Published online Aug 23, 2013. doi: 10.5494/wjh.v3.i3.18
Revised: August 21, 2013
Accepted: August 22, 2013
Published online: August 23, 2013
Processing time: 106 Days and 2.9 Hours
Metabolic syndrome is a growing research area. The underlying mechanisms of metabolic syndrome are still not very clear. Insulin resistance, obesity, inflammation and oxidative stress may play an important role in the pathogenesis of metabolic syndrome. The role of adipose tissue dysfunction is emphasized during the development of obesity. Adipose tissue is identified as a complex endocrine organ and its metabolic functions extend well beyond the classical actions of thermoregulation and of storage and release of fatty acids. Chronic low-grade inflammation activated by the immune system in adipose tissue is a key contributing factor to type 2 diabetes mellitus and cardiovascular diseases. Visceral obesity results in cell autonomous impairment in insulin signaling that leads to insulin resistance. Chronic inflammation in adipose tissue has gained acceptance as a lead promoter of insulin resistance in obesity. Furthermore, obesity creates oxidative stress conditions in adipose tissue that not only correlates with insulin resistance but is also causative in its development. Oxidative stress may be a mechanistic link between several components of metabolic syndrome and cardiovascular diseases, through its role in inflammation and its ability to disrupt insulin-signaling. The study around adipose tissue dysfunction will help to understand the pathogenesis of metabolic syndrome and may bring effective therapy in treatment of metabolic syndrome related diseases. Therefore, this review mainly focuses on the roles of adipose tissue dysfunction in inflammation, insulin resistance, and oxidative stress in the pathogenesis of metabolic syndrome.
Core tip Metabolic syndrome is a growing research area. Insulin resistance, obesity, inflammation and oxidative stress may play an important role in the pathogenesis of metabolic syndrome. The role of adipose tissue dysfunction is emphasized during the development of obesity in recent years. The study around adipose tissue dysfunction will help to understand the pathogenesis of metabolic syndrome and may bring effective therapy in treatment of metabolic syndrome related diseases. Therefore, this review mainly focuses on the roles of adipose tissue dysfunction in inflammation, insulin resistance, and oxidative stress in the pathogenesis of metabolic syndrome.
- Citation: Zhao D, Liu H. Adipose tissue dysfunction and the pathogenesis of metabolic syndrome. World J Hypertens 2013; 3(3): 18-26
- URL: https://www.wjgnet.com/2220-3168/full/v3/i3/18.htm
- DOI: https://dx.doi.org/10.5494/wjh.v3.i3.18
Metabolic syndrome (MetS) is associated with the mortality and mobility in cardiovascular diseases (CVD). The underlying mechanisms of MetS are still not very clear. Insulin resistance, obesity, inflammation and oxidative stress may play an important role in the pathogenesis of MetS. The roles of adipose tissue dysfunction in obesity have been addressed in recent years. Several mechanisms including insulin resistance, sub-inflammatory state, over activity of renin-angiotensin-aldosterone system (RAAS), oxidative stress, autonomic dysregulation as well as mechanical compression on the kidneys are all activated by obesity[1]. Therefore, this review mainly focuses on the roles of adipose tissue in inflammation, insulin resistance, and oxidative stress in the pathogenesis of MetS.
The prevalence of obesity has increased throughout the last three decades due to genetic, metabolic, behavioral, environmental[2], and epigenetics[3,4] factors. Excess fat is no longer associated with wealth, but is instead recognized as a risk factor for many diseases, such as type 2 diabetic (T2DM), CVD, fatty liver disease and some forms of cancer. Adipose tissue is increasingly being identified as a vital, complex endocrine organ, and not simply as a fat store[5]. It has become increasingly clear that adipose tissue is a much more complex organ than was initially considered and that its metabolic functions extend well beyond the classical actions of thermoregulation and of storage and release of fatty acids[6]. Furthermore, obesity is associated with an increased mortality and morbidity for CVD and adipose tissue is recognized as an important player in obesity-mediated CVD[7].
The discovery of leptin in 1994 sparked dramatic new interest in the study of white adipose tissue (WAT)[8]. As a key endocrine organ, adipose tissue releases multiple bioactive substances, known as adipose-derived secreted factors or adipokines, which have proinflammatory or anti-inflammatory activities[9]. These adipokines include leptin, free fatty acids (FFA), tumour necrosis factor-α (TNF-α), interleukin-6 (IL-6), C-reactive protein, resistin, angiotensinogen and adiponectin (ApN)[10,11]. Retinol binding protein 4 (RBP4) is a recently identified adipokine suggested to link obesity with its comorbidities, especially insulin resistance, T2DM, and certain components of the MetS[12]. It is also abundantly clear that the dysregulation of adipokine secretion and action that occurs in obesity plays a fundamental role in the development of a variety of cardiometabolic disorders, including the MetS, T2DM, inflammatory disorders, and vascular disorders, that ultimately lead to coronary heart disease[6]. The link between obesity, inflammation and insulin resistance indicates the important secretory role of adipose tissue[13,14]. In addition, adipocytokine have been proposed as additional molecules able to modulate kidney function[15]. Epidemiological studies have also repeatedly reported the association between insulin resistance and kidney dysfunction in both non diabetic and diabetic subjects[15].
An increase in adipose tissue mass is associated with the augmented secretion of certain adipokines, such as IL-6, TNF-α and resistin, which cause endothelial dysfunction and hemostasis alterations that also favor a prothrombotic state[11]. Obesity and the accompanying MetS are associated with endothelial dysfunction, such as arterial hypertension and atherosclerosis[16]. This detrimental effect of obesity is mediated, in part, by excessive production of the adipose tissue hormone leptin. Under pathological conditions, such as obesity and MetS, the NO-mediated vasodilatory effect of leptin is impaired[16]. ApN is an important white and brown adipose tissue hormone, which is an insulin sensitizing hormone[17] and has anti-inflammatory properties[18]. ApN exerts its action through its receptors AdipoR1, AdipoR2, and T-cadherin[17] and exerts a pivotal role in vascular protection through activation of multiple intracellular signaling cascades[18]. ApN is highly abundant in human serum but its levels are reduced in obesity and are even lower in patients with hepatic steatosis or non-alcoholic steatohepatitis[19,20]. Decreased plasma ApN levels are implicated in the pathogenesis of the MetS and atherosclerosis and may serve as a diagnostic and prognostic biomarker as well as a rational pharmaco-therapeutic target to treat these disorders[18]. The level of leptin increases with the increase in weight gain, while ApN decreases. Measuring circulating levels of leptin and ApN as a screening tool may help recognize those individuals who do not only have obesity as a major risk factor toward developing cardiometabolic disease but also may have an unfavorable “biomarker profile”, putting them at highest risk[21].
