Published online May 23, 2015. doi: 10.5494/wjh.v5.i2.74
Peer-review started: September 28, 2014
First decision: December 17, 2014
Revised: February 19, 2015
Accepted: March 16, 2015
Article in press: March 18, 2015
Published online: May 23, 2015
Processing time: 236 Days and 9.8 Hours
Symplicity-3 hypertension (HTN) was a recently completed clinical trial that was assumed to be the basis for the approved use of renal artery denervation for the treatment of resistant hypertension in the United States. Dramatic reductions in blood pressure had been reported in two clinical trials (Symplicity-1HTN, -2HTN) carried out in Europe, however Symplicity-3HTN did not show a significant reduction of systolic blood pressure in patients with resistant hypertension 6 mo after renal artery denervation as compared with a sham control. (Denervation group, blood pressure reduction: -14 ± 24, Sham control: -12 ± 26 mmHg). In this review we discuss several potential explanations for the failure of efficacy of Symplicity-3HTN taking into account basic and clinical factors which could have played a role in the discrepancy between the European and American experience.
Core tip: The failure of the Symplicity-3 trial which subjected patients to renal artery denervation to significantly reduce resistant hypertension has been ascribed to many factors. In this review, we focus on the lack of a “biomarker” as a major deficiency in achieving the expected efficacy. We also present experimental and clinical evidence to support the importance of a biomarker to acutely predict long term success of renal artery denervation for effective treatment for drug resistant hypertension.
- Citation: Scherlag BJ, Po SS. Symplicity-3 hypertension trial: Basic and clinical insights. World J Hypertens 2015; 5(2): 74-78
- URL: https://www.wjgnet.com/2220-3168/full/v5/i2/74.htm
- DOI: https://dx.doi.org/10.5494/wjh.v5.i2.74
Renal artery denervation as a procedure for patients with resistant forms of hypertension burst onto the clinical scene in 2009[1] and quickly was followed by clinical trials, Symplicity-1 hypertension (HTN)[2] and Symplicity-2HTN[3]. What was predicted to be a reduction of 5 to 10 mmHg turned out to be a mean reduction of systolic pressure by as much as 32 mmHg even for follow-up periods of 2-3 years. When Medtronic sponsored Symplicity-3HTN as a multi-center trial, enrolling 530 patients, in the United States, it was expected that the European extensive experience would be confirmed. One important difference in Symplicity-3HTN was the inclusion of a sham controlled group, which is common in pharmacological trials but unusual for a procedural study due to potential ethical reservations. In any event, the recently published report[4] concluded that the results did not show a significant reduction of systolic blood pressure 6 mo after renal artery denervation (-14 ± 24 mmHg) compared to the sham controls (-12 ± 26 mmHg). However, there was no issue with the safety of the procedure using the Symplicity renal-denervation catheter. In this review we consider the potential factors and their relative importance to explain the striking differences in the outcomes of these Symplicity trials.
Experimental animal studies have shown that sympathetic nerve hyperactivity is a critical component in the initiation and maintenance of systemic hypertension. For example it has been shown that chronic HTN can be induced by chronic electrical stimulation of the left stellate ganglion[5]. In this regard, Smithwick and Thompson[6] reported on 1266 cases of surgical splanchnicetomies performed to treat malignant HTN. There was a successful lowering of blood pressure, however, these methods were associated with high perioperative morbidity and mortality and long-term complications, including bowel, bladder, and erectile dysfunction, in addition to severe postural hypotension. Although this clinical approach was generally abandoned, experimental studies progressed on the neurogenic basis for essential hypertension[7,8]. These ongoing studies were eclipsed by the general acceptance of the concept that hypertension was based on an abnormality of the rennin-angiotensin-aldosterone system[9].
The seminal study which brought the neurogenic basis of hypertension to the forefront was published in 2009 in which sympathetic nerves in the adventitia of the renal arteries were ablated by transvascular application of radiofrequency energy (8-10 watts) caused a marked reduction of blood pressure in patient with drug resistant hypertension. Specifically, Krum et al[1] using a monopolar electrode catheter (Symplicity) performed renal denervation in 45 patients, 5 untreated patients served as controls. Entry blood pressure (BP) averaged 177/101 ± 20/15 mmHg. At 6 mo, the treated patients showed an office-based BP reduction of -22/-11 ± 10/5 mmHg while the 5 controls had BP increases of +14/+9 mmHg. These startling results, that even surprised the initial investigators quickly morphed into 2 prospective randomized controlled trials, simplicity-HTN 1, HTN2 with as similar or greater dramatic results over follow-up periods as long as 3 years.
