simulating inappropriate sinus tachycardia[18]. In 14 anesthetized dogs; 0.3 mg of 10-3 solution of epinephrine was injected into the anterior right ganglionated plexi (ARGP). In eight of the dogs there was a significant increase in the average heart rate of 57 beats/min but no change in systolic blood pressure. In six dogs both heart rate and systolic blood pressure were equally and significantly accentuated and remained elevated for at least 30 min. In addition ventricular arrhythmias were also observed which overwhelmed sinus rhythm. Other studies provided functional evidence of neural connections between ganglionated plexi in the atria which, when stimulated chemically, caused marked sympathetic effects on the ventricles, including ventricular arrhythmias[19].
It should be mentioned that sympathetic afferents may also play a critical role in the marked reduction of SBP in the Group 1 patients after GP ablation and PVI. Ardell[20] in a review of the cardiac neurons that inhabit the GP and the atrial neural network emphasized the afferent connections from these intrinsic cardiac elements to the brainstem. How does this scenario for reduction of BP by renal denervation translate to the present study? We hypothesize that the findings of the present studies suggest that multiple visceral sites, e.g., the heart and renal arteries, which are autonomically innervated can be a source of abnormal sympathetic afferent conduction to central vasomotor centers leading to excessive efferent return to neuro-effector junctions to the same structures. Hyperactivity of GP has been shown to contribute to the propensity for AF by excessive release of cholinergic and adrenergic neurotransmitters via postganglionic axons innervating the PVs and atria[21-23]. Hyperactive GP may also send excessive afferent signals to central sympathetic centers which in turn would increase sympathetic outflow returning to the heart and vasculature. The results would be enhanced propensity for AF and increased ventricular contractility and vasoconstriction, i.e., the neurogenic form of AF and HTN. Ablation of the GP would therefore, have a dual salutary effect by reducing both efferent and afferent activity leading to amelioration of HTN and suppression of AF. This scenario is what was found in the sub-population of patients in the present study with the appropriate biomarkers, i.e., normal LAD dimensions and drug resistant HTN. In this regard a recent case report, described the application of renal artery denervation without PVI in a patient with drug resistant HTN and symptomatic, persistent AF. After a short follow-up of 5 mo the patient is in sinus rhythm with a reduction of blood pressure prior to renal sympathetic denervation (148/80 mmHg) to 111/60 mmHg. Of interest, echocardiography showed a left atrial diameter of 45 mm prior to ablation which was slightly reduced after ablation[24].
We do not know if eliminating anti-hypertensive drugs from those patients with the “neurogenic” form of HTN manifesting normal left atrial dimensions would have resulted in a reduction in SBP than we found. However, in those patients with the “arterial vasoconstrictor” form of HTN manifesting LADs greater than 4 cm, there was no significant change in mean SBP over the same follow-up period, even though they had the same AF ablation procedure and the same salutary outcome, i.e., restoration of sinus rhythm while still on antihypertensive agents. It has been noted by the authors of the Symplicity trials that the extension of renal artery denervation to patients who respond favorably to drugs is problematic. Indeed, Frohlich[25] in a recent editorial indicated that, “Only a small fraction of patients with hypertension have “drug resistant hypertension”…Consequently, the mass extrapolation to all patients with hypertension for…this specialized procedure does not seem appropriate at this time. Therefore, we do not know if patients in Group 2, off drugs, would have also responded with significant reductions of SBP after catheter ablation. A distinct limitation of this study is the small numbers of patients in groups 1 and 2 which requires that the findings be interpreted with caution. Further studies of the co-morbidity (AF and HTN) in a larger cohort of patients will help to corroborate the present findings, particularly if one group contains those having PVI alone or PVI plus GP ablation[14].
Significant differences were found in the mean SBP before ablation and at follow up intervals, with the SBP being lower post GP ablation in patients with AF and HTN with normal LADs. Based on previous experimental and clinical studies we conclude that HTN in Group 1 was sympathetically based, i.e., neurogenic HTN, and drug resistant whereas HTN in Group 2, mainly drug responders, manifested arterial vasoconstriction as the mechanism for HTN. We hypothesize that GP ablation in Group 1 served to reduce afferent and efferent sympathetic enhanced ventricular contractility leading to HTN amelioration. Further studies in patients with hypertension and AF undergoing PVI and GP ablation, using a prospective protocol and a larger sample size, may be required to achieve more definitive results.
We thank Dr. Michael Scherlag for his critical evaluation of the manuscript. We thank Mrs. Andrea Moseley for her aid paration of this report and Wuping Liu for her aid in editing the galley proof.
Hypertension commonly occurs with atrial fibrillation. The authors studied patients who were successfully treated for atrial fibrillation over a period of 1 year. A sub-group of these patients also had a dramatic reduction of blood pressure into the normal range. The authors determined the mechanisms for the concomitant suppression of these two morbidities on the basis of the singular ablation procedure performed by our cardiologists.
Since 2004 the authors' clinical laboratories have used a singular hybrid procedure which combines the isolation of the muscle tissue of the pulmonary veins, the atrial fibrillation origin, from the rest of the atrium with ablation of the nerve clusters at the pulmonary vein-atrial junctions which induce the abnormal activity arising in the pulmonary veins. A retrospective study was performed in patients who underwent this procedure and collected follow-up data at 2 wk to 1 year after the procedure. Of the 33 patients who were in normal heart rhythm throughout the authors found that 21 had no change in blood pressure whereas in 12 their blood pressures were normal. All patients were on similar multiple anti-hypertensive drugs. The authors found that the non-responders had enlarged atria while the responders had normal sized atria before and after the procedure and follow-up. These finding suggested that there was a sub-population of patients whose hypertension was neurally based in the heart while the others had hypertension due to factors outside the heart, i.e., abnormality of the renin-angiotensin-aldosterone system, which caused the arteries to constrict leading to enlargement of the heart chambers.
Only recently has it been shown that patients with forms of hypertension resistant to multiple drug regimens had a neurogenic basis for their condition which could be dramatically reduced by neural ablation procedures. Just as in small population the resistant forms of hypertension represent a small proportion of the general population with high blood pressure which does not respond to multiple drug therapy. The authors suggest that non-invasive methods for determining heart size by ultrasound, particularly the atrial dimensions can be used to categorize patients with drug resistant and drug responsive hypertension thereby foregoing weeks or months of drug trial for the former group.
Hypertension: Abnormally high blood pressure; Atrial Fibrillation: A very rapid and irregular heart rate which can become persistent and can lead to heart failure and strokes; Ablation: A procedure in which an electrode catheter is introduced into the heart which allows the application of radiofrequency energy to create lesion to destroy abnormal heart or nerve tissues.
The authors performed a retrospective study to discuss the significant differences in the mean systolic blood pressure before ablation and at follow up intervals, with the systolic blood pressure being lower post ganglionated plexi ablation in patients with atrial fibrillation and hypertension with normal left atrial dimensions. These observations are interesting, and could be helpful in further clinical studies.
P- Reviewer: Cheng TH, De Ponti R, Letsas K S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Benjamin EJ, Levy D, Vaziri SM, D’Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 1994;271:840-844. [PubMed] |
| 2. | Campese VM. Neurogenic factors and hypertension in renal disease. Kidney Int Suppl. 2000;75:S2-S6. [PubMed] |
| 3. | Dustan HP, Tarazi RC. Cardiogenic hypertension. Annu Rev Med. 1978;29:485-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | DiBona GF. Neural control of the kidney: functionally specific renal sympathetic nerve fibers. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1517-R1524. [PubMed] |
| 5. | Esler M. Sympathetic nervous system moves toward center stage in cardiovascular medicine: from Thomas Willis to resistant hypertension. Hypertension. 2014;63:e25-e32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Goldblatt H, Lynch J, Hanzal RF, Summerville WW. Studies on experimental hypertension: I. the production of persistent elevation of systolic blood pressure by means of renal ischemia. J Exp Med. 1934;59:347-379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1506] [Cited by in RCA: 1289] [Article Influence: 85.9] [Reference Citation Analysis (0)] |
| 7. | Laragh JH. Renal and adrenal factors in hypertension: diagnostic approaches. Bull N Y Acad Med. 1969;45:859-876. [PubMed] |
| 8. | Gregory YH, Lip D, Beavers G, Singh SP, Watson RDS. ABC of atrial fibrillation: Aetiology, pathophysiology and clinical features. BMJ. 1995;311:1425-1428. [RCA] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Esler M. Sympathetic nervous system: contribution to human hypertension and related cardiovascular diseases. J Cardiovasc Pharmacol. 1995;26 Suppl 2:S24-S28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Krum H, Schlaich M, Whitbourn R, Sobotka PA, Sadowski J, Bartus K, Kapelak B, Walton A, Sievert H, Thambar S. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet. 2009;373:1275-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1591] [Cited by in RCA: 1546] [Article Influence: 96.6] [Reference Citation Analysis (0)] |
| 11. | Schlaich MP, Hering D, Sobotka PA, Krum H, Esler MD. Renal denervation in human hypertension: mechanisms, current findings, and future prospects. Curr Hypertens Rep. 2012;14:247-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Kline RL, Mercer PF. Functional reinnervation and development of supersensitivity to NE after renal denervation in rats. Am J Physiol. 1980;238:R353-R358. [PubMed] |
| 13. | Symplicity HTN-1 Investigators. Catheter-based renal sympathetic denervation for resistant hypertension: durability of blood pressure reduction out to 24 months. Hypertension. 2011;57:911-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 582] [Cited by in RCA: 551] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 14. | Pokushalov E, Romanov A, Corbucci G, Artyomenko S, Baranova V, Turov A, Shirokova N, Karaskov A, Mittal S, Steinberg JS. A randomized comparison of pulmonary vein isolation with versus without concomitant renal artery denervation in patients with refractory symptomatic atrial fibrillation and resistant hypertension. J Am Coll Cardiol. 2012;60:1163-1170. [RCA] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 312] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 15. | Scherlag BJ, Nakagawa H, Jackman WM, Yamanashi WS, Patterson E, Po S, Lazzara R. Electrical stimulation to identify neural elements on the heart: their role in atrial fibrillation. J Interv Card Electrophysiol. 2005;13 Suppl 1:37-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 324] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 16. | Katritsis DG, Pokushalov E, Romanov A, Giazitzoglou E, Siontis GC, Po SS, Camm AJ, Ioannidis JP. Autonomic denervation added to pulmonary vein isolation for paroxysmal atrial fibrillation: a randomized clinical trial. J Am Coll Cardiol. 2013;62:2318-2325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 324] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 17. | Tarazi RC, Fouad FM, Ferrario CM. Can the heart initiate some forms of hypertension? Fed Proc. 1983;42:2691-2697. [PubMed] |
| 18. | Scherlag BJ, Yamanashi WS, Amin R, Lazzara R, Jackman WM. Experimental model of inappropriate sinus tachycardia: initiation and ablation. J Interv Card Electrophysiol. 2005;13:21-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Huang MH, Wolf SG, Armour JA. Ventricular arrhythmias induced by chemically modified intrinsic cardiac neurones. Cardiovasc Res. 1994;28:636-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Ardell JL. Structure and Function of the Mammalian Intrinsic Cardiac Neurons. Eds: Armour JA, Ardell JL. Oxford University Press, New York, NY 1994; Chap 5. |
| 21. | Sharifov OF, Fedorov VV, Beloshapko GG, Glukhov AV, Yushmanova AV, Rosenshtraukh LV. Roles of adrenergic and cholinergic stimulation in spontaneous atrial fibrillation in dogs. J Am Coll Cardiol. 2004;43:483-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 229] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 22. | Patterson E, Po SS, Scherlag BJ, Lazzara R. Triggered firing in pulmonary veins initiated by in vitro autonomic nerve stimulation. Heart Rhythm. 2005;2:624-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 412] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 23. | Shen MJ, Choi EK, Tan AY, Han S, Shinohara T, Maruyama M, Chen LS, Shen C, Hwang C, Lin SF. Patterns of baseline autonomic nerve activity and the development of pacing-induced sustained atrial fibrillation. Heart Rhythm. 2011;8:583-589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 24. | Vollmann D, Sossalla S, Schroeter MR, Zabel M. Renal artery ablation instead of pulmonary vein ablation in a hypertensive patient with symptomatic, drug-resistant, persistent atrial fibrillation. Clin Res Cardiol. 2013;102:315-318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Frohlich ED. Renal denervation using an irrigated radiofrequency ablation catheter for management of drug-resistant hypertension: a demonstrated value? JACC Cardiovasc Interv. 2012;5:766-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |