Published online Nov 20, 2013. doi: 10.5493/wjem.v3.i4.50
Revised: October 28, 2013
Accepted: November 7, 2013
Published online: November 20, 2013
Processing time: 145 Days and 4.5 Hours
In consideration of the poor results obtained with conventional treatments, a review of alternative treatments for elderly patients with glioblastoma was researched in this study. The proposal considers the elimination of human cytomegalovirus, modifying the immune response, arresting growths, blocking some signaling pathways, and modulating the effects of oxygen reactive species.
Core tip: It is necessary to reconsider the treatment of elderly patients with glioma, focusing on their life quality when conventional treatment is used, such as, chemotherapy and radiotheraphy, in review of the fact that these treatments cause the patient further suffering. This is a review of othe therapeutic options, including some phase I vaccine trials.
- Citation: Perez-Campos E, Perez JA, Mayoral LPC, Velasco IG, Cruz PH, Olivera PG. Why not change classical treatments for glioblastoma in elderly patients? World J Exp Med 2013; 3(4): 50-55
- URL: https://www.wjgnet.com/2220-315X/full/v3/i4/50.htm
- DOI: https://dx.doi.org/10.5493/wjem.v3.i4.50
Glioblastoma (GBM) is the most aggressive and frequent of all brain tumors. Glioblastoma usually appears between the ages of 45 and 70 years[1]. The goal of surgery is to confirm the diagnosis and reduce the effect of the tumor mass[2]. Survival of elderly patients of more than 71 years, with glioblastoma GBM, is poor. Temozolomide (TMZ) and radiotherapy (RT) improves the average patient survival rate by as much as 10 to 13 mo, in patients of more than 71 years[3].
Stereotactic radiosurgery, whole brain radiation therapy, and surgery, in isolation or in combination[4] with gamma knife, cyberknife, LINAC, stereotactic brachytherapy, boron neutron capture therapy and hadrontherapy, amongst other treatments, have been used as therapy[5,6]. Although all these methods have been reported to improve prognostic indices, when under constant observation, the statistical analyzes of survival are not effective. Radiation therapy has considerable limitations, mainly infiltrating glioma characteristics and neuronal damage.
Although the survival rate is greater when using chemotherapy than when not used[7], the difference in the number of months survived is very low, and the quality of life is much lower when radiation is used rather than with no chemotherapy.
Gliomas show tumor-associated antigens, which should be detectable by the immune system, however, there are shortcomings in the elimination of a tumor.
Gliomas lack clear-cut tumor-rejection antigens for immune targeting by CD8+ T-cells and indicate cancer/testis antigens as NY-ESO-1[8,9]. However, other antigens from human cytomegalovirus (HCMV) have been found.
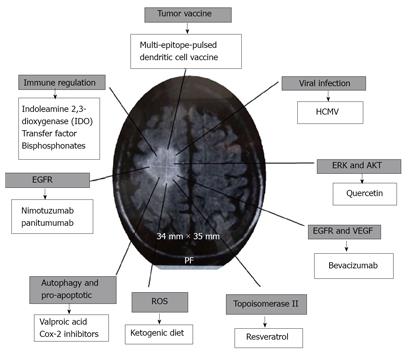
The question is, how can tumor growth be limited without chemotherapy or radiotherapy? Here, some therapeutic options are reviewed, including some phase I vaccine trials, supporting treatment of elderly patients with glioma (Figure 1).
It has been suggested that glioma in elderly patients should be treated as a tumor associated with viral infection[10], this would mean treatment for the elimination of the infectious agent, arresting growths and apoptosis, modifying the immune response and blocking some signaling pathways, which involve metastasis and the modulation of reactive oxygen species (ROS).
Glioma tissues indicate the change in a cascade of a viral protein, typical of replicative HCMV[11], the virus is trophic in glial cells and the HCMV infection remains in between 50% to 90% of adults. The HCMV can be reactivated when there is inflammation and immunosuppression and plays an active role in the pathogenesis of a glioma[12].
In order to clarify the controversy surrounding glioma and HCMV, some researchers have shown the close relationship between HCMV and glioma in the context of mutations related to their existence[13].