The mechanism proposed to explain these findings was suggested to be ablation of sympathetic afferents which after months modulate the vasomotor centers to decrease general sympathetic efferent outflow[10]. This hypothesis was supported by radiotracer dilution studies which showed a 47% spillover of nor-epinephrine within 1 mo of bilateral renal denervation.
The unexpected efficacy failure of Symplicity-3 as reported by Bhatt et al[4] has engendered a flurry of letters to the editor of the New England Journal of Medicine[11] raising multiple concerns regarding the findings reported in the Symplicity-3 trial. It is interesting to note that Dr Bhatt, the lead investigator in the Symplicity-3 trial, in reply to these letters stated: “We agree that various selection criteria and characteristics of our patient population-such as the exclusion of patients with white-coat hypertension, the inclusion of obese patients and a variety of baseline characteristics or medications could account for the null results of this trial, as compared with the findings of previous trials[11]”. Thus, the lead investigator concedes that trial differences could have been the basis of the negative results for Symplicity-3. Many of the same caveats were detailed in a joint consensus statement[12] by respected investigators in the field indicating potential flaws in the Symplicity-3 trial. In a recent report Messerli and Bangalore[13] addressed the possible causes of the failure of the Symplicity-3 trial in light of the dramatic successes of Symplicity-1 and -2. “At first blush, the most likely explanation for the findings of the SYMPLICITY HTN-3 study is the inclusion of a sham-control group. In clinical trials testing interventional procedures and medical devices, sham procedures are seminal, analogous to the use of a placebo in pharmaceutical trials. However, for ethical reasons sham procedures are frowned upon; neither the SYMPLICITY HTN-1 study nor the HTN-2 study had a sham-control cohort. For this reason, placebo effects may well explain all or most of the blood-pressure differences noted in the first two trials. Lack of efficacy could also be caused by incomplete or ineffective denervation. No reliable markers of renal denervation are available, and questions remain as to what exactly the procedure accomplishes. Nevertheless, the ablation catheter used in the SYMPLICITY HTN-3 study was no different from that used in the SYMPLICITY HTN-1 and HTN-2 studies.” In this regard, we suggest that the focus of each of the Simplicity trials on ablating the variable structure of the post-ganglionic axons on the renal artery adventitia provides an important impediment for achieving sympathetic denervation. Indeed, the percent of non-responders in a number of previously reported studies, using the Symplicity approach, ranges from 10%-43%[10,14].
In regard to the lack of biomarkers there is a striking analogy between catheter ablation for atrial fibrillation and catheter ablation for refractory hypertension. Besides the fact that both groups of patients who are candidates for these procedures have failed drug therapy: (1) Both invasive procedures use radiofrequency applications to achieve pulmonary vein isolation (PVI) or renal artery denervation (RAD); (2) It is common that after the procedure AF or high BP is not any different than prior to the procedure. A “blanking” period of various durations ensues before a salutary effect is determined; (3) There is no “biomarker” at the time of the procedure to gauge the success or failure of the intervention; and (4) In both cases neural factors appear to play a critical role in the outcomes.
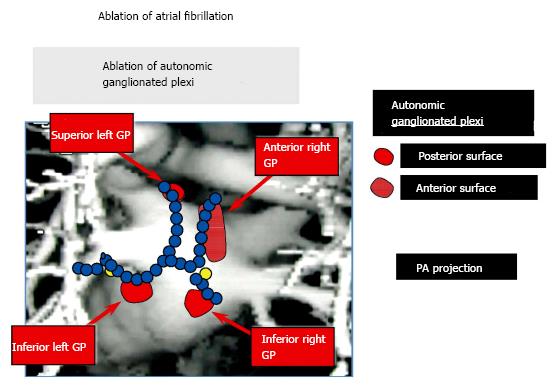
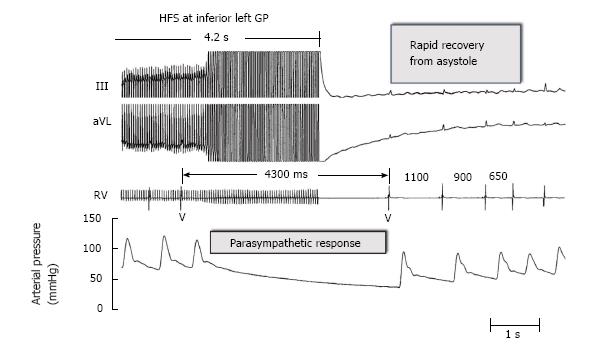
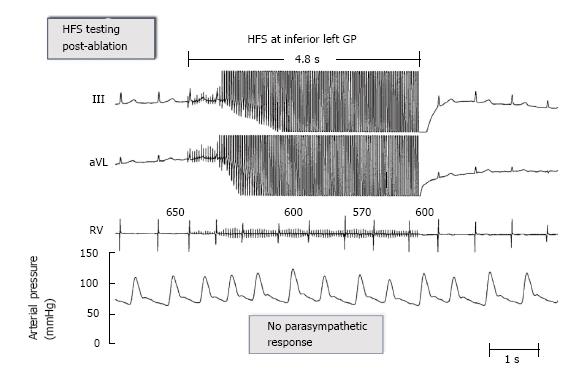
Since 2004[15], in our clinical electrophysiological procedures for AF catheter ablation, we have used additional ablation of clusters of nerves called ganglionated plexi (GP) located at the pulmonary vein-atrial junctions as adjunctive to pulmonary vein isolation (PVI) (Figure 1). High frequency electrical stimulation of these GP invariably leads to marked A-V block induced bradycardia via a parasympathetic effect on the AV node (Figure 2). Destructive radiofrequency current application to the GP causes inability of the same high frequency stimulation to slow the heart rate (Figure 3). A recent study by Katritsis et al[16] consisting of 242 patients who were candidates for catheter ablation were randomized to PVI alone, GP ablation alone or a combination of PVI + GP and followed for 2 years after a single procedure. The success rates were: 44 (56%), 39 (48%), and 61 (74%), respectively.
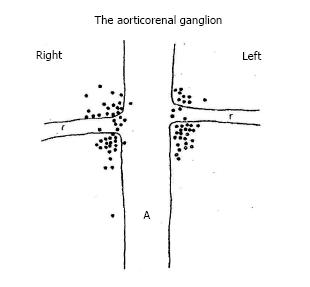
Could an analogous scenario be in play with renal sympathetic denervation? The articorenal ganglion was studied by Doelzel[17] in the dog and Norvell in the human[18]. In the latter study, a detailed dissection of the renal plexus and the aorticorenal area was carried out in 57 adult cadavers of both sexes. Figure 4 shows that the aorticorenal ganglia was found localized in the area of the real artery at the ostium and junction of the aorta. To test the hypothesis that this bilateral ganglion could have a similar biomarker role in renal denervation as the GP contribute to catheter ablation of AF, in the anesthetized dog, we compared the effects of electrical stimulation of sympathetic nerves on the adventitial surface of the renal arteries to similar frequency and intensity applied to the aorticorenal ganglia on heart rate and blood pressure.
Electrical stimulation applied to the renal artery adventitia did not affect the heart rate but significantly increased systolic and diastolic blood pressure (Baseline: 134 ± 24/96 ± 18 mmHg, RAs stimulation: 157 ± 26/114 ± 18 mmHg. Electrical stimulation applied to the aorticorenal ganglia did not affect heart rate but significantly increased systolic and diastolic blood pressure: 207 ± 44/147 ± 26 mmHg, P < 0.05 compared to baseline. In summary, there was a significantly greater effect on both systolic and diastolic BP caused by the same level of electrical stimulation applied to the aorticorenal ganglia than to the adventitial nerves of the renal arteries[18].
Could a similar biomarker be shown in the clinical setting of hypertension? A recent report by Pokushalov et al[19] involved 27 patients (14 randomized to PVI only, and 13 randomized to PVI and renal artery denervation), all of whom were followed for 12 mo after ablation. All had a history of paroxysmal atrial fibrillation and hypertension. “To confirm renal denervation, we used high-frequency stimulation (HFS) before the initial and after each RF delivery within the renal artery. Rectangular electrical stimuli were delivered at the ostium of the targeted renal artery at a frequency of 20 Hz, with an amplitude of 15 V and pulse duration of 10 ms… for 10 s…Renal sympathetic denervation was considered to have been achieved when the sudden increase of blood pressure …was eliminated in the presence of HFS.”
Nine of the 13 patients (69%) treated with PVI with renal denervation were AF-free at the 12-mo post-ablation follow-up examination vs 4 (29%) of the 14 patients in the PVI-only group (P = 0.033). At the end of the follow-up, significant reductions in systolic (from 181 ± 7 to 156 ± 5, P < 0.001) and diastolic blood pressure (from 97 ± 6 to 87 ± 4, P < 0.001) were observed in patients treated with PVI with renal denervation without significant change in the PVI only group.
Although many explanations have been put forward to try to explain the lack of efficacy of the Symplicity-3 trial for renal artery denervation to treat resistant forms of hypertension, it appears that one major reservation has been the lack of a biomarker for the induction of an increase in blood pressure and then after ablation the inability to show the same hypertensive response. A clinical trial to test this acute effect as a predictor of success would negate the reliance on a blanking period (weeks) for central autonomic remodeling to occur in order to determine whether the reduction of blood pressure has been achieved.
We thank Andrea Moseley and Branden Pannell for their help in the preparation of the manuscript. We thank Wuping Liu for her aid in editing the galley proof.
P- Reviewer: Mikolasevic I, Sicari R, Zhao D S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Krum H, Schlaich M, Whitbourn R, Sobotka PA, Sadowski J, Bartus K, Kapelak B, Walton A, Sievert H, Thambar S. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet. 2009;373:1275-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1591] [Cited by in RCA: 1541] [Article Influence: 96.3] [Reference Citation Analysis (0)] |
| 2. | Catheter-based renal sympathetic denervation for resistant hypertension: durability of blood pressure reduction out to 24 months. Hypertension. 2011;57:911-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 582] [Cited by in RCA: 551] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 3. | Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Böhm M. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet. 2010;376:1903-1909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1656] [Cited by in RCA: 1613] [Article Influence: 107.5] [Reference Citation Analysis (0)] |
| 4. | Bhatt DL, Kandzari DE, O’Neill WW, D’Agostino R, Flack JM, Katzen BT, Leon MB, Liu M, Mauri L, Negoita M. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370:1393-1401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1571] [Cited by in RCA: 1590] [Article Influence: 144.5] [Reference Citation Analysis (0)] |
| 5. | Liard JF, Tarazi RC, Ferrario CM, Manger WM. Hemodynamic and humoral characteristics of hypertension induced by prolonged stellate ganglion stimulation in conscious dogs. Circ Res. 1975;36:455-464. [PubMed] |
| 6. | Smithwick RH, Thompson JE. Splanchnicectomy for essential hypertension; results in 1,266 cases. J Am Med Assoc. 1953;152:1501-1504. [PubMed] |
| 7. | DiBona GF, Kopp UC. Neural control of renal function. Physiol Rev. 1997;77:75-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 206] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 8. | Esler M, Jennings G, Lambert G. Noradrenaline release and the pathophysiology of primary human hypertension. Am J Hypertens. 1989;2:140S-146S. [PubMed] |
| 9. | Laragh JH. Renin, angiotensin, aldosterone and hormonal regulation of arterial pressure and salt balance. Introductory remarks. Fed Proc. 1967;26:39-41. [PubMed] |
| 10. | Schlaich MP, Hering D, Sobotka PA, Krum H, Esler MD. Renal denervation in human hypertension: mechanisms, current findings, and future prospects. Curr Hypertens Rep. 2012;14:247-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Bhatt DL, Bakris GL. Renal denervation for resistant hypertension. N Engl J Med. 2014;371:184. [PubMed] [DOI] [Full Text] |
| 12. | Lobo MD, de Belder MA, Cleveland T, Collier D, Dasgupta I, Deanfield J, Kapil V, Knight C, Matson M, Moss J. Joint UK societies’ 2014 consensus statement on renal denervation for resistant hypertension. Heart. 2015;101:10-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Messerli FH, Bangalore S. Renal denervation for resistant hypertension? N Engl J Med. 2014;370:1454-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 14. | Persu A, Jin Y, Azizi M, Baelen M, Völz S, Elvan A, Severino F, Rosa J, Adiyaman A, Fadl Elmula FE. Blood pressure changes after renal denervation at 10 European expert centers. J Hum Hypertens. 2014;28:150-156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 122] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 15. | Scherlag BJ, Nakagawa H, Jackman WM, Yamanashi WS, Patterson E, Po S, Lazzara R. Electrical stimulation to identify neural elements on the heart: their role in atrial fibrillation. J Interv Card Electrophysiol. 2005;13 Suppl 1:37-42. [PubMed] |
| 16. | Katritsis DG, Pokushalov E, Romanov A, Giazitzoglou E, Siontis GC, Po SS, Camm AJ, Ioannidis JP. Autonomic denervation added to pulmonary vein isolation for paroxysmal atrial fibrillation: a randomized clinical trial. J Am Coll Cardiol. 2013;62:2318-2325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 324] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 17. | Dolezel S. Monoaminergic innervation of the kidney. Aorticorenal ganglion--A sympathetic, monoaminergic ganglion supplying the renal vessels. Experientia. 1967;23:109-111. [PubMed] |
| 18. | Norvell JE. The aorticorenal ganglion and its role in renal innervation. J Comp Neurol. 1968;133:101-111. [PubMed] |
| 19. | Pokushalov E, Romanov A, Corbucci G, Artyomenko S, Baranova V, Turov A, Shirokova N, Karaskov A, Mittal S, Steinberg JS. A randomized comparison of pulmonary vein isolation with versus without concomitant renal artery denervation in patients with refractory symptomatic atrial fibrillation and resistant hypertension. J Am Coll Cardiol. 2012;60:1163-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 312] [Article Influence: 24.0] [Reference Citation Analysis (0)] |