Obesity and its comorbidities, including T2DM and CVD, are considered to be a state of chronic low-grade inflammation that can be detected both systemically and within specific tissues[22,23]. This obesity-associated chronic tissue inflammation is a key contributing factor to T2DM and CVD[24]. Furthermore, chronic low-grade inflammation occurring in the adipose tissue of obese individuals is causally linked to the pathogenesis of insulin resistance and the MetS[25]. Pickup et al[26] found that abnormalities of the innate immune system may be a contributor to the hypertriglyceridaemia, low HDL cholesterol, hypertension, glucose intolerance, insulin resistance and accelerated atherosclerosis of T2DM. Their initial studies supported the hypothesis that type 2 diabetes is caused by activated innate immunity and led to research that has uncovered links between insulin resistance, obesity, circulating immune markers, immunogenetic susceptibility, macrophage function and chronic infection[27]. ApN, leptin and other inflammatory proteins have been shown to correlate with insulin resistance and the MetS in adults[22]. A higher inflammation status was significantly correlated with decreases in the levels of antioxidant enzymes, ApN and an increase in the risk of MetS[28]. A number of studies have clearly demonstrated that the immune system and metabolism are highly integrated[24]. This link allows mammals to adapt to changes in their internal and external environments and affects organism-wide function[29]. Obesity-induced inflammation is mainly mediated by tissue resident immune cells, with particular attention being focused on adipose tissue macrophages (ATMs)[30], as accumulating evidence has revealed a critical involvement of inflammatory responses triggered by lesional macrophages in the pathogenesis of MetS[31]. Moreover, Gene silencing of inflammatory cytokines TNF-α or osteopontin in epididymal ATMs of obese mice caused significant improvement in glucose tolerance[32]. These data were consistent with the hypothesis that cytokines produced by ATMs can exacerbate whole-body glucose intolerance[32]. Based on in vitro studies, macrophages can be divided into M1 and M2 classifications[33]. M1 macrophages, also termed ‘‘classically activated macrophages,’’ are highly proinflammatory, secreting the bulk of the cytokines that cause insulin resistance. M2 macrophages, also termed ‘‘alternatively activated macrophages,’’ are not inflammatory and give rise to cytokines that exert anti-inflammatory effects, such as IL-10 and IL-4[33]. The overall macrophage-induced inflammatory state of the tissue is determined by the balance between these different macrophage subpopulations. In the obese state, the balance is clearly tilted toward the proinflammatory macrophage phenotype[34]. Recently, more leukocyte subpopulations have been implicated in obesity, including neutrophils, eosinophils, and mast cells[35]. Neutrophils, which participate in inflammation-induced metabolic disease[36], and mast cells[37] are increased in obese adipose tissue, and studies in mice have indicated that these two cell types can promote insulin resistance. The involvement of multiple leukocyte subpopulations underlines the complexity of obesity-associated AT inflammation[35]. The role of innate immune cells, such as macrophages in AT inflammation has been well demonstrated. In contrast, less is known about the role of lymphocytes[25]. However, more recently, cells of the adaptive immune system, specifically B and T lymphocytes, have emerged as unexpected promoters and controllers of insulin resistance[31], and participate in modulating adipose tissue inflammation during the development of obesity[38]. Furthermore, fluctuations in weight have been associated with worsened metabolic and cardiovascular outcomes[39]. Weight cycling did increase the number of CD4+ and CD8+ T cells in AT, indicating that an exaggerated adaptive immune response in adipose tissue may contribute to metabolic dysfunction during weight cycling, although adipose tissue macrophage number and polarization were not modulated by weight cycling[39]. Molecular mechanisms are complicated in VAT inflammation. Several studies during the past two decades have highlighted the key role of the IκB kinase (IKK)/nuclear factor-κB (NF-κB) pathway in the induction and maintenance of the state of inflammation that underlies metabolic diseases such as obesity and T2DM[40]. Excess adipose tissue is hypothesized to contribute to a state of chronic inflammation which promotes development of insulin resistance as well as other metabolic complications by stimulating NF-κB and Jun N-terminal kinase (JNK) pathways in adipocytes and the liver[2]. JNK in macrophages is required for the establishment of obesity-induced insulin resistance and inflammation[41,42]. Furthermore, bone marrow mesenchymal stem cells from high-fat diet animals showed increased production of IL-1, IL-6, and TNF-α and increased NF-κB and reduced peroxisome proliferator-activated receptor gamma (PPAR-γ) expression[43], suggesting the inflammatory responses during weight gain. In addition, mice with a null mutation for transient receptor potential vanilloid (TRPV4) or wild-type mice treated with a TRPV4 antagonist showed elevated thermogenesis in adipose tissues and were protected from diet-induced obesity, adipose inflammation, and insulin resistance[44]. A causal role for iron in adipocytes as a risk factor for MetS and a role for adipocytes in modulating metabolism through ApN in response to iron stores have also been reported[45], suggesting that adipocyte iron regulates ApN and insulin sensitivity[45].
Excess visceral fat causes local chronic low-grade inflammation and dysregulation of adipocytokines, which contribute to the pathogenesis of the MetS[46]. The amount of visceral adipose tissue (VAT) and the liver fat content are important factors responsible for the link between abdominal obesity and features of the MetS[47]. In addition, visceral fat adiposity also correlates with inflammation in peripheral blood cells[46]. Individuals with MetS have a higher degree of endothelial dysfunction and inflammation compared with individuals with multiple CV risk factors and may therefore have an increased CV risk beyond the contributions of multiple traditional risk factors[48]. The inflammatory profile often observed among sedentary overweight/obese individuals with an excess of VAT/liver fat may be a consequence of a more primary defect in subcutaneous adipose tissue[47]. To address the hypothesis that lowering inflammation will lower vascular event rates, two large-scale placebo controlled trials using targeted anti-inflammatory agents for the secondary prevention of myocardial infarction have been initiated[49]. These inflammatory pathways are potential novel pharmacological targets for the management of obesity-associated insulin resistance[50]. Areas of active investigation focus on the molecular bases of metabolic inflammation and potential pathogenic roles in insulin resistance, diabetes, and CVD. Translating the information gathered from experimental models of insulin resistance and diabetes into meaningful therapeutic interventions is a tantalizing goal with long-term global health implications[23].
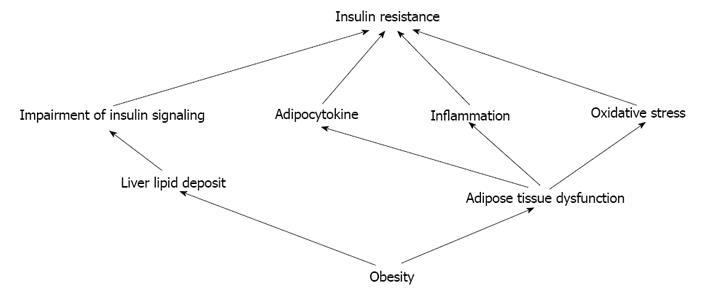
Insulin resistance is a major characteristic of visceral obesity[51]. The association between obesity and insulin resistance is an area of much interest and enormous public health impact[52] (Figure 1). Visceral obesity, but not subcutaneous, results in cell autonomous impairment in insulin signaling that leads to insulin resistance. The mechanisms by which visceral obesity results in insulin resistance may be related to excess lipid accumulation in liver[52]. Furthermore, it is not merely an increased mass of adipose tissue that directly leads to attenuation of insulin action, but rather adipose tissue inflammation activated by the immune system in obese individuals that leads to insulin resistance[53]. VAT is prone to inflammation and inflammatory cytokine production, which also contribute to impairment in insulin signaling[52]. Chronic inflammation in VAT has gained acceptance as a lead promoter of insulin resistance in obesity[54-57]. The chronic state of insulin resistance in established obesity may be largely mediated by macrophage-induced proinflammatory actions, whereas the early-onset insulin resistance during high-fat diet feeding may be more likely related to acute tissue lipid overload[58].
During obesity, many immune cells infiltrate or populate in adipose tissue and promote a low-grade chronic inflammation. Perturbation of inflammation is critically linked to nutrient metabolic pathways and to obesity-associated complications such as insulin resistance and T2DM[59]. A great deal of evidence has pointed to the role of innate immune cells, in particular, adipose tissue macrophages, in the regulation of fat inflammation and glucose homeostasis[56]. An increased accumulation of macrophages occurring in WAT has emerged as a key process in metabolic inflammation[23,55] and insulin resistance in obesity[60]. An association between adipose macrophage content and systemic insulin resistance was reported in diabetic humans[61], suggesting the important role of inflammation in insulin resistance. Furthermore, IFN-γ is a central regulator of macrophage function and play an important role in the regulation of inflammation and glucose homeostasis in obesity though multiple potential mechanisms, including effects on adipogenesis, cytokine expression, and macrophage phenotype[60].
Recently, cells of the adaptive immune system, specifically B and T lymphocytes, have emerged as unexpected promoters and controllers of insulin resistance during the development of obesity[56]. Adipose tissue contains a population of invariant natural killer T (iNKT) cells, whose abundance decreases with increased adiposity and insulin resistance[62]. Adipose tissue-resident iNKT cells maintain healthy adipose tissue through direct interplay with adipocytes and prevent insulin resistance[63]. Activation of NKT cells promotes M2 Macrophage polarization in adipose tissue and improves systemic glucose tolerance via IL-4/STAT6 protein signaling axis in obese adipose tissue[62]. However, activation of iNKT by lipid excess promotes tissue inflammation and insulin resistance in obese mice, suggesting the role of iNKT cells in the complex network linking lipid excess to inflammation in obesity[64]. In addition, iNKT cells do not affect glucose clearance but rather modulate lipid metabolism in both liver and adipose tissue[65]. These effects on lipid metabolism are mainly mediated in the liver[65]. A unique population of VAT-resident regulatory T (Treg) cells was recently implicated in control of the inflammatory state of adipose tissue and, thereby, insulin sensitivity. Unexpectedly, PPAR-γ expression by VAT Treg cells was necessary for complete restoration of insulin sensitivity in obese mice by the thiazolidinedione drug pioglitazone[66]. Obese patients with insulin resistance displayed significantly decreased natural Tregs but an increase in adaptive Tregs in their VAT as compared with lean control subjects, suggesting a potential therapeutic value of Tregs to improve insulin resistance and end organ damage in T2DM by limiting the proinflammatory milieu[67]. Obesity is characterized by circulating immune cells that are activated and insulin resistant, with the T-cell balance polarized towards a pro-inflammatory Th1 phenotype. The loss of insulin-induced suppression of inflammatory phenotypes in circulating immune cells could contribute to the systemic and adipose tissue inflammation[68].
Visceral obesity results in insulin resistance appear to be related to excess lipid accumulation in liver[52]. Visceral obesity is a main risk factor for non-alcoholic fatty liver disease (NAFLD)[19], which is a low-grade chronic inflammatory state[69]. Visceral obesity lead to inappropriate storage of triglycerides in adipocytes and higher concentrations of FFA may add to increased hepatic lipid storage and insulin resistance[19]. The liver is directly exposed to increasing amounts of FFA and pro-inflammatory factors released from visceral fat into the portal vein of obese patients, promoting the development of hepatic insulin resistance, liver steatosis[70] and progressive liver damage[19]. Although liver lipid is closely associated with, and likely to be an important contributor to, (hepatic) insulin resistance[71,72], it may also be in part the consequence of the lipogenic pathway of insulin action being up-regulated by hyperinsulinemia and unimpaired signaling[71]. The 5-lipoxygenase pathway plays a major role in mounting inflammation in hepatic tissue and has emerged as a pathogenic factor in obesity-induced NAFLD. Therefore, modulation of lipoxygenases represents a novel target in the prevention of adipose tissue and hepatic dysfunction related to the MetS[73]. Moreover, insulin resistance and lipotoxicity represent the missing links (beyond the classical cardiovascular risk factors) that help explain the accelerated rate of CVD in type 2 diabetic patients[74]. AMP-activated protein kinase (AMPK) is considered as a master switch in regulating glucose and lipid metabolism. In the liver, activation of AMPK results in decreased production of plasma glucose, cholesterol, triglyceride and enhanced fatty acid oxidation[75]. Interestingly,genetic deletion of the AMPK β1 subunit in mice (referred to herein as β1(-/-) mice) reduced macrophage AMPK activity, acetyl-CoA carboxylase phosphorylation, and mitochondrial content, resulting in reduced rates of fatty acid oxidation. Thus, activation of AMPK β1 and increasing fatty acid oxidation in macrophages may represent a new therapeutic approach for the treatment of insulin resistance[76].
Endoplasmic reticulum (ER) stress in various cells plays an important role in the pathogenesis of several diseases[77]. During the last decade, ER stress has emerged as a new player in the field of obesity, T2DM and insulin resistance, and a considerable number of recent studies have pointed out its role in the onset of insulin resistance[51]. Furthermore, ER stress plays important pathophysiological roles in obesity-induced adipose tissue dysfunction[78]. When the adipocyte endoplasmic reticulum is no longer capable of processing the excess nutrients, the so-called “endoplasmic reticulum stress” develops. This triggers efflux of FFA from adipocytes into the circulation and causes triglyceride overload in skeletal muscle, liver and pancreas[79]. FFA is an important factor that has been implicated in the pathogenesis of insulin resistance[80]. With a positive caloric balance, more FFA is released into the portal system. Excess of circulating FFA, TNF-α and other factors induces insulin resistance[81]. The mechanisms are related to inhibiting insulin signaling through the activation of serin-kinases, which promote a mechanism of serine phosphorylation of insulin receptor substrates, leading to interruption of the downstream insulin receptor signaling[81]. Reducing plasma FFA concentration in obese and T2DM subjects improves insulin sensitivity. Moreover, pharmacologic FFA reduction improves insulin signaling in muscle from insulin resistant subjects. This beneficial effect on insulin action could be related to a decrease in local inflammation[80]. Adipose tissue expansion not only involves enlargement of fat cells, but also the accumulation of inflammatory cells and a shift in the production of adipokines and cytokines[82]. Inflammatory cytokines, ROS and ectopic lipid deposition are the main mediators of insulin resistance and vascular impairment[79]. Impaired insulin signaling on vascular endothelium, atherosclerotic plaque macrophages can alter progression of CVD in the MetS and affect development of microvascular complications of diabetes mellitus[83]. The specific cellular underpinnings or mechanisms of insulin resistance are not clear[84]. The precise causes of insulin resistance are varied, and the relative importance of each is a matter of ongoing research[85]. Advances in understanding of the complex pathophysiology of insulin’s effects on vascular tissues will offer new opportunities for preventing these cardiovascular disorders[83].
The excess activation and the imbalance in the metabolism of oxygen and production of excess free radicals contribute to “oxidative stress” in the heart, vascular and kidney tissue[86]. NADPH oxidase is the enzyme responsible for much of the generation of ·O2− in cardiovascular tissue[86,87], which is comprised of several membrane and cytosolic subunits that mobilize and activate under various agonists such as Ang II, aldosterone as well as fatty acids[86,87]. MetS is associated with high oxidative stress, which is caused by an increased expression of NADPH oxidase and a decreased expression of antioxidant enzymes in the adipose tissue[88]. Obesity creates oxidant conditions that favor the development of comorbid diseases[89]. Oxidative stress in adipose tissue not only correlates with insulin resistance but is also causative in its development[90]. Adipose tissue plays a central role in maintaining metabolic homeostasis under normal conditions[78]. Energy imbalances lead to the storage of excess energy in adipocytes, resulting in both hypertrophy and hyperplasia. These processes are associated with abnormalities of adipocyte function, particularly mitochondrial stress and disrupted endoplasmic reticulum function[89]. Oxidative stress plays a pivotal role in the pathogenesis of the MetS and in the progression of its complications[91]. Oxidative stress may be a mechanistic link between several components of MetS and CVD, through its role in inflammation and its ability to disrupt insulin-signaling[7]. The cross-talk between impaired insulin-signaling and inflammatory pathways enhances both metabolic insulin resistance and endothelial dysfunction, which synergize to predispose to CVD[7].
All components of the RAAS are expressed in and have independent regulation of adipose tissue. This local adipose RAAS exerts important auto/paracrine functions in modulating lipogenesis, lipolysis, adipogenesis as well as systemic and adipose tissue inflammation[92]. The role of the RAAS on the development of insulin resistance and T2DM is an area of growing interest[93]. Excess visceral adiposity contributes to inappropriate activation of the RAAS despite a state of volume expansion and of salt retention that contributes to subclinical elevations of pro-oxidant mechanisms. These adverse effects are mediated by excess generation of ROS and diminished antioxidant defense mechanisms[86]. Extending beyond Ang II as the classical effector peptide, aldosterone has been shown to promote vascular production of oxidative stress through the enzyme complex NADPH oxidase independent of Ang II[86,94,95]. In addition, aldosterone has been shown to potentiate the impact of Ang II impairments in endothelium-dependent relaxation both directly and indirectly through increased vascular oxidative stress resulting in reductions in the bioavailable nitric oxide[86,94-96]. Inappropriate mineralocorticoid receptor activation has been demonstrated to be a causal factor in several pathologic conditions such as vascular inflammation, endothelial dysfunction, insulin resistance and obesity[97].
Oxidative stress is positively associated with VAT as well as diffuse and focal carotid atherosclerosis in apparently healthy men and women[98]. Increased adipose tissue oxygen tension in obese compared with lean men is accompanied by insulin resistance, and inflammation[99]. Moreover, there is a synergistic effect of redox-inflammatory processes to each of the components of the MetS[100]. Using the available plasma oxidative stress biomarkers, many clinical studies have shown the presence of systemic oxidative stress in obese insulin resistant subjects, and its decrease after the successful treatment of obesity[90]. Therefore, the evaluation of oxidative status may allow for the identification of patients at an increased risk of complications[89].
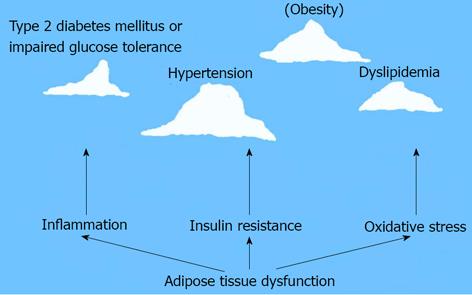
MetS is a growing research area. The roles of VAT dysfunction in pathogenesis of MetS are emphasized in recent years. Adipose tissue dysfunction may lead to insulin resistance, inflammation and oxidative stress, even over activation of RAAS. These pathological mechanisms of MetS are just as the base of iceberg under the sea level. Hypertension, obesity, T2DM or impaired glucose tolerance, and dyslipidemia etc. are observed as the top part of floating iceberg which is above the sea level (Figure 2). The study around VAT dysfunction will help to understand the pathogenesis of MetS and may bring effective therapy in treatment of MetS related diseases.
We apologize for the inability to cite numerous examples of important work in the field.
P- Reviewers Grattagliano I, Guerrero-Romero F, Pavlidis AN, Raghow R, Uehara Y S- Editor Song XX L- Editor A E- Editor Lu YJ
| 1. | Sironi AM, Sicari R, Folli F, Gastaldelli A. Ectopic fat storage, insulin resistance, and hypertension. Curr Pharm Des. 2011;17:3074-3080. [PubMed] |
| 2. | Goran MI, Alderete TL. Targeting adipose tissue inflammation to treat the underlying basis of the metabolic complications of obesity. Nestle Nutr Inst Workshop Ser. 2012;73:49-60; discussion p61-p66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (1)] |
| 3. | Slomko H, Heo HJ, Einstein FH. Minireview: Epigenetics of obesity and diabetes in humans. Endocrinology. 2012;153:1025-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 4. | Herrera BM, Keildson S, Lindgren CM. Genetics and epigenetics of obesity. Maturitas. 2011;69:41-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 178] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 5. | Hassan M, Latif N, Yacoub M. Adipose tissue: friend or foe? Nat Rev Cardiol. 2012;9:689-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 96] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 6. | Harwood HJ. The adipocyte as an endocrine organ in the regulation of metabolic homeostasis. Neuropharmacology. 2012;63:57-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 186] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 7. | Vazzana N, Santilli F, Sestili S, Cuccurullo C, Davi G. Determinants of increased cardiovascular disease in obesity and metabolic syndrome. Curr Med Chem. 2011;18:5267-5280. [PubMed] |
| 8. | Mattu HS, Randeva HS. Role of adipokines in cardiovascular disease. J Endocrinol. 2013;216:T17-T36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 192] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 9. | Scarpellini E, Tack J. Obesity and metabolic syndrome: an inflammatory condition. Dig Dis. 2012;30:148-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 10. | Smith MM, Minson CT. Obesity and adipokines: effects on sympathetic overactivity. J Physiol. 2012;590:1787-1801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 148] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 11. | Pérez PM, Moore-Carrasco R, González DR, Fuentes EQ, Palomo IG. Gene expression of adipose tissue, endothelial cells and platelets in subjects with metabolic syndrome (Review). Mol Med Rep. 2012;5:1135-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Kotnik P, Fischer-Posovszky P, Wabitsch M. RBP4: a controversial adipokine. Eur J Endocrinol. 2011;165:703-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 173] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 13. | Bergmann K, Sypniewska G. Diabetes as a complication of adipose tissue dysfunction. Is there a role for potential new biomarkers? Clin Chem Lab Med. 2013;51:177-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 14. | Bremer AA, Jialal I. Adipose tissue dysfunction in nascent metabolic syndrome. J Obes. 2013;2013:393192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 116] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 15. | De Cosmo S, Menzaghi C, Prudente S, Trischitta V. Role of insulin resistance in kidney dysfunction: insights into the mechanism and epidemiological evidence. Nephrol Dial Transplant. 2013;28:29-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 150] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 16. | Bełtowski J. Leptin and the regulation of endothelial function in physiological and pathological conditions. Clin Exp Pharmacol Physiol. 2012;39:168-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 17. | Shehzad A, Iqbal W, Shehzad O, Lee YS. Adiponectin: regulation of its production and its role in human diseases. Hormones (Athens). 2012;11:8-20. [PubMed] |
| 18. | Vaiopoulos AG, Marinou K, Christodoulides C, Koutsilieris M. The role of adiponectin in human vascular physiology. Int J Cardiol. 2012;155:188-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 19. | Buechler C, Wanninger J, Neumeier M. Adiponectin, a key adipokine in obesity related liver diseases. World J Gastroenterol. 2011;17:2801-2811. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 107] [Reference Citation Analysis (0)] |
| 20. | Matsuzawa Y. Adiponectin: a key player in obesity related disorders. Curr Pharm Des. 2010;16:1896-1901. [PubMed] |
| 21. | Younus S, Rodgers G. Biomarkers associated with cardiometabolic risk in obesity. Am Heart Hosp J. 2011;9:E28-E32. [PubMed] |
| 22. | Aguilar MJ, González-Jiménez E, Antelo A, Perona JS. Insulin resistance and inflammation markers: correlations in obese adolescents. J Clin Nurs. 2013;22:2002-2010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Romeo GR, Lee J, Shoelson SE. Metabolic syndrome, insulin resistance, and roles of inflammation--mechanisms and therapeutic targets. Arterioscler Thromb Vasc Biol. 2012;32:1771-1776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 269] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 24. | Osborn O, Olefsky JM. The cellular and signaling networks linking the immune system and metabolism in disease. Nat Med. 2012;18:363-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1082] [Cited by in RCA: 1171] [Article Influence: 90.1] [Reference Citation Analysis (0)] |
| 25. | Kalupahana NS, Claycombe KJ, Moustaid-Moussa N. (n-3) Fatty acids alleviate adipose tissue inflammation and insulin resistance: mechanistic insights. Adv Nutr. 2011;2:304-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 218] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 26. | Fernández-Real JM, Pickup JC. Innate immunity, insulin resistance and type 2 diabetes. Diabetologia. 2012;55:273-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 27. | Pickup JC, Mattock MB, Chusney GD, Burt D. NIDDM as a disease of the innate immune system: association of acute-phase reactants and interleukin-6 with metabolic syndrome X. Diabetologia. 1997;40:1286-1292. [PubMed] |
| 28. | Chen SJ, Yen CH, Huang YC, Lee BJ, Hsia S, Lin PT. Relationships between inflammation, adiponectin, and oxidative stress in metabolic syndrome. PLoS One. 2012;7:e45693. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 29. | Odegaard JI, Chawla A. Pleiotropic actions of insulin resistance and inflammation in metabolic homeostasis. Science. 2013;339:172-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 494] [Cited by in RCA: 499] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 30. | Lee J. Adipose tissue macrophages in the development of obesity-induced inflammation, insulin resistance and type 2 Diabetes. Arch Pharm Res. 2013;36:208-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 104] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 31. | Miyazaki T, Kurokawa J, Arai S. AIMing at metabolic syndrome. -Towards the development of novel therapies for metabolic diseases via apoptosis inhibitor of macrophage (AIM).-. Circ J. 2011;75:2522-2531. [PubMed] |
| 32. | Aouadi M, Tencerova M, Vangala P, Yawe JC, Nicoloro SM, Amano SU, Cohen JL, Czech MP. Gene silencing in adipose tissue macrophages regulates whole-body metabolism in obese mice. Proc Natl Acad Sci USA. 2013;110:8278-8283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 111] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 33. | Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3290] [Cited by in RCA: 3124] [Article Influence: 208.3] [Reference Citation Analysis (0)] |
| 34. | Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2402] [Cited by in RCA: 2656] [Article Influence: 189.7] [Reference Citation Analysis (0)] |
| 35. | Chmelar J, Chung KJ, Chavakis T. The role of innate immune cells in obese adipose tissue inflammation and development of insulin resistance. Thromb Haemost. 2013;109:399-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 36. | Talukdar S, Oh da Y, Bandyopadhyay G, Li D, Xu J, McNelis J, Lu M, Li P, Yan Q, Zhu Y. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat Med. 2012;18:1407-1412. [PubMed] |
| 37. | Liu J, Divoux A, Sun J, Zhang J, Clément K, Glickman JN, Sukhova GK, Wolters PJ, Du J, Gorgun CZ. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat Med. 2009;15:940-945. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 624] [Cited by in RCA: 573] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 38. | Ballak DB, Stienstra R, Hijmans A, Joosten LA, Netea MG, Tack CJ. Combined B- and T-cell deficiency does not protect against obesity-induced glucose intolerance and inflammation. Cytokine. 2013;62:96-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 39. | Anderson EK, Gutierrez DA, Kennedy A, Hasty AH. Weight cycling increases T-cell accumulation in adipose tissue and impairs systemic glucose tolerance. Diabetes. 2013;62:3180-3188. [PubMed] |
| 40. | Tornatore L, Thotakura AK, Bennett J, Moretti M, Franzoso G. The nuclear factor kappa B signaling pathway: integrating metabolism with inflammation. Trends Cell Biol. 2012;22:557-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 351] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 41. | Han MS, Jung DY, Morel C, Lakhani SA, Kim JK, Flavell RA, Davis RJ. JNK expression by macrophages promotes obesity-induced insulin resistance and inflammation. Science. 2013;339:218-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 520] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 42. | Ferrante AW. Biomedicine. Improving metabolism by throwing out all the JNK. Science. 2013;339:147-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 43. | Cortez M, Carmo LS, Rogero MM, Borelli P, Fock RA. A high-fat diet increases IL-1, IL-6, and TNF-α production by increasing NF-κB and attenuating PPAR-γ expression in bone marrow mesenchymal stem cells. Inflammation. 2013;36:379-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 122] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 44. | Ye L, Kleiner S, Wu J, Sah R, Gupta RK, Banks AS, Cohen P, Khandekar MJ, Boström P, Mepani RJ. TRPV4 is a regulator of adipose oxidative metabolism, inflammation, and energy homeostasis. Cell. 2012;151:96-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 275] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 45. | Gabrielsen JS, Gao Y, Simcox JA, Huang J, Thorup D, Jones D, Cooksey RC, Gabrielsen D, Adams TD, Hunt SC. Adipocyte iron regulates adiponectin and insulin sensitivity. J Clin Invest. 2012;122:3529-3540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 244] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 46. | Yamaoka M, Maeda N, Nakamura S, Kashine S, Nakagawa Y, Hiuge-Shimizu A, Okita K, Imagawa A, Matsuzawa Y, Matsubara K. A pilot investigation of visceral fat adiposity and gene expression profile in peripheral blood cells. PLoS One. 2012;7:e47377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 47. | Després JP. Abdominal obesity and cardiovascular disease: is inflammation the missing link? Can J Cardiol. 2012;28:642-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 48. | Li J, Flammer AJ, Lennon RJ, Nelson RE, Gulati R, Friedman PA, Thomas RJ, Sandhu NP, Hua Q, Lerman LO. Comparison of the effect of the metabolic syndrome and multiple traditional cardiovascular risk factors on vascular function. Mayo Clin Proc. 2012;87:968-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 49. | Ridker PM. Moving beyond JUPITER: will inhibiting inflammation reduce vascular event rates? Curr Atheroscler Rep. 2013;15:295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 50. | Tanti JF, Ceppo F, Jager J, Berthou F. Implication of inflammatory signaling pathways in obesity-induced insulin resistance. Front Endocrinol (Lausanne). 2012;3:181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 128] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 51. | Flamment M, Hajduch E, Ferré P, Foufelle F. New insights into ER stress-induced insulin resistance. Trends Endocrinol Metab. 2012;23:381-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 247] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 52. | Hardy OT, Czech MP, Corvera S. What causes the insulin resistance underlying obesity? Curr Opin Endocrinol Diabetes Obes. 2012;19:81-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 385] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 53. | Patel PS, Buras ED, Balasubramanyam A. The role of the immune system in obesity and insulin resistance. J Obes. 2013;2013:616193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 114] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 54. | Kalupahana NS, Moustaid-Moussa N, Claycombe KJ. Immunity as a link between obesity and insulin resistance. Mol Aspects Med. 2012;33:26-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 186] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 55. | Chatzigeorgiou A, Karalis KP, Bornstein SR, Chavakis T. Lymphocytes in obesity-related adipose tissue inflammation. Diabetologia. 2012;55:2583-2592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 56. | Winer S, Winer DA. The adaptive immune system as a fundamental regulator of adipose tissue inflammation and insulin resistance. Immunol Cell Biol. 2012;90:755-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 57. | Reilly SM, Chiang SH, Decker SJ, Chang L, Uhm M, Larsen MJ, Rubin JR, Mowers J, White NM, Hochberg I. An inhibitor of the protein kinases TBK1 and IKK-ɛ improves obesity-related metabolic dysfunctions in mice. Nat Med. 2013;19:313-321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 310] [Cited by in RCA: 366] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 58. | Lee YS, Li P, Huh JY, Hwang IJ, Lu M, Kim JI, Ham M, Talukdar S, Chen A, Lu WJ. Inflammation is necessary for long-term but not short-term high-fat diet-induced insulin resistance. Diabetes. 2011;60:2474-2483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 399] [Cited by in RCA: 447] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 59. | Sun S, Ji Y, Kersten S, Qi L. Mechanisms of inflammatory responses in obese adipose tissue. Annu Rev Nutr. 2012;32:261-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 215] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 60. | O’Rourke RW, White AE, Metcalf MD, Winters BR, Diggs BS, Zhu X, Marks DL. Systemic inflammation and insulin sensitivity in obese IFN-γ knockout mice. Metabolism. 2012;61:1152-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 127] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 61. | Koppaka S, Kehlenbrink S, Carey M, Li W, Sanchez E, Lee DE, Lee H, Chen J, Carrasco E, Kishore P. Reduced adipose tissue macrophage content is associated with improved insulin sensitivity in thiazolidinedione-treated diabetic humans. Diabetes. 2013;62:1843-1854. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 62. | Ji Y, Sun S, Xu A, Bhargava P, Yang L, Lam KS, Gao B, Lee CH, Kersten S, Qi L. Activation of natural killer T cells promotes M2 Macrophage polarization in adipose tissue and improves systemic glucose tolerance via interleukin-4 (IL-4)/STAT6 protein signaling axis in obesity. J Biol Chem. 2012;287:13561-13571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 172] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 63. | Schipper HS, Rakhshandehroo M, van de Graaf SF, Venken K, Koppen A, Stienstra R, Prop S, Meerding J, Hamers N, Besra G. Natural killer T cells in adipose tissue prevent insulin resistance. J Clin Invest. 2012;122:3343-3354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 169] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 64. | Wu L, Parekh VV, Gabriel CL, Bracy DP, Marks-Shulman PA, Tamboli RA, Kim S, Mendez-Fernandez YV, Besra GS, Lomenick JP. Activation of invariant natural killer T cells by lipid excess promotes tissue inflammation, insulin resistance, and hepatic steatosis in obese mice. Proc Natl Acad Sci USA. 2012;109:E1143-E1152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 155] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 65. | Strodthoff D, Lundberg AM, Agardh HE, Ketelhuth DF, Paulsson-Berne G, Arner P, Hansson GK, Gerdes N. Lack of invariant natural killer T cells affects lipid metabolism in adipose tissue of diet-induced obese mice. Arterioscler Thromb Vasc Biol. 2013;33:1189-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 66. | Cipolletta D, Feuerer M, Li A, Kamei N, Lee J, Shoelson SE, Benoist C, Mathis D. PPAR-γ is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature. 2012;486:549-553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 779] [Cited by in RCA: 940] [Article Influence: 72.3] [Reference Citation Analysis (0)] |
| 67. | Eller K, Kirsch A, Wolf AM, Sopper S, Tagwerker A, Stanzl U, Wolf D, Patsch W, Rosenkranz AR, Eller P. Potential role of regulatory T cells in reversing obesity-linked insulin resistance and diabetic nephropathy. Diabetes. 2011;60:2954-2962. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 214] [Cited by in RCA: 266] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 68. | Viardot A, Heilbronn LK, Samocha-Bonet D, Mackay F, Campbell LV, Samaras K. Obesity is associated with activated and insulin resistant immune cells. Diabetes Metab Res Rev. 2012;28:447-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 69. | Bhatia LS, Curzen NP, Calder PC, Byrne CD. Non-alcoholic fatty liver disease: a new and important cardiovascular risk factor? Eur Heart J. 2012;33:1190-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 345] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 70. | Item F, Konrad D. Visceral fat and metabolic inflammation: the portal theory revisited. Obes Rev. 2012;13 Suppl 2:30-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 177] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 71. | Hocking S, Samocha-Bonet D, Milner KL, Greenfield JR, Chisholm DJ. Adiposity and insulin resistance in humans: the role of the different tissue and cellular lipid depots. Endocr Rev. 2013;34:463-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 195] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 72. | Lagerpusch M, Enderle J, Later W, Eggeling B, Pape D, Müller MJ, Bosy-Westphal A. Impact of glycaemic index and dietary fibre on insulin sensitivity during the refeeding phase of a weight cycle in young healthy men. Br J Nutr. 2013;109:1606-1616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 73. | Martínez-Clemente M, Clària J, Titos E. The 5-lipoxygenase/leukotriene pathway in obesity, insulin resistance, and fatty liver disease. Curr Opin Clin Nutr Metab Care. 2011;14:347-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 74. | DeFronzo RA. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links. The Claude Bernard Lecture 2009. Diabetologia. 2010;53:1270-1287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 621] [Cited by in RCA: 617] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 75. | Misra P. AMP activated protein kinase: a next generation target for total metabolic control. Expert Opin Ther Targets. 2008;12:91-100. [PubMed] |
| 76. | Galic S, Fullerton MD, Schertzer JD, Sikkema S, Marcinko K, Walkley CR, Izon D, Honeyman J, Chen ZP, van Denderen BJ. Hematopoietic AMPK β1 reduces mouse adipose tissue macrophage inflammation and insulin resistance in obesity. J Clin Invest. 2011;121:4903-4915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 289] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 77. | Garg AD, Kaczmarek A, Krysko O, Vandenabeele P, Krysko DV, Agostinis P. ER stress-induced inflammation: does it aid or impede disease progression? Trends Mol Med. 2012;18:589-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 330] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 78. | Kawasaki N, Asada R, Saito A, Kanemoto S, Imaizumi K. Obesity-induced endoplasmic reticulum stress causes chronic inflammation in adipose tissue. Sci Rep. 2012;2:799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 254] [Cited by in RCA: 315] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 79. | Mlinar B, Marc J. New insights into adipose tissue dysfunction in insulin resistance. Clin Chem Lab Med. 2011;49:1925-1935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 80. | Liang H, Tantiwong P, Sriwijitkamol A, Shanmugasundaram K, Mohan S, Espinoza S, Defronzo RA, Dubé JJ, Musi N. Effect of a sustained reduction in plasma free fatty acid concentration on insulin signalling and inflammation in skeletal muscle from human subjects. J Physiol. 2013;591:2897-2909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 81. | Capurso C, Capurso A. From excess adiposity to insulin resistance: the role of free fatty acids. Vascul Pharmacol. 2012;57:91-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 218] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 82. | Eringa EC, Bakker W, van Hinsbergh VW. Paracrine regulation of vascular tone, inflammation and insulin sensitivity by perivascular adipose tissue. Vascul Pharmacol. 2012;56:204-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 83. | Rask-Madsen C, Kahn CR. Tissue-specific insulin signaling, metabolic syndrome, and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2012;32:2052-2059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 238] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 84. | Goodpaster BH. Mitochondrial deficiency is associated with insulin resistance. Diabetes. 2013;62:1032-1035. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 85. | Johnson AM, Olefsky JM. The origins and drivers of insulin resistance. Cell. 2013;152:673-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 494] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 86. | Whaley-Connell A, Sowers JR. Oxidative stress in the cardiorenal metabolic syndrome. Curr Hypertens Rep. 2012;14:360-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 87. | Paravicini TM, Touyz RM. NADPH oxidases, reactive oxygen species, and hypertension: clinical implications and therapeutic possibilities. Diabetes Care. 2008;31 Suppl 2:S170-S180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 502] [Cited by in RCA: 501] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 88. | Peña-Orihuela P, Camargo A, Rangel-Zuñiga OA, Perez-Martinez P, Cruz-Teno C, Delgado-Lista J, Yubero-Serrano EM, Paniagua JA, Tinahones FJ, Malagon MM. Antioxidant system response is modified by dietary fat in adipose tissue of metabolic syndrome patients. J Nutr Biochem. 2013;24:1717-1723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 89. | Codoñer-Franch P, Valls-Bellés V, Arilla-Codoñer A, Alonso-Iglesias E. Oxidant mechanisms in childhood obesity: the link between inflammation and oxidative stress. Transl Res. 2011;158:369-384. [PubMed] |
| 90. | Ruskovska T, Bernlohr DA. Oxidative stress and protein carbonylation in adipose tissue - Implications for insulin resistance and diabetes mellitus. J Proteomics. 2013;Apr 11; [Epub ahead of print]. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 91. | Hopps E, Caimi G. Protein oxidation in metabolic syndrome. Clin Invest Med. 2013;36:E1-E8. [PubMed] |
| 92. | Kalupahana NS, Moustaid-Moussa N. The adipose tissue renin-angiotensin system and metabolic disorders: a review of molecular mechanisms. Crit Rev Biochem Mol Biol. 2012;47:379-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 93. | Motoshima H, Araki E. [RAAS and insulin resistance]. Nihon Rinsho. 2012;70:1542-1549. [PubMed] |
| 94. | Brown NJ. Aldosterone and vascular inflammation. Hypertension. 2008;51:161-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 180] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 95. | Wei Y, Whaley-Connell AT, Habibi J, Rehmer J, Rehmer N, Patel K, Hayden M, DeMarco V, Ferrario CM, Ibdah JA. Mineralocorticoid receptor antagonism attenuates vascular apoptosis and injury via rescuing protein kinase B activation. Hypertension. 2009;53:158-165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 96. | Hirono Y, Yoshimoto T, Suzuki N, Sugiyama T, Sakurada M, Takai S, Kobayashi N, Shichiri M, Hirata Y. Angiotensin II receptor type 1-mediated vascular oxidative stress and proinflammatory gene expression in aldosterone-induced hypertension: the possible role of local renin-angiotensin system. Endocrinology. 2007;148:1688-1696. [PubMed] |
| 97. | Feraco A, Armani A, Mammi C, Fabbri A, Rosano GM, Caprio M. Role of mineralocorticoid receptor and renin-angiotensin-aldosterone system in adipocyte dysfunction and obesity. J Steroid Biochem Mol Biol. 2013;Feb 28; [Epub ahead of print]. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 98. | Lear SA, Sarna LK, Siow TJ, Mancini GB, Siow YL, O K. Oxidative stress is associated with visceral adipose tissue and subclinical atherosclerosis in a healthy multi-ethnic population. Appl Physiol Nutr Metab. 2012;37:1164-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 99. | Goossens GH, Bizzarri A, Venteclef N, Essers Y, Cleutjens JP, Konings E, Jocken JW, Cajlakovic M, Ribitsch V, Clément K. Increased adipose tissue oxygen tension in obese compared with lean men is accompanied by insulin resistance, impaired adipose tissue capillarization, and inflammation. Circulation. 2011;124:67-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 242] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 100. | Bryan S, Baregzay B, Spicer D, Singal PK, Khaper N. Redox-inflammatory synergy in the metabolic syndrome. Can J Physiol Pharmacol. 2013;91:22-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |