Published online May 20, 2021. doi: 10.5493/wjem.v11.i3.30
Peer-review started: March 18, 2021
First decision: May 14, 2021
Revised: May 14, 2021
Accepted: May 20, 2021
Article in press: May 20, 2021
Published online: May 20, 2021
Processing time: 74 Days and 21.8 Hours
Spontaneous posterior vitreous detachment (PVD) is a common age-related condition in which prevalence tends to increase with age. Acute PVD can cause the onset of symptoms that include visual disturbances, myodesopsia and photopsia. The goal of this short review was to provide a quick glance at the important factors related to PVD based on current literature in this field, which includes incidence, symptoms, diagnosis, risk factors, and education for patients with acute symptoms, and treatments. The take home message is that an ophthalmic examination at the onset of symptoms is of utmost importance, considering that irreversible sight-threatening complications can be prevented if diagnosed and treated promptly.
Core Tip: Posterior vitreous detachment (PVD) tends to be a benign condition related to aging. Acute PVD can cause the onset of symptoms like flashes, visual disturbances, and floaters. Current literature has provided new explanations of the mechanisms underlying normal and abnormal PVD. Incidence, prevalence, and risk factors are important in assessing patients. New diagnostic tools like optical coherence tomography have assisted in providing objective evaluation of patients. Treatment with vitrectomy and laser and pharmacological vitreolysis are available, but are seldom considered because they can be invasive and can worsen symptoms. Patients must be educated to seek an ophthalmologic examination that includes a dilated fundus evaluation at the onset of important signs and symptoms, especially those with risk factors, because early diagnosis and treatment can prevent irreversible vision loss.
- Citation: Ramovecchi P, Salati C, Zeppieri M. Spontaneous posterior vitreous detachment: A glance at the current literature. World J Exp Med 2021; 11(3): 30-36
- URL: https://www.wjgnet.com/2220-315x/full/v11/i3/30.htm
- DOI: https://dx.doi.org/10.5493/wjem.v11.i3.30
Spontaneous posterior vitreous detachment (PVD) is a common age-related condition in patients older than 45 years. Among patients aged 50-59 years, there is a prevalence of about 24%, which increases to about 87% those 80-90 years of age[1,2]. Considering that the number of studies reported in literature is relatively extensive, we wanted to provide a quick overview by concentrating mostly, but not exclusively, on studies published in the last 20 years. The aim of our review was to briefly summarize the basic concepts and important factors related to acute PVD, mainly regarding physio-pathogenesis, incidence, risk factor, symptoms, diagnosis, and treatment. We hope that this can serve to remind clinicians in all areas of specialization to send patients for an ophthalmic examination at the onset of symptoms, considering that irreversible sight-threatening complications can be prevented if diagnosed and treated on time.
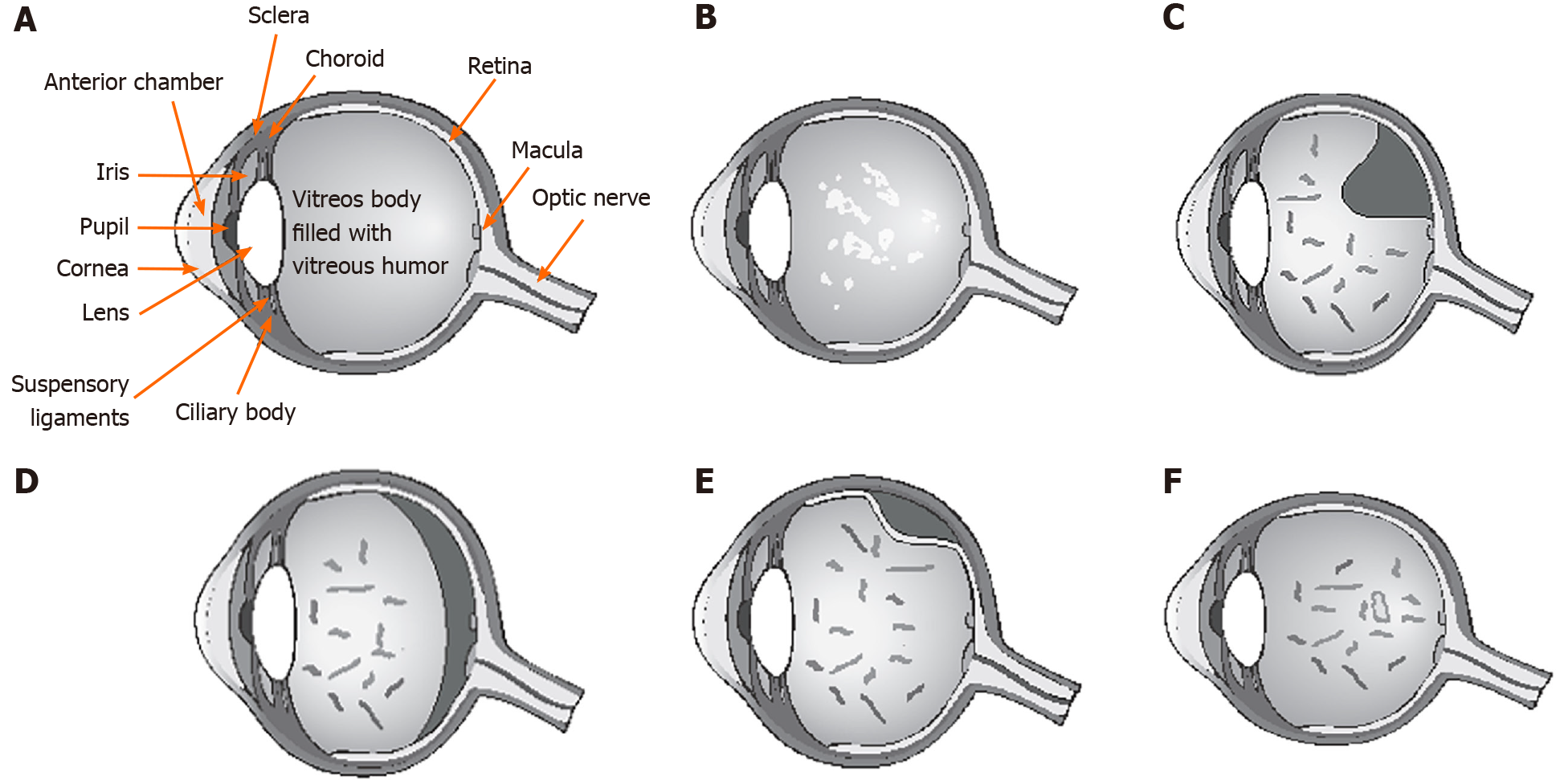
The vitreous humor, the largest anatomic structure in the human eye, is located between the lens and the retina (Figure 1A). It is a transparent gel-like structure composed mostly of water (98%-99%) in addition to hyaluronic acid (0.5%) and collagen type II, a hybrid of types V/XI, and type IX (0.5%)[3]. The collagen component is organized in fibrils of 7-28 nm in diameter, with type V/XI in the core and the other types in the periphery. The vitreous body fills the posterior segment of the eye and is separated from the retina by the internal limiting lamina (ILL), which is composed mostly of type IV collagen. The embryologic origin of the ILL is the same as Bruch’s membrane[4]. The most peripheral part of vitreous is known as vitreous cortex, which is formed from a concentration of collagen fibrils composed mostly of type II collagen, however, other types (IX, V/XI) are also present. Adhesion of the posterior vitreous cortex and the ILL depends on an extracellular matrix that serves as glue. With age, this adhesion weakens, most likely because of biochemical alteration of the components of the extracellular matrix.
Extensive changes of the vitreous body occur with age. The consistency of vitreous changes from a gel phase to a water phase. There is a decrease of gel volume and an increase in liquid volume of human vitreous (Figure 1B). At about 50 years of age, 25% of vitreous is in water phase, which drastically increases to about 62% at 80 years[1]. It is important to note that the adherence of the vitreous is strongest at the retina around the vitreous base (ora serrata), at the optic disc margins, at macula, and around peripheral blood vessels[4].
PVD occurs when there is a sudden separation of the from the internal limiting membrane of the retina, resulting from the liquefaction within the vitreous body (Figure 1B). For PVD to occur, two different events must happen, the liquefaction of vitreous body (synchisis) and the weakening of adhesion between vitreous posterior cortex and the ILL[5,6]. When uncomplicated PVD occurs, the liquid vitreous tends to move toward the retrocortical prepapillar and premacular space, thus causing the vitreous body to collapse (syneresis) in anterior fashion (Figure 1C and D)[7]. That event can develop in several anomalous ways when, for various causes, there is a change in the balance between the degree of gel liquefaction and the weakening of vitreoretinal adhesion. If there is insufficient weakening of the vitreoretinal adhesion in the presence of vitreous liquefaction, abnormal traction at the vitreoretinal interface can result, leading to deleterious effects on the retina (Figure 1E) and vitreous, including hemorrhages, retinal tears, retinal detachment (RD), vitreo-macular traction syndrome, macular pucker, and macular holes[3,7,8].
Women seem to be affected by a faster progression of vitreous detachment. Postmenopausal women may be more prone to PVD because of a lack of estrogen[9], which may have a protective effect against PVD[10]. Studies have shown that up to 20% of PVDs can be asymptomatic in the early stages and thus not detected clinically[8]. When the separation of vitreous from optic disc margins is complete, patients tend to present symptoms. The most common symptoms are photopsia (flashes) and myodesopsia (or floaters), which are perceived as linear dark shadows, moving cob webs or spots that move within the back of the eye (Figure 1F)[4-7]. Floaters tend to be mobile and more evident against a bright background. Symptoms are variable and can remain for months or years after uncomplicated PVD[7].
It is of utmost importance that patients with acute symptoms, which include worsening of floaters and/or photopsia, undergo an ophthalmic examination. Flashes of light are rapid, often located in the temporal quadrant, and are considered to be caused by the stimulation of the retina from vitreoretinal traction and pulling. Studies have shown that the risk of damage of the retina with the onset of symptoms ranges from 8.2% to 47.65%[10,11]. Flashes and/or floaters tended to be associated with the onset or subsequent development of retinal tears[12,13]. The greatest risk factors for retinal tears include the presence of ten or more floaters, cloud-like obscuration in the visual field, and retinal or vitreous hemorrhages[12-16]. Patients may complain of acute blurred vision caused by vitreous hemorrhage, presence of a Weiss ring in the vitreous body (Figure 1F) or big floaters crowding the visual field. In symptomatic PVD, the risk of developing retinal tears and detachment can be as high as 35%, which can increase in the presence of vitreous hemorrhaging[17].
New retinal tears brought on by acute abnormal PVD can lead to RD because the vitreous fluid can enter behind the retina under traction, causing a separation of the neuroretina from retinal pigment epithelium[14-16]. RD may occur within 6 wk in untreated retinal breaks. In that period, it is possible to find new retinal breaks at different locations. Mitry et al[18] reported that the risk of RD was higher in men than in women, with an incidence of 13.09 and 7.41 per 100.000, respectively[18]. Numerous studies suggest re-examining the patient within 6 wk[14,15]. That time period may be too long, considering that retinal tears can appear within a shorter period after the onset of PVD[16-17], thus it is of utmost importance to educate patients regarding the onset of new symptoms after initial diagnosis of PVD. An earlier ophthalmic evaluation may be necessary, especially in patients at increased risk of developing RD, including those with previous retinal tears, high myopia, history of ophthalmic surgery, trauma or laser treatment, pseudophakia/afachia, etc.[14-17].
RD requires immediate surgical intervention to avoid the apoptosis of photoreceptors and irreversible loss of vision[19]. Patients with symptomatic PVD should be directed to an emergency ophthalmology department within 24 h of onset of symptoms. Patients should undergo a complete ophthalmic evaluation, which includes well dilated retinal examination with slit-lamp biomicroscopy, preferably with a superfield Volk lens, Goldmann three-mirror contact lens and/or indirect ophthalmoscopy with scleral indentation to examine the entire 360° of the ora serrata. During the fundus examination, it is possible to find the presence of a Weiss ring[20,21], retinal holes with or without opercula, equatorial breaks, horseshoe tears, RD or vitreous hemorrhages[14-17]. In some cases, a clinically accurate diagnosis may be difficult if PVD is initial or partial, if there are no signs of a Weiss ring, or if the posterior hyaloid is still attached inferiorly. Fundus examination can be difficult if PVD causes a vitreous hemorrhage, which obscures proper diagnosis. B-scan ultrasonography (US) can be used to accurately assess the status of vitreous in vivo and to check the presence and extent of PVD[22]. US is safe, painless and noninvasive, but it is operator dependent and based on the experience and skills of the examiner, thus giving rise to variable and subjective interpretation of results.
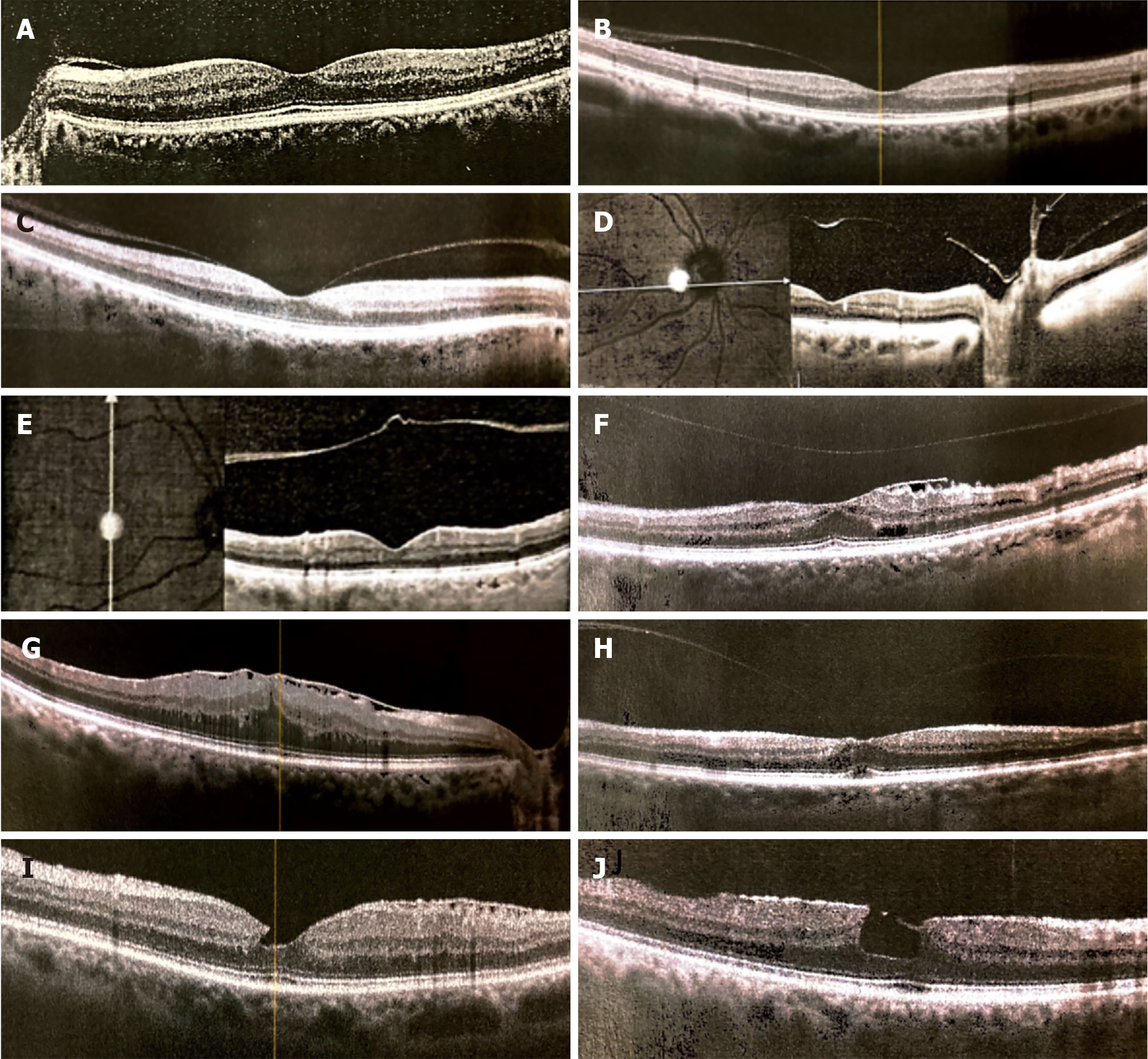
Optical coherence tomography (OCT), is currently in widespread use in the management of retinal pathologies and glaucoma. It can also be used to noninvasively visualize the vitreoretinal interface with high-resolution images in vivo[23]. OCT can accurately show the separation of the posterior vitreous face and retina and can identify a shallow PVD better than slit-lamp biomicroscopy and B-scan US. Thanks to the routine clinical use of OCT, it has been shown that PVD usually starts as a vitreous RD around the fovea[21,24]. Kakehashi et al[25] proposed a classification of PVD into five stages based on OCT, and ranging from nonexistence of PVD in stage 0 to complete PVD with a prominent Weiss ring on slit-lamp biomicroscopy in stage 4[25]. The various stages of PVD can be seen in OCT scans (Figure 2A-E), which are normally prescribed in the management of patients with glaucoma or retinopathies, some of which derive from abnormal PVD, like macular pucker (Figure 2F and G) and macular lamellar holes (Figure 2H-J). The limit of OCT is that this technique cannot visualize the whole vitreous cavity and can give rise to false-positive interpretations of PVD[26]. The diagnostic ability of OCT for detecting PVD, especially if partial, can increase if OCT circumferential peripapillary scan images are added to the transverse OCT images[27].
Uncomplicated PVD is a benign condition with a good prognosis, typically brought on by aging, and is usually not visually threatening. Floaters and myodesopsia normally subside within about 3 mo. Over time, patients develop an adaptation to the visual symptoms and/or floaters may resolve. In many cases, however, floaters can persist for 6 mo or even years[28]. Oral supplements, vitamins and proper systemic hydration can be suggested; however, medical treatment is usually not given for vitreous floaters or for PVD. If no retinal breaks or hemorrhages are found upon initial diagnosis of PVD, it is best to repeat the dilated examination within 2 to 4 wk and then at 3 mo and 6 mo from the onset of symptoms[29]. Closer follow-up is suggested in the presence of mild vitreous hemorrhages, peripheral punctate retinal hemorrhages, and the onset of new or worsening of photopsia and myodesopsia symptoms. Vitreous hemorrhages following symptomatic PVD with photopsia have been shown to be associated with retinal breaks, and thus need to be considered in this manner even if the fundus cannot be clearly assessed in those cases[29]. Patients must be adequately educated and told to seek emergency ophthalmic evaluation in the presence of new signs and symptoms related to possible RD. If a small isolated peripheral retinal break is found, guidelines for tears or breaks, which usually recommend laser treatment, must be followed to avoid RD. Symptomatic breaks after PVD should be immediately treated with laser retinal photocoagulation, which is usually performed by applying two or three rows of confluent burns around the lesion[29]. Retinal breaks can be treated with cryoretinopexy in eyes with small pupils or a hazy cornea. Laser retinopexy is superior to cryoretinopexy, and tends to be more precise, with less risk of epiretinal membrane[29].
In rare cases, floaters can be dense and numerous, which can significantly disturb vision. If symptoms are clinically important and visual acuity levels drop, treatment can be considered. Vitrectomy is the gold-standard surgical treatment for patients with vision-degrading myodesopsia[30]. Of course, that option is used in a very limited number of cases considering the risks associated with this invasive surgery. The other options that are currently being studied involve treatment with Nd:YAG laser vitreolysis[31] and photoablation[30]. However, those treatments are still experimental and not often used in routine clinical practice. Pharmacological vitreolysis with various agents (e.g., hyaluronidase, vitrase, collagenase, ocriplasmin, and others) have been proposed in the literature. However, pharmacologic options tend to be considered for abnormal PVD and treatment for symptomatic vitreomacular adhesions and pucker as opposed to reducing floaters. Studies have reported that pharmacological treatments may actually induce or increase floaters rather than dissolving them[32].
PVD is a common aging-related condition that usually happens twice in a lifetime, once in each eye. The onset of this condition tends to be asymptomatic and without complications; however, acute symptoms need to be assessed quickly. New signs and symptoms like floaters, myodesopsia and flashes can be signs of PVD. An ophthalmologic examination is important to exclude abnormal PVD and acute complications like rhegmatogenous RD, retinal breaks, and vitreal hemorrhaging. PVD may lead to other nonacute conditions that include vitreoretinal interface problems such as epiretinal membranes and macular holes. Such patients need to be assessed and managed with periodical OCT scans and ophthalmic examinations that include best corrected visual acuity and fundus examinations to determine whether or not vitrectomy surgery is needed to address visual decay caused by vitreoretinal interface problems.
PVD is part of the normal aging process. As most degenerative pathologies of aging arise in the presence of complications or abnormal acute conditions, periodic ophthalmic examinations are a must, especially after 65 years of age. Acute symptoms, such as myodesopsia and flashes need quick ophthalmic evaluation because abnormal PVD can lead to irreversible sight-threatening conditions like RD, hemorrhaging, and vitreoretinal traction, which can be avoided, prevented and treated if diagnosed early.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Navea-Tejerina A S-Editor: Fan JR L-Editor: Filipodia P-Editor: Li X
| 1. | Johnson MW. Posterior vitreous detachment: evolution and complications of its early stages. Am J Ophthalmol 2010; 149: 371-82. e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 270] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 2. | Le Goff MM, Bishop PN. Adult vitreous structure and postnatal changes. Eye (Lond). 2008;22:1214-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 224] [Article Influence: 13.2] [Reference Citation Analysis (1)] |
| 3. | Bishop PN. Structural macromolecules and supramolecular organisation of the vitreous gel. Prog Retin Eye Res. 2000;19:323-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 315] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 4. | Sebag J, Hageman GS. Interfaces. Eur J Ophthalmol. 2000;10:1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Sebag J. Anatomy and pathology of the vitreo-retinal interface. Eye (Lond). 1992;6 (Pt 6):541-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 140] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 6. | Sebag J. Age-related differences in the human vitreoretinal interface. Arch Ophthalmol. 1991;109:966-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 144] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 7. | Sebag J. Anomalous posterior vitreous detachment: a unifying concept in vitreo-retinal disease. Graefes Arch Clin Exp Ophthalmol. 2004;242:690-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 313] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 8. | Richardson PS, Benson MT, Kirkby GR. The posterior vitreous detachment clinic: do new retinal breaks develop in the six weeks following an isolated symptomatic posterior vitreous detachment? Eye (Lond). 1999;13 ( Pt 2):237-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Hayashi K, Sato T, Manabe SI, Hirata A. Sex-Related Differences in the Progression of Posterior Vitreous Detachment with Age. Ophthalmol Retina. 2019;3:237-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 10. | Chuo JY, Lee TY, Hollands H, Morris AH, Reyes RC, Rossiter JD, Meredith SP, Maberley DA. Risk factors for posterior vitreous detachment: a case-control study. Am J Ophthalmol. 2006;142:931-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 75] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 11. | Coffee RE, Westfall AC, Davis GH, Mieler WF, Holz ER. Symptomatic posterior vitreous detachment and the incidence of delayed retinal breaks: case series and meta-analysis. Am J Ophthalmol. 2007;144:409-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 65] [Article Influence: 3.6] [Reference Citation Analysis (1)] |
| 12. | Bond-Taylor M, Jakobsson G, Zetterberg M. Posterior vitreous detachment - prevalence of and risk factors for retinal tears. Clin Ophthalmol. 2017;11:1689-1695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 13. | Carrero JL. Incomplete posterior vitreous detachment: prevalence and clinical relevance. Am J Ophthalmol. 2012;153:497-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | van Overdam KA, Bettink-Remeijer MW, Klaver CC, Mulder PG, Moll AC, van Meurs JC. Symptoms and findings predictive for the development of new retinal breaks. Arch Ophthalmol. 2005;123:479-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Schweitzer KD, Eneh AA, Hurst J, Bona MD, Rahim KJ, Sharma S. Predicting retinal tears in posterior vitreous detachment. Can J Ophthalmol. 2011;46:481-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | van Overdam KA, Bettink-Remeijer MW, Mulder PG, van Meurs JC. Symptoms predictive for the later development of retinal breaks. Arch Ophthalmol. 2001;119:1483-1486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Gishti O, van den Nieuwenhof R, Verhoekx J, van Overdam K. Symptoms related to posterior vitreous detachment and the risk of developing retinal tears: a systematic review. Acta Ophthalmol. 2019;97:347-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 18. | Mitry D, Tuft S, McLeod D, Charteris DG. Laterality and gender imbalances in retinal detachment. Graefes Arch Clin Exp Ophthalmol. 2011;249:1109-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Chang CJ, Lai WW, Edward DP, Tso MO. Apoptotic photoreceptor cell death after traumatic retinal detachment in humans. Arch Ophthalmol. 1995;113:880-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 95] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | Akiba J, Ishiko S, Yoshida A. Variations of Weiss's ring. Retina. 2001;21:243-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Johnson MW. Perifoveal vitreous detachment and its macular complications. Trans Am Ophthalmol Soc. 2005;103:537-567. [PubMed] |
| 22. | Mamou J, Wa CA, Yee KM, Silverman RH, Ketterling JA, Sadun AA, Sebag J. Ultrasound-based quantification of vitreous floaters correlates with contrast sensitivity and quality of life. Invest Ophthalmol Vis Sci. 2015;56:1611-1617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 23. | Yannuzzi LA, Ober MD, Slakter JS, Spaide RF, Fisher YL, Flower RW, Rosen R. Ophthalmic fundus imaging: today and beyond. Am J Ophthalmol. 2004;137:511-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 125] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 24. | Uchino E, Uemura A, Ohba N. Initial stages of posterior vitreous detachment in healthy eyes of older persons evaluated by optical coherence tomography. Arch Ophthalmol. 2001;119:1475-1479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 212] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 25. | Kakehashi A, Takezawa M, Akiba J. Classification of posterior vitreous detachment. Clin Ophthalmol. 2014;8:1-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Kim YC, Harasawa M, Siringo FS, Quiroz-Mercado H. Assessment of posterior vitreous detachment on enhanced high density line optical coherence tomography. Int J Ophthalmol. 2017;10:165-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Moon SY, Park SP, Kim YK. Evaluation of posterior vitreous detachment using ultrasonography and optical coherence tomography. Acta Ophthalmol. 2020;98:e29-e35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Kim YK, Moon SY, Yim KM, Seong SJ, Hwang JY, Park SP. Psychological Distress in Patients with Symptomatic Vitreous Floaters. J Ophthalmol. 2017;2017:3191576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 29. | Flaxel CJ, Adelman RA, Bailey ST, Fawzi A, Lim JI, Vemulakonda GA, Ying GS. Posterior Vitreous Detachment, Retinal Breaks, and Lattice Degeneration Preferred Practice Pattern®. Ophthalmology. 2020;127:P146-P181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 30. | Sauvage F, Fraire JC, Remaut K, Sebag J, Peynshaert K, Harrington M, Van de Velde FJ, Xiong R, Tassignon MJ, Brans T, Braeckmans K, De Smedt SC. Photoablation of Human Vitreous Opacities by Light-Induced Vapor Nanobubbles. ACS Nano. 2019;13:8401-8416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 31. | Nguyen JH, Nguyen-Cuu J, Yu F, Yee KM, Mamou J, Silverman RH, Ketterling J, Sebag J. Assessment of Vitreous Structure and Visual Function after Neodymium:Yttrium-Aluminum-Garnet Laser Vitreolysis. Ophthalmology. 2019;126:1517-1526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 32. | Milston R, Madigan MC, Sebag J. Vitreous floaters: Etiology, diagnostics, and management. Surv Ophthalmol. 2016;61:211-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 106] [Article Influence: 10.6] [Reference Citation Analysis (0)] |