In order to reduce the effects of HCMV on the glioma it is possible to use valganciclovir, which targets the DNA polymerase, or the Cox-2 inhibitor celecoxib, and averts HCMV replication by decreasing PGE2 levels[14]. Moreover, infiltrating gliomas in microglia, have been found to be an important source of PGE2, Cox-2 inhibitors and are an alternative, as opposed to glucocorticoids, in peritumoral edema of malignant gliomas[15]. In addition to its anti-inflammatory properties, celecoxib is able to exert a pro-apoptotic effect in vitro and in vivo in the absence of the action of Cox-2 in malignant glioma cells. In fact, it has developed a variant of this substance, 2,5-dimethyl-celecoxib, which is more potently cytotoxic against these cells[16]. The effect of celecoxib is dependent on the existence of p53[17].
One mechanism that could be used in the treatment of gliomas, is the induction of autophagy. Valproic acid is a potent histone deacetylase inhibitor which induces cell differentiation, growth arrest and apoptosis in gliomas and other cancers. Valproic acid induces autophagy in glioma cells, independently from apoptosis[18].
Chloroquine and quinacrine bind tightly to nucleic acids, in particular CG sequences, and reinforce its structural configuration and preventing mutagenesis[19]. Chloroquine also acts as an immunomodulator through the inhibition of phospholipase A2 and the tumor necrosis factor-α (TNF-α)[20]. Chloroquine improves survival in patients with GBM when added to conventional therapy[21,22].
Gliomas show a sequence of events that increase immunosuppressant cytokines, such as interleukin-10 (IL-10), transforming the growth factor-β (TGF-β), prostaglandin E2, inducing regulatory T cells (Treg), and decreasing costimulatory molecules, all results lose the function of effector T cells. Moreover, GBM cells show human leukocyte antigen (HLA) class I molecule mutation. Loss of HLA class I correlate with the grade of tumor and show little response to immunotherapy. NK cells do not have a histocompatibility complex (MHC) restriction. In patients with GBM, the NK cells are depressed, and it has been observed that when NK cells increase there is tumor regression in a recurrent glioma[23]. Glioblastoma stem cells suppress T cell responses in different ways: producing immunosuppressive cytokine that suppress T cell responses and inducing regulatory T cells, which act as a brake on the immune response and eliminate T cells through apoptosis. This is accomplished through the immunosuppressive protein B7-H1 from stem cells, or soluble galectin-3[24]. Gamma-delta T cells (γδ T-cells) are the primary effector cells in the immune response of a high grade glioma[25].
Some GBM subjects have responded similarly in autoimmune diseases, showing anergy to common bacterial antigens, lymphopenia, defective production of antibodies, and abnormal delayed hypersensitivity [26].
In order to modify the immune response in gliomas, the quantity of Tregs, a subclass of lymphocytes with immunosuppressive properties, is increased. It has been noted that indoleamine 2,3-dioxygenase (IDO), which converts tryptophan to kynurenine, increases the activity of Treg, and could be modified by aciclovir[27]. Also, in order to reduce Tregs and improved antitumoral immunity in other tumors, denileukin diftitox is used, which is a recombinant fusion protein of IL-2 and the diphtheria toxin targeting IL-2 receptors (CD25)[28].
Dendritic cells (DCs) have an antigen presentation function, their maturation is critical for the induction of the T cell response. Glioma cells suppress the maturation of DCs[29], Inmunoferon (AM3) promotes the maturation of DCs derived from human monocytes[30], and reduces the concentration of TNF-α and IL-6[31], IL-6 promoting the invasiveness of glioma cells via up-regulation of the STAT3 pathway and fascin-1[32].
Transfer factors are dialyzable products of low molecular weight extracted from the cells involved in the immune system. It has been reported that the transfer factor, in combination with carmustine in experimental malignant glioma, reduces the tumor and increases the CD2+ CD4+, CD8+, NK lymphocytes, and apoptotic tumor cells[33]. γδ T-cells recognize unprocessed nonpeptide compounds known as phospho-antigens and are involved through the mevalonate pathway or 1-deoxy-D-xylulose-5-phosphate, in activating the cytotoxic response and releasing cytokine and chemokines[34,35]. γδ T-cells activation can be induced in vivo by molecules such as zoledronic acid, which induce the accumulation of the T cell Vγ2. The zoledronic acid induces an effective antitumor response. Aminobisphosphonates play dual roles, apparently acting directly against GBM cells and enhancing antitumor activity from Vγ2 T-cells, which is present up to 75% in γδ T-cells[36]. Otherwise bisphosphonates, such as alendronate, increase γδ T-cell activation by interaction with monocytes circulating or macrophage associated tissues[37].
In glioblastomas there are many genomic alterations especially RTK amplification/mutation, NF1 mutation/loss, NFK1B loss, PIK3R1/PIK3CA mutation, PTEN mutation/loss, TP53 loss, CDK2N2A loss, CDKN2B loss, RB1 mutation/loss, and CDK4 amplification[38].
Heterogeneity in glioblastoma suggests that no therapy can be generalized in different types of GBM. In neural/classical type GBM there are mutations in the epidermal growth factor receptor (EGFR) gene. In proneural type GBM frequent mutations occur in p53, in platelet-derived growth factor receptor A, and in isocitrate dehydrogenase 1. Mesenchymal type GBM causes frequent mutation, observed in neurofibromatosis type 1 gene (NF-1)[39].
In neural/classical type GBM, Nimotuzumab could be used, it is a humanized antibody that recognizes EGFR[40] or panitumumab, originally approved for treating colorectal cancer, and it has been used with good results in glioma[41]. In proneural types which have IDH1 mutation, bevacizumab[42] could be used. Bevacizumab is a humanized monoclonal IgG1 antibody that selectively binds with great affinity to human vascular endothelial growth factor. This antibody is being used in phase III randomized trials in combination with temozolomide and radiotherapy, and has also been reported to be of benefit in phase II studies in recurrent glioblastoma [43]. In mesenchymal type NF-1, therapeutic targets use Ras antagonists and ERK antagonists. Also, mTOR dysregulation and PI3K/PKB/mTOR are central regulators of cell proliferation, growth, differentiation, and survival[44], they could logically be used with resveratrol or quercetin[45]. Treatment with low doses of resveratrol inhibits mono-ubiquitination of histone H2B at K120 in senescent glioma cells[46]. Resveratrol reduces TNF-α induced NF-κB, and reduces the effect of urokinase plasminogen activator[47]. Resveratrol acts over topoisomerase II on one of the enzymes found in highly proliferating cells[48]. Quercetin causes a rapid reduction in phosphorylation regulated to kinase (ERK) and Akt signaling. With quercetin the death of human glioma cells is brought about with a mechanism that involves caspase-dependent down-regulation of ERK, Akt, and survivin[49].
ROS are regulators of mitogen-activated protein kinase (MAPK), a family of serine/threonine kinases. An increase in intracellular ROS participates in autophagic execution[50]. The ketogenic diet reduces oxygen reactive species (ROS) in tumor cells, it also induces a total pattern of reversal in gene expression, compared with non-tumorous tissues[51].
Considering that there is a poor immune response to tumor associated antigens (TAAs) various strategies have been proposed to increase the immune response. Amongst them are new experimental options for treatment, for example, cytokine like IL-4, which facilitates an immune response against glioma[52] in a similar way to toll-like receptor (TLR) agonists. One example of this TLR agonist is Imiquimod, which could enhance T-cell responses to intracranial tumors, apart from reducing the number CD4(+)Foxp3(+) cells[53]. Costimulation of B7 molecule[54], blocks the B7-H1/PD-1 pathway with antagonistic antibodies to protect T cell responses[55].
Most immunotherapy attempts have had limited clinical success, with the exception of cellular immunotherapy using dendritic cell vaccines[56]. The multi-epitope-pulsed dendritic cell vaccine can be used for treatment. Dendritic cells are the most potent antigen-presenting cells for naive T cells, and can be obtained ex vivo from blood monocytes[57]. Monocytes are matured with the granulocyte-macrophage colony-stimulating factor (GM-CSF) and IL-4, or IL-6, prostaglandin E2 (PGE2), IL-1β and the TNF-α[58], to obtain denditric cells. Mature DC (mDC) induces antigen-specific T-cell responses when mDC is pulsed with tumor lysate, cancer stem cells, or peptides from TAAs, as reported by Phuphanich et al[59]. These pulsed dendritic cells increase the immune response against tumor cells[59]. Amongst the various TAAs used for pulsed cells are antigens from gliomas or cancer stem cells which are HER2/N, TRP-2, AIM-2 or peptides.
It is more effective if multiple epitopes are used to target and enhance cancer vaccines[60]. The peptides used in the autologous vaccine mDC, could be a combination of peptides, for example, six synthetic class I peptides AIM-2, MAGE1, TRP-2, gp100, HER2/neu, and IL-13Ra2. These were named ICT-107 and were selected from a glioma[58]. This combination of enhanced epitopes is clearly recognised by HLA class I-restricted T cells. This multi-epitope-pulsed dendritic cell vaccine can be administered intradermally at multiple sites.
In the treatment of patients with glioblastoma the use of many forms of therapeutic drugs could cause three main reactions, firstly increasing the brain edema which was a problem for the patient. Secondly, it is believed that brain tumor capillaries could limit the delivery of therapeutic drugs to the brain, and finally, the sum of many therapeutic drugs may easily lead an elderly patient into a delirious state.
There are many regulatory edema molecules in the brain. In the environment of the brain tumor, PGE2, aquaporins, aquaporin 1 (AQP1) and 4 (AQP4) exist. The glioma that infiltrate microglia are an important source of PGE2 and Cox-2, so Cox-2 inhibitors are proposed as an alternative to the use of glucocorticoids in peritumoral edema of malignant gliomas[15].
In short, in order to improve the quality of life in elderly patients with brain tumors, such as glioblastoma, many new treatment options should now be tested.
For recommendations and advice of Alfredo Guzmán Mayoral, Cuahtemoc Matadamas Zarate, Ángel Porras, Socorro Pina Canseco, Juan Molina, and Ruth Martínez. For the technical assistance Charlotte Grundy.
P- Reviewer: Arbab AS, Wang S S- Editor: Song XX L- Editor: A E- Editor: Lu YJ
| 1. | Idoate MA, Echeveste J. [Update on the molecular biology of gliomas: towards a pathomolecular classification of gliomas]. Rev Neurol. 2007;44:217-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | Chen J, Xu T. Recent therapeutic advances and insights of recurrent glioblastoma multiforme. Front Biosci (Landmark Ed). 2013;18:676-684. [PubMed] |
| 3. | Barker CA, Chang M, Chou JF, Zhang Z, Beal K, Gutin PH, Iwamoto FM. Radiotherapy and concomitant temozolomide may improve survival of elderly patients with glioblastoma. J Neurooncol. 2012;109:391-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Ellis TL, Neal MT, Chan MD. The role of surgery, radiosurgery and whole brain radiation therapy in the management of patients with metastatic brain tumors. Int J Surg Oncol. 2012;2012:952345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Iacob G, Dinca EB. Current data and strategy in glioblastoma multiforme. J Med Life. 2009;2:386-393. [PubMed] |
| 6. | Schiffer D. Radiotherapy by particle beams (hadrontherapy) of intracranial tumours: a survey on pathology. Neurol Sci. 2005;26:5-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Stewart LA. Chemotherapy in adult high-grade glioma: a systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet. 2002;359:1011-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 912] [Cited by in RCA: 852] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 8. | Sahin U, Koslowski M, Türeci O, Eberle T, Zwick C, Romeike B, Moringlane JR, Schwechheimer K, Feiden W, Pfreundschuh M. Expression of cancer testis genes in human brain tumors. Clin Cancer Res. 2000;6:3916-3922. [PubMed] |
| 9. | Konkankit VV, Kim W, Koya RC, Eskin A, Dam MA, Nelson S, Ribas A, Liau LM, Prins RM. Decitabine immunosensitizes human gliomas to NY-ESO-1 specific T lymphocyte targeting through the Fas/Fas ligand pathway. J Transl Med. 2011;9:192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Dziurzynski K, Chang SM, Heimberger AB, Kalejta RF, McGregor Dallas SR, Smit M, Soroceanu L, Cobbs CS. Consensus on the role of human cytomegalovirus in glioblastoma. Neuro Oncol. 2012;14:246-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 232] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 11. | Bhattacharjee B, Renzette N, Kowalik TF. Genetic analysis of cytomegalovirus in malignant gliomas. J Virol. 2012;86:6815-6824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 12. | Cobbs CS, Harkins L, Samanta M, Gillespie GY, Bharara S, King PH, Nabors LB, Cobbs CG, Britt WJ. Human cytomegalovirus infection and expression in human malignant glioma. Cancer Res. 2002;62:3347-3350. [PubMed] |
| 13. | Price RL, Song J, Bingmer K, Kim TH, Yi JY, Nowicki MO, Mo X, Hollon T, Murnan E, Alvarez-Breckenridge C. Cytomegalovirus contributes to glioblastoma in the context of tumor suppressor mutations. Cancer Res. 2013;73:3441-3450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 14. | Hawkins C, Croul S. Viruses and human brain tumors: cytomegalovirus enters the fray. J Clin Invest. 2011;121:3831-3833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Badie B, Schartner JM, Hagar AR, Prabakaran S, Peebles TR, Bartley B, Lapsiwala S, Resnick DK, Vorpahl J. Microglia cyclooxygenase-2 activity in experimental gliomas: possible role in cerebral edema formation. Clin Cancer Res. 2003;9:872-877. [PubMed] |
| 16. | Schönthal AH. Exploiting cyclooxygenase-(in)dependent properties of COX-2 inhibitors for malignant glioma therapy. Anticancer Agents Med Chem. 2010;10:450-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Kang KB, Zhu C, Yong SK, Gao Q, Wong MC. Enhanced sensitivity of celecoxib in human glioblastoma cells: Induction of DNA damage leading to p53-dependent G1 cell cycle arrest and autophagy. Mol Cancer. 2009;8:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 85] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 18. | Fu J, Shao CJ, Chen FR, Ng HK, Chen ZP. Autophagy induced by valproic acid is associated with oxidative stress in glioma cell lines. Neuro Oncol. 2010;12:328-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 132] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 19. | Bach MK. Reduction in the frequency of mutation to resistance to cytarabine in L1210 murine leukemic cells by treatment with quinacrine hydrochloride. Cancer Res. 1969;29:1881-1885. [PubMed] |
| 20. | Neale ML, Fiera RA, Matthews N. Involvement of phospholipase A2 activation in tumour cell killing by tumour necrosis factor. Immunology. 1988;64:81-85. [PubMed] |
| 21. | Sotelo J, Briceño E, López-González MA. Adding chloroquine to conventional treatment for glioblastoma multiforme: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2006;144:337-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 396] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 22. | Briceño E, Reyes S, Sotelo J. Therapy of glioblastoma multiforme improved by the antimutagenic chloroquine. Neurosurg Focus. 2003;14:e3. [PubMed] |
| 23. | Ishikawa E, Tsuboi K, Saijo K, Harada H, Takano S, Nose T, Ohno T. Autologous natural killer cell therapy for human recurrent malignant glioma. Anticancer Res. 2004;24:1861-1871. [PubMed] |
| 24. | Wei J, Barr J, Kong LY, Wang Y, Wu A, Sharma AK, Gumin J, Henry V, Colman H, Sawaya R. Glioma-associated cancer-initiating cells induce immunosuppression. Clin Cancer Res. 2010;16:461-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 182] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 25. | Oriol O, Tapia F. Celulas gamma-delta y su función en la respuesta inmunológica; Gamma-delta cell function in the immunological response. Arch Venez Farmacol Ter. 1990;9:88-99. |
| 26. | Nieto-Sampedro M, Valle-Argos B, Gómez-Nicola D, Fernández-Mayoralas A, Nieto-Díaz M. Inhibitors of Glioma Growth that Reveal the Tumour to the Immune System. Clin Med Insights Oncol. 2011;5:265-314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Söderlund J, Erhardt S, Kast RE. Acyclovir inhibition of IDO to decrease Tregs as a glioblastoma treatment adjunct. J Neuroinflammation. 2010;7:44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Higgins JP, Bernstein MB, Hodge JW. Enhancing immune responses to tumor-associated antigens. Cancer Biol Ther. 2009;8:1440-1449. [PubMed] |
| 29. | Kikuchi T, Abe T, Ohno T. Effects of glioma cells on maturation of dendritic cells. J Neurooncol. 2002;58:125-130. [PubMed] |
| 30. | Martín-Vilchez S, Molina-Jiménez F, Alonso-Lebrero JL, Sanz-Cameno P, Rodríguez-Muñoz Y, Benedicto I, Roda-Navarro P, Trapero M, Aragoneses-Fenoll L, González S. AM3, a natural glycoconjugate, induces the functional maturation of human dendritic cells. Br J Pharmacol. 2008;154:698-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | Brieva A, Guerrero A, Alonso-Lebrero JL, Pivel JP. Immunoferon, a glycoconjugate of natural origin, inhibits LPS-induced TNF-alpha production and inflammatory responses. Int Immunopharmacol. 2001;1:1979-1987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Cordova A, Monserrat J, Villa G, Reyes E, Soto MA. Effects of AM3 (Inmunoferon) on increased serum concentrations of interleukin-6 and tumour necrosis factor receptors I and II in cyclists. J Sports Sci. 2006;24:565-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 33. | Li R, Li G, Deng L, Liu Q, Dai J, Shen J, Zhang J. IL-6 augments the invasiveness of U87MG human glioblastoma multiforme cells via up-regulation of MMP-2 and fascin-1. Oncol Rep. 2010;23:1553-1559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 34. | Pineda B, Estrada-Parra S, Pedraza-Medina B, Rodriguez-Ropon A, Pérez R, Arrieta O. Interstitial transfer factor as adjuvant immunotherapy for experimental glioma. J Exp Clin Cancer Res. 2005;24:575-583. [PubMed] |
| 35. | Fiore F, Castella B, Nuschak B, Bertieri R, Mariani S, Bruno B, Pantaleoni F, Foglietta M, Boccadoro M, Massaia M. Enhanced ability of dendritic cells to stimulate innate and adaptive immunity on short-term incubation with zoledronic acid. Blood. 2007;110:921-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 85] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 36. | Lamb LS. Gammadelta T cells as immune effectors against high-grade gliomas. Immunol Res. 2009;45:85-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 37. | Cimini E, Piacentini P, Sacchi A, Gioia C, Leone S, Lauro GM, Martini F, Agrati C. Zoledronic acid enhances Vδ2 T-lymphocyte antitumor response to human glioma cell lines. Int J Immunopathol Pharmacol. 2011;24:139-148. [PubMed] |
| 38. | Gutman D, Epstein-Barash H, Tsuriel M, Golomb G. Alendronate liposomes for antitumor therapy: activation of γδ T cells and inhibition of tumor growth. Adv Exp Med Biol. 2012;733:165-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 39. | Dunn GP, Rinne ML, Wykosky J, Genovese G, Quayle SN, Dunn IF, Agarwalla PK, Chheda MG, Campos B, Wang A. Emerging insights into the molecular and cellular basis of glioblastoma. Genes Dev. 2012;26:756-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 40. | Bartek J, Ng K, Bartek J, Fischer W, Carter B, Chen CC. Key concepts in glioblastoma therapy. J Neurol Neurosurg Psychiatry. 2012;83:753-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 41. | Westphal M, Bach F. Phase III trial of Nimotuzumab for the treatment of newly diagnosed glioblastoma in addition to standard radiation and chemotherapy with temozolamide., ASCO 2010. Cited 2011-01-13. Available from: http: //www.cimab-sa.com/publicaciones/1354225532.PDF. |
| 42. | Berezowska S, Schlegel J. Targeting ErbB receptors in high-grade glioma. Curr Pharm Des. 2011;17:2468-2487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 43. | Lv S, Teugels E, Sadones J, Quartier E, Huylebrouck M, DU Four S, LE Mercier M, DE Witte O, Salmon I, Michotte A. Correlation between IDH1 gene mutation status and survival of patients treated for recurrent glioma. Anticancer Res. 2011;31:4457-4463. [PubMed] |
| 44. | Lino MM, Merlo A. PI3Kinase signaling in glioblastoma. J Neurooncol. 2011;103:417-427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 45. | Gipson TT, Johnston MV. Plasticity and mTOR: towards restoration of impaired synaptic plasticity in mTOR-related neurogenetic disorders. Neural Plast. 2012;2012:486402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 46. | Gao Z, Xu MS, Barnett TL, Xu CW. Resveratrol induces cellular senescence with attenuated mono-ubiquitination of histone H2B in glioma cells. Biochem Biophys Res Commun. 2011;407:271-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 47. | Ryu J, Ku BM, Lee YK, Jeong JY, Kang S, Choi J, Yang Y, Lee DH, Roh GS, Kim HJ. Resveratrol reduces TNF-α-induced U373MG human glioma cell invasion through regulating NF-κB activation and uPA/uPAR expression. Anticancer Res. 2011;31:4223-4230. [PubMed] |
| 48. | Leone S, Basso E, Polticelli F, Cozzi R. Resveratrol acts as a topoisomerase II poison in human glioma cells. Int J Cancer. 2012;131:E173-E178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 49. | Kim EJ, Choi CH, Park JY, Kang SK, Kim YK. Underlying mechanism of quercetin-induced cell death in human glioma cells. Neurochem Res. 2008;33:971-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 50. | Trejo-Solís C, Jimenez-Farfan D, Rodriguez-Enriquez S, Fernandez-Valverde F, Cruz-Salgado A, Ruiz-Azuara L, Sotelo J. Copper compound induces autophagy and apoptosis of glioma cells by reactive oxygen species and JNK activation. BMC Cancer. 2012;12:156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 103] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 51. | Scheck AC, Abdelwahab MG, Fenton KE, Stafford P. The ketogenic diet for the treatment of glioma: insights from genetic profiling. Epilepsy Res. 2012;100:327-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 52. | Faber C, Terao E, Morga E, Heuschling P. Interleukin-4 enhances the in vitro precursor cell recruitment for tumor-specific T lymphocytes in patients with glioblastoma. J Immunother. 2000;23:11-16. [PubMed] |
| 53. | Xiong Z, Ohlfest JR. Topical imiquimod has therapeutic and immunomodulatory effects against intracranial tumors. J Immunother. 2011;34:264-269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 54. | Anderson RC, Anderson DE, Elder JB, Brown MD, Mandigo CE, Parsa AT, Goodman RR, McKhann GM, Sisti MB, Bruce JN. Lack of B7 expression, not human leukocyte antigen expression, facilitates immune evasion by human malignant gliomas. Neurosurgery. 2007;60:1129-1136; discussion 1136. [PubMed] |
| 55. | Wang S, Chen L. Immunobiology of cancer therapies targeting CD137 and B7-H1/PD-1 cosignal pathways. Curr Top Microbiol Immunol. 2011;344:245-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 56. | Avril T, Vauleon E, Tanguy-Royer S, Mosser J, Quillien V. Mechanisms of immunomodulation in human glioblastoma. Immunotherapy. 2011;3:42-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 57. | Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109-1118. [PubMed] |
| 58. | Trepiakas R, Berntsen A, Hadrup SR, Bjørn J, Geertsen PF, Straten PT, Andersen MH, Pedersen AE, Soleimani A, Lorentzen T. Vaccination with autologous dendritic cells pulsed with multiple tumor antigens for treatment of patients with malignant melanoma: results from a phase I/II trial. Cytotherapy. 2010;12:721-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 59. | Phuphanich S, Wheeler CJ, Rudnick JD, Mazer M, Wang H, Nuño MA, Richardson JE, Fan X, Ji J, Chu RM. Phase I trial of a multi-epitope-pulsed dendritic cell vaccine for patients with newly diagnosed glioblastoma. Cancer Immunol Immunother. 2013;62:125-135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 255] [Cited by in RCA: 292] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 60. | Sakakura K, Chikamatsu K, Furuya N, Appella E, Whiteside TL, Deleo AB. Toward the development of multi-epitope p53 cancer vaccines: an in vitro assessment of CD8(+) T cell responses to HLA class I-restricted wild-type sequence p53 peptides. Clin Immunol. 2007;125:43-51. [PubMed] |